Abstract
Objectives
This study investigated the cardioprotective effect of repeated remote ischemic preconditioning (rRIC) on doxorubicin-induced cardiotoxicity in mice.
Background
Doxorubicin is an effective chemotherapeutic agent for a wide range of tumor types but its use and dosing are limited by acute and chronic cardiotoxicity. Remote ischemic conditioning (RIC) is cardioprotective in multiple cardiovascular injury models, but the effectiveness of rRIC in doxorubicin-induced cardiotoxicity has not been fully elucidated.
Methods
rRIC was performed on mice before and after doxorubicin administration. Cardiac function was assessed by echocardiography and myocardial biology was tested by molecular approaches.
Results
Doxorubicin administration induced acute cardiotoxicity, as indicated by reduced cardiac function, reduced myocyte cross-section area and increased extracellular collagen deposition, increased circulating cardiac muscle damage markers, and decreased heart weight. Doxorubicin also adversely affected other organs, including the kidney, liver, and spleen, as evaluated by circulating markers or organ weight loss. rRIC not only abrogated doxorubicin-induced cardiotoxicity (left ventricular ejection fraction, doxorubicin 47.5 ± 1.1%, doxorubicin + rRIC 51.6 ± 0.7%, p = 0.017), but also was associated with multiorgan protection. Within the myocardium, rRIC attenuated doxorubicin-induced cardiomyocyte apoptosis, reduced inflammation, and increased autophagy signaling.
Conclusions
rRIC may be a promising approach to reduce doxorubicin-induced cardiotoxicity.
Key Words: apoptosis, autophagy, cardiac function, doxorubicin, inflammation, multiorgan toxicity, repeated remote ischemic conditioning
Abbreviations and Acronyms: BUN, blood urea nitrogen; rRIC, repeated remote ischemic conditioning
Central Illustration
Anthracyclines are a class of chemotherapeutic drugs used to treat a wide range of hematological malignances and solid tumors, including leukemias, lymphomas, neuroblastoma, soft tissue and bone sarcomas, breast carcinoma, and ovarian carcinoma. Despite decades of use, the exact antiproliferative mechanisms underlying their clinical effectiveness remain to be determined. However, it is well described that doxorubicin leads (either directly or via iron accumulation) to overproduction of reactive oxygen species, which damage vital subcellular components, including DNA, protein, and lipids, leading to cell death (1,2). Furthermore, because of its planar structure, doxorubicin intercalates between adjacent GC base pairs, probably due to specific hydrogen-bond formation between doxorubicin and guanine (3), leading to the formation of DNA adducts with resultant torsional stress and nucleosome destabilization (4). This intercalation also inhibits progression of topoisomerase II involved in DNA replication, transcription, and DNA repair (5). Interfered topoisomerase II results in double-strand DNA breaks and cell death. Other studies have shown that doxorubicin increases intracellular ceramides that arrest the cell cycle via proteolytic activating transcription factor CREB3L1 (cAMP response element binding protein 3-like 1) (6).
Although the anticancer mechanisms continue to be investigated, the antiproliferative effects of doxorubicin clearly also target proliferative noncancer cells, such as stem cells in the bone marrow and hair follicles, commonly leading to hematologic side effects and hair loss during treatment. However, cardiotoxicity has emerged as one of the most concerning adverse effects of the use of doxorubicin in cancer therapy. The risk of cardiomyopathy has been noted to increase with dose, and is estimated to be 7%, 18%, to 65% with cumulative anthracycline doses of 150, 350, and 550 mg/m2, respectively (7). There is considerable interindividual variability, however, with adverse effects at much lower doses seen in some patients. Clinically, cardiac function is usually assessed by echocardiography before and after doxorubicin administration. Strain echocardiography may be a more sensitive measure to detect early cardiotoxicity of doxorubicin (8), and cardiac magnetic resonance imaging also can be used to monitor cardiac function. Previously, serial radionuclide imaging had been used to assess left ventricular ejection fraction, and endomyocardial biopsies were sometimes obtained to characterize the myocardial damage with anthracyclines (9,10).
Several approaches to mitigate cardiotoxicity have been suggested, including: 1) limiting cumulative dose; 2) extended infusion time to reduce peak blood levels; 3) liposomal doxorubicin to increase tumor tissue distribution (11); 4) concomitant treatment with dexrazoxane (12), the only drug approved specifically to prevent doxorubicin-induced cardiotoxicity; and 5) primary or secondary prevention of worsening cardiomyopathy with neurohormonal antagonists. The merits of these approaches have been reviewed elsewhere (13), but improved approaches to reduce doxorubicin cardiotoxicity remain a clinical need.
Remote ischemic conditioning (RIC), induced by repeated cyclic occlusion of blood flow and reperfusion of a limb, protects the heart in various animal models and clinical scenarios of ischemia/reperfusion injury (14), postinfarction ventricular remodeling (15), and sepsis-induced cardiomyopathy (16). Besides the heart, RIC has been shown to be protective in other organs, including liver, brain, kidney, lung, gastrointestinal tract, skeletal muscle, and even skin flaps.
We therefore performed this proof-of-principle study to test the effect of repeated RIC (rRIC) on doxorubicin-induced cardiotoxicity in mice. We additionally performed preliminary studies to assess the potential effects of rRIC on doxorubicin-induced multiorgan dysfunction.
Methods
Animal experiment
All animal protocols were approved by the Institutional Animal Care and Use Committees of the Cincinnati Children’s Hospital Medical Center in accordance with the Animal Welfare Act and Public Health Service Policy on Humane Care and Use of Laboratory Animals. Mice were housed in a fully equipped animal facility, with free access to food and water ad libitum, and on a 12-h dark/light cycle. Male 9-week-old C57 mice were ordered from Charles River Laboratories (Wilmington, Massachusetts). Baseline echocardiography was performed after a 48-h acclimation. Animals with normal heart function (baseline ejection fraction between 50% and 65%) were randomized to receive doxorubicin 10 mg/kg (doxorubicin group, n = 8) or an equivalent volume of saline (vehicle group, n = 8) injected via intraperitoneal injection. RIC was performed 30 min before doxorubicin injection and then repeated at the same time every day for the following 5 days (doxorubicin + rRIC group, n = 8). The mice were euthanized after echocardiography on day 6. Heparinized blood was collected from the inferior caval vein, and subsequently plasma was isolated by centrifugation, aliquoted, snap frozen in liquid nitrogen, and kept at −80°C for biomarker and inflammatory factor assays. Vital organs, including heart, liver, kidney, and spleen, were harvested and weighed. The heart was sliced into 3 pieces transversely after removal of the atriums, and the middle section was embedded in optimal cutting temperature compound for histology, apoptosis, and other immune stains. The remainder was homogenized and prepared for protein analysis.
rRIC
We used a protocol of 4 cycles of 5 min of hindlimb ischemia, induced by tourniquet, followed by 5 min of reperfusion using the method we have described before (17). Ischemia was confirmed by pallor and cyanosis of the lower limb below the tourniquet. As noted previously, this was performed 30 min before doxorubicin injection and then repeated at the same time every day for 5 days.
Western blot
Heart tissue was homogenized in a lysis buffer containing 150 mM NaCl, 50 mM Tris·Cl (pH 7.5), 0.5% deoxycholate, 0.1% sodium dodecyl sulfate, 1% nonidet P-40, PhosSTOP, and Complete Mini. Protein concentration was determined using a Coomassie protein assay kit and bovine serum albumin was used as the standard. Cardiac protein levels were determined by Western blot analysis (mini-gel, 100 μg loading weight) as described previously (18) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the loading control.
