Abstract
Objectives
This study sought to evaluate the safety of continuing trastuzumab in patients with human epidermal growth factor receptor–positive breast cancer who developed mild cardiotoxicity.
Background
Cardiotoxicity is the most common dose-limiting toxicity associated with trastuzumab. Current standard of care is discontinuation of trastuzumab, which can lead to worse cancer outcomes. It is unknown whether it is safe to continue trastuzumab despite mild cardiotoxicity.
Methods
Patients were eligible for this phase I, prospective, single-arm trial if left ventricular ejection fraction (LVEF) was between 40% and the lower limit of normal or if it fell ≥15% from baseline. Participants were treated with angiotensin-converting enzyme (ACE) inhibitors and/or beta-blockers in a cardio-oncology clinic and were followed clinically and with serial echocardiograms for 1 year. The primary outcome was cardiac dose-limiting toxicity, defined as cardiovascular death, LVEF <40% together with any heart failure symptoms, or LVEF <35%.
Results
All 20 participants received ACE inhibitors and/or beta-blockers. A total of 18 participants (90%) received all planned trastuzumab doses. Two (10%) participants developed cardiac dose-limiting toxicity (heart failure with LVEF <40%). Their LVEF and heart failure symptoms improved to nearly normal following permanent trastuzumab discontinuation. There were no deaths. LVEF rose progressively from a mean of 49% at enrollment to 55% at 12 months (p < 0.001).
Conclusions
It may be feasible to continue trastuzumab despite mild cardiotoxicity in the setting of a cardio-oncology clinic, where ACE inhibitors and beta-blockers are administered. Approximately 10% of patients may develop moderate to severe heart failure using this approach. (Safety of Continuing Chemotherapy in Overt Left Ventricular Dysfunction Using Antibodies to Human Epidermal Growth Factor Receptor-2 [SCHOLAR]; NCT02907021)
Key Words: breast cancer, cardiomyopathy, HER2, trastuzumab
Abbreviations and Acronyms: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; cDLT, cardiac dose-limiting toxicity; HER, human epidermal growth factor receptor; LV, left ventricular; LVEF, left ventricular ejection fraction
Central Illustration
Breast cancer is the most common cancer among women. One in 5 women with breast cancer has human epidermal growth factor receptor (HER2)–positive disease (1). Trastuzumab is a monoclonal antibody acting on the HER2 receptor. It results in improved overall survival and reduced risk of recurrent disease in early stage HER2-positive breast cancer 2, 3. Cardiotoxicity, which manifests as impaired left ventricular (LV) function, and in some cases clinical heart failure, is the most common dose-limiting toxicity associated with trastuzumab therapy 4, 5. Trastuzumab cardiotoxicity is often mild, however. In series of patients with trastuzumab cardiotoxicity, trastuzumab did not cause cardiac myocyte necrosis 6, 7, and impaired LV function (as measured by LV ejection fraction [LVEF]) generally returns toward baseline following discontinuation of trastuzumab (2).
Breast cancer clinical practice guidelines recommend withholding or discontinuing trastuzumab if LV function declines by greater than 10% to 15%, or below the lower limit of normal (<54% for women) 8, 9. Although cardiotoxicity can be life-threatening, there is evidence to indicate that incomplete trastuzumab therapy leads to worse cancer outcomes, including higher cancer recurrence rates and higher mortality rates 10, 11. These observations led us to question current guidelines that suggest holding or discontinuing trastuzumab therapy in the presence of LV myocardial injury (and especially when LV injury is mild).
Angiotensin-converting enzyme (ACE) inhibitors and beta-blockers are effective at improving LVEF in nonischemic cardiomyopathy, including chemotherapy-induced LV dysfunction (12). We speculated that the use of ACE inhibitors and beta-blockers could prevent further decline in LV function in patients with trastuzumab cardiotoxicity.
The primary objective of the SCHOLAR (Safety of Continuing Chemotherapy in Overt Left Ventricular Dysfunction Using Antibodies to Human Epidermal Growth Factor Receptor-2) trial was to determine the safety and tolerability of a strategy of continued administration of trastuzumab in combination with therapy for LV myocardial injury (by using ACE inhibitors and beta-blockers) in patients with stage I to III HER2-positive breast cancer and LV injury secondary to trastuzumab. The secondary objective was to measure the change in LVEF with ongoing trastuzumab (coupled with standard-of-care treatments for LV impairment) in patients experiencing mild or moderate cardiotoxicity.
Methods
SCHOLAR is a modified phase I, single-arm clinical trial conducted at the Juravinski Hospital and Cancer Centre in Hamilton, Canada. The study was approved by the Institutional Research Ethics Committee. All participants provided written informed consent.
