Abstract
The discipline of cardio-oncology has expanded at a remarkable pace. Recent developments and challenges to clinicians who practice cardio-oncology were presented at the Global Cardio-Oncology Summit on October 3 to 4, 2019, in São Paulo, Brazil. Here, we present the top 10 priorities for our field that were discussed at the meeting, and also detail a potential path forward to address these challenges. Defining robust predictors of cardiotoxicity, clarifying the role of cardioprotection, managing and preventing thromboembolism, improving hematopoietic stem cell transplant outcomes, personalizing cardiac interventions, building the cardio-oncology community, detecting and treating cardiovascular events associated with immunotherapy, understanding tyrosine kinase inhibitor cardiotoxicity, and enhancing survivorship care are all priorities for the field. The path forward requires a commitment to research, education, and excellence in clinical care to improve our patients' lives.
Key Words: anthracycline, antiangiogenic therapy, bone marrow transplantation, breast cancer, cancer survivorship, immunotherapy, thrombosis, tyrosine kinase inhibitor
Abbreviations and Acronyms: CV, cardiovascular; CVD, cardiovascular disease; DOAC, direct oral anticoagulant; GCOS, Global Cardio-Oncology Summit; GLS, global longitudinal strain; HCT, hematopoietic cell transplantation; ICI, immune checkpoint inhibitor; LVEF, left ventricular ejection fraction; PD-1, programmed cell death 1 or its ligand; PD-L1, programmed cell death ligand 1; TKI, tyrosine kinase inhibitor; VTE, venous thromboembolism
Central Illustration
Highlights
-
•
The discipline of cardio-oncology has enjoyed extensive and complex development at a breathtaking pace.
-
•
Extensive ongoing clinical and basic research continue to shape and formulate the practice of cardio-oncology.
-
•
The path forward for the field needs to be centered on excellence in clinical care, transformative research, and broad education principles.
The development and expansion of cardio-oncology as a discipline in providing care to cancer patients has occurred at a remarkable pace. From the initial beginnings as a concept (1) and then officially as a dedicated society with the formation of the International Cardio-Oncology Society in 2009 (2), cardio-oncology continues to encompass a very broad range of clinical topics. There are basic science and clinical research principles describing disease mechanisms that ultimately explain the efficacy of cancer therapy and also provide insights into clinically evident cardiovascular (CV) toxicity. It also seems apparent that an integrated, comprehensive care model involving a multidisciplinary team of providers is necessary to achieve the best outcome. In response to this broad mandate, the Global Cardio-Oncology Summit (GCOS) is an annual meeting in which scientists, clinicians, and trainees from all over the world come together to learn the best practices and most current research in the discipline of cardio-oncology. This year, International Cardio-Oncology Society Brazil hosted a meeting in São Paulo, with 587 participants, the highest number of attendees to date. The makeup of this engaged audience included 302 cardiologists; 99 oncologists; 101 nurses, physiotherapists, or pharmacists; and 85 primarily researchers in the field. The energy and enthusiasm at this meeting serves as a testament to the vibrancy of the discipline, particularly in Brazil (Figures 1A, 1B, and 2).
Figure 1.
Global Cardio-Oncology Summit 2019 Co-Chairs, Drs. Ludhmila Hajjar and Roberto Kalil Filho
Opening presentations by (A) Dr. Hajjar and (B) Dr. Filho.
Figure 2.
Global Cardio-Oncology Summit 2019 Participants
All the attendees at Global Cardio-Oncology Summit 2019.
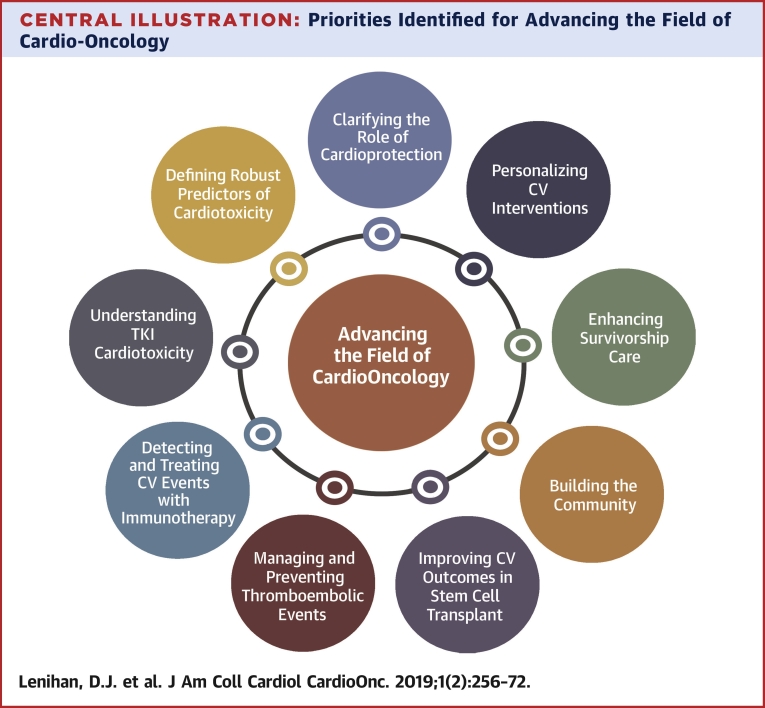
This Proceedings document is intended to highlight some of the most important clinical and research priorities that were discussed in detail at the GCOS Meeting but it is not all inclusive of what was presented (Supplemental Figure 1). The specific topics and priorities highlight the current state of the field as presented in each lecture and wherever possible the strategies to purposefully advance the field are detailed in this document. Cardio-oncology is still in its early stages; the clinical and basic research data that inform our clinical practice decision-making in many ways need to be developed. It is the intended goal of this review summarizing the events at GCOS 2019 to help collectively identify key knowledge gaps that need to be filled to advance our understanding and improve the clinical care of our patients (Central Illustration). The following priorities are discussed in the order of presentation at the international meeting.
Central Illustration.
Priorities Identified for Advancing the Field of Cardio-Oncology
The priorities identified for the discipline of cardio-oncology from the Global Cardio-Oncology Summit 2019 meeting serve as a focus for our collective efforts to advance the field. CV = cardiovascular; TKI = tyrosine kinase inhibitor.
Defining Robust Predictors of Cardiotoxicity
Identifying patients at risk of CV toxicity before initiation of cancer therapy and during treatment, and in survivors, is an important priority in cardio-oncology. This would inform the consideration of alternate but similarly efficacious cancer therapy, guide targeted surveillance strategies, promote primary prevention therapies, and guide the frequency and duration of follow-up after completion of treatment.
Cancer survivors as a whole are at an elevated risk of dying from CV disease (CVD) compared with the general population (3). This risk is especially prominent with Hodgkin and non-Hodgkin lymphomas; leukemia, bladder, lung, testicular, and breast malignancies; and with childhood cancer survivors. Traditional CV risk models such as the Framingham risk score are perceived to be insufficient to identify CV risk, given that they do not consider cancer patients, do not incorporate cancer therapy as a unique contributor to the development of CVD, and do not account for the competing risk of non-CV death (3,4). Currently, risk prediction models for the development of cardiomyopathy do exist in women with HER2+ breast cancer; however, they are not used clinically or have poor discrimination (5,6). A recently published model allows prediction of 5- and 10-year risk of overall CVD in women with breast cancer (7) but still requires external validation and assessment for the ability to specifically discriminate cardiotoxicity risk. In the absence of robust models, clinicians have predominantly relied on the identification of traditional CV risk factors such as diabetes, hypertension, and dyslipidemia, or the presence of prior CVD (8). Unfortunately, this is far from precision medicine.
There has also been an interest in cardiac imaging strategies to predict the risk of cardiomyopathy development. Pretreatment left ventricular ejection fraction (LVEF) and myocardial strain (global longitudinal strain [GLS] and circumferential strain) may identify patients at risk for subsequent cardiomyopathy and major adverse CV events in the context of anthracycline or trastuzumab therapy (9, 10, 11). However, as it relates to anthracyclines, monitoring LVEF alone may not be adequate (12). The most widely studied echocardiographic imaging marker of early dysfunction is GLS. Although early changes in GLS in the setting of anthracycline or trastuzumab use predict the subsequent development of cardiotoxicity as defined by a significant change in LVEF (13,14), prospective studies are needed to determine specific thresholds (15). It is also unclear whether cardioprotective interventions driven by GLS alter outcomes in patients; this is currently the focus of the ongoing SUCCOUR (Strain sUrveillance of Chemotherapy for improving Cardiovascular Outcomes) trial (16). More recently, early diastolic dysfunction also appears to identify patients at risk of cardiotoxicity (17); however, it is unclear if this is superior to GLS. The other important idea that summarizes these data is the concept that a minor change in LVEF or GLS may be detectable with serial testing, but whether this is a clinically relevant finding that requires a specific action, such as holding therapy or adding cardioprotective medications, is an essential knowledge gap we need to fill.
An alternate approach to risk prediction is to use circulating biomarkers to identify early cardiac injury (18). In patients receiving high-dose anthracycline therapy, initial studies demonstrated repeated measurements of troponin I identified patients at risk of developing cardiotoxicity (19) and adverse CV events (20). Initiation of angiotensin-converting enzyme inhibitors in those with elevated troponin prevented a decline in left ventricular function (21). However, the value of troponin measurements to predict cardiotoxicity has not been uniformly demonstrated in other studies (22,23). Another marker that has shown promise in the setting of anthracycline and trastuzumab therapy is myeloperoxidase (10). Other clinically relevant cardiac biomarkers, B-type natriuretic peptide or N-terminal pro–B-type natriuretic peptide, have been investigated and may be important adjuncts to assess for cardiotoxicity and useful tools to assist in risk-stratifying patients. Although there have been several studies than have shown associations between early or persistent rise in natriuretic peptides and cardiotoxicity (24, 25, 26, 27, 28), this has not been a uniform finding (10,24).
