Abstract
Background
Adriamycin-associated cardiomyopathy (ACM) can lead to end-stage heart failure requiring advanced heart failure therapies.
Objectives
This study sought to provide post–cardiac transplant survival data in patients with ACM in the contemporary era of mechanical circulatory support and cardiac transplantation.
Methods
Adults (≥18 years of age) who underwent first-time, single-organ heart transplantation were identified from the United Network for Organ Sharing between October 18, 2008, and October 18, 2018. Cardiomyopathy subtypes that could have been supported with a left ventricular assist device (LVAD) including ACM, dilated cardiomyopathy (DCM), and ischemic cardiomyopathy (ICM) were included. A multivariable Cox regression analysis was performed to determine the association between cardiomyopathy subtype and post–cardiac transplant survival.
Results
This analysis included 18,270 patients (357 with ACM; 10,662 with DCM; and 7,251 with ICM). Heart transplant recipients with ACM were younger, included more women, and had higher pulmonary vascular resistance at the time of listing. Patients with ACM had a lower percentage of durable LVADs at the time of transplant across all years of the study period. Patients with ACM did not experience an increase in post–cardiac transplant mortality compared to those with DCM (adjusted hazard ratio: 0.96; 95% confidence interval: 0.79 to 1.40; p = 0.764) or ICM (adjusted hazard ratio: 0.85; 95% confidence interval: 0.6 to 1.2; p = 0.304).
Conclusions
Patients with ACM who received heart transplants between 2008 and 2018 had similar post–cardiac transplant survival to those with dilated and ischemic cardiomyopathy. Bridge-to-transplant LVAD use remains lower compared to other cardiomyopathy subtypes.
Key Words: adriamycin cardiomyopathy, cardiac transplantation, left ventricular assist device
Abbreviations and Acronyms: ACM, adriamycin-associated cardiomyopathy; CI, confidence interval; DCM, dilated cardiomyopathy; HR, hazard ratio; ICM, ischemic cardiomyopathy; IQR, interquartile range; LVAD, left ventricular assist device; OR, odds ratio; UNOS, United Network for Organ Sharing
Central Illustration
Adriamycin-associated cardiomyopathy (ACM) is a significant cause of morbidity and mortality in cancer survivors and can lead to end-stage heart failure (1). Because many of these patients are young and otherwise healthy, cardiac transplantation can be considered after a period of cancer-free survival. The last published analyses that assessed outcomes of patients with ACM who underwent cardiac transplantation were from 2012 and 2013, and they demonstrated reassuringly similar post-transplant survival compared to other cardiomyopathy subgroups (2,3). However, a subsequent Interagency Registry of Mechanically Assisted Circulatory Support (INTERMACS) study from 2014 demonstrated higher rates of right ventricular failure after left ventricular assist device (LVAD) implantation in the patients with ACM (4). It is possible these data influenced the subsequent bridge-to transplant LVAD utilization in patients with ACM.
Since these publications, the numbers of cancer survivors have increased (5), more patients with ACM have undergone cardiac transplantation in the United States, and the field of mechanical support has evolved. Using the United Network for Organ Sharing (UNOS) registry, we investigated the association between ACM and survival after cardiac transplantation in the contemporary era of continuous flow LVADs. We describe the percentage of patients with ACM who were supported on LVAD at the time of transplant and how this has changed over the last 10 years. We also examined the differences in the primary causes of death after heart transplantation in those with ACM as compared with dilated cardiomyopathy (DCM) and ischemic cardiomyopathy (ICM). We hypothesized that patients with ACM would have similar post–cardiac transplant survival compared to the other cardiomyopathy subtypes. In addition, we hypothesized that bridge-to-transplant LVAD use in the patients with ACM would be less than other cardiomyopathy subtypes during the study period.