Echocardiography
Cardiac function was evaluated by echocardiography by using the Vevo2100 mouse echocardiography system equipped with a 40-MHz high-frequency transducer. Studies were performed under anesthesia induced by isoflurane inhalation. Anesthetic depth was indicated by heart rate, and all images were obtained between 400 and 600 beats/min. Measurements and calculations were made offline by investigators blinded to treatments.
Cardiomyocyte cross-sectional area
After air drying and fixation in ice-cold acetone, the slides were treated with 3.3 U/ml neuraminidase type v for 60 min at room temperature. The slides then were incubated with fluorescein-labeled peanut agglutinin and rhodamine-labeled Griffonia simplicifolia lectin I for 2 h at room temperature. The slides were covered with coverslip in presence of antifade fluorescent mounting medium and sealed with nail polish oil. Four radially oriented microscope fields were selected from each section and photographed under a ×40 objective. Myocyte cross-section area was measured with Image J (National Institutes of Health, Bethesda, Maryland) and 4 images from the same sample were averaged.
Apoptosis
Frozen sections were air dried for 30 min, then fixed in ice-cold acetone for 10 min. The slides were immune-stained with α-smooth muscle actin antibody and a Texas-red conjugated second antibody. Apoptotic nuclei were stained with a TACS TDT-Fluor in situ apoptosis detection kit from RnDSystem. The total nuclei count was made after staining with 4′,6-diamidino-2-phenylindole. Five images were obtained with 3 filters: green for apoptosis, red for α-smooth muscle actin, and blue for 4′,6-diamidino-2-phenylindole under ×20 objective using Nikon (Tokyo, Japan) Eclipse confocal microscopy equipped with NIS-Elements software. The 3 color images were merged together and apoptotic nuclei expressed as number per field.
Myocardial fibrosis
Frozen section slides were air dried for 30 min, then fixed in Bonin’s solution, and stained with Pico-Sirius for 60 min. The slides were washed with acid water and mounted with a resinous medium after dehydration and cleared with xylene. Five images per slide were obtained under ×20 objective and collagen area was analyzed with Image J software, as previously described in detail (18).
Biomarker assays
Circulating biomarkers of organ injury were assayed with the following commercially available kits: uric acid with a Uric Acid Colorimetric/Fluorometric Assay kit; aspartate aminotransferase activity with a Colorimetric Assay kit from Biovision (Milpitas, California); Creatine kinase with an EnzyChrom Creatine Kinase Assay kit from BioAssay System (Hayward, California); and troponin I with a Mouse High Sensitivity Cardiac Troponin I Elisa kit from MyBioSource (San Diego, California).
Circulating inflammatory markers
The concentration of inflammatory factors in plasma was determined with a Mouse Inflammation Array Q1, sandwich enzyme-linked immunosorbent assay–based quantitative array platform from RayBiotech Life (Peachtree Corners, Georgia) following the manufacturer’s directions. The array detects 40 inflammatory factors and each antibody is arrayed on standard glass slide in quadruplicate.
Statistical analysis
Data are expressed as the mean ± SE unless otherwise noted. Differences between 2 groups were analyzed by a 2-tailed t-test, and multiple groups were compared using 1-way analysis of variance and post hoc Tukey’s or Bonferroni-Holm correction. A value of p < 0.05 was regarded as a significant. Analysis was carried out with SAS software version 9.4 (SAS Institute, Cary, North Carolina) and Excel 2016 (Microsoft, Redmond, Washington).
Results
rRIC reduces the inflammatory response to doxorubicin therapy
Doxorubicin is well known to induce a systemic inflammatory reaction (19), which, in turn, can be associated with multiorgan dysfunction. Previous data from our laboratory showed that RIC improved lipopolysaccharide-induced cardiomyopathy and reduces systemic inflammatory responses in a mouse sepsis model (16). As expected, doxorubicin induced a systemic inflammatory reaction in our animals, with 39 of 40 of the inflammatory markers showing proinflammatory directional change (12 of them reaching statistical significance). rRIC reversed these responses in most of the inflammatory factors, although only macrophage inflammatory protein-1γ and tumor necrosis factor receptor-1 reached statistical significance, when compared with doxorubicin alone (Table 1).
Table 1.
rRIC Globally Antagonized Doxorubicin-induced Alteration of Inflammatory Factors
| Vehicle (n = 8) | Dox (n = 8) | Dox/rRIC (n = 8) | p Value | |
