Abstract
Background
Probiotics are among the most commonly used dietary supplements and evidence of their efficacy is increasing. Despite the long historical use of probiotics, some experts suggest that additional research is necessary to understand their potential risks.
Objectives
Main aims of this study were to assess short-term tolerability and safety of a new, high colony-forming unit count, multi-strain probiotic supplement. Exploratory objectives included evaluating effects on gut microbial composition.
Methods
Ten healthy adults were enrolled in a single-arm, open-label study. Over a 10-day period, participants consumed a once daily probiotic capsule (2.1 x 1011 CFU) containing Lactobacillus acidophilus NCFM, Lactobacillus paracasei Lpc-37, Lactobacillus plantarum Lp-115, Lactobacillus rhamnosus GG, Lactobacillus rhamnosus HN001, Bifidobacterium lactis Bi-07, Bifidobacterium lactis Bl-04, and Bifidobacterium lactis HN019. The primary measure of tolerability pertained to whether or not participants completed the study. Secondary safety measures included clinical biomarkers from a routine metabolic panel and a complete blood count. Exploratory measures included stool microbiota counts.
Results
All participants completed the study and there were no serious adverse events. All documented adverse events were prompted by the investigators and the most commonly reported symptoms were gastrointestinal. There was a single instance of a biomarker abnormality in one individual. Overall, decreases in total bilirubin and aspartate aminotransferase, and increases in stool levels of Lactobacillus species, Faecalibacterium prausnitzii, and Akkermansia muciniphila (P < .05) were observed over the course of the study.
Conclusions
The findings of this study suggest the multi-strain probiotic supplement was well-tolerated and most likely safe. Changes in liver function measures suggest the probiotics could potentially impact liver health. Stool microbiota changes suggest the probiotic could potentially impact gut health by affecting levels of intestinal microbiota that have been described as bioindicators of health and potential keystone species. However, additional research is necessary to follow up on the exploratory findings of this preliminary work.
Introduction
Probiotics are among the most commonly used dietary supplements.1 Strains from the genera Lactobacillus and Bifidobacterium are among the most commonly used probiotics.2 Although many probiotics are considered safe, with increasing usage there may be a greater need to assess their safety and efficacy.1-3
Probiotics may impact immune cells, secretion of intestinal antibodies and mucin, and gut microbial composition.4-6 Probiotic effects are believed to be strain-specific and disease-specific, based on strong clinical evidence.7 Published reviews have demonstrated that specific single- or mono-strain probiotics,8,9 as well as specific multi-strain probiotic combinations,10-12 can be beneficial for distinct clinical applications. Potential advantages of multi-strain probiotic supplements include additive and synergistic effects of individual probiotic strains, as well as a broader spectrum of health benefits compared with intake of a single probiotic strain.10 To this end, a new high colony-forming unit (CFU) count combination of 8 strains of Lactobacillus and Bifidobacterium probiotics was formulated into a product intended to support healthy gut microbial composition and overall health and wellness.
For this multi-strain probiotic mixture, the selection of the strains and target CFU counts were based on previous studies that demonstrated health benefits in various clinical populations. Numerous studies have documented immune and gastrointestinal benefits for usage of these probiotics as single strains, as well as some combinations of the strains.13-27 Demonstrated immune benefits of the strains in the formula include reducing the risk of upper respiratory tract infections (during consumption of 2 billion CFU per day Bifidobacterium lactis Bl-04),13 potential immunomodulation post-vaccination (with consumption of 20 billion CFU of single strains, including B. lactis Bl-04 and Lactobacillus plantarum Lp-115),14 and increased natural killer cell activity (following consumption of 5 billion CFU B. lactis HN019 or L. rhamnosus HN001 per day).15
Demonstrated gastrointestinal benefits of the strains in the formula include decreased digestive discomfort and flatulence in adults with constipation (after consumption of a 27.5 billion CFU 5-strain combination of L. acidophilus NCFM, L. paracasei Lpc-37, B. lactis Bl-04, B. lactis Bi-07, and B. lactis HN019),20 reduced pain after colonoscopy (during consumption of a 2-strain combination of 12.5 billion CFU L. acidophilus NCFM and 12.5 billion CFU B. lactis Bi-07),21 and lowered risk of antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea (with consumption of L. rhamnosus GG22 as a single strain, or a 4-strain combination of L. acidophilus NCFM, L. paracasei Lpc-37, B. lactis Bl-04, and B. lactis Bi-07 at a dose of 4.2 billion or 17 billion CFU daily).23
Although probiotics have a long history of use, healthcare practitioners are frequently asked by their patients about effectiveness and side effects of probiotics.2 A meta-analysis by Hempel et al, which included 387 studies that evaluated for adverse events (AEs) associated with probiotic intake, found that probiotics did not increase the risk of AEs.28 However, the quality of AE reporting in probiotic studies varies and some experts suggest that rigorous documentation of AEs related to probiotic consumption is needed.3,28 Furthermore, compared to research on single probiotic strains, there are fewer studies on multi-strain combinations.10,29 Thus, more research on the tolerability, safety, and efficacy of multi-strain probiotics is warranted.
Clinical tolerability relates to the degree to which AEs can be tolerated by a study participant; whether or not participants complete a study or elect to withdraw due to AEs are indicators of tolerability.30 Clinical safety also relates to AEs, including whether they are serious or nonserious.30-32 As shown in Table 1, previously published AE data is available for each of the 8 strains evaluated in the present study.13,16,18-20,24,25,33-43 Most studies included a placebo or control group, and no differences in AEs or adverse outcomes were found when comparing probiotic intake to that of placebo or the control.13,16,19,20,24,25,34-42 However, in a study on the prevention of antibiotic-associated diarrhea, Fox et al reported fewer AEs in the group given probiotics than the group given placebo.43
Table 1.
Summary of Previous Studies That Have Described Adverse Event Data on the Eight Probiotic Strains in the Study Supplement
| Reference | Study population | Adverse event data described | Routine blood tests monitored | No. of strains evaluated | Total daily CFU (billion) | Probiotic strain(s) evaluated | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactobacillus | Bifidobacterium | Other strains | ||||||||||||
| acidophilus | paracasei | plantarum | rhamnosus | animalis subsp. lactis | ||||||||||
| NCFM | Lpc-37 | Lp-115 | HN001 | GG | Bi-07 | Bl-04 | HN019 | |||||||
| Hibberd 201433 | Healthy elderly | ✓ | ✓ | 1 | 20 | ✓ | ||||||||
| Sheih 200118 | Healthy middle-aged adults and elderly | ✓ | 1 | 50 | ✓ | |||||||||
| West 201413 | Healthy adults | ✓ | 1 | 2 | ✓ | |||||||||
| 2 | 10 | ✓ | ✓ | |||||||||||
| Cox 201434 | Healthy adults | ✓ | ✓ | 1 | 2 | ✓ | ||||||||
| 2 | 10 | ✓ | ✓ | |||||||||||
| Forssten 201435 | Healthy adults given antibiotics | ✓ | 1 | 25 | ✓ | ✓ | ||||||||
| Engelbrektson 200936 | Healthy adults given antibiotics | ✓ | 5 | 20.5 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Airaksinen 201920 | Adults with bloating and constipation | ✓ | 5 | 27.5 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Ringel-Kulka 201124 | Adults with functional bowel disorders | ✓ | ✓ | 2 | 200 | ✓ | ✓ | |||||||
| Waller 201137 | Adults with functional gastrointestinal symptoms | ✓ | 1 | 1.8 or 17.2 | ✓ | |||||||||
| Lyra 201625 | Adults with IBS | ✓ | 1 | 1 or 10 | ✓ | |||||||||
| Zhang 201338 | Adults undergoing liver transplantation | ✓ | 6 | 27 | ✓ | ✓ | ✓ | |||||||
| Eggers 201819 | Adults positive for S. aureus carriage | ✓ | 1 | 10 | ✓ | |||||||||
| Wickens 201739 | Pregnant women | ✓ | ✓ | 1 | 6 | ✓ | ||||||||
| Dekker 200940 | Pregnant/nursing women, Infants | ✓ | 1 | 6 | ✓ | |||||||||
| 1 | 9 | ✓ | ||||||||||||
| Mutlu 202041 | Newborns with hyperbilirubinemia | ✓ | 1 | 1 | ✓ | |||||||||
| Mutlu 201842 | Newborns with hyperbilirubinemia | ✓ | ✓ | 1 | 1 | ✓ | ||||||||
| Leyer 200916 | Healthy children | ✓ | 1 | 10 | ✓ | |||||||||
| 2 | 10 | ✓ | ✓ | |||||||||||
| Fox 201543 | Children prescribed antibiotics | ✓ | 3 | 34 | ✓ | ✓ | ||||||||
Abbreviations: IBS, irritable bowel syndrome; S. aureus, Staphylococcus aureus.