An important new application of troponin is in the identification of myocarditis secondary to immune checkpoint inhibitor (ICI) therapy. An increase in high-sensitivity troponin is described as the most consistent finding in patients with suspected myocarditis related to ICI associated myocarditis (29). However, the value of routine troponin as a screening tool appears to be limited even in this clinical scenario (30).
Despite the previous data, there are currently no comprehensive guidelines as to how to integrate clinical information, imaging data, and serum biomarkers to risk stratify patients before and during cancer therapy. Although the recent American Society of Clinical Oncology guidelines suggest that it may be reasonable to use imaging and blood biomarkers during cancer therapy to screen for CV toxicity, specific markers, monitoring timelines, or the implications of abnormal findings are not established at the present time. This is certainly a knowledge gap that needs to be filled (31).
Clarifying the Role of Cardioprotection
Cardioprotective strategies to mitigate cancer treatment related cardiotoxicity have largely focused on breast cancer patients exposed to anthracyclines or trastuzumab (Table 1). Three randomized control trials (PRADA [Prevention of Cardiac Dysfunction during Adjuvant Breast Cancer Therapy], MANTICORE [Multidisciplinary Approach to Novel Therapies in Cardiology Oncology Research], and Guglin et al. [32] ) in women with early-stage breast cancer receiving anthracyclines or trastuzumab reported a statistically significant benefit, measured as an attenuation in LVEF decline, with the addition of cardioprotective medications (candesartan, bisoprolol, carvedilol, lisinopril) (32, 33, 34), while 2 other randomized control trials failed to demonstrate a benefit with these medications (35,36). Notably, in the Guglin et al. (32) study, the benefit was only observed in the patient subgroup receiving both anthracyclines and trastuzumab.
Table 1.
Primary Cardiotoxicity Prevention Trials in Patients With Breast Cancer
| Trial/First Author (Ref. #) | Sample | Intervention | Outcomes | Benefit (Yes/No) | Limitations | Path Forward |
|---|---|---|---|---|---|---|
| PRADA (34) | 130, all anthracycline, 22% trastuzumab | 1:1:1:1, metoprolol, candesartan, metoprolol and candesartan, or placebo | Changes in LVEF by CMR at 10 to 64 weeks | Yes Absolute LVEF change: 2.6% in placebo, 0.8% in candesartan (p = 0.026) |
Lack of follow-up beyond adjuvant therapy period Statistical power for subgroup analyses is limited |
PRADA II (NCT03760588): 300 patients with ESBC receiving anthracycline containing chemo randomized to Entresto or placebo |
| MANTICORE (34) | 94, all trastuzumab, 12–33% anthracycline | 1:1:1 bisoprolol, perindopril, or placebo | Changes in LVEDVI by CMR at 1 yr | Yes Small reduction in LVEF decline with bisoprolol compared with perindopril and placebo (–1% vs. –3% vs. –5%; p = 0.001) |
Limited follow-up period Patients younger and fewer cardiovascular risk factors than average in clinical practice |
Future trials involving subgroups with higher risk factors and that are most likely to benefit from primary prophylaxis therapies |
| Guglin et al. (32) | 468, all trastuzumab, 40% doxorubicin | 1:1:1 carvedilol, lisinopril, or placebo | LVEF >10% or LVEF decline >5% with absolute LVEF <50% | Yes >10% LVEF decline in subset with prior anthracycline exposure: 47% placebo, 31% carvedilol, 37% lisinopril (p = 0.009) |
Randomized to treatment groups post-anthracycline exposure Centers measured LVEF by their preferred method (echocardiography, MUGA) Not powered to compare efficacy of prevention between lisinopril and carvedilol |
Future trials comparing the efficacy of prevention with lisinopril vs. carvedilol and assessing optimum timing and dosing of cardioprotective medications alone and in combination |
| CECCY (35) | 200, all doxorubicin | 1:1 carvedilol or placebo | LVEF >10% decline from baseline to 6 months | No LVEF decline: 13.5% placebo,14.5% carvedilol (p = 1.00) |
Single center study Dose of carvedilol was optimized during chemotherapeutic treatment Interobserver variability may influence repeated LVEF measurements |
Future randomized controlled trials of preventive beta-blockade in larger populations and in patients with higher risk of severe left ventricular dysfunction and heart failure |
| Boekhout et al. (36) | 206, all epirubicin with trastuzumab | 1:1 candesartan or placebo | LVEF decline of >15% or a decrease below the absolute value of 45% | No LVEF decline: 19% in candesartan, 16% in placebo (p = 0.58) |
Small sample size Baseline LVEF values not available in 35.9% of patients |
Future trials exploring relationships between gene polymorphisms of ERBB2 and trastuzumab-related cardiotoxic effects |
CECCY = Carvedilol Effect in Preventing Chemotherapy-Induced Cardiotoxicity; CMR = cardiac magnetic resonance; ESBC = early stage breast cancer; LVEDI = left ventricular end-diastolic volume index; LVEF = left ventricular ejection fraction; MANTICORE = Multidisciplinary Approach to Novel Therapies in Cardiology Oncology Research; MUGA = multigated acquisition scan; PRADA = Prevention of Cardiac Dysfunction during Adjuvant Breast Cancer Therapy.
The interpretation of these trials is limited due to heterogeneity in the study populations with variability in anthracycline and trastuzumab exposure, different definitions of cardiotoxicity, and variable clinical endpoints (33,35). Furthermore, the women included in these studies were younger and with few baseline CV risk factors or comorbidities, and the follow-up was relatively short. In addition, the clinical significance of the modest LVEF changes reported in these studies is uncertain (32,34,36).
A recent meta-analysis of 17 studies (14 in breast cancer) of cardioprotective strategies in adult patients that underwent chemotherapy and neurohormonal blockade (beta-blockers, mineralocorticoid receptor antagonists, or angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) demonstrated that with these therapies, there was a 3.96% (95% confidence interval: 2.90% to 5.02%) smaller decline in LVEF as compared with placebo, but with significant heterogeneity observed in treatment effects across studies, highlighting the need for larger trials of cardioprotective strategies (37).
Where do we go from here?
Future studies assessing cardioprotective strategies should include and stratify patients based on risk of cardiotoxicity (e.g., ≥60 years of age, higher anthracycline exposure, high-dose radiotherapy, cardiac comorbidities) as per the American Society of Clinical Oncology guidelines (31) and ascertain clinically meaningful endpoints, such as heart failure, CV mortality, and ability to complete anticancer treatment. Clinical trials that include an assessment of the attenuation of LVEF declines should use standardized definitions of cardiotoxicity (e.g., American Society of Echocardiography and European Association of Cardiovascular Imaging Expert Consensus) (14).
There are a number of ongoing randomized clinical trials exploring novel cardioprotective strategies including the use of statins, optimization of radiation therapy (proton vs. photon), diet, exercise, and lifestyle interventions. These include the PREVENT (Preventing Anthracycline Cardiovascular Toxicity With Statins) trial (NCT01988571), comparing statin therapy versus placebo in anthracycline-treated patients; the SUCCOUR trial (ACTRN12614000341628); the NIH-funded TACTIC trial (NCT03879629); the PRADA II trial (NCT03760588), comparing sacubitril/valsartan versus placebo in breast cancer patients receiving anthracyclines; the PROACT (Preventing Cardiac Damage in Patients Treated for Breast Cancer: a Phase 3 Randomised, Open Label, Blinded Endpoint, Superiority Trial of Enalapril to Prevent Anthracycline-induced CardioToxicity) trial (NCT03265574); and the Cardiac CARE (The Cardiac CARE Trial–can heart muscle injury related to chemotherapy be prevented?) trial (ISRCTN24439460). Nevertheless, simply defining the role of pharmacologic cardioprotective strategies in this patient population (38) is unlikely to be sufficient. Protecting patients from the adverse CV consequences of cancer therapy will likely require a multipronged approach, including pharmacologic therapy, optimization of diet, physical activity, CV risk factors and comorbid conditions (e.g., diabetes). This strategy is supported by the recent American Heart Association Scientific Statement on Cardio-Oncology Rehabilitation to manage CV outcomes in cancer patients and survivors (39). The optimization of cardioprotective strategies, particularly in high risk populations, should ultimately lead to the prevention or attenuation of cancer therapy related CV toxicity.
Managing and Preventing Thromboembolic Events in Patients With Cancer
Cancer associated thrombotic events, both arterial and venous, are increasingly recognized in specific malignancies and in association with cancer therapies. Patients with cancer have 4- to 7-fold greater risk of venous thromboembolism (VTE), including deep vein thrombosis and pulmonary embolism, when compared with noncancer populations, and the risk of VTE recurrence is as high as 15% per year (40,41). Patients with active malignancy, particularly those with advanced disease or cancer types such as lung, colorectal, and gastric cancer, also face a high short-term risk of arterial thromboembolism (42). Risk factors for arterial and venous thrombosis partially overlap. Many risk factors, such as age, smoking, hypertension, and diabetes, are common to both venous and arterial thrombi (43,44).