|---|---|---|---|---|
| BLC | 23.9 (15.2–28.1) | 52.1 (34.8–108)∗ | 47.6 (33.9–60.4)∗ | 0.004 |
| CD30L | 0.39 (0–1.6) | 0.68 (0–3.0) | 0.22 (0–1.7) | 0.74 |
| Eotaxin | 601 ± 22.6 | 692 ± 17.7 | 746 ± 38.2† | 0.009 |
| Eotaxin-2 | 89.6 (50–127) | 132 (71.5–512) | 129 (118–136) | 0.28 |
| Fas L | 1.9 (0.28–3.6) | 3.5 (1.2–9.1) | 1.8 (0–5.3) | 0.40 |
| G-CSF | 29.4 (17.0–74.0) | 56.1 (18.7–131) | 29.1 (10.4–114) | 0.69 |
| GM-CSF | 3.0 ± 0.46 | 3.5 ± 1.1 | 2.4 ± 0.74 | 0.51 |
| ICAM-1 | 959 ± 144 | 1436 ± 40.9† | 1425 ± 114∗ | 0.016 |
| IFNg | 8.1 (5.6–9.7) | 4.6 (3.3–6.8) | 5.7 (3.2–9.1) | 0.26 |
| IL-1a | 1.4 (0.27–2.5) | 1.4 (0.76–2.7) | 1.7 (1.4–6.4) | 0.39 |
| IL-1b | 13.8 (6.2–16.1) | 6.4 (4.7–12.3) | 11.2 (5.2–17.5) | 0.68 |
| IL-2 | 5.1 (4.1–8.9) | 4.9 (1.0–7.4) | 4.5 (2.5–8.3) | 0.72 |
| IL-3 | 0.32 (0.19–0.50) | 0.16 (0.02–0.25) | 0.02 (0–0.16)∗ | 0.024 |
| IL-4 | 1.8 ± 0.20 | 2.2 ± 0.38 | 1.6 ± 0.43 | 0.85 |
| IL-5 | 5.4 (0–12) | 0.22 (0–7.62) | 1.98 (0–10.8) | 0.78 |
| IL-6 | 3.6 (2.6–6.8) | 4.6 (3.4–14.9) | 3.5 (0.68–6.68) | 0.42 |
| IL-7 | 0 (0–0) | 5.1 (0–57.2)∗ | 0 (0–1.35) | 0.050 |
| IL-10 | 13.2 (7.5–21.9) | 7.3 (2.4–13.3) | 12.8 (7.7–18.6) | 0.34 |
| IL-12p70 | 5.7 (2.6–7.0) | 2.4 (0–4.1) | 1.9 (0–7.4) | 0.14 |
| IL-13 | 3.6 (1.4–10.8) | 10.5 (0–19.9) | 11.7 (2.5–14.5) | 0.67 |
| IL-15 | 0 (0–2.9) | 0 (0–13.1) | 0.78 (0–12.7) | 0.47 |
| IL-17 | 2.6 (2.0–4.1) | 5.5 (3.7–14.6) | 3.8 (3.3–4.6) | 0.08 |
| IL-21 | 0 (0–0.65) | 1.3 (0.56–3.4) | 0.43 (0–2.1) | 0.07 |
| KC | 1.9 (1.6–5.4) | 3.4 (2.4–4.6) | 2.6 (2.0–5.0) | 0.55 |
| Leptin | 283 ± 51.6 | 173 ± 47.5 | 269 ± 41.0 | 0.14 |
| LIX | 67.5 (36.5–88.9) | 57 (49.1–74.4) | 69.4 (49.1–112.0) | 0.80 |
| MCP-1 | 4.9 (3.5–8.4) | 9.6 (6.9–13.4) | 7.6 (6.1–9.2) | 0.18 |
| MCP-5 | 8.1 (5.4–9.5) | 30.3 (17.5–63.0)† | 21.3 (15.6–24.9)† | 0.001 |
| MCSF | 0.38 (0.24–0.65) | 0.37 (0.31–0.63) | 0.66 (0.47–1.00) | 0.28 |
| MIG | 348 ± 93.8 | 273.3 ± 45.4 | 278 ± 55.6 | 0.82 |
| MIP-1a | 2.6 ± 0.22 | 2.0 ± 0.16 | 3.3 ± 0.48‡ | 0.023 |
| MIP-1g | 1,200 (1,120–1,250) | 1,060 (985–1,130) | 1,050 (946–1,160) | 0.050 |
| PF4 | 3,436 ± 105 | 3,212 ± 211 | 3,360 ± 170 | 0.64 |
| RANTES | 2.9 (2.6–3.2) | 3.2 (2.7–3.4) | 2.6 (0.67–3.0) | 0.40 |
| TARC | 4.8 (3.5–6.0) | 7.9 (6.7–8.7)∗ | 5.7 (5.1–7.7) | 0.007 |
| TCA-3 | 10.8 ± 0.36 | 21.2 ± 3.35∗ | 15.5 ± 2.6 | 0.015 |
| TIMP-1 | 365 (301–430) | 1,130 (923–1,260)† | 828 (786–1,100)† | <0.001 |
| TNF-α | 3.8 (1.5–4.8) | 3.7 (1.8–5.4) | 2.1 (1.5–5.3) | 0.95 |
| TNF RI | 998 ± 32.4 | 1,366 ± 46.2† | 1,112 ± 74.7‡ | 0.005 |
| TNF RII | 69.3 (58.4–81.0) | 115 (102–169)∗ | 144 (94.3–186.0)† | 0.009 |
Values are median (interquartile range [Q1 and Q3]) or mean ± SE. Multiplex inflammatory array quantified 40 inflammatory factors, and in 28 factors rRIC abrogated doxorubicin-induced alteration. Concentrations in pg/ml.
BLC = B lymphocyte chemoattractant (CXCL13); CD30L = tumor necrosis factor ligand superfamily member 8 (TNFSF8, CD30 ligand); Fas L = Fas ligand (CD95L, CD178); G-CSF = granulocyte colony-stimulating factor; GM-CSF = granulocyte-macrophage colony-stimulating factor; ICAM = intercellular adhesion molecule (CD54); IFN = interferon; IL = interleukin; KC = keratinocyte-derived chemokine (C-X-C motif chemokine receptor 1, CXCL1); LIX = lipopolysaccharide-induced CXC chemokine; MCP = monocyte chemoattractant protein (CCL2); MCSF = macrophage colony-stimulating factor; MIG = monokine induced by gamma (CXCL9); MIP = macrophage inflammatory protein (CCL3); PF = platelet factor (CXCL4); RANTES = regulated upon activation, normal T cell expressed and secreted (CCL5); TARC = thymus and activation regulated chemokine (CCL17); TCA-3 = CCL1, I-309; TIMP = tissue inhibitor matrix metalloproteinase; TNF = tumor necrosis factor; TNFR = tumor necrosis factor receptor.
p < 0.05.
p < 0.01 versus vehicle.
p < 0.05 versus doxorubicin alone.
rRIC preserves organ weight
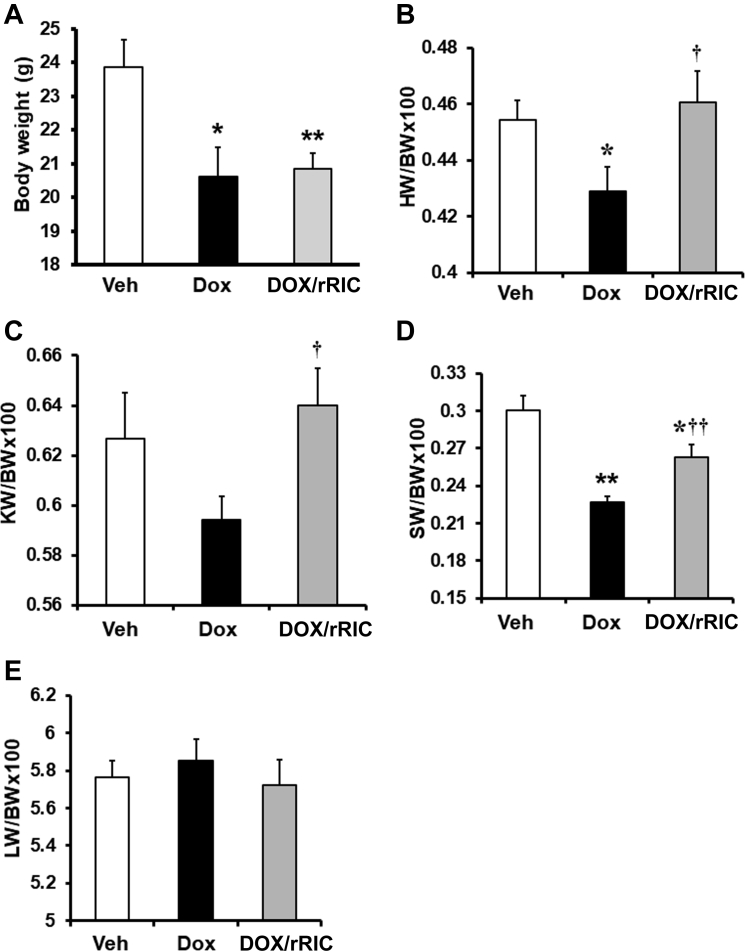
We found that rRIC had little effect on doxorubicin-induced body weight loss (Figure 1A), but significantly attenuated the doxorubicin-induced decrease in heart weight corrected for body weight (Figure 1B). Although doxorubicin reduced kidney weight, it failed to reach statistical significance compared with vehicle; however, kidney weight was significantly improved by rRIC when the doxorubicin and doxorubicin + rRIC groups were compared (Figure 1C). The significant effect of doxorubicin on spleen weight was also partially abrogated by rRIC (Figure 1D), but the liver was unaffected by doxorubicin and, as such, it was unchanged in the doxorubicin + rRIC group (Figure 1E).