Methods
Cohort
The UNOS registry contains no patient identifiers and is publicly available. For this reason, our UNOS work has been granted an exception by the University of Minnesota Institutional Review Board. To find the era within UNOS that included only contemporary, continuous flow LVAD devices, the device type file was sorted to determine the timepoint in which older-generation (noncontinuous flow) devices were no longer identified in the database (2010). The study period was then limited to a 10-year span between October 18, 2008, and October 18, 2018, when UNOS listing criteria changed. The analysis was limited to adults who were ≥18 years of age at listing and undergoing a first-time cardiac, single-organ transplantation. Patients with physiology that could be supported with an LVAD, including DCM, ICM, and the study group (ACM), were included in the analysis. We excluded UNOS patients with a previous heart transplant, congenital heart disease, restrictive cardiomyopathy, hypertrophic cardiomyopathy, or valvular heart disease. UNOS patients with biventricular assist devices, right ventricular assist devices, or total artificial hearts at the time of cardiac transplant were also excluded.
Primary exposure and outcome
The primary outcome was time to all-cause mortality after cardiac transplantation. Time was modeled from the date of cardiac transplantation to the date of last follow-up or death. All patients with follow-up times after transplant of >5 years were censored at 5 years. The date of last follow-up for the study population was June 6, 2019. Patients were divided into groups based on primary cardiomyopathy subtype (ACM, ICM, or DCM), which was considered the primary exposure variable. Patients were classified as having ACM if the thoracic diagnosis variable was coded as “dilated myopathy: Adriamycin.”
Statistical analysis
Normally distributed continuous data are presented as mean and SD. Non-normally distributed data are presented as median (interquartile range [IQR]). Categorical variables are presented as number and percentage. Baseline characteristics among patient groups (ACM, DCM, and ICM) were compared with 1-way analysis of variance for normally distributed variables or the Kruskal-Wallis test for non-normally distributed variables. Chi-square tests were used to compare categorical variables. To assess the unadjusted association between Adriamycin cardiomyopathy and post-transplant mortality, a Kaplan-Meier analysis was performed. Survival between the cardiomyopathy subtypes was compared with log-rank tests (ACM vs. ICM, ACM vs. DCM) with Bonferroni correction. Because the ACM and DCM survival curves crossed at 2.5 years, log-rank tests were repeated before and after the 2.5-year mark for this comparison. Survival probabilities at 30 days, 1 year, 3 years, and 5 years after transplant by cardiomyopathy subtype were calculated, accounting for censoring at the last follow-up visit.
To determine the adjusted association between ACM and post-transplant mortality, a multivariable Cox regression analysis was performed. The following pre-specified potential confounders of the relationship between ACM and post-transplant mortality were included in the model: recipient age, sex, race, initial UNOS status, pulmonary vascular resistance, female donor to male recipient, ischemic time, prior cardiothoracic surgery, and diabetes. The proportional hazards assumption was validated by visually inspecting scaled Schoenfeld residuals for each variable. Linearity assumptions were validated by visually inspecting plots of continuous covariates against martingale residuals of the null Cox proportional hazards model. The analysis was then repeated, limited to only patients who did not have an LVAD at the time of transplant. Lastly, a sensitivity analysis was performed excluding patients in the ICM and DCM subgroups with a history of cancer. The purpose of these additional analyses was to ensure the robustness of the results excluding LVAD patients and to prevent confounding by cancer in comparing the ACM group to others.
The percentage of patients with each cardiomyopathy type who were bridged with LVADs at the time of transplant was graphically displayed by year for visual comparison. To evaluate the time trend and association with cardiomyopathy type, a logistic regression model adjusted for time, cardiomyopathy type, and the interaction between time and cardiomyopathy type was performed. Causes of death at the 30-day, 1-year, 3-year, and 5-year timepoints by cardiomyopathy type were also plotted for visual comparison. All comparisons were 2-sided, and a p value of <0.05 was considered to be statistically significant. Analyses were performed using R, version 4.0.2 (R Core Team, Vienna, Austria).