Clinical safety can also be assessed by monitoring blood tests.30 Routine clinical blood tests have been monitored during previous studies that evaluated some of the probiotic strains in the 8-strain combination. Intake of L. acidophilus NCFM in combination with B. lactis Bi-07,24,34 or intake of B. lactis Bl-04 as a single strain,34 was not associated with adverse changes in laboratory values (CMP, CBC). In a study of L. rhamnosus GG, blood counts and liver function tests were monitored; although there were isolated instances of laboratory values that were outside of reference ranges, intake of L. rhamnosus GG was still considered safe.33 Furthermore, some of the probiotic strains that were evaluated in the present study were researched previously in vulnerable populations including infants,40-42 children,43 pregnant and breastfeeding women,39,40,44 and elderly people.15,18,33 The probiotics did not adversely impact birth outcomes,39 infant growth,40 or symptoms of postpartum depression and anxiety.44 Furthermore, each of the 8 probiotic strains has a generally recognized as safe (GRAS) notice on file with the United States Food and Drug Administration (FDA), including GRAS notices 231 (L. rhamnosus GG), 288 (L. rhamnosus HN001), 357 (L. acidophilus NCFM), 445 (B. lactis Bi-07, Bl-04, and HN019), 722 (L. plantarum Lp-115), and 736 (L. paracasei Lpc-37).45
More clinical research on multi-strain probiotics is needed10 and although there is no evidence that the 8 strains in the probiotic combination evaluated in this study were harmful, no previous studies had evaluated the tolerability and safety of the 8 strains together in a single formula. Thus, the main aims of this study were to assess the short-term tolerability and safety of a strain-identified probiotic formula administered orally as a daily high CFU count capsule. Furthermore, considering that probiotics may impact gut microbial composition, changes to gut microbiota were also explored.
Methods
Study Design
A single-arm, open-label study to assess the short-term tolerability and safety of a multi-strain probiotic supplement based on study completion, assessment for AEs, and assessment for changes in routine clinical laboratory biomarkers was implemented. The primary measure of tolerability was defined as participant completion of the study without withdrawal due to AEs. Secondary measures included routine biomarkers on a nonfasting comprehensive metabolic panel (CMP) and a complete blood count (CBC). Exploratory measures included stool microbiota CFU counts and stool levels of short-chain fatty acids (SCFA). This study was conducted according to the principles of the Declaration of Helsinki. Prior to initiation, this study was approved by Aspire IRB (IRB # 520190208) and registered at ClinicalTrials.gov (NCT04044144).
Study Intervention
The studied supplement was a professional-grade, strain-identified probiotic formula supplied by Metagenics, Inc (Gig Harbor, WA) in bottles containing 14 capsules. Participants were asked to take 1 capsule per day, with food, over a 10-day period. The supplement contained L. acidophilus NCFM, L. paracasei Lpc-37, L. plantarum Lp-115, L. rhamnosus HN001, L. rhamnosus GG, B. animalis subsp. lactis Bi-07, B. animalis subsp. lactis Bl-04, B. animalis subsp. lactis HN019, microcrystalline cellulose, magnesium stearate, and silicon dioxide in a hydroxypropyl methylcellulose capsule (see Table 2).
Table 2.
Study Supplement Probiotic Strains and Label Claims
| Genus | Species (and subspecies) | Strain | Billion CFU per capsule |
|---|---|---|---|
| Lactobacillus | acidophilus | NCFM | 12.5 |
| Lactobacillus | paracasei | Lpc-37 | 10 |
| Lactobacillus | plantarum | Lp-115 | 20 |
| Lactobacillus | rhamnosus | HN001 | 5 |
| Lactobacillus | rhamnosus | GG | 20 |
| Bifidobacterium | animalis subspecies lactis | Bi-07 | 12.5 |
| Bifidobacterium | animalis subspecies lactis | Bl-04 | 20 |
| Bifidobacterium | animalis subspecies lactis | HN019 | 5 |
The probiotic blend’s minimum high quantity CFU/g was designed to achieve the label claim based on available stability data. The probiotic blend was encapsulated and bottled according to good manufacturing practices and tested for contaminants, including Salmonella, Listeria, Staphylococcus aureus, Enterococci, Coliforms, and E. coli, and residual solvents and heavy metals (arsenic, cadmium, lead, and mercury), and ensured all values were lower than established acceptable levels. Testing of a representative capsule yielded 2.1 x 1011 CFU at the study end point as measured through third party analysis (Element Materials Technology, Portland, OR, USA).
Participants and Recruitment
Adults aged 21-75 years were recruited to the Personalized Lifestyle Medicine Center in Gig Harbor, WA (USA). Target enrollment was 10 individuals. Recruitment approaches included flyers and contacting individuals who had previously participated in research studies at this same facility. Participants provided written informed consent before participation in the study. All participants attended a baseline visit and a study completion visit.
Prospective participants were eligible if healthy and free of chronic disease. Prospective participants were excluded for the following: known history of chronic bowel disease, liver disease, kidney disease, cardiovascular disease, diabetes, prediabetes, hypoglycemia, seizure disorder, psychiatric illness, human immunodeficiency virus, tuberculosis, hepatitis B, hepatitis C, gastrointestinal surgery within the previous 10 years, or alcoholism; malignancy within the last 5 years; hospitalization within 3 months prior to screening; active gastrointestinal symptoms and/or infections; use of probiotics or antibiotics; smoking or use of nicotine-containing products; women who were lactating, pregnant, or planning pregnancy during the study period; known intolerance or allergy to ingredients in the study supplement; or participating in another research study within 28 days prior to screening. Participants were asked to maintain their usual diet and exercise patterns for the duration of the study.
Data Collection
Participants were queried for AEs at the study completion visit using a 51-question health symptom questionnaire. The questionnaire included 50 questions to prompt for symptoms in the following categories: gastrointestinal (n = 18), general (n = 9), head/eyes/ears/nose/throat (n = 8), genitourinary (n = 5), cardiopulmonary (n = 4), integumentary (n = 3), musculoskeletal (n = 2), and psychological (n = 1). The questionnaire also included 1 open-ended question to allow for reporting of unprompted AEs. To allow for additional spontaneous reporting of AEs, participants were encouraged to contact study staff with any concerns between study visits.
Per the protocol, AEs were defined as any untoward medical occurrence in the clinical investigation that may or may not have a causal relationship with the study supplement. Therefore, an AE could be an unfavorable and unintended sign, symptom, or disease temporally associated with the study, whether or not related to the study supplement. AEs were classified as serious or nonserious based on FDA and Federal Food, Drug, and Cosmetic (FD&C) Act definitions.31,32 AEs were considered serious if a participant outcome had included: a life-threatening experience, inpatient hospitalization, disability or incapacity, death, a congenital anomaly or birth defect, or a medical or surgical intervention to prevent these outcomes. All other AEs were designated as nonserious.