Figure 1.
rRIC Reduced Doxorubicin-Induced Multiorgan Weight Loss
At end of the experiment, body weight of the animals was measured (A), organs were harvested and weighed, and corrected by body weight. The tested organs include heart (B), kidney (C), spleen (D), and liver (E). *p < 0.05 and **p < 0.01 versus vehicle, whereas †p < 0.05 and ††p < 0.01 versus doxorubicin alone. n = 8 in each of the groups (vehicle, doxorubicin, doxorubicin + rRIC). BW = body weight; Dox = doxorubicin; Dox/RIC = doxorubicin + remote ischemic conditioning; HW = heart weight; KW = kidney weight; LW = liver weight; rRIC = repeated remote ischemic conditioning; SW = spleen weight; Veh = vehicle.
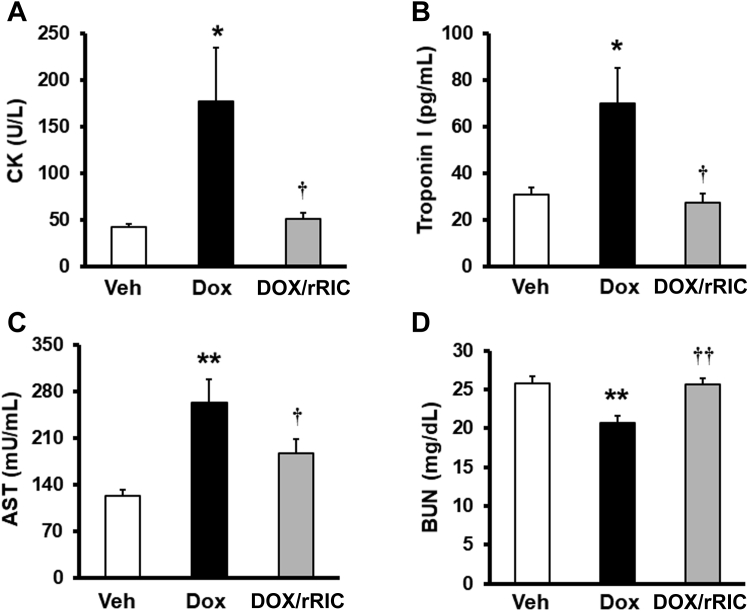
rRIC improves circulating biomarker evidence of multiorgan dysfunction
We next examined circulating markers of organ dysfunction. These data are shown in Figure 2. As a marker of predominantly skeletal muscle damage, creatinine kinase levels after doxorubicin treatment were increased up to 4-fold compared with vehicle, and this increase was completely abrogated by rRIC (Figure 2A). Cardiac troponin I release showed a similar profile to that of creatinine kinase. Doxorubicin led to significantly increased troponin I levels and, again, rRIC completely abrogated this effect (Figure 2B). Doxorubicin also significantly increased circulating aspartate aminotransferase, and rRIC restored levels to near normal (Figure 2C). The changes in blood urea nitrogen (BUN) (Figure 2D) were perhaps unexpected. Although there was no difference between controls and the doxorubicin + rRIC groups, BUN in the doxorubicin treated animals fell. We speculate that this may relate to a decrease in skeletal muscle mass evidenced by the raised creatine kinase levels, and plasma BUN being closely related to muscle mass in the absence of renal dysfunction. Overall, these data suggest that besides cardiotoxicity, doxorubicin also has detrimental effects on skeletal muscle, liver, and spleen, which was reduced by rRIC.
Figure 2.
rRIC Inhibited Doxorubicin-Induced Alteration of Circulating Markers of Multiorgan Dysfunction
Circulating multiorgan markers were tested with commercially available assay kits. The tested markers include creatinine kinase (CK) (A), troponin I (B), aspartate aminotransferase (AST) (C), and blood urea nitrogen (BUN) (D). *p < 0.05 and **p < 0.01 versus vehicle, whereas †p < 0.05 versus doxorubicin alone. n = 8 in each of the groups (vehicle, doxorubicin, doxorubicin + rRIC). Abbreviations as in Figure 1.
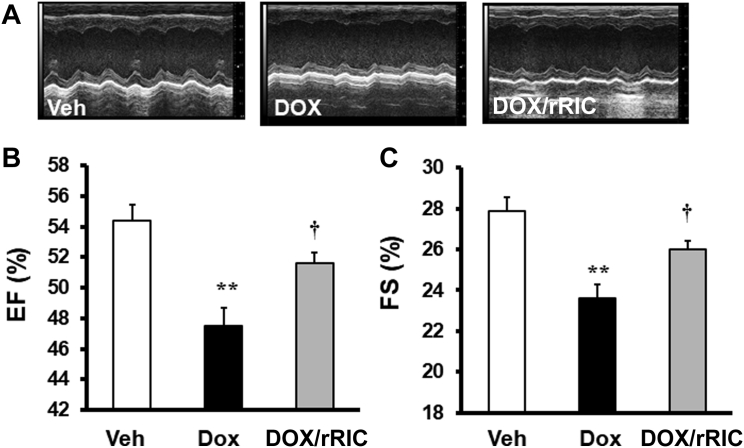
rRIC preserves cardiac function
High-dose doxorubicin (10 mg/kg) decreased cardiac function as evaluated by echocardiography. Both ejection fraction and fractional shortening were significantly reduced compared with vehicle-treated animals. rRIC significantly abrogated this decline in cardiac function, as indicated by improved ejection fraction (doxorubicin 47.5 ± 1.1%, doxorubicin + rRIC 51.6 ± 0.7%, p = 0.017) and fractional shortening (vehicle 27.9 ± 0.7, doxorubicin 23.6 ± 0.7, doxorubicin + rRIC 26.0 ± 0.5, p < 0.02) at 5 days (Figure 3).
Figure 3.
rRIC Abrogated Doxorubicin-Induced Cardiac Function Decline
Echocardiography was performed under isoflurane anesthesia. Representative m-mode 2-dimensional images (A). Ejection fraction (EF) (B) and fraction shortening (FS) (C). **p < 0.01 versus vehicle, †p< 0.05 versus doxorubicin alone. n = 8 in each of the groups (vehicle, doxorubicin, doxorubicin + rRIC). Abbreviations as in Figure 1.
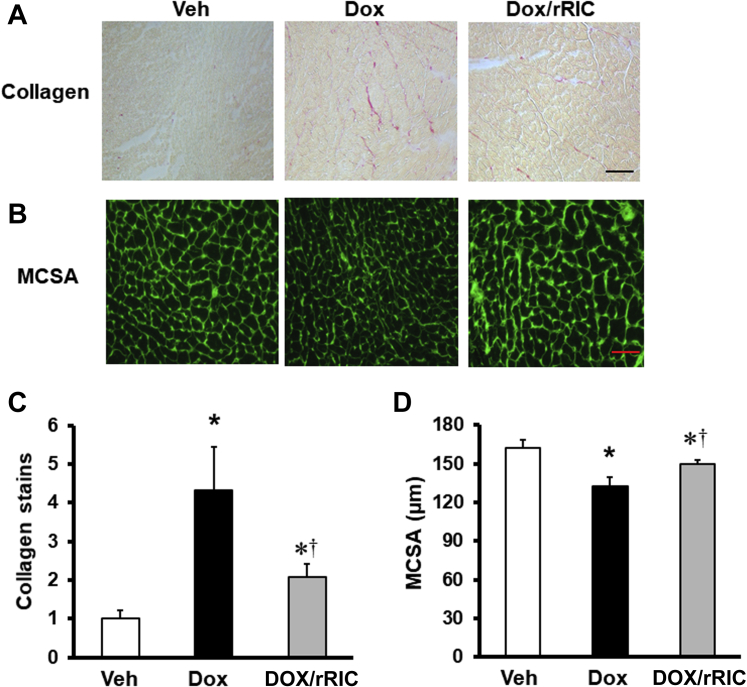
rRIC reduces myocardial fibrosis
Myocyte cross-sectional area was reduced by doxorubicin, and this reduction was significantly reversed by rRIC (Figure 4). Doxorubicin also led to a 4-fold increase in collagen content in the extracellular matrix, and this was significantly reduced by rRIC.
