Two distinct types of systemic amyloidosis, light chain (AL) and transthyretin (ATTR), account for >95% of diagnosed cardiac amyloidosis (CA) (1). AL arises from a clonal population of plasma cells that produce misfolded immunoglobulin light chains that aggregate in organs such as the heart, kidneys, peripheral and autonomic nerves, liver, and gastrointestinal tract (1). ATTR arises from the liver-derived protein transthyretin (TTR) that becomes kinetically unstable, misfolds, and aggregates into amyloid fibrils. This can occur due to a hereditary point mutation (ATTRv for “variant”) or is acquired as a form known as wild-type (ATTRwt) (1), which is most commonly associated with aging. ATTRv affects the peripheral and autonomic nerves and/or the heart, with the phenotype varying depending on the mutation, whereas ATTRwt exhibits a more cardiac-dominant phenotype (1).
The traditional method used to diagnose amyloidosis is histological confirmation of the affected organ using Congo red or thioflavin S staining (2). Further characterization of the amyloid fibril protein content is made using immunohistochemistry (IHC) or liquid chromatography tandem mass spectrometry (LC-MS/MS) (2). Determination of the type of amyloidosis is crucial because the prognosis and treatment significantly differ between AL and ATTR amyloidosis (1). Current analytical techniques are very good at distinguishing the 2 types; however, occasionally, there can be diagnostic confusion by identifying the presence of multiple precursor proteins in an amyloid deposit, including reports of co-deposition of AL and TTR (3, 4, 5).
Herein, we present 2 cases with clinical and histological evidence of both ATTR and AL-CA occurring concurrently.
Case 1
An 89-year-old Eastern European woman with a history of hypertension and stage 4 chronic kidney disease presented with heart failure. Initial echocardiography revealed a preserved ejection fraction of 60% with thickened left ventricular walls and a mitral inflow pattern suggestive of restrictive cardiomyopathy. Electrocardiography (ECG) showed sinus rhythm with occasional premature atrial complexes. Due to a high suspicion for cardiac amyloidosis, work-up with nuclear 99m-technetium pyrophosphate scintigraphy (99mTc-PYP) was pursued. This showed a semi quantitative visual score of grade 3 with diffuse cardiac uptake on single-photon emission computed tomography-computed tomography that was highly suggestive of ATTR-CA. However, simultaneous laboratory work-up for AL amyloidosis showed a lambda restricted M protein present on both serum and urine immunofixation, along with serum free kappa light chains of 20.6 mg/l and serum free lambda of 531.3 mg/l with a highly abnormal ratio of 0.04, which indicated a lambda monoclonal gammopathy.
Because of the potential diagnosis of ATTR-CA or AL-CA, endomyocardial biopsy was obtained. IHC stains displayed a striking positivity for TTR in a nodular pattern within the interstitium and weak positivity in a peri-myocytic linear pattern around the myocytes for a lambda AL. Laser capture microdissection and LC-MS/MS identified both TTR and lambda AL as the main amyloidogenic proteins in the sample. Subsequent bone marrow biopsy revealed a monoclonal lambda plasma cell neoplasm that represented approximately 20% to 30% of the bone marrow cellularity without evidence of amyloid deposits. Concurrent diagnosis of lambda AL-CA and ATTR-CA was established. TTR genetic testing revealed no mutation, consistent with ATTRwt-CA.
Antiplasma cell therapy was recommended to treat the AL component of CA. At the time of diagnosis, no treatments were available for ATTR-CA. The patient opted for palliative care, was discharged to hospice, and died 5 months after initial onset of symptoms.
Case 2
An 85-year-old Russian man presented with heart failure with acute volume overload and an NT-pro-B-type natriuretic peptide level of 7,913 pg/ml, which required admission for intravenous diuresis. ECG showed normal sinus rhythm, but chest radiographs revealed a lytic lesion in the left scapula, prompting further investigation. Echocardiography revealed increased left ventricular septal and posterior wall thickness and grade III diastolic dysfunction. Cardiac magnetic resonance displayed diffuse delayed myocardial enhancement of the left ventricle with overall features suggestive of amyloidosis, and the patient was referred to an amyloidosis specialist.
Upon initial work-up for CA, 99mTc-PYP showed grade 3 cardiac uptake suggestive of ATTR-CA. However, monoclonal protein testing, which was ordered at the same time, showed a lambda M protein present on serum and urine immunofixation, along with serum free kappa of 26.3 mg/l and free lambda of 365.5 mg/l with a highly abnormal ratio of 0.07, which indicated a lambda monoclonal gammopathy. Bone marrow biopsy revealed a monoclonal lambda plasma cell neoplasm representing approximately 10% of the bone marrow cellularity with trace amyloid deposition. An endomyocardial biopsy was performed to reconcile the findings, and IHC stains displayed nodular deposits positive for TTR and distinct perimyocytic staining positive for lambda AL. The IHC results were confirmed by LC-MS/MS, establishing a diagnosis of concurrent lambda AL-CA and ATTR-CA (Figure 1). TTR genetic testing showed no mutation, consistent with ATTRwt-CA.