Vital signs were measured and nonfasting blood samples were obtained by venipuncture at both the baseline and study completion visits. Participants were instructed to collect a stool sample at home within 48 hours prior to the baseline visit and within 24 hours before or after the study completion visit. To assess adherence, participants were given paper logs to track intake of the study supplement. Logs and unused probiotic supplement were returned at the study completion visit; a pill count of returned capsules was performed.
Blood and Stool Sample Analysis
Whole blood and serum samples were sent to the lab the day of collection for a CMP and CBC. Stool specimens were shipped to Genova Diagnostics (Asheville, NC, USA) within 24 hours of collection; gut microbiota were quantified using 16S ribosomal RNA gene PCR and concentrations of SCFA were measured using gas chromatography-mass spectrometry (GC-MS) as described by Chen et al.46
Statistical Analysis
Descriptive statistics are provided for participant demographics and adherence to the probiotic supplement. The number and percentage of participants who reported any symptom (prompted or in response to an open-ended question) was recorded. The incidence of new-onset abnormal values (outside of the laboratory reference range) on the CBC panel and CMP was reported. CBC and CMP descriptive statistics are reported at baseline and study completion; changes were analyzed using paired t tests. Microbiota PCR data were log transformed prior to calculation of geometric mean percent change and analyzed using paired t tests. Statistically significant (P < .05) microbiota parameters were subsequently evaluated using a Wilcoxon signed rank analysis, a more conservative test, to confirm statistical significance. In the case of microbiota PCR data that were outside of laboratory detection limits, the extreme detectable value was imputed for analysis. Changes in the ratio of Firmicutes to Bacteroidetes and in SCFA were analyzed using paired t tests. Given the small sample size of the study, effect size as Cohen’s d (d) was calculated between baseline and the study end point for all continuous measures. Cohen’s d was calculated as (day 10 mean – baseline mean)/(baseline standard deviation) and interpreted as small (d = 0.20), medium (d = 0.50), or large (d ≥ 0.80) effect size. Statistical analyses were performed using the software R, version 3.6.0.47
Results
Participant Characteristics
Thirteen individuals were assessed for eligibility. One did not meet eligibility criteria, 2 had scheduling conflicts, and 10 were enrolled in the study. Demographic parameters of the participants are described in Table 3. All participants completed the study.
Table 3.
Participant Demographics at Baseline (N = 10)
| Mean ± SD or n (%) | |
|---|---|
| Age (y) | 45.0 ± 17.1 |
| Sex | |
| Female | 8 (80%) |
| Male | 2 (20%) |
| Race | |
| White | 9 (90%) |
| Black | 1 (10%) |
| Ethnicity | |
| Not Hispanic or Latino | 10 (100%) |
| Weight (lb) | 200.5 ± 43.3 |
| Body mass index (kg/m2) | 32.0 ± 6.3 |
| Resting heart rate (b/min) | 65.1 ± 9.5 |
| Systolic blood pressure (mm Hg) | 129.5 ± 17.6 |
| Diastolic blood pressure (mm Hg) | 73.8 ± 7.9 |
Abbreviations: y, years; lb, pounds; kg, kilograms; m, meter; b/min, beats per minute; mmHg, millimeters of mercury; SD, standard deviation.
Adherence to the Probiotic Supplement Protocol
Participant adherence to the probiotic protocol was quantified using 2 methods; findings were consistent between the 2 approaches and indicated high adherence. An end-of-study capsule count indicated participants consumed a mean of 99% ± 0.038% of dosages. Logs completed by the participants to track their intake of capsules were consistent with participants consuming a mean of 100 ± 0.0% of dosages.
Adverse Events
No serious AEs were reported during the study. The participants collectively reported a total of 22 nonserious AEs at the study completion visit. All documented AEs were prompted/reported upon query; none were unexpected/spontaneously reported. More than two-thirds of symptoms were gastrointestinal related. Table 4 summarizes all AEs reported by the participants. The most common symptom reported by participants was an increase in bowel movement frequency. Two of these participants voluntarily provided additional information and described the change in bowel movement frequency as an improvement in bowel regularity.
Table 4.
Expected and Unexpected Adverse Events
| Symptoms Reported at the Study Completion Visit | n | % of Study Participants |
|---|---|---|
| Expected adverse events (prompted) | 22 (total) | 100% |
| Gastrointestinal | ||
| Increased bowel movement frequency | 4 | 40% |
| Loose stools | 3 | 30% |
| Flatulence | 3 | 30% |
| Diarrhea | 1 | 10% |
| Bloating | 1 | 10% |
| Abdominal rumbling | 1 | 10% |
| Nausea | 1 | 10% |
| Decreased appetite | 1 | 10% |
| Other | ||
| Difficulty falling asleep or staying asleep | 2 | 20% |
| Headache | 1 | 10% |
| Rhinorrhea | 1 | 10% |
| Sore throat | 1 | 10% |
| Increased energy | 1 | 10% |
| Muscle aches or pain | 1 | 10% |
| Unexpected adverse events (spontaneously reported) | 0 | 0% |
Blood Biomarkers: Metabolic Panel and Complete Blood Count
The incidence of new-onset abnormal values on the CMP and CBC was monitored. At the study end point, there was one abnormality in a single individual. In this participant, mean platelet volume (MPV) increased by 0.1 femtoliters (fL), from 12.5 to 12.6, and shifted to outside of the laboratory reference range (7.5-12.5 fL). All additional CMP and CBC values (329 out of 330 individual study participant data points) were within normal reference ranges at the study end point. Statistically significant changes over the course of the study included the following: total bilirubin decreased by 21.4% (P = .045, d = -0.76), aspartate aminotransferase (AST) decreased by 6.9% (P = .018, d = -0.34), and red cell distribution width (RDW) increased by 1.4% (P = .017, d = 0.32). All baseline and study completion CMP and CBC data are summarized in Table 5 and Table 6.
Table 5.
Comprehensive Metabolic Panel
| Laboratory reference rangea | Baseline | Day 10 | Mean % Δ | P valueb | Cohen’s d | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| Glucose, non-fasting (mg/dL) | 65-139 | 90.3 | 16.6 | 95.4 | 10.7 | 7.1 % | .201 | 0.31 |
| BUN (mg/dL) | 7-25 | 15.4 | 4.7 | 14.4 | 2.7 | 0.6 % | .495 | -0.21 |
| Creatinine (mg/dL) | 0.50-1.10, 0.50-0.99 (Fc), 0.60-1.35 (M) | 0.8 | 0.2 | 0.8 | 0.1 | 0.0 % | .701 | -0.07 |
| eGFR Non-African American (mL/min/1.73m2)d | ≥60 | 94.4 | 12.3 | 94.4 | 9.5 | 0.8 % | >.99 | 0.00 |
| eGFR African American (mL/min/1.73m2)e | ≥60 | 97 | 113 | 16.5 % | ||||
| Sodium (mmol/L) | 135-146 | 138.4 | 0.8 | 138.5 | 1.4 | 0.1 % | .832 | 0.12 |
| Potassium (mmol/L) | 3.5-5.3 | 4.3 | 0.3 | 4.3 | 0.2 | 0.3 % | >.99 | 0.00 |
| Chloride (mmol/L) | 98-110 | 103.6 | 2.0 | 104.3 | 2.1 | 0.7 % | .333 | 0.36 |
| Carbon dioxide (mmol/L) | 19-30 (F), 20-31 (M) | 27.3 | 2.8 | 25.9 | 2.6 | -4.6 % | .148 | -0.50 |
| Calcium (mg/dL) | 8.6-10.2, 8.6-10.3, 8.6-10.4c | 9.3 | 0.4 | 9.3 | 0.2 | 0.3 % | .941 | 0.02 |
| Protein (g/dL) | 6.1-8.1 | 6.9 | 0.2 | 7.0 | 0.2 | 2.1 % | .173 | 0.59 |
| Albumin (g/dL) | 3.6-5.1 | 4.4 | 0.2 | 4.5 | 0.2 | 2.9 % | .074 | 0.49 |
| Globulin (g/dL) | 1.9-3.7 | 2.5 | 0.2 | 2.5 | 0.3 | 0.6 % | .726 | 0.12 |
| Albumin/globulin ratio | 1.0-2.5 | 1.8 | 0.2 | 1.8 | 0.3 | 2.0 % | .343 | 0.22 |
| Total bilirubin (mg/dL) | 0.2-1.2 | 0.6 | 0.2 | 0.5 | 0.2 | -21.4% | .045 | -0.76 |
| Alkaline phosphatase (U/L) | 33-115, 33-130 (Fc), 40-115 (M) | 62.2 | 23.0 | 60.2 | 23.0 | -3.3 % | .090 | -0.09 |
| Aspartate aminotransferase, AST (U/L) | 10-30 or 10-35 (Fc), 10-40 (M) | 19.4 | 3.8 | 18.1 | 4.1 | -6.9 % | .018 | -0.34 |
| Alanine aminotransferase, ALT (U/L) | 6-29 (F), 9-46 (M) | 19.3 | 9.9 | 19.4 | 9.0 | 2.6 % | .922 | 0.01 |
aQuest Diagnostics (Seattle, WA)
bP values calculated using paired t test.