Figure 4.
rRIC Protected Against Doxorubicin-induced Cardiotoxicity at the Tissue Level
Extracellular collagen was stained with Sirius Red (A), quantified with Image J, and expressed as fold change versus vehicle (C). Representative picture for myocyte cross-section area (MCSA) (B) and quantified in (D). *p < 0.05 versus vehicle, whereas †p < 0.05 versus doxorubicin alone. Scale bar in A is 20 μm and 25 μm in B. n = 8 in each of the groups (vehicle, doxorubicin, doxorubicin + rRIC). Abbreviations as in Figure 1.
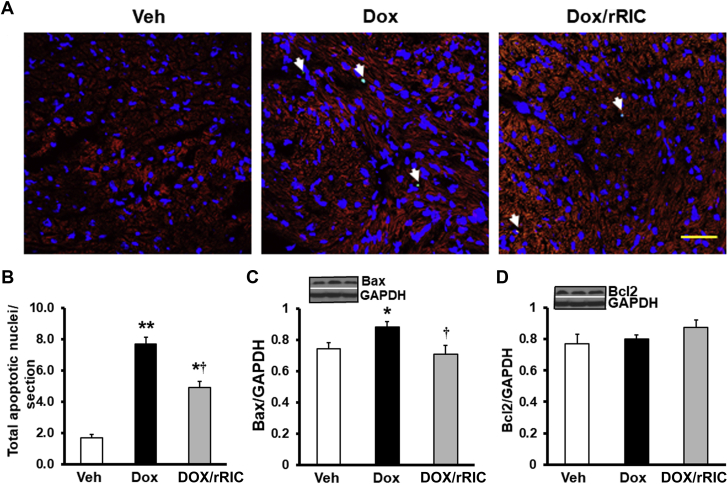
rRIC reduces doxorubicin-induced myocyte apoptosis
Figure 5 shows that doxorubicin led to a marked increase in cardiac myocyte apoptosis when compared with vehicle. This was significantly reduced by rRIC (Figures 5A and 5B). Western blot analysis showed that rRIC also blocked doxorubicin-induced Bax overexpression (Figure 5C), but Bcl2 was not affected (Figure 5D), suggesting that modification of Bax signaling is, at least in part, involved in the beneficial effect of rRIC on doxorubicin-induced cardiomyocyte apoptosis.
Figure 5.
rRIC Abrogated Doxorubicin-Induced Cardiomyocyte Apoptosis
Apoptosis was assayed by TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) stain (A) and quantified (B) by Image J expressed as apoptotic nuclei per image. Apoptotic protein Bax (C) and Bcl2 (D) were quantified by Western blot and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as loading control. *p < 0.05 and **p < 0.01 versus vehicle, whereas †p < 0.05 versus doxorubicin alone. Scale bar is 25 μm. n = 8 in each of the groups (vehicle, doxorubicin, doxorubicin + rRIC). Abbreviations as in Figure 1.
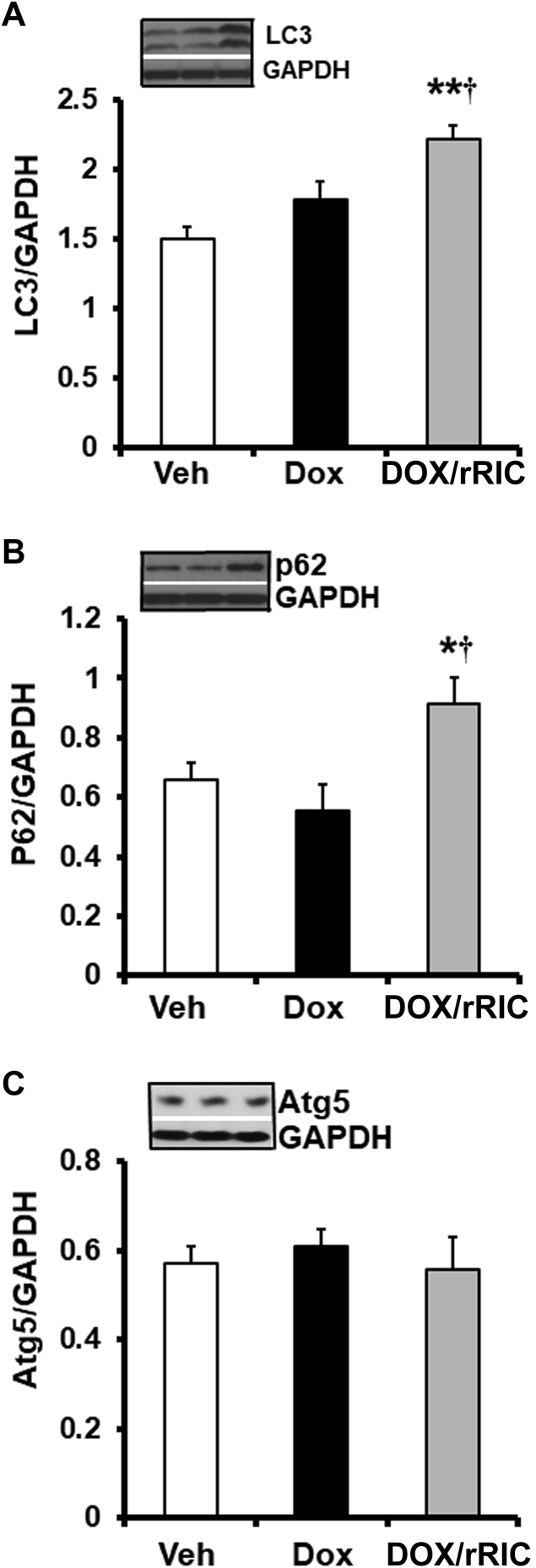
rRIC increases myocardial pro-autophagy signaling
Autophagy flux has been shown impaired by doxorubicin (20,21), and previous studies from our laboratory and others showed that myocardial autophagy signaling is enhanced by RIC (22, 23, 24). We therefore examined autophagy signaling in our animals. As shown in Figure 6, doxorubicin had minimal effect on autophagy proteins LC3 and p62/SQSTM1, but rRIC significantly increased their expression. The other measured autophagy protein Atg5 was not affected by doxorubicin or concurrent rRIC. This enhanced autophagy signaling may contribute to the beneficial effects of rRIC on doxorubicin-induced cardiotoxicity and warrants future study.
Figure 6.
rRIC Increased Cardiac Autophagy Proteins
Autophagy proteins were quantified by Western blot analysis as described under Methods. Cardiac LC3 (A), p62/SQSTM1 (B), and Atg5 (C) were quantified and expressed as the ratio over loading control glyceraldehyde-3-phosphate dehydrogenase (GAPDH). *p < 0.05 versus vehicle and ††p < 0.01 versus doxorubicin alone. n = 8 in each of the groups (vehicle, doxorubicin, doxorubicin + rRIC). Abbreviations as in Figure 1.