The Khorana Risk Score utilizes the type of cancer, blood counts, and body mass index to predict risk of VTE in cancer patients. Recent randomized clinical trials have provided data for the prophylactic management of anticoagulation for VTE in high-risk ambulatory cancer patients with Khorana Risk Score ≥2 (Table 2), including the CASSINI (Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer) (45) and AVERT (Apixaban to Prevent Venous Thromboembolism in Patients with Cancer) (46) randomized clinical trials. These studies suggest that direct oral anticoagulants (DOACs) could be used in the high-risk setting, and the American Society of Clinical Oncology clinical practice guidelines have been updated in 2019 to reflect these findings (47). Although routine pharmacologic thromboprophylaxis is not offered to all cancer patients, those who have a Khorana Risk Score ≥2 before starting a new systemic chemotherapy regimen may be considered for thromboprophylaxis with apixaban, rivaroxaban, or low-molecular-weight heparin, provided that there are no significant risk factors for bleeding and no drug-drug interactions with concomitant systemic anticancer therapy is anticipated (47). Recent randomized trials have provided additional evidence to support the noninferiority of DOACs compared with low-molecular-weight heparin for the treatment of VTE, including the HOKUSAI VTE (Edoxaban versus Warfarin for the Treatment of Symptomatic Venous Thromboembolism) (48), SELECT-D (Comparison of an Oral Factor Xa Inhibitor With Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial) (49), and ADAM-VTE (Apixaban and Dalteparin in Active Malignancy Associated Venous Thromboembolism Trial) (50) trials. Additional considerations need to be taken into account when choosing the proper anticoagulant therapy in cancer patients, including alterations in DOAC metabolism, drug-drug interactions, renal impairment, and thrombocytopenia.
Table 2.
Recent Trials Involving Novel Therapies for Anticoagulation
| Study | Population | Comparators | Primary Efficacy Endpoint | Primary Safety Endpoint |
|---|---|---|---|---|
| Primary prevention | ||||
| CASSINI trial: double-blind RCT (45) | 841 high-risk ambulatory cancer patients | Rivaroxaban 10 mg vs. placebo for 6 months | DVT or PE or VTE-related death HR: 0.66, 95% CI: 0.40–1.09 |
Major bleeding 1.0% vs. 2.0% HR: 1.96, 95% CI: 0.59–6.49 |
| AVERT trial: double-blind RCT (46) | 574 high-risk ambulatory cancer patients | Apixaban 2.5 mg twice daily vs. placebo For 6 months |
Objectively documented VTE 4.2% vs. 10.2%; HR: 0.41, 95% CI: 0.26–0.65 |
Major bleeding 3.5% vs. 1.8% HR: 2.00, 95% CI: 1.01–3.95 |
| Treatment of VTE | ||||
| HOKUSAI VTE: open-label, noninferiority (48) | 1,050 cancer patients with acute symptomatic or incidental VTE | LMWH 5 days + edoxaban 60 mg vs. dalteparin Treated for 6 months |
Recurrent VTE or major bleeding 12.8% vs. 13.5% HR: 0.97, 95% CI: 0.70–1.36 |
Major bleeding 6.9% vs. 4.0%, HR: 1.77, 95% CI: 1.03 to 3.04 |
| SELECT-D: open-label trial (49) | 406 patients with active cancer and symptomatic PE or DVT | Rivaroxaban vs. dalteparin Treated for 6 months |
VTE recurrence rate 4% vs. 11% HR: 0.43, 95% CI: 0.19–0.99 |
Major bleeding 6% vs. 4% HR: 1.83, 95% CI: 0.68–4.96 |
| ADAM-VTE trial (50) | 300 patients with cancer-associated VTE | Apixaban 10 mg twice daily for 7 days then 5 mg twice daily vs. dalteparin | VTE recurrence rate 0.7% vs. 6.3% HR: 0.099, 95% CI: 0.013–0.78 |
Major bleeding 0% vs. 1.4% |
ADAM-VTE = Apixaban and Dalteparin in Active Malignancy Associated Venous Thromboembolism Trial; AVERT = Apixaban to Prevent Venous Thromboembolism in Patients with Cancer; CASSINI = Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer; CI = confidence interval; DVT = deep vein thrombosis; HOKUSAI VTE = Edoxaban versus Warfarin for the Treatment of Symptomatic Venous Thromboembolism; HR = hazard ratio; LMWH = low-molecular-weight heparin; PE = pulmonary embolism; RCT = randomized clinical trial; SELECT-D = Anticoagulation Therapy in Selected Cancer Patients at Risk of Recurrence of Venous Thromboembolism; VTE = venous thromboembolism.
Although recent data have shed some insight on the safety and effectiveness of DOACs in cancer patients both as primary prophylaxis and as treatment of VTE, several areas need future investigation. Among them is the optimal anticoagulant management of patients with cancer who are admitted for minor procedures or short chemotherapy infusion, as well as the optimal duration of post-operative anticoagulation in patients with cancer.
As it relates to arterial thromboembolism management, there are no guidelines specific to patients with cancer. In recent clinical trials such as the DAPT (Dual Antiplatelet Therapy) trial and the LEADERS FREE (Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk) trial, 10% to 12% of patients undergoing percutaneous coronary intervention had a history of cancer (51, 52, 53). For cancer patients, the Society of Cardiovascular Angiography and Interventions (54) advocates for the use of radial access or a small needle kit femoral access, careful review of the appropriate use criteria for the need of percutaneous coronary intervention, liberal use of fractional flow reserve in indeterminate cases, and intravascular ultrasound–guided percutaneous coronary intervention. Cancer patients also have a higher risk of stent thrombosis, as malignancy is a potent predictor of late stent thrombosis (55). The other frequent concern in cancer is thrombocytopenia. A platelet cutoff of >50,000/μl for coronary artery bypass grafting, >30,000/μl for dual antiplatelet therapy, and >10,000/μl for aspirin is advised by the Society of Cardiovascular Angiography and Interventions. Certainly, shaping recommendations for dual antiplatelet therapy, the role of percutaneous coronary intervention, and clarifying the bleeding risk in the cancer population is of critical importance.
Improving CV Outcomes in Stem Cell Transplantation
There are currently 200,000 hematopoietic cell transplantation (HCT) survivors in the United States today, a number that will exceed 500,000 by 2030. HCT survivors continue to have substantially higher mortality rates compared with the general population (56, 57, 58). In particular, the risk of CV-related mortality is more than twice that of the general population (57, 58, 59), and the magnitude of risk increases with time (59). However, examining CVD-associated mortality alone underestimates the true burden of CVD morbidities after HCT. HCT survivors have a 4-fold higher risk of developing CVD compared with the general population (60). Among HCT survivors, the median age at first CV event such as myocardial infarction is ∼15 years earlier than would be expected in the general population (61). The markedly increased risk of CVD, coupled with the development of complications earlier than would be expected in the general population, suggests the presence of an accelerated CV aging phenotype in HCT survivors.
Biologic aging involves multiple complex changes in structure and function that lead to decreased reserve capacity across virtually all organ systems, with increased vulnerability to age-related diseases (62). Similarly, we can envision CVD in HCT patients as being induced by pathologic perturbations that over time resulting in a decline in CV reserve and accelerated aging (62,63). To understand the pathophysiology of accelerated CV aging in HCT patients, it is important to deconstruct the organ-specific injuries that occur before, during and after HCT, ultimately contributing to depletion of CV reserves over time.
Cardiac
It is well-established that cancer treatments (e.g., anthracycline chemotherapy, radiation) can lead to alterations in the heart and vasculature (61). For HCT survivors treated with anthracyclines before HCT, additional exposures such as high-dose cyclophosphamide during conditioning may further compromise cardiomyocyte structure and function (61). Studies have shown that cumulative anthracycline dose ≥250 mg/m2 is associated with a 10-fold risk of heart failure in HCT survivors (odds ratio: 9.9; p < 0.01) (61,64), a dose threshold that is markedly lower than conventionally recognized cutoffs (350 to 450 mg/m2) in non-HCT cancer populations (65). Radiation can cause direct myocardial injury, or result in endothelial cell proliferation and atherosclerosis (66). Among allogeneic HCT patients, graft-versus-host disease can lead to additional microvascular disease, because of endothelial infiltration of alloreactive cytotoxic T lymphocytes (67), suggesting an immunological mechanism for accelerated arterial disease (67).
Pulmonary
Pulmonary disease in these patients can further contribute to decreased exercise intolerance and deconditioning. Nearly one-half of all patients undergoing allogeneic HCT will develop acute pulmonary toxicity, and pulmonary complications account for up to 40% of transplant-related deaths within the first year after HCT (68,69). Pulmonary complications can be due to injuries sustained during HCT or noninfectious complications that develop after HCT (69). In a recently study, there was a high prevalence (35%) of previously undiagnosed diffusion capacity defects in very long-term HCT survivors, corresponding to a >5-fold risk compared with age-matched noncancer controls (odds ratio: 5.2; p < 0.01) (70).
Musculoskeletal
Chronic corticosteroid exposure, traumatic brain injury, prolonged inactivity, or poor nutritional status can lead to abnormal body composition that manifests as an increase in total percent fat mass and sarcopenia (71). Studies have shown that pre-HCT sarcopenia is associated with 2-fold risk of CVD-related and nonrelapse mortality in HCT survivors (72). Moreover, patients who survive HCT often do not adhere to national exercise recommendations, and have significant declines in physical activity levels from pre- to post-HCT (73). Patients with graft-versus-host disease are more likely to demonstrate physical inactivity due to the disproportionate muscle atrophy.
Hematologic
Persistent post-HCT abnormalities such as myelodysplastic syndrome, myelofibrosis, or bone marrow failure (69), although rare, may further contribute to decline in CV reserve over time.
Traditional CV risk factors such as hypertension, diabetes, and dyslipidemia are important modifiers of CVD risk in the general population (74). Studies have shown that 32% of HCT survivors have multiple (≥2) CV risk factors, compared with only 21% in the general population (61,75), and that each risk factor confers an incremental risk of CVD over time. CVD incidence is highest (15%) among HCT survivors with multiple risk factors who have also been exposed to cardiotoxic therapies. Other health conditions such as thyroid dysfunction, chronic kidney disease, and gonadal dysfunction are established CVD risk factors in the general population (74), and are highly prevalent in HCT survivors (76).