cReference range age-dependent;
deGFR non-African American participants (n = 9);
eeGFR African American participant (n = 1)
Abbreviations: Δ, change; dL, deciliter; F, female; g, gram; M, male; m, meter; mg, milligrams; min, minutes; mL, milliliter; mmol, millimoles; L, liter; SD, standard deviation; U, units.
Table 6.
Complete Blood Count (CBC) Panel
| Laboratory reference rangea | Baseline | Day 10 | Mean % Δ | P valueb | Cohen’s d | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| White blood cell count (thousand/μL) | 3.8-10.8 | 6.4 | 1.8 | 6.4 | 1.1 | 1.9% | .900 | -0.02 |
| Red blood cell count (million/μL) | 3.80-5.10 (F), 4.20-5.80 (M) | 4.6 | 0.4 | 4.6 | 0.4 | -0.2% | .830 | -0.03 |
| Hemoglobin (g/dL) | 11.7-15.5 (F), 13.2-17.1 (M) | 13.8 | 0.9 | 13.9 | 0.9 | 0.4% | .798 | 0.05 |
| Hematocrit (%) | 35.0-45.0 (F), 38.5-50.0 (M) | 40.8 | 2.5 | 40.9 | 2.7 | 0.1% | .984 | 0.00 |
| Mean corpuscular volume, MCV (fL) | 80.0-100.0 | 88.5 | 4.3 | 88.7 | 4.2 | 0.3% | .405 | 0.05 |
| Mean corpuscular hemoglobin, MCH (pg) | 27.0-33.0 | 30.0 | 1.5 | 30.2 | 1.5 | 0.5% | .164 | 0.11 |
| Mean corpuscular hemoglobin conc., MCHC (g/dL) | 32.0-36.0 | 33.9 | 0.4 | 34.0 | 0.5 | 0.3% | .409 | 0.27 |
| Red cell distribution width, RDW (%) | 11.0-15.0 | 12.5 | 0.6 | 12.7 | 0.7 | 1.4% | .017 | 0.32 |
| Platelet count (thousand/μL) | 140-400 | 291.3 | 46.0 | 285.3 | 42.5 | -1.5% | .505 | -0.13 |
| Mean platelet volume, MPV (fL) | 7.5-12.5 | 10.5 | 0.9 | 10.6 | 0.9 | 0.6% | .573 | 0.06 |
| Absolute neutrophils (cells/μL) | 1500-7800 | 3664.9 | 1288.5 | 3577.9 | 1051.8 | -0.1% | .705 | -0.07 |
| Absolute lymphocytes (cells/μL) | 850-3900 | 2038.0 | 569.2 | 2039.2 | 348.6 | 5.2% | .994 | 0.00 |
| Absolute monocytes (cells/μL) | 200-950 | 516.3 | 163.4 | 559.9 | 117.0 | 12.6% | .269 | 0.27 |
| Absolute eosinophils (cells/μL) | 15-500 | 157.4 | 90.9 | 146.0 | 90.5 | -1.3% | .515 | -0.13 |
| Absolute basophils (cells/μL) | 0-200 | 45.9 | 22.7 | 54.9 | 17.4 | 51.0% | .107 | 0.40 |
| Neutrophils (%) | N/A | 56.5 | 7.1 | 55.2 | 8.1 | -2.1% | .519 | -0.18 |
| Lymphocytes (%) | N/A | 32.0 | 6.2 | 32.7 | 7.0 | 3.0% | .711 | 0.11 |
| Monocytes (%) | N/A | 8.1 | 1.5 | 8.9 | 1.5 | 10.3% | .059 | 0.52 |
| Eosinophils (%) | N/A | 2.7 | 1.7 | 2.4 | 1.6 | -0.8% | .403 | -0.15 |
| Basophils (%) | N/A | 0.7 | 0.4 | 0.9 | 0.2 | 47.3% | .230 | 0.29 |
aQuest Diagnostics (Seattle, WA)
bP values calculated using paired t test.
Abbreviations: Δ, change; dL, deciliter; F, female; fL, femtoliter; g, gram; M, male; N/A, not applicable; pg, picogram; SD, standard deviation; μL, microliter.
Stool Analyses
CFU counts of the majority of stool microbiota did not change over the study period (Table 7). However, significant increases with medium or large effect sizes were observed in Lactobacillus species (P = .003, d = 1.45), F. prausnitzii (P = .025, d = 0.91), Akkermansia muciniphila (P = .045, d = 0.71), and Ruminococcus species (P = .034, d = 0.80). Desulfovibrio piger decreased (P = .028, d = -0.54). Bifidobacterium species also increased, but the change was not significant (P = .106, d = 0.52). Upon subsequent evaluation using the more conservative Wilcoxon signed rank test, the changes in Ruminococcus species and D. piger were no longer significant (P = .059 for both); however, the increases in Lactobacillus species, F. prausnitzii, and A. muciniphila remained significant (P = .014, P = .029, and P = .030, respectively). The ratio of Firmicutes to Bacteroidetes increased from 10.3 ± 8.0 to17.5 ± 4.7 (P = .008). Shifts in stool levels of SCFA were not significant (data not presented).
Table 7.