Discussion
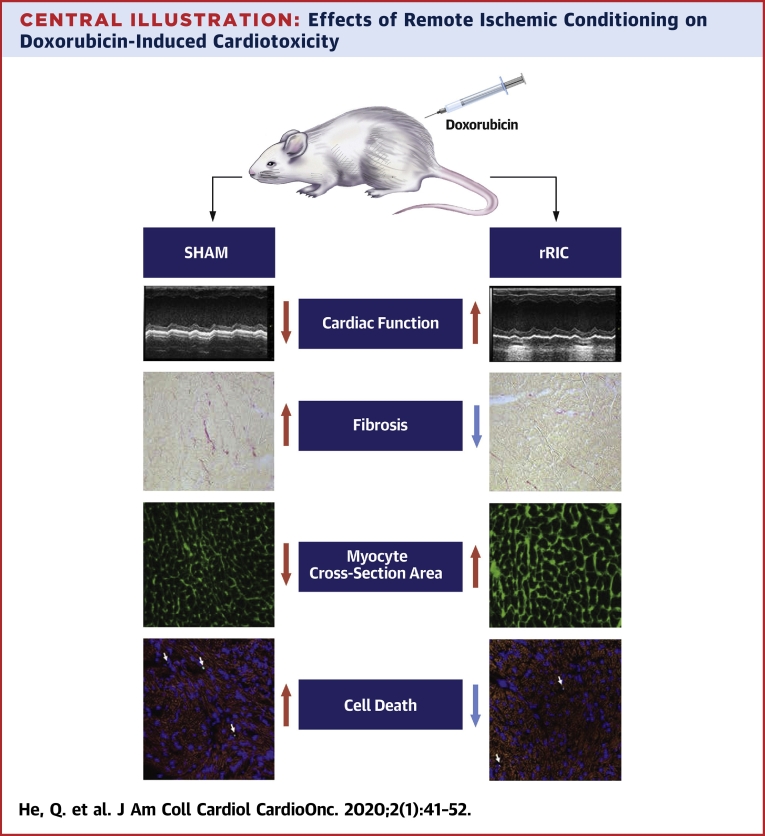
This study demonstrates, for the first time, that rRIC ameliorates acute doxorubicin-induced cardiotoxicity in vivo. rRIC not only improves cardiac function, but our data also suggest multiorgan protection. In terms of the cardioprotection, rRIC: 1) reduced doxorubicin-induced troponin I elevations, suggesting reduced myocardial damage; 2) improved cardiac function evaluated by echocardiography; 3) modified myocardial structural responses as indicated by improved cardiomyocyte cross-sectional area and reduced fibrosis; and 4) increased cardiac pro-autophagy signaling and reduced cardiomyocyte apoptosis (Central Illustration).
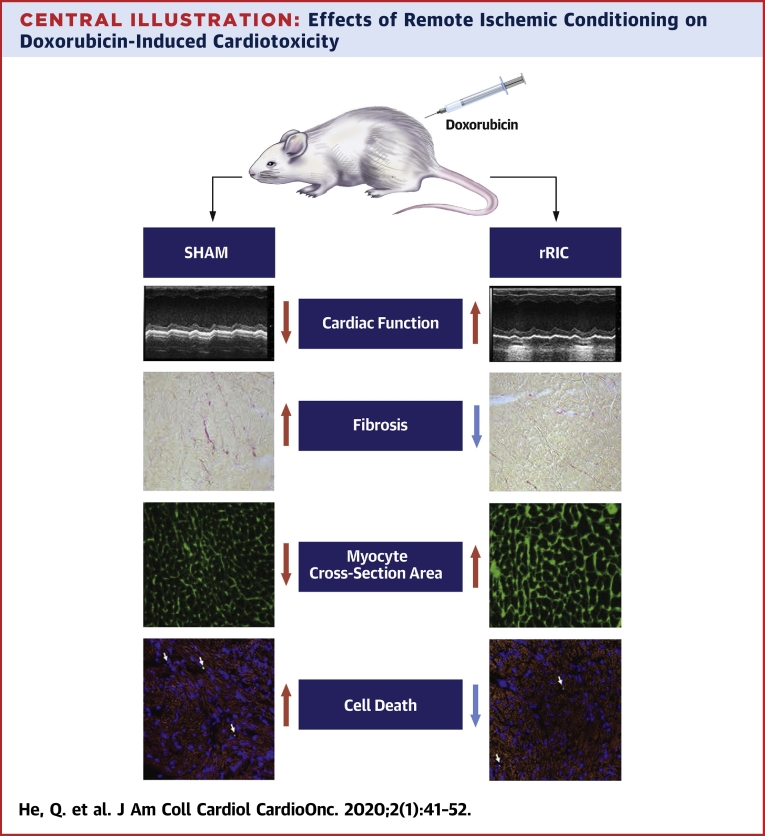
Central Illustration.
Effects of Remote Ischemic Conditioning on Doxorubicin-Induced Cardiotoxicity
Injection of doxorubicin caused decline of cardiac function, increased cardiac fibrosis, decreased myocyte cross-section area, and increased cardiomyocyte apoptosis. Remote ischemic conditioning abrogated doxorubicin-induced cardiotoxicity. rRIC = repeated remote ischemic conditioning.
As discussed earlier, the mechanisms of the beneficially cytotoxic effects of doxorubicin on cancer cells continues to be explored and debated (25, 26, 27). Similarly, the exact mechanisms of the cardioprotective effects of RIC remain to be fully delineated. That said, it is clear that the effects of RIC go beyond simple induction of ischemia resistance, or modification of reperfusion injury, as it has been shown to be effective in other models of injury that do not involve ischemia-reperfusion injury, such as contrast-induced nephropathy (28,29) and sepsis-induced cardiomyopathy (16,30). The mechanisms underlying these latter effects also remain to be fully elucidated, but almost certainly involve reduction of oxidative stress and inflammation (30,31).
Although it is difficult to provide a comprehensive mechanistic explanation for the benefits of rRIC in doxorubicin-induced cardiotoxicity, our data suggest substantial benefits that, given the potential clinical impact, would warrant future investigation. One of the most notable effects of rRIC was the complete abrogation of the 4-fold increase in cardiomyocyte apoptosis induced by doxorubicin. Doxorubicin-induced apoptosis has been well described in both cardiac myocytes (32) and cancer cells (33). Our data showed that doxorubicin significantly induced Bax, the earliest identified pro-apoptotic member of the Bcl-2 protein family. This increase in Bax was rescued completely by rRIC and consequently the number of apoptotic nuclei was markedly reduced by rRIC. It is worth noting, however, that rRIC only partially reduced the number of doxorubicin-induced apoptotic nuclei, despite rescuing Bax, suggesting Bax is only one of several apoptotic mechanisms at play. Interestingly, neither doxorubicin nor rRIC had an effect on Bcl2 expression, but the effect of rRIC to reduce apoptosis in the absence of changes in Bcl2 signaling is compatible with previous observations of the effect of RIC to reduce apoptosis after ischemia-reperfusion injury (34).