Ultimately, CV reserve capacity is determined by the integrative capacity of multiple organ systems and is impacted by modifiable comorbidities that emerge with age. Primary prevention is the most effective strategy to reduce long-term CVD risk, but effective interventions are lacking, or have not been evaluated in a rigorous prospective manner. There is an important need for such studies. In the meantime, management of modifiable risk factors can reduce long-term CVD risk in HCT survivors. These efforts will require ongoing collaboration among hematologists or oncologists, cardiologists, primary care providers, and advanced care practitioners.
Personalizing Cardiovascular Interventions
With improved oncologic outcomes along with an aging population, ischemic heart disease and valvular heart disease are increasingly significant CV issues facing cancer patients and survivors (77,78). Nevertheless, there are few studies evaluating the procedural management of these diseases in cancer patients, and a collaborative and nuanced approach is needed to achieve optimal outcomes. Assessment and optimization of CV risk factors in cancer patients at risk for adverse CV events are essential, especially if they have known CV risk factors or underlying CVD.
Multiple cancer therapeutics are associated with accelerated coronary artery disease, leading to ischemic heart disease including tyrosine kinase inhibitors (TKIs) used to treat chronic myeloid leukemia such as nilotinib and ponatinib (79,80). In addition, multiple agents are associated with worsening metabolic and lipid abnormalities, which can lead to ischemic events, including aromatase inhibitors and androgen deprivation therapies (81,82). The association with long-term coronary disease after chest radiation is well established (83,84). Nevertheless, the pathophysiology leading to these events is still being elucidated. In addition, shared risk factors for both cancer and CVD are increasingly recognized (77). Genetic abnormalities, including clonal hematopoiesis of indeterminate potential, may also play a role in the development of these disease states. Somatic mutations in blood and bone marrow cells are associated with both an increased risk of both hematologic malignancies and atherosclerotic vascular disease (85). This is a likely to be important target for future interventions for both cancer and heart disease.
Approximately 5% of all acute coronary syndromes occur in patients with cancer and management of these events can be quite challenging, often with outcomes inferior to the general population (86). For example, 2 recent studies using the National Inpatient Sample and evaluating percutaneous interventions in patients with leukemia or lymphoma reported increased post-procedural adverse events (87,88). Various reasons include increased bleeding complications as well as an elevated incidence of concomitant left ventricular dysfunction, either stress-mediated or from chemotherapy. Moreover, atherosclerotic lesions because of radiation are often complex and challenging, and occur at ostial and proximal locations of epicardial vessels (89). Nevertheless, interventions can be performed safely and effectively in cancer patients, even when platelets are as low as 30,000/μl (54). As the number of patients with active cancer and associated acute coronary syndromes is expected to increase, research priorities need to focus on informing the optimal treatment strategies to minimize risk and maximize benefit and enhancing our understanding of the molecular mechanism of coronary disease in this unique population.
Building the Community
Cardio-oncology emphasizes balancing cardiac risk with oncology treatments. The cardio-oncology team requires collaboration from both oncology and hematology specialists and cardiologists, with an emphasis on continuing the most appropriate cancer therapy while optimizing heart health. Additionally, for many cancer survivors, there is continued need to incorporate CV risk reduction with the management of hypertension, hypercholesterolemia, and diabetes (90,91).
Training clinicians within cardio-oncology remains vital. In a recent survey of the Accreditation Council for Graduate Medical Education–accredited cardiology fellowships, 51% of institutions had a dedicated cardio-oncology service, up from 27% in 2014. Although U.S.-based fellowships with a dedicated cardio-oncology program exist, these are predominantly in the Northeast and West Coast, suggesting disparities in research and clinical training. To enhance and grow the cardio-oncology community, there needs to be an emphasis on education and training. Internationally, cardio-oncology services and fellowships have grown, with established programs throughout South America, Europe, and Asia. These efforts have begun with dedicated cardio-oncology fellowships, guidelines on cardio-oncology training (92), and support from the American College of Cardiology, European Society of Cardiology, European Society of Medical Oncology, and the American Society of Clinical Oncology (31). Further development of a board certification in cardio-oncology may also enhance and delineate expertise within this field.
Building the cardio-oncology community requires a joint effort among cardiology and oncology, with an emphasis on enhancing the research as well as improving patient care. Continued research on strategies to personalize care in cardio-oncology, as well as growing the cardio-oncology workforce will reinforce the management of CV disease in the rapidly growing cancer survivor community.
Detecting and Treating Adverse CV Events Associated With Immunotherapy
ICIs represent a paradigm shift in cancer care, leveraging the immune system to identify and target cancer cells. These immune checkpoints, such as cytotoxic T-lymphocyte antigen 4 and programmed cell death 1 (PD-1) or its ligand, programmed cell death ligand 1 (PD-L1), are proteins on the surface of regulatory T cells that have a key role in regulating the immune system (93).
In 2015, ICIs were approved for 9 cancer indications. By 2019, ICIs were approved for 29 cancer indications and, at present, there are 2,000 ICIs in various cancer stages in over 3,000 active clinical trials (94). The use of ICIs will expand from late stage disease to adjuvant and neoadjuvant use in patients with a longer anticipated survival (95). Immune-related adverse events are generally low grade and manageable, especially if recognized early.
Myocarditis from ICIs is uncommon, but is a potentially fatal immune-mediated adverse event. The first specific report of myocarditis during treatment with a PD-1/PD-L1 inhibitor occurred in 2014 (96). Since then, numerous cases of ICI-associated myocarditis with PD-1/PD-L1 inhibitor therapy have been published (97,98). Beyond myocarditis, additional complications have been reported (Table 3).
Table 3.
CV Events Associated With ICIs and Potential Management Strategies
| Potential CV Events Related to a Checkpoint Inhibitor | Methods for Diagnosis | Potential Initial Approach to Treatment | Potential Additional Therapies if Stable and not Responding to Initial Approach | Potential Additional Therapies if Unstable |
|---|---|---|---|---|
| Myocarditis |
|
|
|
|
| Pericarditis |
|
|
Methylprednisone 1 g/day for 3.5 days
|
|
| Takutsubo cardiomyopathy |
|
|
|
|
| Dilated cardiomyopathy |
|
|
|
This table details the cardiovascular (CV) toxicities associated with immune checkpoint inhibitor (ICIs) and potential management strategies. Many of the management strategies listed for toxicities other than myocarditis are extrapolated from the myocarditis literature or based on small case series or reports.
CMR = cardiac magnetic resonance; ECG = electrocardiogram; IVIG = intravenous immune globulin; LVEF = left ventricular ejection fraction; VTE = venous thromboembolism.
The incidence of myocarditis has been reported to be 0.09% to ∼1% (29,97). However, the true incidence of ICI-associated myocarditis may be underestimated due to the wide range of clinical presentations, challenges in diagnosis, and a general lack of awareness of this condition. Myocarditis presentation, typically within the first 3 months of therapy initiation, can vary from an asymptomatic increase in serum troponin to sudden cardiac death (29). Data have consistently shown that the outcomes with myocarditis are poor with a mortality rate that varies from 25% to 50% (99,100).
There are limited data on the utility of cardiac testing when there is a clinical suspicion for myocarditis. Currently, many perform an electrocardiogram and troponin measurement before ICI. However, the absence of either being abnormal does not exclude the diagnosis. Echocardiography is a standard initial imaging test, although the LVEF can also be normal in over 50% of cases (29). There are no published data on the use of GLS in the assessment of ICI myocarditis. Cardiac magnetic resonance is the gold-standard imaging test for the assessment of myocarditis; however, in a published series of 30 cases, late gadolinium enhancement was noted in only 23% and myocardial edema in 33% (99). Biopsy with use of the Dallas criteria is the gold-standard diagnostic test, but is invasive and associated with risk and sampling error. The consistent pathological finding is the presence of a lymphocyte-predominant T cell–rich infiltrate that is CD4 and CD8 positive (101).
The current first line management of myocarditis with ICIs is cessation of therapy and administration of corticosteroids (102) (Table 3), as specified in a number of expert consensus and best practice guidance statements (102, 103, 104). However, it is important to note that there have been no studies specifically comparing treatment strategies for ICI-associated myocarditis, particularly to guide the dose and time of initiation of corticosteroids. These studies are clearly needed. Alternative immunosuppressive approaches for the management of ICI-associated myocarditis have included the use of mycophenolate, monoclonal antibodies to CD52, plasma exchange, cytotoxic T-lymphocyte–associated antigen 4 agonists, antithymocyte globulin, and infliximab (105). However, the support for these approaches is limited to case reports and associated publication bias. Similarly, management of other cardiovascular complications is based on extrapolation of the myocarditis literature, or other case series and reports (Table 3).
It is reasonable to hypothesize, given the key role that the immune system plays in CVD, that the presentations of ICI cardiac toxicity may extend beyond these current definitions. Specifically, each of these checkpoints has been implicated in the development of atherosclerosis and basic data suggest that blockade of these critical regulators leads to accelerated atherosclerosis (106). There are no clinical data on the role of ICIs in the development of atherosclerosis, and in contrast to the hypothesis and the basic science data, initial imaging data suggest that ICIs may reduce atherosclerosis (107).
In summary, ICIs hold tremendous promise for extending the lives of patients with cancer. As ICI use expands, it is critical to undertake efforts to understand and mitigate the risks of these uncommon but life-threatening cardiac toxicities.
Understanding Multi-targeted TKI Cardiotoxicity
Over the last decade, there has been a paradigm shift in the approach to cancer therapy, moving away from nonspecific cytotoxic chemotherapy to treatments that target abnormal intracellular signaling pathways which are fundamental to the development and progression of cancer. Many of these abnormalities are due to mutated or overexpressed protein kinases that regulate the cell cycle leading to uninhibited growth and metastasis. TKIs are a class of therapeutics that target these abnormal proteins (108), for which we are only beginning to recognize the vast array of possible CV toxicities (Table 4).
