Corresponding Author

Key Words: anthracycline, cardiotoxicity, global longitudinal strain
Anthracycline cardiotoxicity (AC) is one of the most concerning cancer therapeutic adverse events and can be a limiting factor in treating hematological malignancies, breast cancers, and other solid tumors (1, 2, 3). One of the “holy grails” of cardio-oncology is to be able to predict which patients will develop cardiotoxicity. Several studies have identified clinical variables associated with increased risk of AC (4, 5, 6, 7, 8, 9); however, several different definitions of AC have been proposed. Early definitions used clinical evidence of heart failure (HF) as per Von Hoff et al. (10), but this has changed to various arbitrary left ventricular ejection fraction (LVEF) cutoffs (11,12), elevations in troponin levels (7), and more recently changes in global longitudinal strain (GLS) (11). Unfortunately, there is not a clear consensus on a standardized definition for either clinical or for research purposes. The mechanism of AC is likely multifactorial, including impairment in mitochondrial function, increased burden of free radicals (12), a genetic component, and potentially alterations in topoisomerase 2-beta expression, which increase myocyte apoptosis (13). It has been suggested that patients with traditional cardiovascular risk factors may have less contractile reserve, making them more vulnerable to develop left ventricular systolic dysfunction with anthracycline use (14). This has been shown by many studies of AC prediction, despite limitations in a lack of consensus defining AC.
In the study by Kang et al. (15) published in this issue of JACC: CardioOncology, the investigators took on the task of developing a risk score to predict symptomatic HF related to AC by using retrospective data on 450 patients with acute myelocytic leukemia and acute lymphocytic leukemia. In this cohort, 40 (8.9%) patients developed symptomatic HF over a median follow-up of 16 months, with consideration for noncardiac death as a competing event. Using a multivariable Fine and Gray’s subdistribution hazard regression model analysis, they found 6 baseline variables to be predictive of symptomatic HF. The investigators developed a 21-point risk score using these variables, each weighted based on their clinical relevance: age >60 years (1 point), cumulative anthracycline dose ≥250 mg/m2 (2 points), presence of AML (4 points), pre-existing cardiovascular disease (4 points), baseline LVEF <50% (4 points), and baseline GLS absolute value of >−15% (greater impairment) (6 points). With this risk score, they were able to separate the patient population into low (0 to 6 points; risk: 0.9%), moderate (7 to 13 points; risk: 18.8%), or high risk (14 to 21 points; risk: 65%) to develop symptomatic HF following treatment with anthracycline. Some of these baseline risk factors, such as age, cardiovascular disease, and anthracycline dose are present in the American Society of Clinical Oncology Clinical Practice Guideline of Prevention and Monitoring in Survivors of Adult Cancers (16). The authors acknowledged the need to validate this score in a separate prospective cohort. Of note, chronic HF was part of the pre-existing cardiovascular disease definition, but because symptomatic HF endpoint was not categorized as either a new event or an exacerbation, the accuracy of risk estimation in patients without pre-existing HF may be affected.
One of the strengths of this study is the use of symptomatic HF as an endpoint, rather than subclinical AC. Other approaches used to improve on the outcome definition were the exclusion of septic patients with a decline in left ventricular systolic function, use of noncardiac death as a competing risk, and deeming transfer to hospice as noncardiac death. Studying these populations can be difficult if cancer morbidity and competing cancer-related death are not separated from cardiovascular outcomes. For example, it is well known that cancer patients can be more prone to develop stress-induced cardiomyopathy, a phenomenon that may be seen in hematological malignancies in the presence of sepsis (17,18).
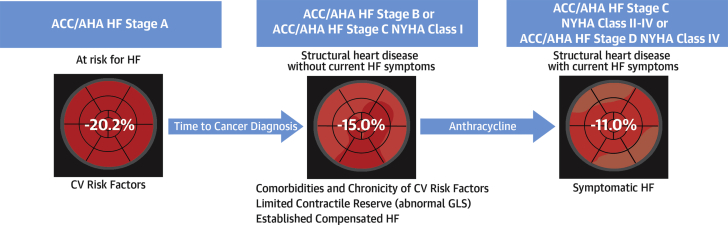
Anthracycline use in clinical practice in the setting of a low baseline LVEF is quite uncommon (baseline prevalence of LVEF <53% was 7% in this cohort), and perhaps the inclusion of this covariate could be less useful in widespread clinical practice, but in tertiary centers with complex and selected cases, this may occur more often. LVEF was an important risk predictor, but less so in comparison with GLS. Limitations of LVEF derived from echocardiography should be kept in mind given the wider interobserver variability (19). GLS continues to be more robust than LVEF in these types of clinical studies (20,21). It weighted the most in this risk score, which is congruent with the hypothesis that baseline vulnerability from subclinical impairment of myocardial mechanics before treatment with anthracycline increases the risk of toxicity (Figure 1) (22). The work by Kang et al. (15) is a move in the right direction, with regard to studying AC by using well-established and clinically relevant cardiovascular endpoints such as symptomatic HF.