Similarly, the current data are consistent with our previous studies showing that rRIC enhances autophagy signaling (14,22). In this study, we showed that rRIC enhanced autophagy protein expression including LC3 I/II and p62/SGSTM1. Autophagy is a highly regulated process of cell death. It is a multistep process including recruitment to the autophagosomal membrane of p62/SQSTM1 ubiquitinated denatured protein, LC3 II, and other autophagy proteins. Elevation of LC3 and p62/SQSTM1 in general indicates increased autophagosome formation, suggesting ether increased autophagy activity or blockade of autophagosome clearance. The process of autophagy can be inhibited at multiple steps and leads to cell stress and death. Doxorubicin has been showed to interfere with the late steps of autophagy leading to transient autophagosome accumulation, which returns to normal levels by 7 days after doxorubicin injection (20,35). Our data, showing no residual autophagy activation 6 days after doxorubicin injection, are compatible with these prior observations; however, continued RIC leads to persistent pro-autophagy signaling, perhaps contributing to the cardioprotection we observed. The involvement of apoptosis and autophagy in rRIC-diminished doxorubicin-induced cardiotoxicity is consistent with the recent report by Gertz et al. (36). In their study, a higher dose of doxorubicin was used (20 mg/kg) and only 1 episode of RIC, consisting of 3 cycles of 5-min occlusion and 5-min reperfusion, was used, 1 h before therapy. Their data showed that this RIC abrogated doxorubicin-induced apoptosis, and enhanced autophagy signaling (36). Interestingly, in contrast to our findings, despite the higher dose of doxorubicin, there was no significant change in cardiac function in their animals, although long-term survival was enhanced, perhaps reflecting some of the multiorgan benefits that we additionally showed in our study.
The relationship between the modification of systemic inflammatory responses and the reduction in autophagocytic and apoptotic cell death we observed must remain speculative. Cardiac dysfunction can be induced as part of the systemic inflammatory response, and we have previously shown that mortality associated with this “sepsis-induced” cardiomyopathy can be reduced by rRIC (16). Sepsis-induced cardiomyopathy tends to be transient, however, and sustained myocardial structural changes are rare, unlike what is seen with doxorubicin-induced cardiotoxicity. Conversely, widespread cell death from any cause can induce a systemic inflammatory reaction, and it may be reasonable to suggest that our observations are more consistent with rRIC, primarily affording protection against cytotoxic cell death, with resulting reduced systemic inflammatory responses, rather than vice versa.
Finally, we showed that rRIC not only provided myocardial protection, but also positively influenced other organs, in terms of preventing reductions in size and improving some aspects of their function. The underlying mechanisms of, and potential clinical impact of, these “off-target” benefits remain to be seen, but again may be important areas for future investigation.
Study limitations
Given the lack of prior studies regarding the potential role of this strategy of rRIC in doxorubicin-induced cardiotoxicity, this “proof-of-principle” study has necessary limitations. We only measured the effects of rRIC at a single time point, 6 days after administration. We therefore have no data regarding any effect on early responses, and no longer-term data showing that the improvements we observed are sustained beyond the period of rRIC administration. The improved structural integrity of the myocardium, with improved cardiomyocyte cross-sectional area, and reduced fibrosis, are encouraging, but clearly further studies, focused on improving our mechanistic understanding, and confirming long-term benefit, are required.
Given that rRIC can be induced noninvasively using a blood pressure cuff, its translation into clinical trials has been quite rapid, but the impact despite encouraging preclinical and proof-of-principle clinical trials has been disappointing in larger studies (37). Multiple factors for lack of clinical effect have been postulated, including older patient age, the effect of comorbidities, and the concomitant effect of drugs that may inhibit the rRIC effect or themselves induce a “conditioned” state (38, 39, 40). These factors may be less of a problem in the sometimes younger population of patients in whom doxorubicin is used for chemotherapy, but our data cannot address a potential concern of the effect of rRIC on the beneficial cytotoxicity that underlies its benefits as a cancer therapeutic. Further studies of this potential adverse effect of rRIC in a tumor-bearing model are clearly needed, and careful attention is also needed to understand the potential effects of rRIC on tumor efficacy in any early-phase clinical trials. Future experimental studies also should be designed to elucidate the mechanisms of rRIC in preventing doxorubicin cardiotoxicity, as they may identify new targets for the development of specific therapeutic agents.
Conclusions
These data show that rRIC reduces doxorubicin cardiotoxicity in a mouse model of acute injury. These preliminary data suggest that further mechanistic studies of the acute and chronic effects of rRIC are warranted.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Dose-dependent cardiotoxicity of doxorubicin limits its use in patients with cancer. The mechanisms of doxorubicin cardiotoxicity are not fully understood and effective approaches to reduce cardiotoxicity are an important clinical need. Our data in mice show rRIC is an effective noninvasive strategy to protect the heart and other organs from doxorubicin toxicity.
TRANSLATIONAL OUTLOOK: We demonstrated that rRIC protects doxorubicin-induced cardiotoxicity in a mouse model. Further studies of the acute and chronic effects of rRIC, and confirmation that the anticancer effects of doxorubicin are unaffected by rRIC, are necessary prior to clinical translation.
Acknowledgments
The authors acknowledge Victoria Moore and Chrissy Schulte from the Cardiovascular Imaging Core Research Laboratory of Cincinnati Children’s Hospital for their support in echocardiography.
Footnotes
Financial support was from the Cincinnati Children’s Research Fund. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
References
- 1.Ichikawa Y., Ghanefar M., Bayeva M. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest. 2014;124:617–630. doi: 10.1172/JCI72931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim S.Y., Kim S.J., Kim B.J. Doxorubicin-induced reactive oxygen species generation and intracellular Ca2+ increase are reciprocally modulated in rat cardiomyocytes. Exp Mol Med. 2006;38:535–545. doi: 10.1038/emm.2006.63. [DOI] [PubMed] [Google Scholar]
- 3.Jawad B., Poudel L., Podgornik R., Steinmetz N.F., Ching W.Y. Molecular mechanism and binding free energy of doxorubicin intercalation in DNA. Phys Chem Chem Phys. 2019;21:3877–3893. doi: 10.1039/c8cp06776g. [DOI] [PubMed] [Google Scholar]
- 4.Yang F., Teves S.S., Kemp C.J., Henikoff S. Doxorubicin, DNA torsion, and chromatin dynamics. Biochim Biophys Acta. 2014;1845:84–89. doi: 10.1016/j.bbcan.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyu Y.L., Kerrigan J.E., Lin C.P. Topoisomerase IIbeta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res. 2007;67:8839–8846. doi: 10.1158/0008-5472.CAN-07-1649. [DOI] [PubMed] [Google Scholar]
- 6.Denard B., Lee C., Ye J. Doxorubicin blocks proliferation of cancer cells through proteolytic activation of CREB3L1. Elife. 2012;1 doi: 10.7554/eLife.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swain S.M., Whaley F.S., Ewer M.S. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 8.Charbonnel C., Convers-Domart R., Rigaudeau S. Assessment of global longitudinal strain at low-dose anthracycline-based chemotherapy, for the prediction of subsequent cardiotoxicity. Eur Heart J Cardiovasc Imaging. 2017;18:392–401. doi: 10.1093/ehjci/jew223. [DOI] [PubMed] [Google Scholar]