Levels of Stool Microbiota
| Phylum | Microbiota (CFU/g stool) | Untransformed Data | Log Transformed Data | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Day 10 | Baseline | Day 10 | Geometric mean % Δ | P valuea | Cohen’s d | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||
| Actinobacteria | Bifidobacterium spp. | 1.6E+09 | 1.9E+09 | 3.0E+09 | 3.3E+09 | 20.1 | 2.3 | 21.3 | 1.1 | 222.9% | .106 | 0.52 |
| Bifidobacterium longum | 1.1E+08 | 1.4E+08 | 9.3E+07 | 1.7E+08 | 17.3 | 2.2 | 17.6 | 1.1 | 43.6% | .567 | 0.16 | |
| Collinsella aerofaciens | 9.3E+08 | 9.4E+08 | 1.2E+09 | 1.2E+09 | 19.7 | 2.0 | 20.0 | 2.4 | 34.0% | .547 | 0.15 | |
| Bacteroidetes | Bacteroides-Prevotella group | 9.4E+08 | 5.4E+08 | 8.4E+09 | 6.9E+08 | 20.5 | 0.6 | 20.7 | 0.7 | 25.8% | .283 | 0.36 |
| Bacteroides vulgatus | 5.9E+09 | 4.8E+09 | 8.4E+09 | 7.7E+09 | 22.2 | 0.8 | 22.4 | 1.1 | 19.7% | .692 | 0.22 | |
| Barnesiella spp. | 9.9E+07 | 1.7E+08 | 1.1E+08 | 1.7E+08 | 15.9 | 2.7 | 16.3 | 2.8 | 48.9% | .519 | 0.15 | |
| Odoribacter spp. | 1.6E+08 | 1.5E+08 | 1.5E+08 | 1.3E+08 | 17.2 | 3.3 | 17.9 | 2.0 | 101.2% | .188 | 0.21 | |
| Prevotella spp. | 2.1E+07 | 2.1E+07 | 2.2E+07 | 1.5E+07 | 16.4 | 1.1 | 16.6 | 0.9 | 27.2% | .632 | 0.22 | |
| Euryarchaeota | Methanobrevibacter smithii | 7.1E+07 | 1.3E+08 | 3.9E+07 | 8.1E+07 | 14.0 | 3.4 | 14.6 | 2.9 | 71.0% | .398 | 0.16 |
| Firmicutes | Anaerotruncus colihominis | 1.9E+07 | 2.0E+07 | 1.9E+07 | 1.8E+07 | 16.1 | 1.4 | 16.4 | 0.9 | 43.2% | .311 | 0.26 |
| Butyrivibrio crossotus | 1.1E+05 | 1.0E+05 | 1.5E+05 | 2.7E+05 | 10.5 | 2.3 | 10.0 | 2.6 | -35.7% | .762 | -0.20 | |
| Clostridium spp. | 5.8E+09 | 9.4E+09 | 5.7E+09 | 6.7E+09 | 21.2 | 1.9 | 21.9 | 1.1 | 99.7% | .262 | 0.36 | |
| Coprococcus eutactus | 8.5E+06 | 8.9E+06 | 1.6E+07 | 1.3E+07 | 14.9 | 2.0 | 16.2 | 0.9 | 268.2% | .110 | 0.67 | |
| Faecalibacterium prausnitzii | 2.1E+09 | 2.4E+09 | 7.5E+09 | 6.4E+09 | 20.6 | 2.0 | 22.4 | 0.9 | 504.4% | .025b | 0.91 | |
| Lactobacillus spp. | 1.4E+09 | 1.2E+09 | 9.6E+09 | 7.3E+09 | 20.4 | 1.6 | 22.7 | 0.7 | 924.0% | .003b | 1.45 | |
| Pseudoflavonifractor spp. | 2.5E+08 | 2.2E+08 | 2.7E+08 | 1.9E+08 | 18.7 | 1.5 | 19.0 | 1.3 | 33.7% | .586 | 0.20 | |
| Roseburia spp. | 3.0E+09 | 2.0E+09 | 4.2E+09 | 2.4E+09 | 21.5 | 1.0 | 22.0 | 0.5 | 67.6% | .221 | -0.54 | |
| Ruminococcus spp. | 1.6E+08 | 1.3E+08 | 5.8E+08 | 7.3E+08 | 18.4 | 1.3 | 19.5 | 1.3 | 174.5% | .034 | 0.80 | |
| Veillonella spp. | 9.8E+06 | 8.2E+06 | 1.9E+07 | 1.6E+07 | 15.4 | 1.6 | 16.3 | 1.2 | 135.0% | .220 | 0.52 | |
| Fusobacteria | Fusobacterium spp. | 8.6E+04 | 1.3E+05 | 9.5E+04 | 9.3E+04 | 9.5 | 2.6 | 11.0 | 1.1 | 342.7% | .102 | 0.57 |
| Proteobacteria | Desulfovibrio piger | 1.2E+07 | 3.3E+07 | 8.5E+07 | 2.4E+07 | 12.4 | 3.2 | 10.6 | 3.1 | -82.4% | .028 | -0.54 |
| Escherichia coli | 5.5E+07 | 6.1E+07 | 3.6E+08 | 8.7E+08 | 17.0 | 1.6 | 17.5 | 2.2 | 61.0% | .478 | 0.29 | |
| Oxalobacter formigenes | 7.8E+06 | 9.1E+07 | 8.2E+06 | 8.2E+06 | 15.3 | 1.1 | 15.6 | 0.8 | 35.7% | .397 | 0.27 | |
| Verrucomicrobia | Akkermansia muciniphila | 1.1E+07 | 1.7E+07 | 2.0E+07 | 1.9E+07 | 13.8 | 3.1 | 16.0 | 1.7 | 805.2% | .045b | 0.71 |
aP values calculated using paired t test.
bP values confirmed with a Wilcoxon signed rank test (P < .05).
Abbreviations: Δ, change; CFU, colony-forming units; g, gram; SD, standard deviation; spp., species.
Discussion
Main objectives of this study were to prospectively assess the short-term tolerability and safety of a new 8-strain probiotic supplement in healthy adults. This study demonstrated participants consumed the probiotic supplement daily for 10 days without withdrawal, without adverse impacts on routine biomarkers, and without serious AEs. The tolerability and safety findings of the present study are consistent with multiple previous studies on the 8 probiotic strains administered in the present study, which reported that oral intake by healthy participants and various clinical populations was not associated with serious AEs or adverse changes in routine laboratory biomarkers.13,16,18-20,24,25,33-43
In the present study, all documented AEs were prompted by the investigators, were nonserious, and were most commonly gastrointestinal (which was the most common type of symptom queried). The symptom most frequently documented as an AE per the protocol was increased bowel movement frequency; however, 2 of the participants who reported this change described it as an improvement in bowel regularity. The change in bowel movement frequency is in alignment with previously reported human subject data on 6 of the strains in the formula. A study in healthy elderly people demonstrated that daily intake of 10-11 billion CFU L. acidophilus NCFM with a prebiotic resulted in an increase in bowel movement frequency.48 Upon post hoc analysis, Airaksinen et al demonstrated that in adults with bloating and constipation, supplementation with a 5-strain combination of L. acidophilus NCFM, L. paracasei Lpc-37, B. lactis Bl-04, B. lactis Bi-07, and B. lactis HN019 resulted in a significant increase in bowel movement frequency.20 In a systematic review, Skórka et al reported that L. rhamnosus GG intake is associated with a higher defecation frequency when administered in infant formula.49
Furthermore, the number of AEs per participant documented in this study (22 in 10 participants) was similar to a single-arm, open-label study of L. rhamnosus GG by Hibberd et al (in which 47 AEs were documented in 15 participants); despite the seemingly high incidence rate of AEs, Hibberd et al concluded that L. rhamnosus GG was well-tolerated and safe.33 In the present study and the Hibberd et al study,33 nonserious AEs were documented in 100% of participants. Consistent with many previous studies on single strains13,16,18,19,25,33-35,37,39-42 and combinations of strains in the 8-strain study supplement13,16,20,24,34,36,38,43 which did not yield evidence that the probiotic strains may be harmful, the tolerability and safety findings of the present study suggest that when the 8 GRAS probiotic strains are combined and taken as a multi-strain formula, they are still well-tolerated and likely safe.