Results
Baseline characteristics
The study cohort included 18,270 patients who underwent cardiac transplantation between October 18, 2008, and October 18, 2018 (ACM: 357; DCM: 10,662; ICM: 7,251). The median follow-up for the groups was as follows: ACM: 1,126 days (IQR: 424 to 2,190 days), DCM: 1,100 days (IQR: 387 to 2,153 days), and ICM: 1,283 days (IQR: 401 to 2,201 days). Baseline characteristics by cardiomyopathy subtype are presented in Table 1. As compared to the ICM group, patients with ACM were younger, more likely to be female, and less likely to be diabetic and had lower body mass indexes. They were less likely to have an LVAD at listing and at the time of transplant, although they had a similar degree of temporary support at the time of transplant. Patients with ACM had higher pulmonary vascular resistance and slightly longer ischemic times, and they were less likely to have a male recipient with a female donor. The percentage of patients in each cardiomyopathy subtype group receiving induction immunosuppression was similar.
Table 1.
Baseline Characteristics of Patients Receiving Transplants Between 2008 and 2018 by Cardiomyopathy Subtype
| ACM (n = 357) | DCM (n = 10,662) | ICM (n = 7,251) | p Value | |
|---|---|---|---|---|
| Age at listing, yrs | 51 ± 13 | 50 ± 13 | 59 ± 8 | <0.001 |
| Male | 84 (24) | 7,425 (70) | 6,294 (87) | <0.001 |
| White | 242 (68) | 6,104 (57) | 5,555 (77) | <0.001 |
| Diabetes | 70 (20) | 2,276 (21) | 2,537 (35) | <0.001 |
| Creatinine, mg/dl | 1.16 ± 0.59 | 1.23 ± 0.61 | 1.29 ± 0.53 | <0.001 |
| Body mass index, kg/m2 | 25.3 ± 4.6 | 27.4 ± 5.0 | 27.7 ± 4.5 | <0.001 |
| PVR, WU | 3.0 ± 1.9 | 2.56 ± 1.86 | 2.42 ± 1.69 | <0.001 |
| Ischemic time, h | 3.2 ± 1.0 | 3.09 ± 1.03 | 3.16 ± 1.05 | <0.001 |
| Female donor to male recipient | 23 (6) | 1,302 (12) | 1,270 (18) | <0.001 |
| LVAD at transplant | 124 (35) | 5,014 (47) | 3,332 (46) | <0.001 |
| LVAD at listing | 85 (24) | 3,076 (29) | 2,274 (31) | <0.001 |
| Induction immunosuppression | 175 (49.0) | 5276 (49.5) | 3490 (48.1) | 0.206 |
| Temporary mechanical support | 38 (11) | 1,050 (10) | 699 (10) | 0.771 |
| Total bilirubin, mg/dl | 0.7 (0.5–1.1) | 0.7 (0.5–1.1) | 0.7 (0.5–1.0) | <0.001∗ |
Values are mean ± SD, n (%), or median (interquartile range). Categorical variables were compared with chi-square tests. Continuous variables were compared with 1-way analysis of variance for normally distributed variables or the Kruskal-Wallis test for non-normally distributed variables.
ACM = adriamycin-associated cardiomyopathy; DCM = dilated cardiomyopathy; ICM = ischemic cardiomyopathy; LVAD = left ventricular assist device; PVR = pulmonary vascular resistance.
Kruskal-Wallis test.
Cancer history
In the ACM group, breast cancer and hematologic malignancies were the most common, accounting for 44% and 25% of all malignancies, respectively. Nineteen percent were coded as having “other cancers”; 1% had skin, genitourinary, and liver cancer; and in 11%, the cancer type was unknown. Cancer history was noted in 6.7% of patients in the DCM group and 6.9% of the ICM group.