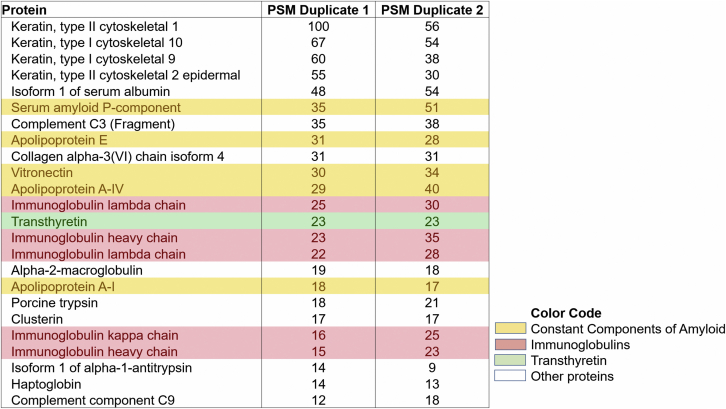
Figure 1.
PSM Matches From LC-MS/MS Analysis of Endomyocardial Biopsy From Case 2
Protein spectrum matches (PSM) convey information on the relative abundance of particular proteins. In most cases of amyloid, a few proteins are always identified and in relatively abundant quantities: serum P component, apolipoprotein E, apolipoprotein A1, apolipoprotein AIV, and vitronectin (yellow). Further examination identifies multiple proteins with amyloidogenic properties, including transthyretin (green) and the immunoglobulin light chains (red). The identification of transthyretin in the samples, at a PSM comparable to that of the other amyloid-associated proteins, is evidence that the amyloid has a significant transthyretin component. Immunoglobulin heavy and light chains are present in virtually all patient samples, a consequence of serum contamination. However, in this case, the light chains are present at concentrations similar to those of other amyloid-associated proteins. Although kappa immunoglobulin light chains are significantly more abundant than lambda in most patients, in this case, lambda light chains dominated, providing another piece of evidence that amyloid has a significant lambda light chain component. PSM duplicates 1 and 2 represent duplicate testing results from the same patient. LC-MS/MS = liquid chromatography with tandem mass spectrometry.
Antiplasma cell therapy with bortezomib was initiated to treat the AL component of the CA. Like the previous patient, no treatments were available for ATTR-CA at the time of diagnosis. One day after receiving his fourth bortezomib infusion, the patient decompensated and was admitted for heart failure. Following a palliative care consultation, the patient was discharged to hospice and died.
Discussion
These 2 cases represent the rare concurrence of clinical and histological evidence of AL-CA and ATTR-CA (3, 4, 5). Co-deposition of 2 types of amyloid protein has been previously reported but reports of cardiac ATTR and AL co-deposition are exceedingly rare (3, 4, 5). Concomitant ATTR and AL cardiac amyloidosis have been described in 2 patients by Siddiqi et al. (5) and in a case report by Mahmood et al. (4). Liepnieks and Benson reported an autopsy finding of ATTR and AL co-deposition in a patient diagnosed with ATTR-CA (3).
In our report, the work-up of both patients entailed simultaneous 99mTc-PYP scan and laboratory testing for AL amyloidosis. However, most guidelines for the diagnostic work-up of suspected CA recommend testing for monoclonal protein first and, if abnormal, pursuing the pathway of bone marrow and fat pad or endomyocardial biopsy. In this pathway, there is typically no need for 99mTc-PYP in the setting of abnormal monoclonal protein. However, in some clinical scenarios, a clinician may opt to pursue both 99mTc-PYP and monoclonal laboratory testing simultaneously, particularly if the patient is an older adult with a higher pre-test likelihood of ATTR-CA.
Initially, both cases were presumed to be ATTR-CA because of the high-grade cardiac uptake on nuclear scintigraphy and the patients’ ages. However, abnormal monoclonal protein testing necessitated an endomyocardial biopsy to reconcile the findings. These cases highlight the importance of completeness of the diagnostic work-up for CA, because the existence of one disease process does not preclude the existence of the other. In addition, it is known that AL-CA can occasionally lead to significant cardiac uptake on nuclear scintigraphy (6).
The observation that >95% of CA cases are due to either ATTR or AL was pivotal in the development of the nonbiopsy diagnostic method for ATTR-CA, which is a less invasive strategy compared with traditional histological confirmation (6). Nonbiopsy diagnosis of ATTR-CA can be reliably made when there is a semi quantitative visual score of grade 2 or 3 cardiac uptake on nuclear scintigraphy coupled with a normal serum free light chain ratio and no M protein on serum and urine immunofixation to rule out AL amyloidosis (6). In cases in which AL amyloidosis cannot be ruled out due to an abnormal serum free light chain ratio and/or an M protein on immunofixation, endomyocardial biopsy is recommended for confirmation of the amyloid diagnosis and type. As these cases demonstrated, a presumptive diagnosis of ATTR-CA with positive nuclear scintigraphy alone risks missing the diagnosis of AL-CA in the setting of an underlying abnormal free light chain ratio or presence of an M protein on serum or urine immunofixation.