Interestingly, the liver function parameters bilirubin and AST decreased significantly over the course of the study. The mechanisms for how probiotics may impact liver health are unclear, but supporting homeostasis of the gut-liver axis (the relationship between the gut, the gut microbiota, and the liver) has been proposed.50-52 Probiotics may enhance intestinal barrier function, prevent uptake of endotoxin (bacterial lipopolysaccharide) and hepatotoxins, and reduce inflammation of the liver.50,52 In a liver injury mouse model, L. rhamnosus GG decreased bilirubin and increased intestinal and hepatic activation of the farnesoid X receptor (FXR),53 which regulates intestinal permeability and pro-inflammatory cytokine production.54 Two recent studies in human newborns with hyperbilirubinemia demonstrated that oral L. rhamnosus GG intake decreased bilirubin levels within 36-72 hours.41,42 A study in Holstein calves showed L. rhamnosus GG mitigated increases in liver enzymes induced by aflatoxin, a potent hepatotoxin.55 A study in healthy adults demonstrated oral supplementation with a 2-strain combination of L. acidophilus NCFM and B. lactis Bi-07 was associated with a pre- to post-intervention decrease in AST.34 Furthermore, a recent systematic review and meta-analysis demonstrated that some probiotics decrease liver enzymes, including AST, particularly in individuals with liver disease; the authors concluded probiotics may represent a therapy for improving liver function tests.50 The changes in liver function parameters in the present study suggest the multi-strain probiotic supplement could impact liver health. Strains that could be involved include L. rhamnosus GG,41,42,53,55 L. acidophilus NCFM,34 and B. lactis Bi-07,34 and the mechanism may have involved modulation of the gut-liver axis.50-52
Changes in commensal stool microbiota were explored during the study. The significant increase in Lactobacillus spp., as well as a nonsignificant increase in Bifidobacterium spp., was not unexpected given that the probiotic supplement contained 5 strains of lactobacilli and 3 strains of bifidobacteria. However, stool counts of microbiota not present in the probiotic supplement, F. prausnitzii and A. muciniphila, also increased significantly. These changes are notable given that F. prausnitzii56 and A. muciniphila57,58 have been described as potential keystone species, signifying they may be critical for maintaining the organization and diversity of the gut ecosystem through biotic interactions with other species.59 F. prausnitzii and A. muciniphila have each been described as potential bioindicators of health because low abundance of these species is associated with many inflammatory and/or metabolic diseases.60,61 Of timely relevance, a recently published observational study of patients hospitalized with laboratory-confirmed SARS-CoV-2 infection found stool abundance of F. prausnitzii was inversely correlated with COVID-19 disease severity.62
Based on findings from previous studies, the increases in F. prausnitzii and A. muciniphila could potentially be related to probiotic strains in the investigated supplement. In children, L. rhamnosus GG intake was associated with increased stool counts of F. prausnitzii.63 In healthy adults, intake of L. rhamnosus HN001, in combination with a strain of bifidobacteria, was associated with increased stool levels of A. muciniphila.64 It is also possible the increases in A. muciniphila and F. prausnitzii were related to the decreases in AST. A study of overweight adults demonstrated supplementation with A. muciniphila significantly decreased AST levels,65 and a rodent study demonstrated intragastric inoculation with F. prausnitzii decreased AST levels.66
F. prausnitzii is one of the most abundant bacterial species in the gut and has been described as the most important butyrate-producing commensal bacteria.61,67 Growth of F. prausnitzii is fueled by acetate, a butyrate precursor produced by carbohydrate-fermenting microbiota, including lactobacilli and bifidobacteria.68,69 The enhanced growth of F. prausnitzii in the presence of bifidobacteria has been well-established in coculture experiments.68,70,71 It is possible the increase in stool levels of F. prausnitzii observed over the course of this study was mechanistically related to and potentially fueled by metabolites produced by probiotic strains in the supplement.
Interestingly, the increases in F. prausnitzii and A. muciniphila could be interrelated and associated with microbial cross-feeding, which involves the production of intermediate fermentation products by one microbial species, then subsequent utilization of those metabolites by another microbial species.72,73 F. prausnitzii and A. muciniphila are both inhabitants of the protective layer of mucus that coats the gut mucosa.57,74 Within the mucous layer, F. prausnitzii may share a metabolic network with A. muciniphila, a mucolytic species that degrades and ferments the human glycoprotein mucin, the main component of intestinal mucus, into acetate and other metabolites.57,74 F. prausnitzii also utilizes acetate produced by mucolytic microbiota, including A. muciniphila, within the intestinal mucous layer.57,74 Coculture experiments of F. prausnitzii and A. muciniphila have demonstrated syntrophic growth of these species.57,74 Therefore, it is possible the increases in F. prausnitzii and A. muciniphila were mechanistically related to trophic interactions between the 2 species.
Furthermore, it is possible that growth of F. prausnitzii fuels the growth of mucolytic microbiota, including A. muciniphila, because one of the key functions of butyrate, of which F. prausnitzii is a main producer, is stimulation of mucin and mucus production by intestinal goblet cells.61,75 Preclinical studies have demonstrated F. prausnitzii regulates goblet cell mucin production, and butyrate regulates mucin gene translation and colonic mucus production.76-79 Potential cross-feeding interactions and potential effects of the multi-strain probiotic supplement in the gut are summarized in the Figure.
Figure.
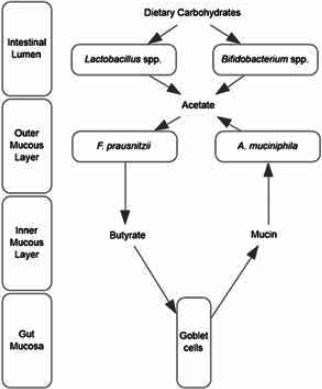
Potential Cross-Feeding Interactions between Microbiota in the Multi-Strain Probiotic Supplement, Endogenous Mucosa-Associated Gut Microbiota, Bacterial Metabolites, and the Host Gut Mucosa
This study had both strengths and limitations. Strengths include full retention of study participants, excellent adherence to the study supplement by the participants, and evaluation of a strain-identified probiotic formula; in the eighth of a recent series of probiotics review articles, Finley et al described how the lack of identification of probiotic strains on labels of professional and commercial probiotic products makes it challenging for health care practitioners and consumers to determine which products contain clinically-researched probiotic strains.80 The small sample size and lack of an untreated group are limitations of this study. However, some experts suggest early phase complementary medicine investigations should use small sample sizes and untreated groups are not always required.81,82 A randomized controlled follow-up study of the multi-strain probiotic combination could be implemented to further investigate the exploratory outcomes of the present study.
Conclusions
This work adds to the evidence base on multi-strain probiotic supplement clinical tolerability and safety. The results of this study are particularly relevant to health care practitioners who currently recommend multi-strain probiotics to their patients, including the studied 8-strain probiotic combination. Additional results of this study, specifically the changes in liver function parameters and commensal microbiota known to predominantly inhabit the intestinal mucosal barrier, were particularly novel and mechanistically interesting. However, these findings cannot be generalized to all multi-strain probiotic combinations because effects may be strain-specific and/or related to synergistic effects of the 8-strain probiotic combination. In summary, the findings of this study indicate that the multi-strain probiotic combination was well-tolerated by healthy adults, the most common side effect was expected (increased bowel movement frequency), and the probiotic combination was most likely safe when taken short-term. Based on preliminary stool microbiota results, additional research is necessary to further evaluate potential interactions and mechanisms between the probiotic strains and endogenous gut microbiota.
Footnotes
Author Contributions
Drs Ryan and Patno contributed equally to this paper.
Author Disclosure Statement
J. Ryan reports grants from Metagenics, Inc during the conduct of the study. N. Patno is an employee of Metagenics, Inc.
Funding/Support
This work was supported by Metagenics, Inc.
Additional Contributions
The authors thank Dr. Joseph Lamb, the Medical Director at Personalized Lifestyle Medicine Center, and Kim Koch, the Clinical Research Coordinator, for their instrumental contributions to this work. The authors also thank Douglas Allen Hanes, PhD, for assistance with data analysis.