Eligibility criteria
Patients were eligible for inclusion if they fulfilled all the following criteria: 1) stage I to III HER2-positive breast cancer (defined as immunohistochemical staining for HER2 protein as 3+ or immunohistochemistry score of 2+ and in situ hybridization with an amplification ratio of ≥2.0); 2) current adjuvant therapy with trastuzumab; and 3) LV myocardial injury as evidenced by either: a) LVEF between 40% and 54%, corresponding to the lower limit of normal in women (13); or b) LVEF within normal limits (i.e., ≥54% in women) and an absolute fall in LV ejection fraction of ≥15% from baseline. Patients were excluded if any of the following criteria were fulfilled: 1) current New York Heart Association functional class III or IV heart failure; 2) systolic blood pressure <90 mm Hg; 3) current use of both an ACE inhibitor or angiotensin receptor blocker and a beta-blocker; 4) contraindications to both ACE inhibitor angiotensin receptor blocker and beta-blocker therapy; or 5) refusal to consent to participate in the study.
Study procedures
Initially, a cohort of 5 participants was to be enrolled. These patients all continued to receive trastuzumab every 3 weeks for 1 year or 18 cycles. All participants were also to be prescribed standard-of-care treatment for patients with LV systolic dysfunction, including a beta-blocker, and an ACE inhibitor at the maximum doses tolerated. Cardiac dose-limiting toxicity (cDLT) was defined as the occurrence of any of the following: 1) cardiovascular death; 2) LVEF <40% together with any heart failure symptoms; or 3) LVEF <35%. If at any time 1 or more of the first 5 participants developed cDLT, de-escalation would occur. De-escalation would involve a change in the eligibility criteria to exclude patients with an LVEF <45% and patients with New York Heart Association functional class II, III, or IV heart failure. A further 5 patients would then be recruited. If 2 or more of the second 5 participants developed cDLT after de-escalation, the intervention would be considered unsafe, and the study would be closed. If the intervention was considered safe, by using either the initial eligibility criteria or the de-escalated eligibility criteria, the study would be closed after 20 participants had completed follow-up. If at any time during the study >20% of participants developed cDLT, the intervention would be considered unsafe, and the study would be closed.
Trastuzumab would be discontinued if any of the following occurred: 1) cDLT; 2) the participant requested trastuzumab discontinuation; 3) there was evidence of rapid cancer progression; 4) unacceptable noncardiac trastuzumab toxicity developed; or 5) all initially planned trastuzumab doses were completed.
Study visits
Consecutive, potentially eligible patients were referred to the cardio-oncology service by their medical oncologist, where they were screened for recruitment. Following the provision of informed consent, participants were prescribed an ACE inhibitor or a beta-blocker by the cardiologist. After the baseline visit, where cardiac medications were initiated, cardiology visits were mandated at 3 ± 1 weeks, 6 ± 1 weeks, 3 months ± 1 week, 6 months ± 1 week, 9 months ± 1 week, and 12 months ± 1 week following the baseline visit. At each visit, information on heart failure symptoms and signs and medication use was collected. Additional visits were permitted if clinically indicated.
Study intervention
The goal was to initiate both an ACE inhibitor and a beta-blocker in each participant. To avoid first-dose hypotension, it was recommended that the initiation of the ACE inhibitor and the beta-blocker be staggered. The participant was instructed to commence taking 1 drug immediately and the other drug subsequently if the participant had not observed severe dizziness or syncope. The choice of which of the 2 drugs to start first was left to the discretion of the treating cardiologist.
For the ACE inhibitor, if the systolic blood pressure was at least 90 mm Hg and there was no contraindication to ramipril, ramipril was prescribed at an initial dose of 1.25 to 2.5 mg once daily. The dose of ramipril was assessed at each study visit and titrated accordingly up to a target dose of 10 mg once daily. Each increase in dose was individualized; for instance, if the participant has a systolic blood pressure of >150 mm Hg, the ramipril dose was increased by 2.5 to 5 mg at a time, whereas if the participant had a systolic blood pressure between 100 and 110 mm Hg, smaller ramipril dose increases of 1.25 mg at a time were recommended. Changes in ACE inhibitor dose could be made at protocol-mandated study visits or at additional visits if clinically indicated. If the participant developed symptomatic hypotension or the systolic blood pressure was <90 mm Hg, the dose of ramipril was reduced to the highest dose that did not result in symptomatic hypotension or systolic blood pressure <90 mm Hg. If intractable cough developed while the participant was taking ramipril, candesartan was prescribed instead. The initial dose of candesartan was 4 mg once daily and was titrated up to a target dose of 32 mg once daily according to a similar approach as for ramipril. Serum potassium and creatinine concentrations were measured within 6 weeks of ACE inhibitor initiation. The ACE inhibitor was discontinued if the serum potassium concentration was ≥5.5 mmol/l or if the serum creatinine concentration doubled from baseline.