Utilization of LVADs and heart transplantation
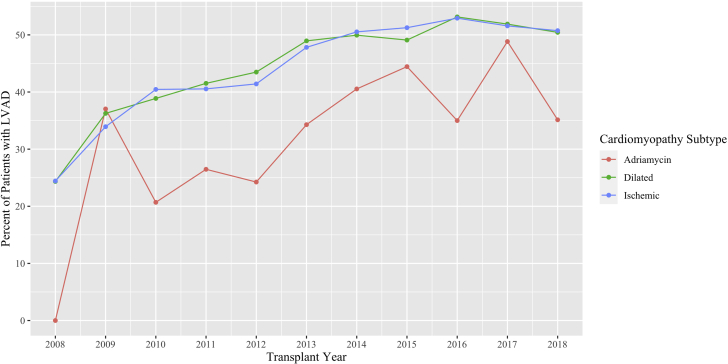
In 2008, 24% of patients with DCM or ICM had an LVAD at the time of transplant. By 2018, 50% of patients with ICM or DCM had an LVAD at the time of transplant. The odds of LVAD at the time of transplant increased significantly over the study period (odds ratio [OR]: 1.11; 95% confidence interval [CI]: 1.02 to 1.20; p = 0.0112) (Figure 1). Furthermore, there were significant differences in the odds of having an LVAD at time of transplant between the ACM and DCM groups (OR: 1.91; 95% CI: 1.16 to 3.20; p = 0.012) and between the ACM and ICM groups (OR: 1.80; 95% CI: 1.09 to 3.03; p = 0.023). The rate of increase in LVAD use, however, was not different across cardiomyopathy subtypes, and there was no interaction between time and cardiomyopathy subtype on the odds of LVAD use.
Figure 1.
Percentage of Patients Supported With an LVAD at the Time of Cardiac Transplantation
The use of left ventricular assist devices (LVADs) in patients with adriamycin-associated cardiomyopathy was less compared to the dilated and ischemic cardiomyopathy groups, despite an increase in the utilization of bridge-to-transplant LVADs in all groups.
Survival
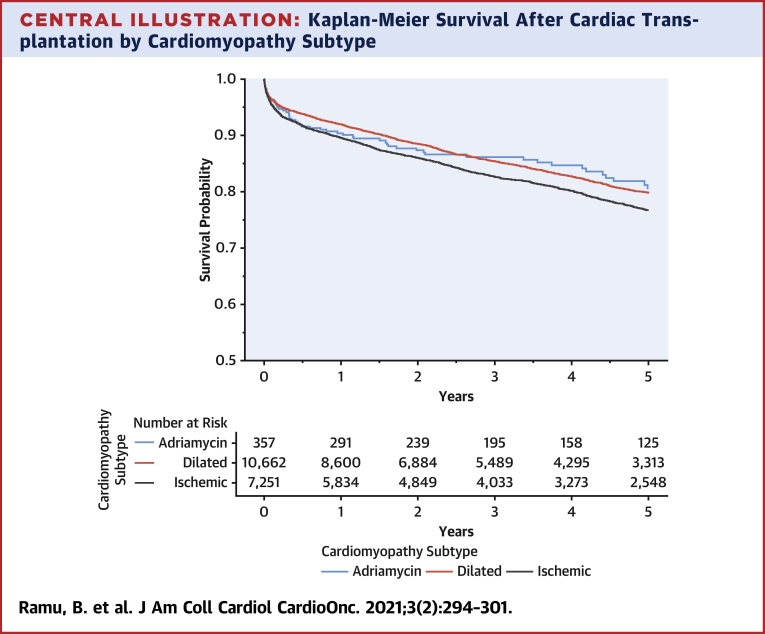
Short-term and long-term survival post–heart transplant were similar in the ACM, DCM, and ICM groups (Central Illustration), without statistically significant differences (ACM vs. DCM: p = 1.00; ACM vs. ICM: p = 0.470 by Bonferroni-corrected log-rank tests). The comparisons between ACM and DCM did not differ before or after the 2.5-year time period. In the multivariable analysis, patients with ACM did not experience an increase in post–cardiac transplant mortality compared to those with DCM (adjusted hazard ratio [HR]: 0.96; 95% CI: 0.79 to 1.40; p = 0.764) or ICM (adjusted HR: 0.85; 95% CI: 0.60 to 1.20; p = 0.304). HRs for all multivariable covariates are displayed in Table 2. When limiting the multivariable analysis to patients who did not have an LVAD at the time of transplant, ACM was not associated with increased post-transplant mortality (ACM vs. DCM: adjusted HR: 1.08; 95% CI: 0.80 to 1.50; p = 0.651; ACM vs. ICM: adjusted HR: 0.97; 95% CI: 0.70 to 1.40; p = 0.884). In sensitivity analysis excluding patients who had prior cancer history in the DCM (6.7%) and ICM groups (6.9%), ACM was not associated with statistically increased post-transplant mortality (ACM vs. DCM: adjusted HR: 1.04; 95% CI: 0.78 to 1.40; p = 0.775; ACM vs. ICM: adjusted HR: 1.15; 95% CI: 0.85 to 1.55; p = 0.369).