Trial Registration
ClinicalTrials.gov Identifier: NCT04044144
References
- 1.Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. National Center for Health Statistics, US Dept of Health and Human Services; February10, 2015;79. [PMC free article] [PubMed] [Google Scholar]
- 2.Islam SU. Clinical uses of probiotics. Medicine. February2016;95(5):e2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015;60(Suppl 2):S129-S134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Adv Nutr. 2019;10:S49-S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, Gómez-Llorente C, Gil A. Probiotic mechanisms of action. Ann Nutr Metab. 2012;61(2):160-174. [DOI] [PubMed] [Google Scholar]
- 6.Scott KP, Antoine JM, Midtvedt T, van Hemert S. Manipulating the gut microbiota to maintain health and treat disease. Microb Ecol Health Dis. 2015;26:25877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McFarland LV, Evans CT, Goldstein EJ. Strain-specificity and disease-specificity of probiotic efficacy: a systematic review and meta-analysis. Front Med (Lausanne). 2018;5:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McFarland LV. Efficacy of single-strain probiotics versus multi-strain mixtures: systematic review of strain and disease specificity. Dig Dis Sci. 2020April9. [DOI] [PubMed] [Google Scholar]
- 9.Ouwehand AC, Invernici MM, Furlaneto FA, Messora MR. Effectiveness of multistrain versus single-strain probiotics: current status and recommendations for the future. J Clin Gastroenterol. Nov-Dec 2018;52;S35-S40. [DOI] [PubMed] [Google Scholar]
- 10.Chapman CM, Gibson GR, Rowland I. Health benefits of probiotics: are mixtures more effective than single strains? Eur J Nutr. 2011;50(1):1-17. [DOI] [PubMed] [Google Scholar]
- 11.McFarland LV, Huang Y, Wang L, Malfertheiner P. Systematic review and meta-analysis: multi-strain probiotics as adjunct therapy for Helicobacter pylori eradication and prevention of adverse events. United European Gastroenterol J. 2016August;4(4):546-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leite GS, Resende AS, West NP, Lancha AH, Jr. Probiotics and sports: a new magic bullet? Nutrition. 2019April;60:152-160. [DOI] [PubMed] [Google Scholar]
- 13.West NP, Horn PL, Pyne DB, et al. Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals. Clin Nutr. 2014August;33(4):581-587. [DOI] [PubMed] [Google Scholar]
- 14.Paineau D, Carcano D, Leyer G, et al. Effects of seven potential probiotic strains on specific immune responses in healthy adults: a double-blind, randomized, controlled trial. FEMS Immunol Med Microbiol. 2008;53(1):107-113. [DOI] [PubMed] [Google Scholar]
- 15.Gill HS, Rutherfurd KJ, Cross ML. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: an investigation of age-related immunological changes. J Clin Immunol. 2001;21(4):264-271. [DOI] [PubMed] [Google Scholar]
- 16.Leyer GJ, Li S, Mubasher ME, Reifer C, Ouwehand AC. Probiotic effects on cold and influenza-like symptom incidence and duration in children. Pediatrics. 2009;124(2):e172-179. [DOI] [PubMed] [Google Scholar]
- 17.Gill HS, Rutherfurd KJ, Cross ML, Gopal PK. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am J Clin Nutr. 2001;74(6):833-839. [DOI] [PubMed] [Google Scholar]
- 18.Sheih YH, Chiang BL, Wang LH, Liao CK, Gill HS. Systemic immunity-enhancing effects in healthy subjects following dietary consumption of the lactic acid bacterium Lactobacillus rhamnosus HN001. J Am Coll Nutr. 2001;20(2 Suppl):149-156. [DOI] [PubMed] [Google Scholar]
- 19.Eggers S, Barker AK, Valentine S, Hess T, Duster M, Safdar N. Effect of Lactobacillus rhamnosus HN001 on carriage of Staphylococcus aureus: results of the impact of probiotics for reducing infections in veterans (IMPROVE) study. BMC Infect Dis. 2018;18(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Airaksinen K, Yeung N, Lyra A, et al. The effect of a probiotic blend on gastrointestinal symptoms in constipated patients: a double blind, randomised, placebo controlled 2-week trial. Benef Microbes. 2019;10(6):617-627. [DOI] [PubMed] [Google Scholar]
- 21.D’Souza B, Slack T, Wong SW, et al. Randomized controlled trial of probiotics after colonoscopy. ANZ J Surg. 2017;87(9):E65-E69. [DOI] [PubMed] [Google Scholar]
- 22.Capurso L. Thirty years of Lactobacillus rhamnosus GG: a review. J Clin Gastroenterol. 2019;53(March)(suppl 1):S1-S41. [DOI] [PubMed] [Google Scholar]
- 23.Ouwehand AC, DongLian C, Weijian X, et al. Probiotics reduce symptoms of antibiotic use in a hospital setting: a randomized dose response study. Vaccine. 2014;32(4):458-463. [DOI] [PubMed] [Google Scholar]
- 24.Ringel-Kulka T, Palsson OS, Maier D, et al. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. J Clin Gastroenterol. 2011;45(6):518-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyra A, Hillilä M, Huttunen T, et al. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol. 2016;22(48):10631-10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders ME, Klaenhammer TR. Invited review: the scientific basis of Lactobacillus acidophilus NCFM functionality as a probiotic. J Dairy Sci. 2001;84(2):319-331. [DOI] [PubMed] [Google Scholar]
- 27.Lahtinen SJ, Forssten S, Aakko J, et al. Probiotic cheese containing Lactobacillus rhamnosus HN001 and Lactobacillus acidophilus NCFM® modifies subpopulations of fecal lactobacilli and Clostridium difficile in the elderly. Age (Omaha). 2012;34(1):133-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hempel S, Newberry S, Ruelaz A, et al. Safety of probiotics used to reduce risk and prevent or treat disease. Evid Rep Technol Assess (Full Rep). 2011;(200):1-645. [PMC free article] [PubMed] [Google Scholar]
- 29.Korada SK, Yarla NS, Mishra V, et al. Single probiotic versus multiple probiotics - a debate on current scenario for alleviating health benefits. Curr Pharm Des. 2018;24(35):4150-4153. [DOI] [PubMed] [Google Scholar]
- 30.Shader RI. Safety Versus Tolerability. Clin Ther. 2018;40(5):672-673. [DOI] [PubMed] [Google Scholar]
- 31.U.S. Food & Drug Administration: What is a Serious Adverse Event? AccessedMarch4, 2020. https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event
- 32.Serious Adverse Event Reporting for Dietary Supplements. AccessedMarch4, 2020. https://uscode.house.gov/view.xhtml?req=granuleid:USC-prelim-title21-section379aa-1&num=0&edition=prelim
- 33.Hibberd PL, Kleimola L, Fiorino AM, et al. No evidence of harms of probiotic Lactobacillus rhamnosus GG ATCC 53103 in healthy elderly - A phase I open label study to assess safety, tolerability and cytokine responses. PLoS One. 2014;9(12):1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cox AJ, West NP, Horn PL, et al. Effects of probiotic supplementation over 5 months on routine haematology and clinical chemistry measures in healthy active adults. Eur J Clin Nutr. 2014;68(11):1255-1257. [DOI] [PubMed] [Google Scholar]
- 35.Forssten S, Evans M, Wilson D, Ouwehand AC. Influence of a probiotic mixture on antibiotic induced microbiota disturbances. World J Gastroenterol. 2014;20(33):11878-11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engelbrektson A, Korzenik JR, Pittler A, et al. Probiotics to minimize the disruption of faecal microbiota in healthy subjects undergoing antibiotic therapy. J Med Microbiol. 2009;58(5):663-670. [DOI] [PubMed] [Google Scholar]
- 37.Waller PA, Gopal PK, Leyer GJ, et al. Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scand J Gastroenterol. 2011;46(9):1057-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Chen J, Wu J, Chalson H, Merigan L, Mitchell A. Probiotic use in preventing postoperative infection in liver transplant patients. Hepatobiliary Surg Nutr. 2013;2(3):142-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wickens KL, Barthow CA, Murphy R, et al. Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: a randomised controlled trial. Br J Nutr. 2017;117(6):804-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dekker JW, Wickens K, Black PN, et al. Safety aspects of probiotic bacterial strains Lactobacillus rhamnosus HN001 and Bifidobacterium animalis subsp. lactis HN019 in human infants aged 0-2 years. Int Dairy J. 2009;19(3):149-154. [Google Scholar]