For the beta-blocker, carvedilol (or if the participant was intolerant to carvedilol, then bisoprolol) was recommended. If the systolic blood pressure was at least 90 mm Hg, the heart rate was at least 55 beats/min, and there was no contraindication to carvedilol, carvedilol was prescribed at an initial dose of 3.125 to 6.25 mg twice daily. Titration of carvedilol dose followed a similar approach to titration of the ACE inhibitor, except that in addition to the lower threshold of acceptable systolic blood pressure of 90 mm Hg, a lower threshold of acceptable resting heart rate was 55 beats/min. The target doses of carvedilol and bisoprolol were 25 mg twice daily and 10 mg daily, respectively.
Evaluation of left ventricular ejection fraction
In all participants, LVEF was measured at 6 ± 1 weeks, 3 months ± 1 week, 6 months ± 1 week, 9 months ± 1 week, and 12 months ± 1 week from baseline. Extra LVEF measurements were permissible at the physician’s discretion.
Three-dimensional echocardiography was the preferred modality by which LVEF was measured. If 3-dimensional echocardiography was not possible for technical reasons, 2-dimensional echocardiography was the modality by which LVEF was measured. Measurement of LVEF was performed by a clinical cardiologist who was not necessarily a study investigator.
Outcomes
The primary safety outcome was the development of cDLT. The efficacy outcome was the number of trastuzumab cycles completed after enrollment as a proportion of the originally planned number of trastuzumab cycles. The secondary safety outcomes included symptomatic hypotension, symptomatic bradycardia, hyperkalemia (defined as serum potassium concentration ≥5.5 mmol/l), acute kidney injury, and LVEF expressed as a continuous variable.
Statistical analysis
Continuous variables are presented as mean ± SD. Categorical variables are presented as a number (percentage). For the primary outcome we report the frequency of the occurrence of cDLT. Change in LVEF over time was evaluated by linear mixed-effects models, with the participant included as a random effect to account for the increased likelihood that each individual’s LVEFs were correlated with their other LVEFs. An independent covariance structure was assumed. Because many participants had extra echocardiograms performed (in excess of those prescribed by the protocol), we analyzed LVEFs by grouping them by when they were measured relative to the enrollment echocardiogram (0 to 3, 3 to 6, 6 to 9, 9 to 12, and 12 to 24 months after enrollment). We also conducted an analysis in which LVEFs were grouped according to whether they were measured while participants were still receiving trastuzumab or after trastuzumab completion. Pairwise comparisons of LVEFs between different time points were performed with p values corrected for multiple comparisons by using Bonferroni’s adjustment. Any p values <0.05 were considered statistically significant. Statistical analysis was performed using STATA software version 15.1 (StataCorp., College Station, Texas).
Results
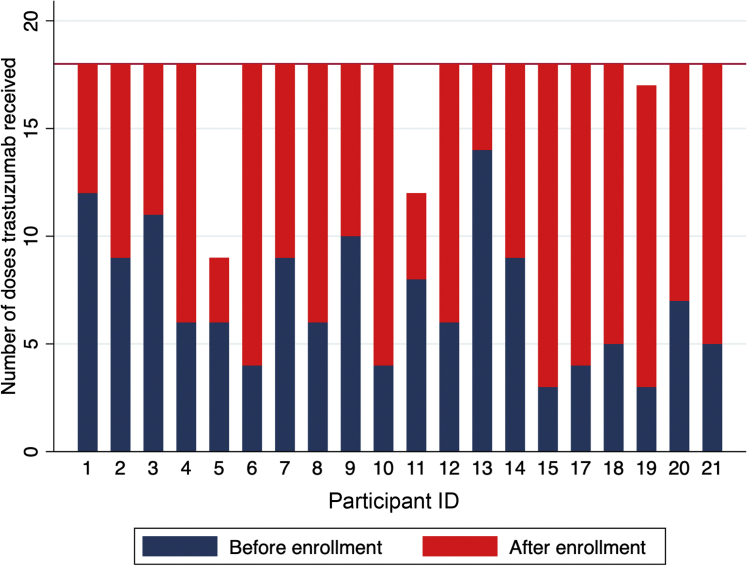
A total of 20 consecutive eligible patients consented to participate. There were no eligible patients identified who declined participation. Participants’ baseline characteristics are presented in Table 1. All patients studied were women. Before enrollment, the mean anthracycline dose administered was 223 mg/m2, and 16 (80%) of the cohort received dose-dense doxorubicin, cyclophosphamide, and paclitaxel (AC/T), whereas the remainder received doxorubicin and cyclophosphamide (AC) alone. The median time from the last dose of anthracycline to the initiation of trastuzumab was 34 days (range 21 to 147 days). The median (25th to 75th percentile) number of days from measurement of the index reduced LVEF and enrollment was 32 days (range 20 to 57 days). All SCHOLAR participants were in New York Heart Association functional class I at the baseline visit. No participant had a history of heart failure, coronary revascularization, pacemaker, implantable defibrillator, or cardiac surgery before to initiating trastuzumab. The mean LVEF following AC chemotherapy and before any trastuzumab exposure was 60 ± 3%. The mean LVEF at the time of enrollment was 49 ± 2%. The number of trastuzumab doses received before study enrollment is presented in Figure 1. The mean number of trastuzumab doses received before enrollment was 7 ± 3 (range 2 to 13). No SCHOLAR participant was taking both an ACE inhibitor and a beta-blocker at enrollment, although 4 (20%) were taking 1 of these medications.
