Corresponding Author

Key Words: cardiac fibrosis, chimeric antigen receptor, fibroblast activation protein
Pathological cardiac fibrosis accompanies nearly all forms of heart failure and contributes to mechanical dysfunction of the diseased heart. After myocardial infarction, the fibrotic response is a critical defense against myocardial rupture, but adverse remodeling subsequently contributes to progression of heart failure. Fibrosis results, at least in part, from inflammation and immune activation that accompanies cardiac stress, ischemia, infarction, or exposure to a host of toxic agents. Therapeutic approaches to intervene in the inflammation-fibrosis axis have been the focus of intense research and clinical investigation, albeit with generally disappointing results to date (1). At present, there are essentially no effective therapies for established cardiac fibrosis.
Emerging results from animal studies suggest that targeting cardiac fibroblasts, even after fibrosis and heart failure are established, is an approach worthy of further investigation. At first glance, this is not an intuitive conclusion because large portions of an established cardiac scar are composed of extracellular matrix, which may in some cases be “fixed” and unresponsive to destruction of the fibroblasts that originally produced the matrix. However, we are learning that scar tissue is often actively maintained by ongoing production and concomitant resorption processes. Diseases such as scurvy, in which old wounds can reopen due to breakdown of existing scar, teach that scar is not immutable. Fibroblasts themselves, as well as myocytes, macrophages, and other cells, make enzymes that degrade matrix including metalloproteases, collagenases, and endopeptidases. Thus, mouse models in which cardiac fibroblasts are destroyed using genetic approaches (including the expression of diphtheria toxin in activated fibroblasts) after scar has already formed reveal the possibility in at least some circumstances of rapid resorption of existing scar and improved cardiac function as a result (2).
Based on these observations, we recently tested the efficacy of engineered T cells targeted to seek and destroy activated fibroblasts in the diseased heart (3). One of the most exciting advances in cancer therapy in recent times has been the advent of cell-based therapies to target leukemia and other malignant cell types (4). Patients with acute lymphoblastic leukemia who did not respond to all conventional therapies and have little hope of survival have been treated with a single dose of engineered T cells and have achieved lasting remission. In these cases, the patients’ own T cells are removed from the body, exposed ex vivo to a lentivirus encoding a chimeric antigen receptor (CAR) that recognizes the CD19 molecule expressed on all B cells (including leukemic B cells), and reinfused with functional expression of the engineered CAR. Rapid tumor lysis ensues, frequently resulting in release of potent cytokines that can induce hyperactivation of the immune system, a life-threatening complication known as cytokine release syndrome (CRS), which can include cardiac toxicity, as reviewed by Ghosh et al. (5) in this issue of JACC: CardioOncology. With appropriate supportive therapy, patients often emerge disease-free (although requiring ongoing immunoglobulin therapy due to the lack of B cells) and long-lasting clones of engineered T cells can provide ongoing surveillance against tumor recurrence.
The opportunity to extend this approach to treat fibrotic diseases is attractive, although it is necessary to identify antigens expressed by pathological, activated fibroblasts that can allow them to be distinguished from quiescent, physiological fibroblasts that are necessary for tissue integrity and homeostasis. A growing body of work using powerful tools of single cell and single nucleus analysis coupled with genetic lineage tracing in mouse models suggests that there are many types of fibroblasts in the heart and in other tissues, and that fibroblasts induced to proliferate, to secrete extracellular matrix, and to modulate the immune response can be identified through gene expression characteristics and cell surface markers (6,7).
To identify an appropriate antigenic target for CAR T therapy in human fibrotic hearts, we analyzed RNA sequencing data from diseased explanted human hearts obtained at the time of transplantation and compared these with healthy controls. We identified fibroblast-specific transcripts that were expressed in hearts of patients with hypertrophic and dilated cardiomyopathies, but not in healthy control hearts. Among these candidates, Fibroblast Activation Protein (FAP) showed the greatest difference between control and diseased specimens (3).
FAP is a transmembrane and secreted glycoprotein with prolyl endopeptidase activity that has been extensively studied. It is expressed at very low levels or not at all in most healthy tissues, but expression is up-regulated in many activated fibroblasts associated with injury and tumor stroma. FAP is expressed by fibroblasts in hearts of patients with cardiomyopathy and in scar associated with infarction (8). It is also expressed by fibroblasts in the cirrhotic liver, arthritic joints, and other diseased tissues. CAR T cells targeting FAP have been developed for use in cancer, including pancreatic cancer where tumor stroma is a dominant feature of disease, and in mesothelioma (9). Several patients in Europe have received FAP CAR T cells without reported complications (NCT01722149), although the results of these trials have not been reported in the literature.