Figure 1.
Vulnerability to Develop Symptomatic Heart Failure With Exposure to Anthracycline
Baseline CV risk factors and disease and impaired myocardial mechanics, as quantified by GLS, seem to identify vulnerable patients who could develop symptomatic HF following treatment with anthracycline. ACCF/AHA HF stages from Yancy et al. (22). ACCF = American College of Cardiology Foundation; AHA = American Heart Association; CV = cardiovascular; GLS = Global longitudinal strain; HF = heart failure; NYHA = New York Heart Association.
Footnotes
The authors have reported that they have no relationship relevant to the contents of this paper to disclose.
References
- 1.Durand M., Lacaria K., Sidsworth M., Davis M.K., Sanford D. Management of cardiovascular health in acute leukemia: a national survey. Leuk Lymphoma. 2019;60:2982–2992. doi: 10.1080/10428194.2019.1613539. [DOI] [PubMed] [Google Scholar]
- 2.Upshaw J.N., Ruthazer R., Miller K.D. Personalized decision making in early stage breast cancer: applying clinical prediction models for anthracycline cardiotoxicity and breast cancer mortality demonstrates substantial heterogeneity of benefit-harm trade-off. Clin Breast Cancer. 2019;19:259–267.e1. doi: 10.1016/j.clbc.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bini I., Asaftei S.D., Riggi C. Anthracycline-induced cardiotoxicity in patients with paediatric bone sarcoma and soft tissue sarcoma. Cardiol Young. 2017;27:1815–1822. doi: 10.1017/S1047951117001536. [DOI] [PubMed] [Google Scholar]
- 4.Kotwinski P., Smith G., Cooper J. Body surface area and baseline blood pressure predict subclinical anthracycline cardiotoxicity in women treated for early breast cancer. PLoS One. 2016;11 doi: 10.1371/journal.pone.0165262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferraro M.P., Gimeno-Vazquez E., Subirana I. Anthracycline-induced cardiotoxicity in diffuse large B-cell lymphoma: NT-proBNP and cardiovascular score for risk stratification. Eur J Haematol. 2019;102:509–515. doi: 10.1111/ejh.13234. [DOI] [PubMed] [Google Scholar]
- 6.Goel S., Liu J., Guo H. Decline in left ventricular ejection fraction following anthracyclines predicts trastuzumab cardiotoxicity. JACC Heart Failure. 2019;7:795–804. doi: 10.1016/j.jchf.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Cardinale D., Sandri M.T., Colombo A. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109:2749–2754. doi: 10.1161/01.CIR.0000130926.51766.CC. [DOI] [PubMed] [Google Scholar]
- 8.Sawaya H., Sebag I.A., Plana J.C. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5:596–603. doi: 10.1161/CIRCIMAGING.112.973321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawaya H., Sebag I.A., Plana J.C. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011;107:1375–1380. doi: 10.1016/j.amjcard.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Von Hoff D.D., Layard M.W., Basa P. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 11.Plana J.C., Galderisi M., Barac A. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Gewirtz D.A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S., Liu X., Bawa-Khalfe T. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 14.Jones D.N., Jordan J.H., Meléndez G.C. Frequency of transition from stage A to stage B heart failure after initiating potentially cardiotoxic chemotherapy. JACC Heart Fail. 2018;6:1023–1032. doi: 10.1016/j.jchf.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang Y., Assuncao B.L., Denduluri S. Symptomatic heart failure in acute leukemia patients treated with anthracyclines. J Am Coll Cardiol CardioOnc. 2019;1:208–217. doi: 10.1016/j.jaccao.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armenian S.H., Lacchetti C., Barac A. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 17.Giza D.E., Lopez-Mattei J., Vejpongsa P. Stress-induced cardiomyopathy in cancer patients. Am J Cardiol. 2017;120:2284–2288. doi: 10.1016/j.amjcard.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Cammann V.L., Sarcon A., Ding K.J. Clinical features and outcomes of patients with malignancy and takotsubo syndrome: observations from the International Takotsubo Registry. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.010881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellenger N.G., Davies L.C., Francis J.M., Coats A.J.S., Pennell D.J. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2000;2:271–278. doi: 10.3109/10976640009148691. [DOI] [PubMed] [Google Scholar]
- 20.Oikonomou E.K., Kokkinidis D.G., Kampaktsis P.N. Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol. 2019 Aug 21 doi: 10.1001/jamacardio.2019.2952. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thavendiranathan P., Poulin F., Lim K.-D., Plana J.C., Woo A., Marwick T.H. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63:2751–2768. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 22.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]