Table 1.
Participant Characteristics (N = 20)
| Age, yrs | 59 ± 11 |
| Ethnicity | |
| Caucasian | 17 (85) |
| African | 1 (5) |
| Persian | 1 (5) |
| Indigenous | 1 (5) |
| Hypertension | 5 (25) |
| Systolic blood pressure, mm Hg | 130 ± 17 |
| Diastolic blood pressure, mm Hg | 78 ± 9 |
| Heart rate, beats/min | 83 ± 14 |
| Hyperlipidemia | 3 (15) |
| Diabetes | 1 (5) |
| Smoking | |
| Never | 14 (75) |
| Current | 1 (5) |
| Former | 5 (25) |
| Alcohol | |
| Never | 6 (30) |
| Current | 12 (60) |
| Former | 2 (10) |
| Weight, kg | 81 ± 21 |
| Body mass index, kg/m2 | 30 ± 7 |
| Laterality | |
| Left | 9 (45) |
| Right | 9 (45) |
| Bilateral | 2 (10) |
| Cancer stage | |
| I | 4 (20) |
| IIa | 6 (30) |
| IIb | 4 (20) |
| IIIa | 5 (25) |
| IIIb | 1 (5) |
| Estrogen receptor positive | 15 (75) |
| Progesterone receptor positive | 10 (50) |
| Surgery | |
| Mastectomy | 13 (65) |
| Breast conserving | 7 (35) |
| Radiotherapy | 17 (65) |
| Chemotherapy | 20 (100) |
| Hormonal therapy | |
| Tamoxifen | 3 (15) |
| Aromatase inhibitor | 11 (55) |
| Cardiovascular medications | |
| ACE inhibitor | 2 (10) |
| Beta-blocker | 2 (10) |
| Diuretic agent | 2 (10) |
| Statin | 3 (15) |
| Aspirin | 2 (10) |
Values are mean ± SD or n (%).
ACE = angiotensin-converting enzyme; SCHOLAR = Safety of Continuing Chemotherapy in Overt Left Ventricular Dysfunction Using Antibodies to Human Epidermal Growth Factor Receptor-2.
Figure 1.
Number of Trastuzumab Doses Received Before and After Enrollment, by Participant ID
Participants #5 and #11 discontinued trastuzumab permanently because of cardiac dose-limiting toxicity. Participant #19 completed treatment after 12 months of trastuzumab, amounting to 17 cycles; there was no plan to administer 18 cycles to this participant.
Cardiovascular therapies
The use rates of ACE inhibitors and beta-blockers are displayed in Table 2. This demonstrates high adherence to at least 1 cardiovascular medication. Average blood pressure and heart rate fell following the initiation of cardiovascular medications (Table 2). A total of 4 participants (20%) achieved the target dose of ACE inhibitor or angiotensin receptor blocker (ARB), and 5 (25%) achieved the target dose of beta-blocker.
Table 2.
Use Rates of ACE Inhibitors or ARB and Beta-Blockers, Blood Pressure, and Heart Rate Over Time
| Time Period | Neither ACE Inhibitor/ARB nor Beta-Blocker | Either ACE Inhibitor/ARB or Beta-Blocker | Both ACE Inhibitor or ARB and Beta-Blocker | Blood Pressure (Systolic/Diastolic), mm Hg | Heart Rate, beats/min |
|---|---|---|---|---|---|
| Baseline | 16 (80) | 4 (20) | 0 (0) | 130 ± 17/78 ± 9 | 83 ± 14 |
| 3 weeks | 0 (0) | 7 (35) | 13 (65) | 127 ± 12/76 ± 11 | 71 ± 13 |
| 6 weeks | 0 (0) | 3 (15) | 17 (85) | 120 ± 15/73 ± 9 | 72 ± 11 |
| 3 months | 0 (0) | 1 (5) | 19 (95) | 120 ± 16/75 ± 9 | 75 ± 11 |
| 6 months | 0 (0) | 2 (10) | 18 (90) | 120 ± 14/74 ± 9 | 68 ± 10 |
| 9 months | 2 (10) | 3 (15) | 14 (75) | 119 ± 14/70 ± 12 | 70 ± 9 |
| 12 months | 2 (15) | 4 (25) | 10 (60) | 121 ± 13/76 ± 8 | 73 ± 12 |
Values are n (%) or mean ± SD.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker.