LC-MS/MS relies on the purification of proteins from histological sections, followed by their digestion with trypsin and analysis by LC-MS/MS. It is important to emphasize that analysis by LC-MS/MS is semi quantitative and does not allow a precise quantification of each protein, but the protein spectrum matches (PSMs) convey information on the relative abundance of particular proteins. In most cases of amyloidosis, a few proteins are always identified and present in relatively abundant quantities: serum P component, apolipoprotein E, apolipoprotein A1, apolipoprotein AIV, and vitronectin. The identification of these proteins in the cases presented was not only a confirmation of the initial diagnosis but also indicative of significant amounts of amyloid in the sample obtained by microdissection.
In our cases, further examination of the mass spectrometry data identified multiple proteins with amyloidogenic properties, including TTR and immunoglobulin lambda. The identification of TTR in the samples, at a PSM comparable to that of the other amyloid-associated proteins, was evidence that the amyloid had a significant TTR component. Immunoglobulin heavy chains and light chains are present in virtually all patient samples, which is a consequence of serum contamination. However, in this case, the immunoglobulin light chains were present at concentrations similar to those of other amyloid-associated proteins. Although kappa immunoglobulin light chains are significantly more abundant than lambda light chains in most patients, in the cases illustrated, lambda light chains dominated, providing another piece of evidence that amyloid has a significant lambda AL component.
Conclusions
These cases represented the rare presentation of clinical and histological ATTR- and AL-CA occurring concurrently. Although a dominant amyloidosis type can often be identified, AL and ATTR arise from independent pathological mechanisms, and the existence of one does not preclude the existence of the other. As awareness for CA increases with the emergence of new therapies (7, 8, 9, 10), it is important to be vigilant in the work-up and complete the diagnostic process.
Footnotes
This work was supported by the Cleveland Clinic Term Chair in Amyloid Heart Disease. Dr. Hanna has served on advisory boards for Pfizer, Alynam, Eidos, and Akcea. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
References
- 1.Donnelly J.P., Hanna M. Cardiac amyloidosis: an update on diagnosis and treatment. Cleve Clin J Med. 2017;84:12 Suppl 3:12–26. doi: 10.3949/ccjm.84.s3.02. [DOI] [PubMed] [Google Scholar]
- 2.Tan C.D., Rodriguez E.R. Cardiac amyloidosis. In: Picken M., Herrera G., Dogan A., editors. Amyloid and Related Disorders. Current Clinical Pathology. Humana Press; Totowa, NJ: 2015. pp. 391–411. [Google Scholar]
- 3.Liepnieks J.J., Benson M.D. Codeposition of transthyretin and immunoglobulin lambda light chain in senile cardiac (ATTR) amyloidosis. In: Grateu G., Kyle R.A., Skinner M., editors. Proceedings of Xth International Symposium on Amyloidosis. CRC Press; Washington, DC: 2004. pp. 332–333. [Google Scholar]
- 4.Mahmood S., Gilbertson J.A., Rendell N. Two types of amyloid in a single heart. Blood. 2014;124:3025–3027. doi: 10.1182/blood-2014-06-580720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sidiqi M.H., McPhail E.D., Theis J.D. Two types of amyloidosis presenting in a single patient: a case series. Blood Cancer J. 2019;9:30. doi: 10.1038/s41408-019-0193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillmore J.D., Maurer M.S., Falk R.H. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133:2404–2412. doi: 10.1161/CIRCULATIONAHA.116.021612. [DOI] [PubMed] [Google Scholar]
- 7.Sperry B.W., Ikram A., Hachamovitch R. Efficacy of chemotherapy for light-chain amyloidosis in patients presenting with symptomatic heart failure. J Am Coll Cardiol. 2016;67:2941–2948. doi: 10.1016/j.jacc.2016.03.593. [DOI] [PubMed] [Google Scholar]
- 8.Khouri J., Kin A., Thapa B. Daratumumab proves safe and highly effective in AL amyloidosis. Br J Haematol. 2019;185:342–344. doi: 10.1111/bjh.15455. [DOI] [PubMed] [Google Scholar]
- 9.Maurer M.S., Schwartz J.H., Gundapaneni B. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–1016. doi: 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]
- 10.Buxbaum J.N. Oligonucleotide drugs for transthyretin amyloidosis. N Engl J Med. 2018;379:82–85. doi: 10.1056/NEJMe1805499. [DOI] [PubMed] [Google Scholar]