Graphical abstract
Key Words: cancer, cardio-oncology, cardiovascular, clinical trials, oncology
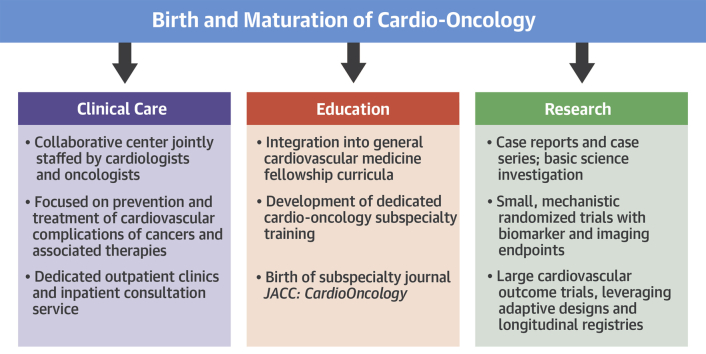
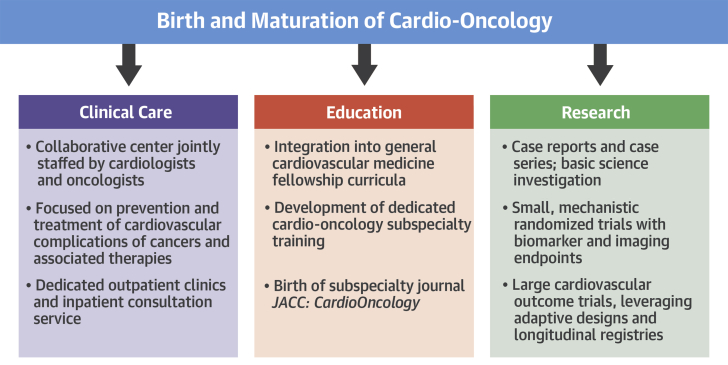
Few areas of medicine have seen as rapid growth as we have witnessed in cardio-oncology. Every major medical center is trying to start a program and is recruiting dedicated faculty. Patient demand for these specialized services is increasing dramatically. Approximately one-half of cardiovascular trainees now receive cardio-oncology exposure as part of their core curriculum, and subspecialty fellowships are being launched 1, 2. Research productivity in cardio-oncology is in an exponential phase. Reflecting this momentous progress is the birth of JACC: CardioOncology—an exciting development (Figure 1).
Figure 1.
The Birth and Maturation of Cardio-Oncology
Advances in clinical care delivery, education, and research in cardio-oncology are all in an exponential phase of growth.
The collaborative nature of the field, with cardiologists and oncologists working side by side, has helped propel the understanding of areas of overlap. Case reports and case series of cardiovascular complications of cancer therapies have shined a light on an area that was previously underappreciated. Small randomized trials have been conducted that help advance our understanding of how to provide cardioprotection to cancer patients receiving various treatments (3). Basic investigation is paving the way for future diagnostics and therapeutics. A solid scientific foundation is being laid for the field of cardio-oncology.
An example of the rapid flux of information in cardio-oncology has been with immune checkpoint inhibitors (4). These drugs have been a major advance in oncology, although it is now well recognized that potentially fatal cardiac complications such as myocarditis can occur. In addition, other cardiovascular issues may arise, including Takotsubo syndrome, arrhythmias, and vasculitis. Thus, a broad understanding of cardiovascular medicine, importantly, including the vascular part, is quite essential for the future cardio-oncologist to identify and treat these patients. There is also a pressing need for clinical trials to explore the prevention and management of cardiovascular toxicities from cancer drugs.
From a public health perspective, there are several shared risk factors for cancer and cardiovascular disease, such as eating processed meats, smoking, obesity, and pollution. New threats emerge, such as the scourge of vaping, which appears to be gaining popularity among our youth at an alarming rate. Although these factors can be influenced on an individual level, they also need to be countered with a population health approach. There also appear to be shared pathways toward cardiovascular disease and cancer, perhaps not surprising given overlapping risk factors. The role of inflammation in both diseases has long been appreciated, and several scientists and companies are currently developing drugs that target specific pathways involved with the inflammatory response. Again, clinical trials at the individual level and possibly the population level would inform our care recommendations for primordial, primary, secondary, and tertiary cardiovascular and oncologic prevention.
As well, novel cardiovascular risk factors appear to be emerging from the oncology world. Clonal hematopoiesis of indeterminate potential is an intriguing entity that appears to be common and may provide 1 of the links between inflammation and cardiovascular disease 5, 6. A challenge is what to do now with the increasing numbers of patients identified as having clonal hematopoiesis of indeterminate potential, which is clearly an area in need of thoughtful collaboration among cardiologists, oncologists, and basic scientists, as well as clinical trialists.
In this issue of JACC: CardioOncology, a distinguished group of cardio-oncologists has put forth a blueprint for designing cardio-oncology studies funded by the National Institutes of Health to address the cardiovascular toxicities of contemporary cancer treatment (7). They outline a transformative vision to enhance the rigor of one critical aspect of cardio-oncology research. Indeed, the next step in the maturation of cardio-oncology will be the conduct of large, randomized, multicenter studies—the same approach that has catapulted our evidence-based knowledge in other aspects of cardiology over the past 2 decades.
However, trials in this space will require creativity. Adaptive designs, currently more frequent in oncology than cardiology, would be one way of making clinical trials more efficient (8). Establishment of longitudinal registries of patients being treated for various cancers and then using them as platforms for randomized clinical trials is another approach that has gained some traction in other areas and would appear to be worthwhile in cardio-oncology 9, 10.