Outcomes
Primary safety outcome
There were no deaths observed among SCHOLAR participants. Two participants (10%; 95% confidence interval: 1% to 32%) developed cDLT. These participants’ LVEFs fell to a trough of 28% and 26% 15 and 6 weeks after enrollment, respectively. These findings were associated with the development of New York Heart Association functional class III and class IV heart failure symptoms, respectively. In 1 patient, the decline in LV function occurred at the same time a hormone-secreting adrenocortical carcinoma was detected. The other patient was 65 years of age and was a reformed smoker with a history of hypertension but was not diabetic and did not have known coronary artery disease. Following permanent trastuzumab discontinuation, their respective LVEFs recovered to 56% and 47%, and both patients had symptoms improved to New York Heart Association functional class I.
Efficacy outcome
The number of trastuzumab doses administered before and following enrollment for each participant is displayed in Figure 1. Following enrollment, all participants received additional doses of trastuzumab. In all cases, except the 2 where cDLT occurred, all a priori planned trastuzumab doses were administered (Figure 1). Thus, 90% (95% confidence interval: 68% to 99%) of participants received the planned number of trastuzumab doses.
Secondary safety outcomes
One participant developed a single episode of symptomatic hypotension while taking both an ACE inhibitor and a beta-blocker during follow-up. This episode resolved spontaneously without requiring the discontinuation of either the ACE inhibitor or the beta-blocker. Another participant experienced an episode of dizziness and withheld her beta-blocker temporarily until symptoms resolved and then resumed taking the drug at a lower dose. No participants developed symptomatic bradycardia, acute kidney injury, or hyperkalemia.
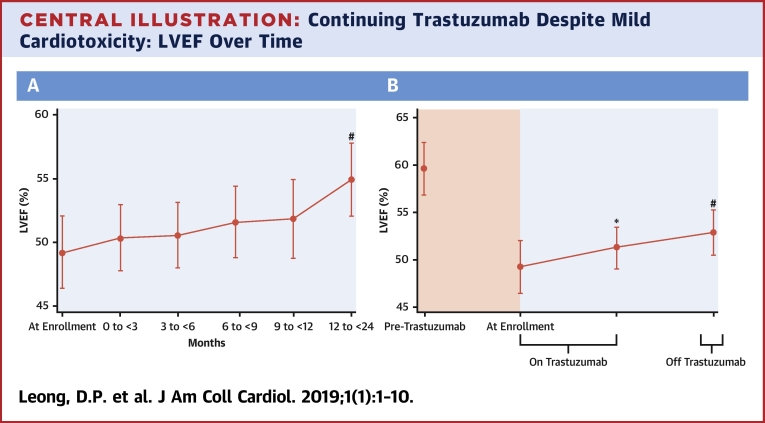
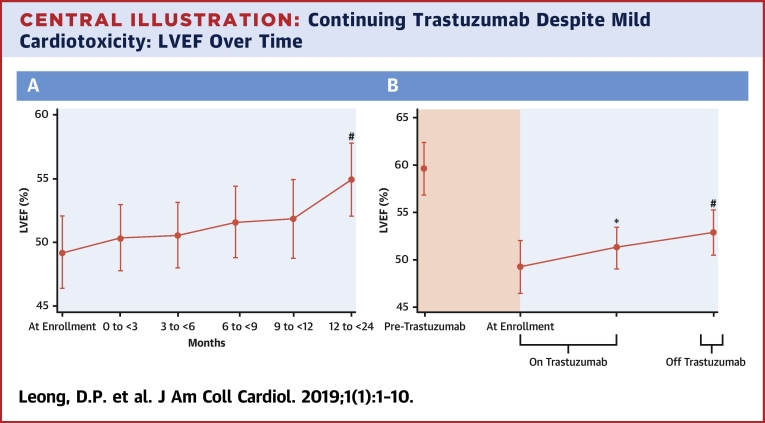
Central Illustrations A and B display the average LVEF, and Figure 2 is a spaghetti plot of individual LVEFs. There was a progressive increase in mean LVEF following the initiation of cardiovascular medications. As compared with the LVEF at the time of enrollment into SCHOLAR, the 12-month LVEF was statistically significantly higher by 5.6% (p < 0.001). However, the LVEF at 12 months failed to return to the same level as the pre-trastuzumab LVEF (a 4.8% decrease; p = 0.040).