Table 4.
Small Molecule Tyrosine Kinase Inhibitors and CV Considerations
| Class/Target | Specific Agents | Cancer Control Mechanisms | Cardiovascular Toxicities | Proposed Mechanism of Cardiotoxicity | Cardiovascular Treatment Considerations | Ref. # |
|---|---|---|---|---|---|---|
| ALK inhibitors | Alectinib; crizotinib | Inhibition of ALK activity leading to decreased cell proliferation and angiogenesis | Bradycardia; QT prolongation | Decrease If (pacemaker current) in sinoatrial nodal cells | Avoid nodal blocking agents | (120,121) |
| BCR-ABL | Imatinib; nilotinib, dasatinib, bosutinib; ponatinib | Target BCR-ABL fusion protein, c-Kit, and platelet-derived growth factor receptors | Ischemic vascular disease; hypertension; hyperlipidemia; hyperglycemia; QT prolongation; pulmonary hypertension; pleural effusion; pericardial effusion | Accelerated atherosclerosis; endothelial dysfunction; thrombotic microangiopathy | Statins; antihyperglycemics | (80,110,111) |
| BTK inhibitor | Acalabrutinib; ibrutinib | Inhibition of the BTK pathway | Atrial fibrillation; ventricular arrhythmias; hypertension | PI3K–Akt pathway, atrial fibrosis, dysregulated calcium handling | Avoid drugs that interact with the CYP 3A4 system (i.e., dihydropyridine calcium-channel blockers) and p-glycoprotein (i.e., dabigatran) | (112,113,115,116) |
| BRAF inhibitor | Dabrafenib; vemurafenib | Selective inhibition of B-raf which blocks cellular proliferation | QT Prolongation | N/A | N/A | (122) |
| MEK inhibitor | Binimetinib; combimetinib; trametinib | Allosteric inhibition of MEK affecting the MAPK pathway | Hypertension; heart failure/left ventricular dysfunction; QT prolongation | Inhibition of ERK1/2 activation in the heart | N/A | (123,124) |
| VEGF inhibitor | Axitinib; cabozantanib; lenvatinib; pazopanib; regorafenib; sorafenib; sunitinib; vandetanib |
Inhibits vascular endothelial growth factor receptors | Hypertension; left ventricular dysfunction; QT prolongation | Decreased nitric oxide bioavailability; microvascular rarefaction; increased endothelin-1 | ACE inhibitors/angiotensin receptor blockers; dihydropyridine calcium-channel blockers for hypertension | (125) |
ACE = angiotensin-converting enzyme; ALK = anaplastic lymphoma kinase; MAPK = mitogen-activated protein kinase; VEGF = vascular endothelial growth factor.
Many of the cardiotoxicities observed with TKIs are related to the on-target inhibition of the multiple tyrosine kinase receptors these agents affect, including downstream, off-target effects. Inhibitors of the vascular endothelial growth factor signaling pathway prevent angiogenesis, however blocking this pathway also decreases nitric oxide bioavailability leading to vasoconstriction and hypertension. TKIs developed to treat chronic myeloid leukemia are associated with vascular toxicities ranging from myocardial infarction and stroke to systemic and pulmonary hypertension, potentially from effects unrelated BCR-ABL (79,109). Although we have increased our recognition of these problems, we still lack a comprehensive understanding of the mechanism of these toxicities. Nilotinib-induced vascular events may be related to accelerated atherosclerosis and vascular endothelial dysfunction (110), whereas ponatinib-associated events may be due to thrombotic microangiopathy (111).
Ibrutinib, a unique kinase inhibitor that target the Bruton’s tyrosine kinase is associated with increased rates of arrhythmias, especially atrial fibrillation, with a reported incidence of 10% to 15% (112,113). Given frequent drug-drug interactions as well the effects of ibrutinib on the platelet collagen receptor glycoprotein VI pathway leading to enhanced bleeding complications, the ability to effectively treat ibrutinib atrial fibrillation can also be quite challenging (114,115). A more thorough understanding of these molecular mechanisms will likely improve the management options. There is also an increased risk of ventricular arrhythmias and sudden cardiac death, unrelated to QT prolongation (116,117).
Developing strategies to mitigate cardiotoxicity is essential particularly with TKIs, as many of these are chronic oral therapies that are given for months to years if treatment response is maintained. Unfortunately, cardiotoxicities ranging from QT prolongation to hypertension and left ventricular dysfunction can be reasons for treatment interruption or discontinuation. Although current recommendations focus on general CV risk factor modification, these are based primarily on expert opinion rather than on data (80). Prospective clinical studies with a CV focus will be the fundamental mechanism for improving CV care offered to patients treated with TKIs and advancing the field of cardio-oncology.
Enhancing Survivorship Care
With remarkable progress in cancer diagnosis and therapy, there are growing numbers of adult cancer survivors, with >16 million current survivors in the United States currently and >26 million projected by 2040. The late effects of treatment involve not only the heart, but also multiple organ systems and the sequelae of the psychological stress associated with cancer. Among the major challenges for the survivorship population is access to comprehensive and personalized care delivery by providers adept and knowledgeable about the late effects of cancer therapies.
Although interrelated, building a survivorship clinic is both philosophically and fundamentally different than starting a cardio-oncology clinic. Specific issues include the scope of disease, scope of care, personnel, and the choice of care models (Table 5). In 2006, the Institute of Medicine presented the following 6 concepts that drive the care for cancer survivors, including: 1) surveillance for recurrence; 2) screening for new cancers; 3) identification or interventions of consequences of cancer and its treatment; 4) providing emotional support; 5) health promotion strategies; and 6) coordination between all of the involved caregivers-oncologists, primary care physicians, and a wide spectrum of specialists. Moreover, because all survivors may not have access to even a basic survivorship program, there is an emerging interest in web-based self- management care delivery, with several reliable and proven programs.
Table 5.
Roadmap to Building a Survivorship Clinic
| 1. | Identify your position in the community – Are you a community hospital or an academic medical center, and is there a comprehensive cancer center? |
| 2. | Understand the cancer survivor population in your catchment area – Will you be encountering disease specific adult survivors or young adult/adolescent and adult survivors of pediatric cancer? |
| 3. | Describe the current practice landscape – Is anyone providing survivorship care and what are their gaps that you can collaboratively provide for more complex or coordinated care? |
| 4. | Design a model that is feasible with the personnel, space and financial resources that you have – What gaps need to be filled to begin a program and what other institutional support, such as oncology and other multidisciplinary medical specialists, exists? |
| 5. | Decide whether to start with long term cancer survivors or an immediate transition from cancer treatment – Do you want to start with the care of patients who are immediately transitioning from care completion or at a later stage from treatment completion? |
Five key questions to guide the development of a survivorship clinic.
Unfortunately, various barriers exist that prevent the widespread implementation of survivorship care, including the lack of dedicated and trained providers. Given the limited workforce, the use of nurses, nurse practitioners or physician assistants as the primary care providers in collaboration with a physician who is trained and committed to cancer survivorship may be a viable option for the delivery or care Additional obstacles include the “5 P’s”: physical barriers including space and resource availability; patient barriers including a lack of knowledge of lifelong risk; prior treatment and financial toxicity; priorities of the institution, as there has to be philosophical, financial, and resource commitment of the health care organization to ensure success; and payment—reimbursement should be commensurate with effort (in our experience the average new survivor patient visit takes 122 min and the average return visit takes 91 min). Working to overcome these barriers and implement them into practice is certainly a priority for the field.
Advancing the Field of Cardio-Oncology
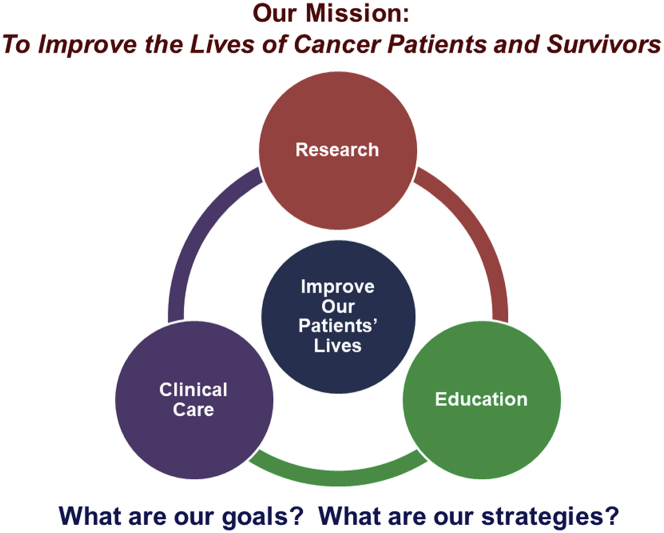
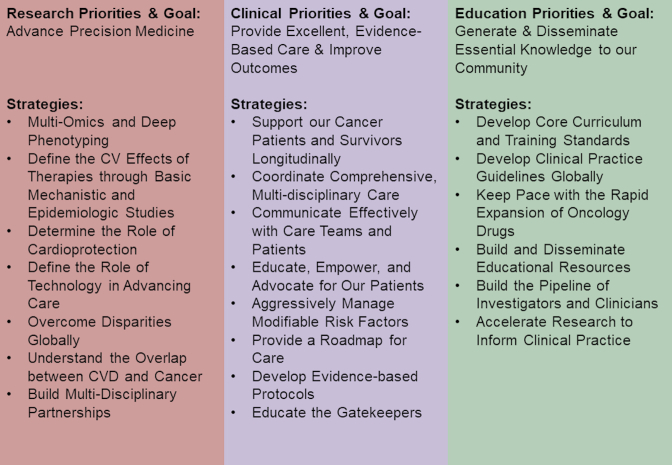
In the last session of the GCOS meeting, Dr. Ky provided her perspective on how to advance the field of cardio-oncology. Part of this included a historical perspective on the history of cardio-oncology, beginning first with a case series describing the CV complications of anthracyclines from the 1960s. Since then, the field has seen tremendous growth in the development of newer cancer therapies and changes in cancer treatment paradigms, as well as the epidemiology of cancer, which altogether have important implications for CVD. The global burden of CVD and cancer was also noted, as these 2 disease states are the 2 major causes of morality worldwide, with 9.5 million deaths due to cancer and 17.7 million deaths due to CVD (118). To advance the field, Dr. Ky proposed that the mission needs to be focused on improving the lives of cancer patients and survivors (Figure 3). To accomplish this mission, goals and strategies were proposed in the following 3 domains: research, clinical care, and education. Major goals in each of these domains include advancement of precision medicine; provision of excellent, evidence-based patient care focused on improving outcomes; and the generation and dissemination of knowledge to our multidisciplinary community. Potential strategies to accomplish these goals are noted in Figure 4. Dr. Ky concluded with a statement reflective of her tireless devotion and commitment to excellence, rigor, and the strengthening of our community through JACC: CardioOncology (119).