Central Illustration.
Kaplan-Meier Survival After Cardiac Transplantation by Cardiomyopathy Subtype
Short-term and long-term survival post–heart transplant were similar in the adriamycin-associated cardiomyopathy, dilated cardiomyopathy, and ischemic cardiomyopathy groups. Log-rank p values: adriamycin-associated cardiomyopathy vs. dilated cardiomyopathy: p = 1.00; Adriamycin-associated cardiomyopathy vs. ischemic cardiomyopathy: p = 0.470.
Table 2.
Multivariable Associations Between Cardiomyopathy Subtype and Post–Cardiac Transplant Survival
| Hazard Ratio | 95% CI |
|||
|---|---|---|---|---|
| Lower | Upper | p Value | ||
| ACM | Reference | |||
| DCM | 1.05 | 0.78 | 1.40 | 0.764 |
| ICM | 1.17 | 0.87 | 1.58 | 0.304 |
| Recipient age, per year | 1.00 | 1.00 | 1.00 | 0.740 |
| Male | 0.92 | 0.84 | 1.01 | 0.092 |
| White race | 0.83 | 0.76 | 0.90 | <0.001 |
| Female to male donor | 1.14 | 1.02 | 1.27 | 0.017 |
| Status 1A | Reference | |||
| Status 1B | 0.98 | 0.89 | 1.08 | 0.694 |
| Status 2 | 1.04 | 0.93 | 1.16 | 0.484 |
| Prior cardiothoracic surgery | 1.28 | 1.18 | 1.39 | <0.001 |
| Ischemic time per hour | 1.11 | 1.07 | 1.15 | <0.001 |
| Recipient diabetes | 1.18 | 1.09 | 1.29 | <0.001 |
| Pulmonary vascular resistance per 1 WU | 1.02 | 1.00 | 1.04 | 0.074 |
| LVAD at transplant | 1.16 | 1.07 | 1.26 | <0.001 |
Hazard ratios are per 1-U change for continuous variables.
CI = confidence interval; other abbreviations as in Table 1.
Cause of death
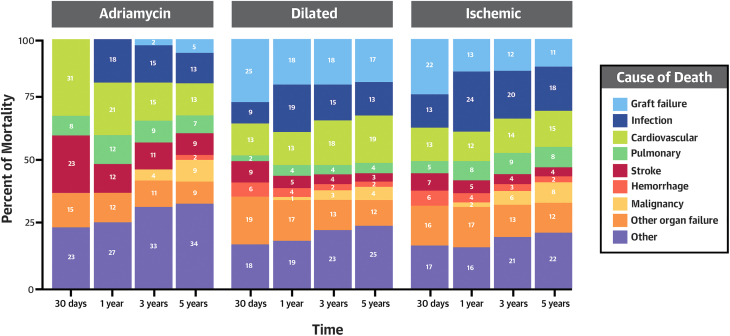
The percentage of deaths by cause at each timepoint (30 days, 1 year, 3 years, and 5 years) within each cardiomyopathy subgroup is visually displayed in Figure 2. Of the deaths that occurred by 30 days, the ACM group had a higher percentage that were cardiovascular (ACM: 30.8%; DCM: 12.7%; ICM: 13.4%) and stroke related (ACM: 23.1%; DCM: 8.7%; ICM: 7.0%). At the 1-year, 3-year, and 5-year timepoints, death from graft failure was higher in the DCM and ICM groups. For example, at year 3, the cause of death was graft failure for 18% of DCM, 12% of ICM, and 2.2% of ACM recipients.