Central Illustration.
Continuing Trastuzumab Despite Mild Cardiotoxicity: LVEF Over Time
(A) Left ventricular ejection fraction (LVEF) progressively increased despite ongoing trastuzumab in individuals with mild trastuzumab cardiotoxicity when trastuzumab was accompanied by the administration of an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker and/or a beta-blocker. #p < 0.001 as compared with the enrollment left ventricular ejection fraction indicating significant improvement in left ventricular ejection fraction as compared with the left ventricular ejection fraction at enrollment. (B) Left ventricular ejection fraction while continuing trastuzumab and after completion of trastuzumab. *p = 0.53 compared with left ventricular ejection fraction at enrollment; #p = 0.025 compared with left ventricular ejection fraction at enrollment.
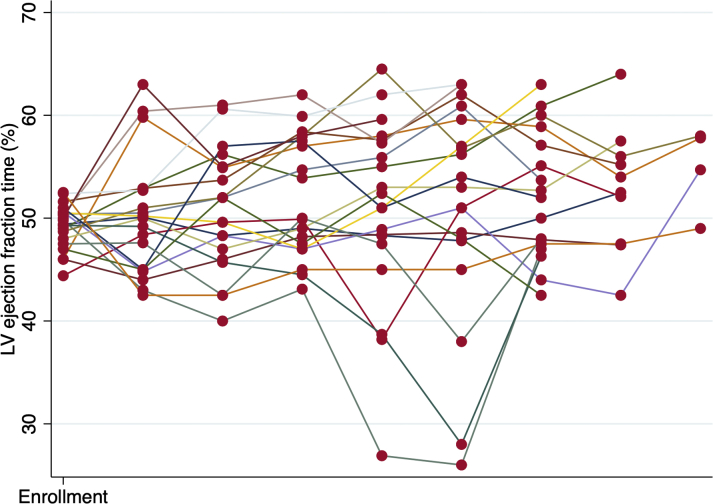
Figure 2.
LVEF Over Time Stratified by Participant
This spaghetti plot demonstrates that all but 2 participants exhibited stable left ventricular ejection fraction (LVEF) while continuing to receive trastuzumab and that the 2 participants who experienced deterioration in left ventricular ejection fraction had improvement in left ventricular ejection fraction after permanently discontinuing trastuzumab.
The average LVEF grouped according to whether the participants were on or off trastuzumab is displayed in the Central Illustration. Compared with enrollment, there was no significant fall in LVEF during trastuzumab (and ACE inhibitor or ARB and/or beta-blocker) treatment. Following trastuzumab completion, there was a significant rise in LVEF.
Discussion
The major findings from SCHOLAR are that in women with early-stage breast cancer who develop mild cardiotoxicity secondary to trastuzumab, a strategy of continuing trastuzumab in combination with therapy for LV dysfunction (cardio-oncology clinic assessment and ACE inhibitor or ARB and beta-blocker therapy) is associated with a 10% incidence of acute moderate to severe heart failure (Central Illustration). However, this strategy did lead to the uninterrupted use of trastuzumab in 90% of individuals, no patient died of a cardiovascular complication, and all patients had improved LVEFs after enrollment.
Cumulative dose dependency of trastuzumab
Current standard of care is to hold or discontinue trastuzumab in the setting of impaired LV function secondary to trastuzumab. However, there is evidence to suggest that trastuzumab duration <12 months leads to worse cancer outcomes. A recent chart review of 2,401 patients aiming to complete 17 cycles of trastuzumab showed that there was an 11.25% absolute increase in the risk of breast cancer recurrence (17.5% vs. 6.25%) in patients who had an interruption in their course of treatment compared with those who did not (14). In PHARE (Protocol of Herceptin Adjuvant With Reduced Exposure, a Randomised Comparison of 6 Months vs. 12 Months in All Women Receiving Adjuvant Herceptin), an open-label trial in which 3,384 patients with HER2-positive early-stage breast cancer were randomized to 6 or 12 months of trastuzumab, disease-free survival was 28% better (95% confidence interval: 5% to 56%) in those receiving 12 months of therapy (10). A systematic review identified 7,614 patients in 4 trials that randomized participants to 12 months of trastuzumab versus shorter durations (11). The study demonstrated that 12 months of trastuzumab therapy resulted in better overall survival (hazard ratio: 1.28; p = 0.040) and cancer-free survival (hazard ratio: 1.24; p = 0.005). A subsequent trial, PERSEPHONE (Trastuzumab in Treating Women With HER2-Positive Early Breast Cancer), did demonstrate noninferiority of 6 versus 12 months of trastuzumab therapy (15). However, the noninferiority margins in this trial were wide, and many patients and physicians may not be willing to conclude noninferiority on the basis of a 3% absolute decrease in disease-free survival. Moreover, a high proportion of patients in PERSEPHONE were at lower risk of cancer recurrence. Therefore, these findings are not sufficient to negate earlier data, and it is desirable to complete a full 12 months of trastuzumab for HER2-positive early breast cancer. In addition, the majority of SCHOLAR participants developed cardiotoxicity within 6 months of trastuzumab, so our findings would be relevant for a substantial proportion of trastuzumab recipients even if the treatment goal was 6 months of trastuzumab.