Figure 3.
Paradigm for Advancing the Field of Cardio-Oncology
At Global Cardio-Oncology Summit 2019, Dr. Ky presented a paradigm on how to continue to advance the field of cardio-oncology that is focused on the ultimate goal of improving the lives of our cancer patients and survivors.
Figure 4.
Specific Strategies for Advancing the Field of Cardio-Oncology
At Global Cardio-Oncology Summit 2019, Dr. Ky delineated specific strategies to help advance the field of cardio-oncology. CVD = cardiovascular disease.
Footnotes
Dr. Lenihan has received research support from Myocardial Solutions, Inc.; and has received consulting fees from Pfizer, Roche, Acorda, Bristol-Myers Squibb, Lilly, and Prothena. Dr. Fradley has received research grant support from Medtronic. Dr. Dent has received honoraria from Novartis Canada. Dr. Neilan has received consulting fees from H3-Biomedicine, Bristol-Myers Squibb, Parexel, Aprea Therapeutics, and Intrinsic Imaging. Dr. Melloni has received consulting fees from Amgen, AstraZeneca, Bristol-Myers Squibb, Ferring Pharmaceuticals, GlaxoSmithKline, Intra-Cellular Therapies, Luitpold Pharmaceuticals, Merck and Co., Roche Group, Sanofi, St. Jude Medical, and Pfizer. Dr. Ky has received research support from the NIH; and has received consulting support from Bristol-Myers Squibb. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Anju Nohria, MD, served as Guest Editor for this paper.
Appendix
For a supplemental figure, please see the online version of this paper.
Appendix
References
- 1.Cardinale D., Sandri M.T., Martinoni A. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol. 2000;36:517–522. doi: 10.1016/s0735-1097(00)00748-8. [DOI] [PubMed] [Google Scholar]
- 2.Lenihan D.J., Cardinale D., Cipolla C.M. The compelling need for a cardiology and oncology partnership and the birth of the International CardiOncology Society. Prog Cardiovasc Dis. 2010;53:88–93. doi: 10.1016/j.pcad.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Strongman H., Gadd S., Matthews A. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet. 2019;394:1041–1054. doi: 10.1016/S0140-6736(19)31674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Law W., Johnson C., Rushton M., Dent S. The Framingham risk score underestimates the risk of cardiovascular events in the HER2-positive breast cancer population. Curr Oncol. 2017;24:e348–e353. doi: 10.3747/co.24.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ezaz G., Long J.B., Gross C.P., Chen J. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc. 2014;3:e000472. doi: 10.1161/JAHA.113.000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romond E.H., Jeong J.H., Rastogi P. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30:3792–3799. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel-Qadir H., Thavendiranathan P., Austin P.C. Development and validation of a multivariable prediction model for major adverse cardiovascular events after early stage breast cancer: a population-based cohort study. Eur Heart J. 2019 Jul 18 doi: 10.1093/eurheartj/ehz460. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Thavendiranathan P., Abdel-Qadir H., Fischer H.D. Risk-imaging mismatch in cardiac imaging practices for women receiving systemic therapy for early-stage breast cancer: a population-based cohort study. J Clin Oncol. 2018;36:2980–2987. doi: 10.1200/JCO.2018.77.9736. [DOI] [PubMed] [Google Scholar]
- 9.Narayan H.K., Finkelman B., French B. Detailed echocardiographic phenotyping in breast cancer patients: associations with ejection fraction decline, recovery, and heart failure symptoms over 3 years of follow-up. Circulation. 2017;135:1397–1412. doi: 10.1161/CIRCULATIONAHA.116.023463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ky B., Putt M., Sawaya H. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809–816. doi: 10.1016/j.jacc.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demissei B.G., Finkelman B.S., Hubbard R.A. Cardiovascular function phenotypes in response to cardiotoxic breast cancer therapy. J Am Coll Cardiol. 2019;73:248–249. doi: 10.1016/j.jacc.2018.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardinale D., Colombo A., Bacchiani G. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 13.Sawaya H., Sebag I.A., Plana J.C. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5:596–603. doi: 10.1161/CIRCIMAGING.112.973321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plana J.C., Galderisi M., Barac A. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Oikonomou E.K., Kokkinidis D.G., Kampaktsis P.N. Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol. 2019 Aug 21 doi: 10.1001/jamacardio.2019.2952. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Negishi T., Thavendiranathan P., Negishi K., Marwick T.H., investigators S Rationale and design of the strain surveillance of chemotherapy for improving cardiovascular outcomes: the SUCCOUR trial. J Am Coll Cardiol Img. 2018;11:1098–1105. doi: 10.1016/j.jcmg.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Upshaw J.N., Finkelman B., Hubbard R.A. Comprehensive assessment of changes in left ventricular diastolic function with contemporary breast cancer therapy. J Am Coll Cardiol Img. 2019 Sep 18 [E-pub ahead of print] [Google Scholar]
- 18.Riddell E., Lenihan D. The role of cardiac biomarkers in cardio-oncology. Curr Probl Cancer. 2018;42:375–385. doi: 10.1016/j.currproblcancer.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Cardinale D., Sandri M.T., Martinoni A. Myocardial injury revealed by plasma troponin I in breast cancer treated with high-dose chemotherapy. Ann Oncol. 2002;13:710–715. doi: 10.1093/annonc/mdf170. [DOI] [PubMed] [Google Scholar]
- 20.Cardinale D., Sandri M.T., Colombo A. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109:2749–2754. doi: 10.1161/01.CIR.0000130926.51766.CC. [DOI] [PubMed] [Google Scholar]
- 21.Cardinale D., Colombo A., Sandri M.T. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114:2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 22.Onitilo A.A., Engel J.M., Stankowski R.V., Liang H., Berg R.L., Doi S.A. High-sensitivity C-reactive protein (hs-CRP) as a biomarker for trastuzumab-induced cardiotoxicity in HER2-positive early-stage breast cancer: a pilot study. Breast Cancer Res Treat. 2012;134:291–298. doi: 10.1007/s10549-012-2039-z. [DOI] [PubMed] [Google Scholar]
- 23.Morris P.G., Chen C., Steingart R. Troponin I and C-reactive protein are commonly detected in patients with breast cancer treated with dose-dense chemotherapy incorporating trastuzumab and lapatinib. Clin Cancer Res. 2011;17:3490–3499. doi: 10.1158/1078-0432.CCR-10-1359. [DOI] [PubMed] [Google Scholar]
- 24.Skovgaard D., Hasbak P., Kjaer A. BNP predicts chemotherapy-related cardiotoxicity and death: comparison with gated equilibrium radionuclide ventriculography. PLoS One. 2014;9:e96736. doi: 10.1371/journal.pone.0096736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Iuliis F., Salerno G., Taglieri L. Serum biomarkers evaluation to predict chemotherapy-induced cardiotoxicity in breast cancer patients. Tumour Biol. 2016;37:3379–3387. doi: 10.1007/s13277-015-4183-7. [DOI] [PubMed] [Google Scholar]
- 26.Lenihan D.J., Stevens P.L., Massey M. The utility of point-of-care biomarkers to detect cardiotoxicity during anthracycline chemotherapy: a feasibility study. J Card Fail. 2016;22:433–438. doi: 10.1016/j.cardfail.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Cornell R.F., Ky B., Weiss B.M. Prospective study of cardiac events during proteasome inhibitor therapy for relapsed multiple myeloma. J Clin Oncol. 2019;37:1946–1955. doi: 10.1200/JCO.19.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardinale D., Sandri M.T., Colombo A. Prevention of atrial fibrillation in high-risk patients undergoing lung cancer surgery: the PRESAGE trial. Ann Surg. 2016;264:244–251. doi: 10.1097/SLA.0000000000001626. [DOI] [PubMed] [Google Scholar]
- 29.Mahmood S.S., Fradley M.G., Cohen J.V. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee Chuy K., Oikonomou E.K., Postow M.A. Myocarditis surveillance in patients with advanced melanoma on combination immune checkpoint inhibitor therapy: the Memorial Sloan Kettering Cancer Center experience. Oncologist. 2019;24:e196–e197. doi: 10.1634/theoncologist.2019-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armenian S.H., Lacchetti C., Barac A. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 32.Guglin M., Krischer J., Tamura R. Randomized trial of lisinopril versus carvedilol to prevent trastuzumab cardiotoxicity in patients with breast cancer. J Am Coll Cardiol. 2019;73:2859–2868. doi: 10.1016/j.jacc.2019.03.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gulati G., Heck S.L., Ree A.H. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 x 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37:1671–1680. doi: 10.1093/eurheartj/ehw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pituskin E., Mackey J.R., Koshman S. Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research (MANTICORE 101-Breast): a randomized trial for the prevention of trastuzumab-associated cardiotoxicity. J Clin Oncol. 2017;35:870–877. doi: 10.1200/JCO.2016.68.7830. [DOI] [PubMed] [Google Scholar]
- 35.Avila M.S., Ayub-Ferreira S.M., de Barros Wanderley M.R., Jr. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J Am Coll Cardiol. 2018;71:2281–2290. doi: 10.1016/j.jacc.2018.02.049. [DOI] [PubMed] [Google Scholar]