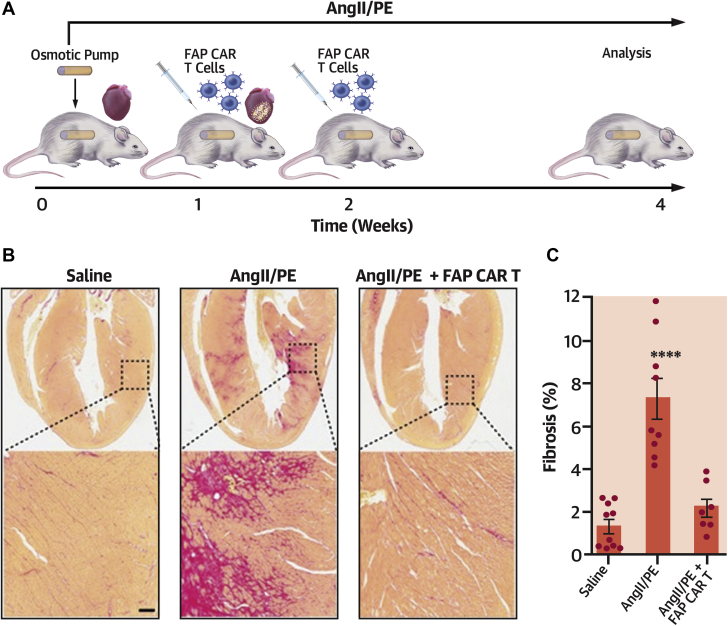
A mouse model of hypertensive heart disease that has been well validated in the literature involves chronic infusion of angiotensin II and phenylephrine via implanted osmotic pumps over a 2-week period. These animals develop significant cardiac fibrosis and signs of cardiac dysfunction. Treatment with FAP CAR T cells, delivered after the appearance of cardiac fibrosis, dramatically attenuated fibrosis at 4 weeks and improved function when compared with animals treated with T cells that had not been engineered to recognize FAP (Figure 1) (3). Infused FAP CAR T cells accumulated in the heart within 1 day of delivery. Treated animals did not exhibit signs of CRS, such as fever, lethargy, or other untoward reactions, and only mild changes in serum cytokines or gene expression abnormalities indicative of cardiotoxicity were recorded. This is likely due to the fact that the overall burden of cell lysis in this model is far lower than in leukemia, where CRS has been observed, and perhaps because fibroblasts contain fewer cytokines released upon cell lysis than lymphocytes.
Figure 1.
FAP CAR T Cells Can Target Cardiac Fibrosis
(A) Schematic of experiments for FAP CAR T cell targeting of cardiac fibroblasts. (B) Picro-Sirius red staining of heart coronal sections in mice treated with saline (left), AngII/PE (center), or AngII/PE and FAP CAR T cells (right) to evaluate fibrosis (red). (Bottom) Magnification of left ventricular fibrosis. Scale bar, 100 μm. (C) Quantification of cardiac fibrosis. ****p < 0.0001; one-way ANOVA between groups, p < 0.0001; Tukey test was used for post-hoc multiple comparisons; n = 10, 9, and 7 biologically independent mice, from left to right. Reproduced with permission from Aghajanian et al. (3). ANGII/PE = angiotensin II and phenylephrine; ANOVA = analysis of variance; CAR = chimeric antigen receptor; FAP = fibroblast activation protein.
Interestingly, animals treated with FAP CAR T cells were able to heal skin wounds at an identical rate to control animals, despite the fact that some activated fibroblasts in the skin express FAP. This is likely due to the fact that many activated fibroblasts in the skin do not express FAP and are able to compensate for those that are ablated by FAP CAR T cells. This result is consistent with the notion that there are many types of fibroblasts in the body, with populations characterized by different markers of activation. Indeed, in the hearts treated with angiotensin and phenylephrine we noted a population of perivascular fibroblasts that form a “cuff” around small coronary arteries that do not express FAP and are not removed by FAP CAR T therapy. The clinical implications of this finding are yet to be determined, but the existence of multiple populations of activated fibroblasts raises the prospect of developing an array of engineered T cells targeting different populations relevant to specific diseases or tissues.