Safety of continuing trastuzumab despite manifest cardiotoxicity
In clinical trials, the rate of trastuzumab discontinuation, which is largely the result of cardiotoxicity, ranges from 8.5% to 31.4% (16). Rates of premature discontinuation of trastuzumab secondary to cardiotoxicity in the nonclinical trial setting are as high as 25% (7). Therefore, the strategy of cardio-oncology referral and initiation of cardiovascular therapies has the potential to enable continuation of trastuzumab in the majority of patients with an acceptable level of risk of heart failure. This finding has significant implications for many trastuzumab recipients.
In a retrospective study of 573 patients with HER2-positive breast cancer who were treated with adjuvant trastuzumab, 16% developed cardiotoxicity (defined by an absolute decrease in LVEF of ≥10% to <55% or an absolute decrease of ≥16%) (17). Of these patients with cardiotoxicity, trastuzumab was continued uninterrupted in 31 patients, all of whom were asymptomatic, with LVEF ranging from 50% to 63%. None of these patients developed heart failure during follow-up. Of note, the patients who received uninterrupted trastuzumab in this study were younger and had a higher LVEF at baseline than did patients who developed cardiotoxicity and had interrupted trastuzumab therapy. Whether it is safe to continue trastuzumab in all asymptomatic patients regardless of potential risk factors is unknown. We have recently reported our experience in continuing trastuzumab despite mild trastuzumab cardiotoxicity in the context of a cardio-oncology service (18). Among 18 women who elected to continue trastuzumab, 1 (6%) developed heart failure. All others were able to continue trastuzumab successfully. We designed SCHOLAR as a prospective study to extend these retrospective findings.
SCHOLAR is, to our knowledge, 1 of only 2 trials that have prospectively evaluated the safety of continuing trastuzumab despite cardiotoxicity. SAFE-HEaRT (Cardiac Safety Study in Patients With HER2-Positive Breast Cancer) (19) is another prospective single-arm trial with the same objective, although it differed from SCHOLAR in that it included patients with metastatic disease. In SAFE-HEaRT, 29 patients treated with trastuzumab and 2 patients treated with ado-trastuzumab emtansine with a mean baseline LVEF of 45% were included. Of the 30 of these participants who were evaluable, 90% completed the planned HER2-targeted therapy, and 10% developed a clinical cardiac event or LVEF fall to ≤35%. SCHOLAR also demonstrates that although it may be feasible to continue trastuzumab in many patients who develop mild cardiotoxicity, there is an important risk of progressive deterioration in LV systolic function and of clinical heart failure. Because they are single-arm trials, neither SCHOLAR nor SAFE-HEaRT can evaluate whether any cancer therapeutic benefit outweighs the risk of heart failure when adopting a strategy of continuing trastuzumab despite mild cardiotoxicity. A randomized controlled trial assessing the risks of heart failure and the benefits with respect to rates of trastuzumab completion is needed to better understand whether the strategy of continuing trastuzumab despite mild cardiotoxicity is advisable. We believe that such a trial is particularly important because LVEF did not normalize during follow-up in the SAFE-HEaRT trial and did not return to levels observed before the initiation of trastuzumab in SCHOLAR. It is therefore plausible that the approach taken in these 2 trials could cause myocardial injury that does not recover and that predisposes patients to future heart failure.
Study limitations
Follow-up was limited to 1 year, so late changes in LVEF are not known. We did not evaluate whether an ACE inhibitor and a beta-blocker can be discontinued if LVEF returns to normal after trastuzumab discontinuation. The intervention in SCHOLAR was administered in a dedicated cardio-oncology clinic at a single center. Further research is necessary to evaluate whether this intervention is feasible in other settings. The sample size of this study is small. Further research is needed to evaluate the net clinical benefit of the strategy implemented in SCHOLAR. This study is unable to fully inform the risk-to-benefit ratio of the strategy used; data on breast cancer outcomes will be important to understand whether the risk of heart failure associated with this strategy is acceptable.