- 36.Boekhout A.H., Gietema J.A., Milojkovic Kerklaan B. Angiotensin II-receptor inhibition with candesartan to prevent trastuzumab-related cardiotoxic effects in patients with early breast cancer: a randomized clinical trial. JAMA Oncol. 2016;2:1030–1037. doi: 10.1001/jamaoncol.2016.1726. [DOI] [PubMed] [Google Scholar]
- 37.Vaduganathan M., Hirji S.A., Qamar A. Efficacy of neurohormonal therapies in preventing cardiotoxicity in patients with cancer undergoing chemotherapy. J Am Coll Cardiol CardioOnc. 2019;1:54–65. doi: 10.1016/j.jaccao.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dent S., Melloni C., Ivars J., Sammons S., Kimmick G. Cardiotoxicities of modern treatments in breast cancer. Curr Treat Options Cardiovasc Med. 2019;21:34. doi: 10.1007/s11936-019-0738-z. [DOI] [PubMed] [Google Scholar]
- 39.Gilchrist S.C., Barac A., Ades P.A. Cardio-Oncology Rehabilitation to Manage Cardiovascular Outcomes in Cancer Patients and Survivors: a scientific statement from the American Heart Association. Circulation. 2019;139:e997–e1012. doi: 10.1161/CIR.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandala M., Falanga A., Roila F., Group E.G.W. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22 Suppl 6:vi85–vi92. doi: 10.1093/annonc/mdr392. [DOI] [PubMed] [Google Scholar]
- 41.Aronson D., Brenner B. Arterial thrombosis and cancer. Thromb Res. 2018;164 Suppl 1:S23–S28. doi: 10.1016/j.thromres.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Navi B.B., Reiner A.S., Kamel H. Risk of Arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926–938. doi: 10.1016/j.jacc.2017.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chew H.K., Wun T., Harvey D., Zhou H., White R.H. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166:458–464. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 44.Timp J.F., Braekkan S.K., Versteeg H.H., Cannegieter S.C. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122:1712–1723. doi: 10.1182/blood-2013-04-460121. [DOI] [PubMed] [Google Scholar]
- 45.Khorana A.A., Soff G.A., Kakkar A.K. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med. 2019;380:720–728. doi: 10.1056/NEJMoa1814630. [DOI] [PubMed] [Google Scholar]
- 46.Carrier M., Abou-Nassar K., Mallick R. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711–719. doi: 10.1056/NEJMoa1814468. [DOI] [PubMed] [Google Scholar]
- 47.Key N.S., Khorana A.A., Kuderer N.M. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2019 Aug 5 doi: 10.1200/JCO.19.01461. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Raskob G.E., van Es N., Verhamme P. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378:615–624. doi: 10.1056/NEJMoa1711948. [DOI] [PubMed] [Google Scholar]
- 49.Young A.M., Marshall A., Thirlwall J. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D) J Clin Oncol. 2018;36:2017–2023. doi: 10.1200/JCO.2018.78.8034. [DOI] [PubMed] [Google Scholar]
- 50.McBane R., 2nd, Wysokinski W.E., Le-Rademacher J.G. Apixaban and dalteparin in active malignancy associated venous thromboembolism: the ADAM VTE trial. J Thromb Haemost. 2019 Oct 20 doi: 10.1160/TH17-03-0193. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 51.Mauri L., Kereiakes D.J., Yeh R.W. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371:2155–2166. doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Urban P., Meredith I.T., Abizaid A. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med. 2015;373:2038–2047. doi: 10.1056/NEJMoa1503943. [DOI] [PubMed] [Google Scholar]
- 53.Wang F., Gulati R., Lennon R.J. Cancer history portends worse acute and long-term noncardiac (but not cardiac) mortality after primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. Mayo Clin Proc. 2016;91:1680–1692. doi: 10.1016/j.mayocp.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 54.Iliescu C.A., Grines C.L., Herrmann J. SCAI Expert Consensus Statement: Evaluation, Management, and Special Considerations of Cardio-Oncology Patients in the Cardiac Catheterization Laboratory (Endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista) Catheter Cardiovasc Interv. 2016;87:E202–E223. doi: 10.1002/ccd.26379. [DOI] [PubMed] [Google Scholar]
- 55.Gori T., Polimeni A., Indolfi C., Raber L., Adriaenssens T., Munzel T. Predictors of stent thrombosis and their implications for clinical practice. Nat Rev Cardiol. 2019;16:243–256. doi: 10.1038/s41569-018-0118-5. [DOI] [PubMed] [Google Scholar]
- 56.Bhatia S., Francisco L., Carter A. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhatia S., Robison L.L., Francisco L. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105:4215–4222. doi: 10.1182/blood-2005-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wingard J.R., Majhail N.S., Brazauskas R. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29:2230–2239. doi: 10.1200/JCO.2010.33.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chow E.J., Mueller B.A., Baker K.S. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann Intern Med. 2011;155:21–32. doi: 10.7326/0003-4819-155-1-201107050-00004. [DOI] [PubMed] [Google Scholar]
- 60.Chow E.J., Baker K.S., Flowers M.E. Influence of metabolic traits and lifestyle factors on cardiovascular disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:S226–S227. [Google Scholar]
- 61.Armenian S.H., Chow E.J. Cardiovascular disease in survivors of hematopoietic cell transplantation. Cancer. 2014;120:469–479. doi: 10.1002/cncr.28444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koelwyn G.J., Khouri M., Mackey J.R., Douglas P.S., Jones L.W. Running on empty: cardiovascular reserve capacity and late effects of therapy in cancer survivorship. J Clin Oncol. 2012;30:4458–4461. doi: 10.1200/JCO.2012.44.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kelsey C.R., Scott J.M., Lane A. Cardiopulmonary exercise testing prior to myeloablative allo-SCT: a feasibility study. Bone Marrow Transplant. 2014;49:1330–1336. doi: 10.1038/bmt.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Armenian S.H., Sun C.L., Francisco L. Late congestive heart failure after hematopoietic cell transplantation. J Clin Oncol. 2008;26:5537–5543. doi: 10.1200/JCO.2008.17.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carver J.R., Shapiro C.L., Ng A. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25:3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 66.Lee P.J., Mallik R. Cardiovascular effects of radiation therapy: practical approach to radiation therapy-induced heart disease. Cardiol Rev. 2005;13:80–86. doi: 10.1097/01.crd.0000131188.41589.c5. [DOI] [PubMed] [Google Scholar]
- 67.Biedermann B.C., Sahner S., Gregor M. Endothelial injury mediated by cytotoxic T lymphocytes and loss of microvessels in chronic graft versus host disease. Lancet. 2002;359:2078–2083. doi: 10.1016/S0140-6736(02)08907-9. [DOI] [PubMed] [Google Scholar]
- 68.Lee S.J., Seaborn T., Mao F.J. Frequency of abnormal findings detected by comprehensive clinical evaluation at 1 year after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15:416–420. doi: 10.1016/j.bbmt.2008.12.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wingard J.R., Vogelsang G.B., Deeg H.J. Stem cell transplantation: supportive care and long-term complications. Hematology Am Soc Hematol Educ Program. 2002:422–444. doi: 10.1182/asheducation-2002.1.422. [DOI] [PubMed] [Google Scholar]
- 70.Armenian S.H., Landier W., Francisco L. Long-term pulmonary function in survivors of childhood cancer. J Clin Oncol. 2015;33:1592–1600. doi: 10.1200/JCO.2014.59.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baker K.S., Chow E., Steinberger J. Metabolic syndrome and cardiovascular risk in survivors after hematopoietic cell transplantation. Bone Marrow Transplant. 2012;47:619–625. doi: 10.1038/bmt.2011.118. [DOI] [PubMed] [Google Scholar]
- 72.Armenian S.H., Yang D., Berano Teh J. Impact of sarcopenia on adverse outcomes after allogeneic hematopoietic cell transplantation. J Natl Cancer Inst. 2019 Feb 14 doi: 10.1093/jnci/djy231. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith S.R., Haig A.J., Couriel D.R. Musculoskeletal, neurologic, and cardiopulmonary aspects of physical rehabilitation in patients with chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21:799–808. doi: 10.1016/j.bbmt.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 74.Greenland P., Alpert J.S., Beller G.A. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–e103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Armenian S.H., Sun C.L., Vase T. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120:4505–4512. doi: 10.1182/blood-2012-06-437178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun C.L., Francisco L., Kawashima T. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood. 2010;116:3129–3139. doi: 10.1182/blood-2009-06-229369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koene R.J., Prizment A.E., Blaes A., Konety S.H. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reicher-Reiss H., Jonas M., Goldbourt U., Boyko V., Modan B. Selectively increased risk of cancer in men with coronary heart disease. Am J Cardiol. 2001;87:459–462. doi: 10.1016/s0002-9149(00)01405-3. A6. [DOI] [PubMed] [Google Scholar]
- 79.Douxfils J., Haguet H., Mullier F., Chatelain C., Graux C., Dogne J.M. Association between BCR-ABL tyrosine kinase inhibitors for chronic myeloid leukemia and cardiovascular events, major molecular response, and overall survival: a systematic review and meta-analysis. JAMA Oncol. 2016;2:625–632. doi: 10.1001/jamaoncol.2015.5932. [DOI] [PubMed] [Google Scholar]