Figure 2.
Causes of Death After Cardiac Transplant at Each Timepoint After Transplant by Cardiomyopathy Subtype
The causes of death in the adriamycin-associated cardiomyopathy, dilated cardiomyopathy, and ischemic cardiomyopathy groups are displayed.
Discussion
Here, we present an analysis of outcomes of patients with ACM who underwent heart transplant in the contemporary era of heart transplant and mechanical circulatory support. Patients with ACM were younger, predominantly women, and had higher pulmonary vascular resistance than those in the DCM and ICM groups. Patients with ACM were less likely to have an LVAD at the time of cardiac transplant, despite a general increase in bridging LVADs in the 10-year period among all groups. The survival of patients with ACM after transplant was similar to that of those with DCM and ICM (HRs very close to 1.00) and with p values that did not reach statistical significance in both the unadjusted and adjusted analyses. Our results show that the overall outcomes of patients with ACM who receive heart transplant were similar to the other groups.
These findings are consistent with the previous International Society of Heart and Lung Transplantation registry report by Oliveira et al. (2) published in 2012. This analysis spanned from 2000 to 2008, included 232 patients with chemotherapy induced cardiomyopathy, and found no statically significant increase in post-transplant mortality in the ACM group (2). Post-transplant malignancy was slightly higher in the ACM group; however, this was driven by skin cancers and not recurrence of the primary malignancy. Post-transplant malignancy deaths at 5 years were similar among patient groups in our analysis. Our report is also similar to the UNOS analysis of 453 patients with ACM who received cardiac transplants between 1987 and 2011 published by Lenneman et al. (3), where no difference in post-transplant survival was found after up to 10 years of follow-up.
In our analysis, patients with ACM were less often treated with a bridge to transplantation with LVAD, despite an overall increase in LVAD use during the study period. This may be related to the INTERMACS registry study, published in 2014, showing that patients with ACM who underwent destination therapy LVADs were more likely to need right ventricular assist devices (19% vs. 11% vs. 6%, respectively; p = 0.006) as compared to patients with other causes of DCM or ICM (4).
Our study adds to previous published reports by demonstrating the safety of heart transplantation in patients with ACM in the contemporary era of advanced heart failure therapies. In addition, we demonstrate a lower use of bridge-to-transplant LVADs despite the general increase in LVAD use during that period. Not surprisingly, patients in the ACM group had higher pulmonary vascular resistance at listing, which may be related to less LVAD use in this group. We postulate that this may also explain the signal of higher cardiovascular deaths at 30 days observed in the ACM group.
Continued study of the ACM population who require advanced therapies will be important (6). Despite enhanced knowledge of the mechanism of Adriamycin-associated cardiac toxicity and enhanced tools and protocols for early detection, the number of survivors who experience cardiac toxicity is increasing (7). In some datasets, patients with ACM have been shown to have worse outcomes compared to those with other causes of cardiomyopathy (8) and, thus, may be more likely to need consideration for advanced heart failure therapies (9). In a more contemporary analysis, similar outcomes were observed between patients with ACM compared to a matched DCM population (10). It may be that earlier recognition or contemporary guideline-directed medical therapy explains some of this improvement in outcomes. Some of the questions that remain include the following: Do patients with ACM exhibit more right ventricular failure after LVAD with the most contemporary continuous flow pump? Is there a certain LV cavity size that predicts right ventricular failure after LVAD? Should post-transplant immunosuppression be altered in patients with ACM? Because detailed information about patients’ cancers cannot be obtained from UNOS and because cancer diagnoses and treatment plans are increasingly tailored, datasets that capture this level of granularity are needed to inform decision making around the safety of proceeding with transplantation and immunosuppression.