Conclusions
It may be feasible to continue trastuzumab uninterrupted in patients with mild cardiotoxicity, when trastuzumab is administered in combination with cardioprotective medication in a cardiology clinic that can perform intensive monitoring, such as a cardio-oncology service. The risk of moderate to severe heart failure is approximately 10%, and the patients observed improved following trastuzumab discontinuation.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Breast cancer patients treated with trastuzumab who have LVEF declines and New York Heart Association functional class I heart failure may continue trastuzumab with the careful institution of cardiologist-prescribed heart failure medications, including ACE inhibitors, ARBs, and/or beta-blockers.
TRANSLATIONAL OUTLOOK: Continuation of trastuzumab with ACE inhibitors, ARBs, and/or beta-blockers is associated with a risk of clinical heart failure. Additional research and larger studies are necessary to confirm these findings and to establish the risk-to-benefit ratio of this approach.
Footnotes
Support for SCHOLAR was provided by the Hamilton Health Sciences Strategic Research Initiative. Dr. Leong is supported by the Heart and Stroke Foundation of Canada. Dr. Mukherjee has received honoraria for serving on the advisory boards for Roche. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Hudziak R.M., Schlessinger J., Ullrich A. Increased expression of the putative growth factor receptor p185HER2 causes transformation and tumorigenesis of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1987;84:7159–7163. doi: 10.1073/pnas.84.20.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slamon D., Eiermann W., Robert N. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romond E.H., Perez E.A., Bryant J. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 4.Ayres L.R., de Almeida Campos M.S., de Oliveira Gozzo T. Trastuzumab induced cardiotoxicity in HER2 positive breast cancer patients attended in a tertiary hospital. Int J Clin Pharm. 2015;37:365–372. doi: 10.1007/s11096-015-0070-y. [DOI] [PubMed] [Google Scholar]
- 5.Guglin M., Hartlage G., Reynolds C., Chen R., Patel V. Trastuzumab-induced cardiomyopathy: not as benign as it looks? A retrospective study. J Card Fail. 2009;15:651–657. doi: 10.1016/j.cardfail.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Ewer M.S., Lippman S.M. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol. 2005;23:2900–2902. doi: 10.1200/JCO.2005.05.827. [DOI] [PubMed] [Google Scholar]
- 7.Ewer M.S., Vooletich M.T., Durand J.B. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820–7826. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 8.Mackey J.R., Clemons M., Cote M.A. Cardiac management during adjuvant trastuzumab therapy: recommendations of the Canadian Trastuzumab Working Group. Curr Oncol. 2008;15:24–35. doi: 10.3747/co.2008.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamo C.E., Bloom M.W., Cardinale D. Cancer therapy-related cardiac dysfunction and heart failure: part 2: prevention, treatment, guidelines, and future directions. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pivot X., Romieu G., Debled M. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 2013;14:741–748. doi: 10.1016/S1470-2045(13)70225-0. [DOI] [PubMed] [Google Scholar]
- 11.Gyawali B., Niraula S. Duration of adjuvant trastuzumab in HER2 positive breast cancer: overall and disease free survival results from meta-analyses of randomized controlled trials. Cancer Treat Rev. 2017;60:18–23. doi: 10.1016/j.ctrv.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Vejpongsa P., Yeh E.T. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol. 2014;64:938–945. doi: 10.1016/j.jacc.2014.06.1167. [DOI] [PubMed] [Google Scholar]
- 13.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Gibson J., Yao R.J., Davis M., Simmons C.E. The impact of mild left ventricular dysfunction on trastuzumab use and oncologic outcomes in early stage breast cancer therapy. J Clin Oncol. 2017;35 [Google Scholar]
- 15.Earl H.M., Hiller L., Vallier A.L. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet. 2019;393:2599–2612. doi: 10.1016/S0140-6736(19)30650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montserrat M., Leveque D., Barthelemy P., Bergerat J.P. Duration of adjuvant trastuzumab treatment in routine practice. Anticancer Res. 2012;32:4585–4588. [PubMed] [Google Scholar]
- 17.Yu A.F., Yadav N.U., Eaton A.A. Continuous trastuzumab therapy in breast cancer patients with asymptomatic left ventricular dysfunction. Oncologist. 2015;20:1105–1110. doi: 10.1634/theoncologist.2015-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barron C.C., Alhussein M.M., Kaur U. An evaluation of the safety of continuing trastuzumab despite overt left ventricular dysfunction. Curr Oncol. 2019;26 doi: 10.3747/co.26.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynce F., Barac A., Geng X. Prospective evaluation of the cardiac safety of HER2-targeted therapies in patients with HER2-positive breast cancer and compromised heart function: the SAFE-HEaRt study. Breast Cancer Res Treat. 2019;175:595–603. doi: 10.1007/s10549-019-05191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]