The paradigm of cross-disciplinary collaboration for the execution of trials already has a precedent in cardiology with the large cardiovascular outcome trials that have been performed in patients with diabetes mellitus. These trials, initially mandated by the US Food and Drug Administration to ensure cardiovascular safety of diabetes drugs, have ushered in a new era of therapeutics that significantly reduce important cardiovascular events, even including cardiovascular mortality. Beyond the contributions to research and novel therapeutics, these collaborations have improved dialogue between cardiologists and endocrinologists involved in these trials, with positive spillover effects into daily patient care. Cardio-oncology would do well to borrow this paradigm of research and clinical collaboration, and every indication is that from its genesis, the field of cardio-oncology has already done so.
There may even be value in setting regulatory obligations that all new oncology drugs have a similarly rigorous evaluation of cardiovascular complications performed after approval, as the diabetes drugs now have. Indeed, industry must play a pivotal role in facilitating cardiovascular toxicity studies at an even earlier stage, in phase I to III oncology clinical trials (i.e., preapproval and preclinical). Although these steps will raise the ultimate costs of drug development and because patients treated for cancer are living longer, it will be necessary to generate these data, and the burden cannot fall entirely on the National Institutes of Health alone. Because event rates will be low, large numbers of patients with extended duration of follow-up will be necessary for any postapproval studies. Registry-based trials may be particularly well-suited to perform these evaluations efficiently and cost effectively. As the time horizon for follow-up may exceed the period of interest of an industry sponsor, these types of evaluations may be best coordinated by governmental entities such as the National Institutes of Health and the Food and Drug Administration, collaborating with professional societies including the American College of Cardiology, the American Heart Association, and oncology societies as well.
A related issue is to ensure inclusion of sufficient numbers of patients with a history of cancer in cardiovascular trials because currently many protocols exclude patients with a history of cancer, even if not active. This creates a real data void for such patients. Moving forward, cardiovascular trials should make dedicated efforts to include this neglected but growing demographic of cancer survivors.
Parallel advances in biomarkers, imaging, and genetics should allow greater personalization of cancer therapies and in lockstep, pharmacological approaches to try and prevent adverse short- and long-term cardiovascular consequences. As the field of cardio-oncology matures, the demand for greater degrees of evidence, including randomized clinical trials, will predictably increase as well. With the launch of JACC: CardioOncology, that evidence will now have a home.
Footnotes
Dr. Bhatt is on the advisory board for Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, and Regado Biosciences; is on the Board of Directors for Boston VA Research Institute, Society of Cardiovascular Patient Care, and TobeSoft; is chair for the American Heart Association Quality Oversight Committee; is on the Data Monitoring Committee for Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute, NCDR-ACTION Registry Steering Committee, and the VA CART Research and Publications Committee; has received honoraria from the American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor-in-Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), and Clinical Cardiology (Deputy Editor); has received research funding from Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; has received royalties from Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); is the site co-investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), and Svelte; is a trustee of the American College of Cardiology; and has unfunded research for FlowCo, Fractyl, Merck, Novo Nordisk, PLx Pharma, and Takeda.
References
- 1.Hayek S.S., Ganatra S., Lenneman C. Preparing the cardiovascular workforce to care for oncology patients: JACC review topic of the week. J Am Coll Cardiol. 2019;73:2226–2235. doi: 10.1016/j.jacc.2019.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campia U., Moslehi J.J., Amiri-Kordestani L. Cardio-oncology: vascular and metabolic perspectives: a scientific statement from the American Heart Association. Circulation. 2019;139:e579–e602. doi: 10.1161/CIR.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kheiri B., Abdalla A., Osman M. Meta-analysis of carvedilol for the prevention of anthracycline-induced cardiotoxicity. Am J Cardiol. 2018;122:1959–1964. doi: 10.1016/j.amjcard.2018.08.039. [DOI] [PubMed] [Google Scholar]
- 4.Ball S., Ghosh R.K., Wongsaengsak S. Cardiovascular toxicities of immune checkpoint inhibitors. J Am Coll Cardiol. 2019;74:1714–1727. doi: 10.1016/j.jacc.2019.07.079. [DOI] [PubMed] [Google Scholar]
- 5.Libby P., Sidlow R., Lin A.E. Clonal hematopoiesis: crossroads of aging, cardiovascular disease, and cancer: JACC review topic of the week. J Am Coll Cardiol. 2019;74:567–577. doi: 10.1016/j.jacc.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khetarpal S.A., Qamar A., Bick A.G. Clonal hematopoiesis of indeterminate potential reshapes age-related CVD: JACC review topic of the week. J Am Coll Cardiol. 2019;74:578–586. doi: 10.1016/j.jacc.2019.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minasian L., Dimond E., Davis M. The evolving design of NIH-funded cardio-oncology studies to address cancer treatment-related cardiovascular toxicity. J Am Coll Cardiol CardioOnc. 2019;1:105–113. doi: 10.1016/j.jaccao.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatt D.L., Mehta C. Adaptive designs for clinical trials. N Engl J Med. 2016;375:65–74. doi: 10.1056/NEJMra1510061. [DOI] [PubMed] [Google Scholar]
- 9.Bhatt D.L. Advancing the care of cardiac patients using registry data: going where randomized clinical trials dare not. JAMA. 2010;303:2188–2189. doi: 10.1001/jama.2010.743. [DOI] [PubMed] [Google Scholar]
- 10.Bhatt D.L., Drozda J.P., Jr., Shahian D.M. ACC/AHA/STS statement on the future of registries and the performance measurement enterprise: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and The Society of Thoracic Surgeons. J Am Coll Cardiol. 2015;66:2230–2245. doi: 10.1016/j.jacc.2015.07.010. [DOI] [PubMed] [Google Scholar]