- 9.Choi B.W., Berger H.J., Schwartz P.E. Serial radionuclide assessment of doxorubicin cardiotoxicity in cancer patients with abnormal baseline resting left ventricular performance. Am Heart J. 1983;106:638–643. doi: 10.1016/0002-8703(83)90080-7. [DOI] [PubMed] [Google Scholar]
- 10.Friedman M.A., Bozdech M.J., Billingham M.E., Rider A.K. Doxorubicin cardiotoxicity. Serial endomyocardial biopsies and systolic time intervals. JAMA. 1978;240:1603–1606. doi: 10.1001/jama.240.15.1603. [DOI] [PubMed] [Google Scholar]
- 11.Wong A.D., Ye M., Ulmschneider M.B., Searson P.C. Quantitative analysis of the enhanced permeation and retention (EPR) effect. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narayan H.K., Putt M.E., Kosaraju N. Dexrazoxane preferentially mitigates doxorubicin cardiotoxicity in female children with sarcoma. Open Heart. 2019;6 doi: 10.1136/openhrt-2019-001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vejpongsa P., Yeh E.T. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol. 2014;64:938–945. doi: 10.1016/j.jacc.2014.06.1167. [DOI] [PubMed] [Google Scholar]
- 14.Li J., Rohailla S., Gelber N. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol. 2014;109:423. doi: 10.1007/s00395-014-0423-z. [DOI] [PubMed] [Google Scholar]
- 15.White S.K., Frohlich G.M., Sado D.M. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol Intv. 2015;8:178–188. doi: 10.1016/j.jcin.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Honda T., He Q., Wang F., Redington A.N. Acute and chronic remote ischemic conditioning attenuate septic cardiomyopathy, improve cardiac output, protect systemic organs, and improve mortality in a lipopolysaccharide-induced sepsis model. Basic Res Cardiol. 2019;114:15. doi: 10.1007/s00395-019-0724-3. [DOI] [PubMed] [Google Scholar]
- 17.Kharbanda R.K., Mortensen U.M., White P.A. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–2883. doi: 10.1161/01.cir.0000043806.51912.9b. [DOI] [PubMed] [Google Scholar]
- 18.He Q., Wang F., Honda T., James J., Li J., Redington A. Loss of miR-144 signaling interrupts extracellular matrix remodeling after myocardial infarction leading to worsened cardiac function. Sci Rep. 2018;8:16886. doi: 10.1038/s41598-018-35314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L., Chen Q., Qi H. Doxorubicin-induced systemic inflammation is driven by upregulation of toll-like receptor TLR4 and endotoxin leakage. Cancer Res. 2016;76:6631–6642. doi: 10.1158/0008-5472.CAN-15-3034. [DOI] [PubMed] [Google Scholar]
- 20.Li D.L., Wang Z.V., Ding G. Doxorubicin blocks cardiomyocyte autophagic flux by inhibiting lysosome acidification. Circulation. 2016;133:1668–1687. doi: 10.1161/CIRCULATIONAHA.115.017443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdullah C.S., Alam S., Aishwarya R. Doxorubicin-induced cardiomyopathy associated with inhibition of autophagic degradation process and defects in mitochondrial respiration. Sci Rep. 2019;9:2002. doi: 10.1038/s41598-018-37862-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rohailla S., Clarizia N., Sourour M. Acute, delayed and chronic remote ischemic conditioning is associated with downregulation of mTOR and enhanced autophagy signaling. PLoS One. 2014;9 doi: 10.1371/journal.pone.0111291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie Y., Jiang D., Xiao J. Ischemic preconditioning attenuates ischemia/reperfusion-induced kidney injury by activating autophagy via the SGK1 signaling pathway. Cell Death Dis. 2018;9:338. doi: 10.1038/s41419-018-0358-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y., Shen J., Xiong X. Remote ischemic preconditioning protects against liver ischemia-reperfusion injury via heme oxygenase-1-induced autophagy. PLoS One. 2014;9 doi: 10.1371/journal.pone.0098834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taymaz-Nikerel H., Karabekmez M.E., Eraslan S., Kirdar B. Doxorubicin induces an extensive transcriptional and metabolic rewiring in yeast cells. Sci Rep. 2018;8:13672. doi: 10.1038/s41598-018-31939-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renu K., Abilash V.G., Pichiah P.B.T., Arunachalam S. Molecular mechanism of doxorubicin-induced cardiomyopathy - An update. Eur J Pharmacol. 2018;818:241–253. doi: 10.1016/j.ejphar.2017.10.043. [DOI] [PubMed] [Google Scholar]
- 27.Patel A.G., Kaufmann S.H. CANCER How does doxorubicin work? Elife. 2012;1 doi: 10.7554/eLife.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou F., Song W., Wang Z. Effects of remote ischemic preconditioning on contrast induced nephropathy after percutaneous coronary intervention in patients with acute coronary syndrome. Medicine (Baltimore) 2018;97:e9579. doi: 10.1097/MD.0000000000009579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Er F., Nia A.M., Dopp H. Ischemic preconditioning for prevention of contrast medium-induced nephropathy: randomized pilot RenPro Trial (Renal Protection Trial) Circulation. 2012;126:296–303. doi: 10.1161/CIRCULATIONAHA.112.096370. [DOI] [PubMed] [Google Scholar]
- 30.Kim Y.H., Yoon D.W., Kim J.H., Lee J.H., Lim C.H. Effect of remote ischemic post-conditioning on systemic inflammatory response and survival rate in lipopolysaccharide-induced systemic inflammation model. J Inflamm (Lond) 2014;11:16. doi: 10.1186/1476-9255-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lotfollahi H., Mohammadi M., Ghaffari S. Effect of remote ischemic post-conditioning on oxidative stress in blood of STEMI patients treated with primary angioplasty. J Cardiovasc Thorac Res. 2016;8:113–118. doi: 10.15171/jcvtr.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao L., Zhang B. Doxorubicin induces cardiotoxicity through upregulation of death receptors mediated apoptosis in cardiomyocytes. Sci Rep. 2017;7:44735. doi: 10.1038/srep44735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pilco-Ferreto N., Calaf G.M. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. Int J Oncol. 2016;49:753–762. doi: 10.3892/ijo.2016.3558. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura M., Wang N.P., Zhao Z.Q. Preconditioning decreases Bax expression, PMN accumulation and apoptosis in reperfused rat heart. Cardiovasc Res. 2000;45:661–670. doi: 10.1016/s0008-6363(99)00393-4. [DOI] [PubMed] [Google Scholar]
- 35.Shi J., Surma M., Wei L. Disruption of ROCK1 gene restores autophagic flux and mitigates doxorubicin-induced cardiotoxicity. Oncotarget. 2018;9:12995–13008. doi: 10.18632/oncotarget.24457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gertz Z.M., Cain C., Kraskauskas D. Remote ischemic pre-conditioning attenuates adverse cardiac remodeling and mortality following doxorubicin administration in mice. J Am Coll Cardiol CardioOnc. 2019;1:221–234. doi: 10.1016/j.jaccao.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hausenloy D.J., Kharbanda R.K., Moller U.K. Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): a single-blind randomised controlled trial. Lancet. 2019;394:1415–1424. doi: 10.1016/S0140-6736(19)32039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hausenloy D.J., Botker H.E. Why did remote ischaemic conditioning not improve clinical outcomes in acute myocardial infarction in the CONDI-2/ERIC-PPCI trial? Cardiovasc Res. 2019;115:e161–e163. doi: 10.1093/cvr/cvz242. [DOI] [PubMed] [Google Scholar]
- 39.Ludman A.J., Yellon D.M., Hausenloy D.J. Cardiac preconditioning for ischaemia: lost in translation. Dis Model Mech. 2010;3:35–38. doi: 10.1242/dmm.003855. [DOI] [PubMed] [Google Scholar]
- 40.Abete P., Testa G., Cacciatore F. Ischemic preconditioning in the younger and aged heart. Aging Dis. 2011;2:138–148. [PMC free article] [PubMed] [Google Scholar]