These proof-of-concept results in mice are encouraging, although multiple hurdles exist before first-in-human clinical trials can be initiated. Unlike cancer, where immune surveillance and long-term persistence of targeted T cells is an advantage and where every last cancer cell should be destroyed, fibrotic disorders demand only an overall reduction in disease burden to achieve clinical benefit. Persistence of anti-fibroblast T cells is likely to be associated with untoward effects and complications. A treated patient who has a subsequent myocardial infarction, for example, might be prone to myocardial rupture. A short-lived, transient CAR T cell is far more attractive, or one in which activity could be regulated with a “switch” or small-molecule regulator, such as those being developed in multiple laboratories. A short-lived CAR T cell can be produced by engineering the cells with messenger RNA instead of virally encoded DNA, thus avoiding the need for a viral vector and the possibility of genomic integration (10). A messenger RNA–generated CAR T cell could be delivered in doses as needed to produce clinical benefit, perhaps by infusion into a coronary artery to produce maximal “first pass” effect. Such “second-generation” CAR T cells will have to be developed against FAP or other fibroblast-specific antigens and validated in animal models before moving to the clinic.
The ability to assess cardiac fibrotic burden and the expression of the target antigen (e.g., FAP) will also be important tools to facilitate clinical translation. FAP–positron emission tomography (PET) probes are already in development and identify robust signal in the hearts of animals after myocardial infarction but not in healthy controls (11) and these probes have also been used in humans. FAP-PET imaging may be useful to screen for potential non-cardiac effects of FAP CAR T therapy due to FAP-expressing scar in non-cardiac tissues, and for monitoring the effectiveness of FAP CAR T therapy. Although it is early days, the prospect of using immunotherapy and engineered immune cells to treat cardiac disease, and other forms of fibrotic illness, represents a new and exciting frontier in medicine.
Footnotes
Dr. Epstein reports a U.S. Provisional Patent Application No. 62/563,323, filed September 26, 2017 entitled: “Treatment of Heart Disease via CAR-T Cell Immunotherapy.”
The author attests they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
References
- 1.Mann D.L., McMurray J.J., Packer M. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 2.Kaur H., Takefuji M., Ngai C.Y. Targeted ablation of periostin-expressing activated fibroblasts prevents adverse cardiac remodeling in mice. Circ Res. 2016;118:1906–1917. doi: 10.1161/CIRCRESAHA.116.308643. [DOI] [PubMed] [Google Scholar]
- 3.Aghajanian H., Kimura T., Rurik J.G. Targeting cardiac fibrosis with engineered T cells. Nature. 2019;573:430–433. doi: 10.1038/s41586-019-1546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.June C.H., O'Connor R.S., Kawalekar O.U., Ghassemi S., Milone M.C. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh A.K., Chen D.H., Guha A., Mackenzie S., Walker J.M., Roddie C. CAR T-cell therapy–related cardiovascular outcomes and management: systemic disease or direct cardiotoxicity? J Am Coll Cardiol CardioOnc. 2020;2:97–109. doi: 10.1016/j.jaccao.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanisicak O., Khalil H., Ivey M.J. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun. 2016;7:12260. doi: 10.1038/ncomms12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tallquist M.D., Molkentin J.D. Redefining the identity of cardiac fibroblasts. Nat Rev Cardiol. 2017;14:484–491. doi: 10.1038/nrcardio.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tillmanns J., Hoffmann D., Habbaba Y. Fibroblast activation protein alpha expression identifies activated fibroblasts after myocardial infarction. J Mol Cell Cardiol. 2015;87:194–203. doi: 10.1016/j.yjmcc.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Petrausch U., Schuberth P.C., Hagedorn C. Re-directed T cells for the treatment of fibroblast activation protein (FAP)-positive malignant pleural mesothelioma (FAPME-1) BMC Cancer. 2012;12:615. doi: 10.1186/1471-2407-12-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster J.B., Barrett D.M., Kariko K. The emerging role of in vitro-transcribed mRNA in adoptive T cell immunotherapy. Mol Ther. 2019;27:747–756. doi: 10.1016/j.ymthe.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varasteh Z., Mohanta S., Robu S. Molecular imaging of fibroblast activity after myocardial infarction using a (68)Ga-labeled fibroblast activation protein inhibitor, FAPI-04. J Nucl Med. 2019;60:1743–1749. doi: 10.2967/jnumed.119.226993. [DOI] [PMC free article] [PubMed] [Google Scholar]