Study limitations
Details about patients’ prior cancer history are not obtainable from UNOS. For example, time from initial cancer diagnosis and history of radiation therapy do not exist in UNOS, but these are important additions to the dataset for future analyses. The ACM group may have higher rates of late cancer that we did not detect, given the 5-year follow-up in this analysis. It is likely that the patients with ACM were in a durable remission before cardiac transplantation, and our findings may not be generalizable to patients who are more recently treated and who develop this complication. Lastly, given the high number of competing causes of death after transplant as well as the relatively small number of patients in the ACM group, we were not able to perform a competing risk analysis to specifically assess cancer-specific death as a separate outcome.
Conclusions
Patients with ACM who received heart transplantation between 2008 and 2018 had similar post-transplant survival to those with DCM and ICM. Bridge-to-transplant LVAD use remains lower than for other cardiomyopathy subtypes.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: The present study demonstrated that heart transplantation, in the contemporary era of advanced heart failure therapies, is safe in patients with end-stage heart failure due to ACM. Survival was similar compared to those with DCM and ICM. Patients with ACM received fewer bridge-to-transplant LVADs despite an overall increase in LVAD use during the study period.
TRANSLATIONAL OUTLOOK: Further studies are needed to evaluate the impact of conventional and newer cancer therapies on outcomes of patients with end-stage heart failure. In addition, understanding the risk of right ventricular failure with newer-generation LVADs in this patient population is important.
Funding Support And Author Disclosures
Dr. Tedford is a consultant for Medtronic, Aria CV Inc., Acceleron, Arena Pharmaceuticals, and United Therapeutics; has served on a steering committee for Medtronic and Abbott; has served on a research advisory board for Abiomed; and has performed hemodynamic core lab work for Actelion and Merck, unrelated to this paper. Dr. Cogswell has served on the Medtronic Heart Failure advisory board; and on the Speakers Bureau for Abbott Lab, unrelated to this manuscript; and her husband works for Medtronic. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank the administrators at UNOS for their assistance in preparing the Standard Transplant Analysis and Research files.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Oliveira G.H., Qattan M.Y., Al-Kindi S., Park S.J. Advanced heart failure therapies for patients with chemotherapy-induced cardiomyopathy. Circ Heart Fail. 2014;7:1050–1058. doi: 10.1161/CIRCHEARTFAILURE.114.001292. [DOI] [PubMed] [Google Scholar]
- 2.Oliveira G.H., Hardaway B.W., Kucheryavaya A.Y., Stehlik J., Edwards L.B., Taylor D.O. Characteristics and survival of patients with chemotherapy-induced cardiomyopathy undergoing heart transplantation. J Heart Lung Transplant. 2012;31:805–810. doi: 10.1016/j.healun.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Lenneman A.J., Wang L., Wigger M. Heart transplant survival outcomes for Adriamycin-dilated cardiomyopathy. Am J Cardiol. 2013;111:609–612. doi: 10.1016/j.amjcard.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliveira G.H., Dupont M., Naftel D. Increased need for right ventricular support in patients with chemotherapy-induced cardiomyopathy undergoing mechanical circulatory support: outcomes from the INTERMACS registry (Interagency Registry for Mechanically Assisted Circulatory Support) J Am Coll Cardiol. 2014;63:240–248. doi: 10.1016/j.jacc.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 5.Armenian S.H., Lacchetti C., Barac A. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 6.Hamo C.E., Bloom M.W., Cardinale D. Cancer therapy-related cardiac dysfunction and heart failure: part 2: prevention, treatment, guidelines, and future directions. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plana J.C., Galderisi M., Barac A. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Felker G.M., Thompson R.E., Hare J.M. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 9.Mukku R.B., Fonarow G.C., Watson K.E. Heart failure therapies for end-stage chemotherapy-induced cardiomyopathy. J Card Fail. 2016;22:439–448. doi: 10.1016/j.cardfail.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Fornaro A., Olivotto I., Rigacci L. Comparison of long-term outcome in anthracycline-related versus idiopathic dilated cardiomyopathy: a single centre experience. Eur J Heart Fail. 2018;20:898–906. doi: 10.1002/ejhf.1049. [DOI] [PubMed] [Google Scholar]