- 80.Moslehi J.J., Deininger M. Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol. 2015;33:4210–4218. doi: 10.1200/JCO.2015.62.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abdel-Qadir H., Amir E., Fischer H.D. The risk of myocardial infarction with aromatase inhibitors relative to tamoxifen in post-menopausal women with early stage breast cancer. Eur J Cancer. 2016;68:11–21. doi: 10.1016/j.ejca.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 82.Gupta D., Lee Chuy K., Yang J.C., Bates M., Lombardo M., Steingart R.M. Cardiovascular and metabolic effects of androgen-deprivation therapy for prostate cancer. J Oncol Pract. 2018;14:580–587. doi: 10.1200/JOP.18.00178. [DOI] [PubMed] [Google Scholar]
- 83.Darby S.C., Ewertz M., McGale P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 84.van Nimwegen F.A., Schaapveld M., Cutter D.J. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol. 2016;34:235–243. doi: 10.1200/JCO.2015.63.4444. [DOI] [PubMed] [Google Scholar]
- 85.Jaiswal S., Natarajan P., Silver A.J. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iannaccone M., D'Ascenzo F., Vadala P. Prevalence and outcome of patients with cancer and acute coronary syndrome undergoing percutaneous coronary intervention: a BleeMACS substudy. Eur Heart J Acute Cardiovasc Care. 2018;7:631–638. doi: 10.1177/2048872617706501. [DOI] [PubMed] [Google Scholar]
- 87.Potts J., Mohamed M.O., Lopez Mattei J.C. Percutaneous coronary intervention and in-hospital outcomes in patients with leukemia: a nationwide analysis. Catheter Cardiovasc Interv. 2019 Aug 13 doi: 10.1002/ccd.28432. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 88.Borovac J.A., Kwok C.S., Iliescu C. Percutaneous coronary intervention and outcomes in patients with lymphoma in the United States (Nationwide Inpatient Sample [NIS] Analysis) Am J Cardiol. 2019;124:1190–1197. doi: 10.1016/j.amjcard.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 89.Reed G.W., Masri A., Griffin B.P., Kapadia S.R., Ellis S.G., Desai M.Y. Long-term mortality in patients with radiation-associated coronary artery disease treated with percutaneous coronary intervention. Circ Cardiovasc Interv. 2016;9:e003483. doi: 10.1161/CIRCINTERVENTIONS.115.003483. [DOI] [PubMed] [Google Scholar]
- 90.Thavendiranathan P., Grant A.D., Negishi T., Plana J.C., Popovic Z.B., Marwick T.H. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61:77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 91.Suh E., Daugherty C.K., Wroblewski K. General internists' preferences and knowledge about the care of adult survivors of childhood cancer: a cross-sectional survey. Ann Intern Med. 2014;160:11–17. doi: 10.7326/M13-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lenihan D.J., Hartlage G., DeCara J. Cardio-oncology training: a proposal from the International Cardioncology Society and Canadian Cardiac Oncology Network for a new multidisciplinary specialty. J Card Fail. 2016;22:465–471. doi: 10.1016/j.cardfail.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 93.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tang J., Shalabi A., Hubbard-Lucey V.M. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol. 2018;29:84–91. doi: 10.1093/annonc/mdx755. [DOI] [PubMed] [Google Scholar]
- 95.Eggermont A.M.M., Blank C.U., Mandala M. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 96.Heery C.R., Coyne G.H.O.S., Madan R.A. Phase I open-label, multiple ascending dose trial of MSB0010718C, an anti-PD-L1 monoclonal antibody, in advanced solid malignancies. J Clin Oncol. 2014;32:3064. [Google Scholar]
- 97.Johnson D.B., Balko J.M., Compton M.L. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Neilan T.G., Rothenberg M.L., Amiri-Kordestani L. Myocarditis associated with immune checkpoint inhibitors: an expert consensus on data gaps and a call to action. Oncologist. 2018;23:874–878. doi: 10.1634/theoncologist.2018-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Escudier M., Cautela J., Malissen N. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136:2085–2087. doi: 10.1161/CIRCULATIONAHA.117.030571. [DOI] [PubMed] [Google Scholar]
- 100.Awadalla M., Golden D.L.A., Mahmood S.S. Influenza vaccination and myocarditis among patients receiving immune checkpoint inhibitors. J Immunother Cancer. 2019;7:53. doi: 10.1186/s40425-019-0535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Norwood T.G., Westbrook B.C., Johnson D.B. Smoldering myocarditis following immune checkpoint blockade. J Immunother Cancer. 2017;5:91. doi: 10.1186/s40425-017-0296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brahmer J.R., Lacchetti C., Schneider B.J. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Puzanov I., Diab A., Abdallah K. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Haanen J., Carbonnel F., Robert C. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 105.Guiney T.E., Lopes M.S., Kalra M.K., Mooradian M.J., Neilan T.G., Stone J.R. Case 30-2019: a 65-year-old woman with lung cancer and chest pain. N Engl J Med. 2019;381:1268–1277. doi: 10.1056/NEJMcpc1900423. [DOI] [PubMed] [Google Scholar]
- 106.Gotsman I., Grabie N., Dacosta R., Sukhova G., Sharpe A., Lichtman A.H. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Invest. 2007;117:2974–2982. doi: 10.1172/JCI31344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gelsomino F., Fiorentino M., Zompatori M. Programmed death-1 inhibition and atherosclerosis: can nivolumab vanish complicated atheromatous plaques? Ann Oncol. 2018;29:284–286. doi: 10.1093/annonc/mdx718. [DOI] [PubMed] [Google Scholar]
- 108.Krause D.S., Van Etten R.A. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353:172–187. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- 109.Aghel N., Lipton J.H., Atenafu E.G., Kim D.D.H., Delgado D.H. Cardiovascular events after exposure to nilotinib in chronic myeloid leukemia: long-term follow-up. Clin Lymphoma Myeloma Leuk. 2017;17:870–878.e1. doi: 10.1016/j.clml.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 110.Hadzijusufovic E., Albrecht-Schgoer K., Huber K. Nilotinib-induced vasculopathy: identification of vascular endothelial cells as a primary target site. Leukemia. 2017;31:2388–2397. doi: 10.1038/leu.2017.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Latifi Y., Moccetti F., Wu M. Thrombotic microangiopathy as a cause of cardiovascular toxicity from the BCR-ABL1 tyrosine kinase inhibitor ponatinib. Blood. 2019;133:1597–1606. doi: 10.1182/blood-2018-10-881557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fradley M.G., Gliksman M., Emole J. Rates and risk of atrial arrhythmias in patients treated with ibrutinib compared with cytotoxic chemotherapy. Am J Cardiol. 2019;124:539–544. doi: 10.1016/j.amjcard.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 113.Wiczer T.E., Levine L.B., Brumbaugh J. Cumulative incidence, risk factors, and management of atrial fibrillation in patients receiving ibrutinib. Blood Adv. 2017;1:1739–1748. doi: 10.1182/bloodadvances.2017009720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ganatra S., Sharma A., Shah S. Ibrutinib-associated atrial fibrillation. J Am Coll Cardiol Img EP. 2018;4:1491–1500. doi: 10.1016/j.jacep.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 115.Levade M., David E., Garcia C. Ibrutinib treatment affects collagen and von Willebrand factor-dependent platelet functions. Blood. 2014;124:3991–3995. doi: 10.1182/blood-2014-06-583294. [DOI] [PubMed] [Google Scholar]
- 116.Guha A., Derbala M.H., Zhao Q. Ventricular arrhythmias following ibrutinib initiation for lymphoid malignancies. J Am Coll Cardiol. 2018;72:697–698. doi: 10.1016/j.jacc.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Salem J.E., Manouchehri A., Bretagne M. Cardiovascular toxicities associated with ibrutinib. J Am Coll Cardiol. 2019;74:1667–1678. doi: 10.1016/j.jacc.2019.07.056. [DOI] [PubMed] [Google Scholar]
- 118.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ky B. JACC: CardioOncology: poised to serve a maturing, collaborative field. J Am Coll Cardiol CardioOnc. 2019;1:131–132. doi: 10.1016/j.jaccao.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Armanious M.A., Mishra S., Fradley M.G. Electrophysiologic toxicity of chemoradiation. Curr Oncol Rep. 2018;20:45. doi: 10.1007/s11912-018-0691-0. [DOI] [PubMed] [Google Scholar]
- 121.Ou S.H., Tang Y., Polli A., Wilner K.D., Schnell P. Factors associated with sinus bradycardia during crizotinib treatment: a retrospective analysis of two large-scale multinational trials (PROFILE 1005 and 1007) Cancer Med. 2016;5:617–622. doi: 10.1002/cam4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guha A., Armanious M., Fradley M.G. Update on cardio-oncology: Novel cancer therapeutics and associated cardiotoxicities. Trends Cardiovasc Med. 2019;29:29–39. doi: 10.1016/j.tcm.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 123.Abdel-Rahman O., ElHalawani H., Ahmed H. Risk of selected cardiovascular toxicities in patients with cancer treated with MEK inhibitors: a comparative systematic review and meta-analysis. J Glob Oncol. 2015;1:73–82. doi: 10.1200/JGO.2015.000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Banks M., Crowell K., Proctor A., Jensen B.C. Cardiovascular effects of the MEK inhibitor, trametinib: a case report, literature review, and consideration of mechanism. Cardiovasc Toxicol. 2017;17:487–493. doi: 10.1007/s12012-017-9425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pandey A.K., Singhi E.K., Arroyo J.P. Mechanisms of VEGF (vascular endothelial growth factor) inhibitor-associated hypertension and vascular disease. Hypertension. 2018;71:e1–e8. doi: 10.1161/HYPERTENSIONAHA.117.10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.