- 41.Mutlu M, Aslan Y, Kader Ş, Aktürk Acar F. Preventive effects of probiotic supplementation on neonatal hyperbilirubinemia caused by isoimmunization. Am J Perinatol. 2020;37(11):1173-1176. [DOI] [PubMed] [Google Scholar]
- 42.Mutlu M, Irmak E, Aslan Y, Kader Ş. Effects of Lactobacillus rhamnosus GG as a probiotic on neonatal hyperbilirubinemia. Turk J Pediatr. 2018;60(5):482-487. [DOI] [PubMed] [Google Scholar]
- 43.Fox MJ, Ahuja KD, Robertson IK, Ball MJ, Eri RD. Can probiotic yogurt prevent diarrhoea in children on antibiotics? A double-blind, randomised, placebo-controlled study. BMJ Open. 2015;5(1):e006474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slykerman RF, Hood F, Wickens K, et al. Probiotic in pregnancy study group . effect of lactobacillus rhamnosus hn001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. EBioMedicine. 2017;24:159-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.GRAS Notice Inventory. AccessedOctober22, 2020. https://www.fda.gov/food/generally-recognized-safe-gras/gras-notice-inventory
- 46.Chen L, Reynolds C, David R, Peace Brewer A. Development of an index score for intestinal inflammation-associated dysbiosis using real-world stool test results. Dig Dis Sci. 2020;65(4):1111-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.eea.europa.eu/data-and-maps/indicators/oxygen-consuming-substances-in-rivers/r-development-core-team-2006 [Google Scholar]
- 48.Ouwehand AC, Tiihonen K, Saarinen M, Putaala H, Rautonen N. Influence of a combination of Lactobacillus acidophilus NCFM and lactitol on healthy elderly: intestinal and immune parameters. Br J Nutr. 2009;101(3):367-375. [DOI] [PubMed] [Google Scholar]
- 49.Skórka A, Pieścik-Lech M, Kołodziej M, Szajewska H. To add or not to add probiotics to infant formulae? An updated systematic review. Benef Microbes. 2017;8(5):717-725. [DOI] [PubMed] [Google Scholar]
- 50.Khalesi S, Johnson DW, Campbell K, et al. Effect of probiotics and synbiotics consumption on serum concentrations of liver function test enzymes: a systematic review and meta-analysis. Eur J Nutr. 2018;57(6):2037-2053. [DOI] [PubMed] [Google Scholar]
- 51.Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 2020;72(3):558-577. [DOI] [PubMed] [Google Scholar]
- 52.Gratz SW, Mykkanen H, El-Nezami HS. Probiotics and gut health: a special focus on liver diseases. World J Gastroenterol. 2010;16(4):403-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ren L, Song Q, Liu Y, Zhang L, Hao Z, Feng W. Probiotic Lactobacillus rhamnosus GG prevents progesterone metabolite epiallaopregnanolone sulfate-induced hepatic bile acid accumulation and liver injury. Biochem Biophys Res Commun. 2019;520(1):67-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stojancevic M, Stankov K, Mikov M. The impact of farnesoid X receptor activation on intestinal permeability in inflammatory bowel disease. Can J Gastroenterol. 2012;26(9):631-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang LY, Liu S, Zhao XJ, et al. Lactobacillus rhamnosus GG modulates gastrointestinal absorption, excretion patterns, and toxicity in Holstein calves fed a single dose of aflatoxin B1. J Dairy Sci. 2019;102(2):1330-1340. [DOI] [PubMed] [Google Scholar]
- 56.Miquel S, Martín R, Lashermes A, et al. Anti-nociceptive effect of Faecalibacterium prausnitzii in non-inflammatory IBS-like models. Sci Rep. 2016;6:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belzer C, Chia LW, Aalvink S, et al. Microbial metabolic networks at the mucus layer lead to diet-independent butyrate and vitamin B12 production by intestinal symbionts. mBio. 2017;8(5):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chia LW, Hornung BV, Aalvink S, et al. Deciphering the trophic interaction between Akkermansia muciniphila and the butyrogenic gut commensal Anaerostipes caccae using a metatranscriptomic approach. Antonie Van Leeuwenhoek. 2018;111(6):859-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trosvik P, de Muinck EJ. Ecology of bacteria in the human gastrointestinal tract—identification of keystone and foundation taxa. Microbiome. 2015;3:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verhoog S, Taneri PE, Roa Díaz ZM, et al. Dietary factors and modulation of bacteria strains of akkermansia muciniphila and faecalibacterium prausnitzii: a systematic review. Nutrients. 2019;11(7):1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferreira-Halder CV, Faria AV, Andrade SS. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract Res Clin Gastroenterol. 2017;31(6):643-648. [DOI] [PubMed] [Google Scholar]
- 62.Zuo T, Zhang F, Lui GC, et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159(3):944-955:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bruzzese E, Callegari ML, Raia V, et al. Disrupted intestinal microbiota and intestinal inflammation in children with cystic fibrosis and its restoration with Lactobacillus GG: a randomised clinical trial. PLoS One. 2014;9(2):e87796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toscano M, De Grandi R, Stronati L, De Vecchi E, Drago L. Effect o. Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 on the healthy gut microbiota composition at phyla and species level: a preliminary study. World J Gastroenterol. 2017;23(15):2696-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Depommier C, Everard A, Druart C, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25(7):1096-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Munukka E, Rintala A, Toivonen R, et al. Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. ISME J. 2017;11(7):1667-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walker AW, Ince J, Duncan SH, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5(2):220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. 2016;7(June):979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moens F, Verce M, De Vuyst L. Lactate- and acetate-based cross-feeding interactions between selected strains of lactobacilli, bifidobacteria and colon bacteria in the presence of inulin-type fructans. Int J Food Microbiol. 2017;241:225-236. [DOI] [PubMed] [Google Scholar]
- 70.Moens F, Weckx S, De Vuyst L. Bifidobacterial inulin-type fructan degradation capacity determines cross-feeding interactions between bifidobacteria and Faecalibacterium prausnitzii. Int J Food Microbiol. 2016;231:76-85. [DOI] [PubMed] [Google Scholar]
- 71.Rios-Covian D, Gueimonde M, Duncan SH, Flint HJ, de los Reyes-Gavilan CG. Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiol Lett. 2015;362(21):1-7. [DOI] [PubMed] [Google Scholar]
- 72.Rowland I, Gibson G, Heinken A, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57(1):1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.D’Souza G, Shitut S, Preussger D, Yousif G, Waschina S, Kost C. Ecology and evolution of metabolic cross-feeding interactions in bacteria. Nat Prod Rep. 2018;35(5):455-488. [DOI] [PubMed] [Google Scholar]
- 74.Lopez-Siles M, Enrich-Capó N, Aldeguer X, et al. Alterations in the abundance and co-occurrence of Akkermansia muciniphila and Faecalibacterium prausnitzii in the colonic mucosa of inflammatory bowel disease subjects. Front Cell Infect Microbiol. 2018;8(September):281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cornick S, Tawiah A, Chadee K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers. 2015;3(1-2):e982426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wrzosek L, Miquel S, Noordine ML, et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11(5):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27(2):104-119. [DOI] [PubMed] [Google Scholar]
- 78.Canani RB, Di Costanzo M, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17(12):1519-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jung TH, Park JH, Jeon WM, Han KS. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr Res Pract. 2015;9(4):343-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Finley HJ, Gasta MG, Dolan KE, et al. Probiotics and disease: A comprehensive summary - Part 8, gastrointestinal and genitourinary disorders. Integr Med. 2018;17(1):38-48. [PMC free article] [PubMed] [Google Scholar]
- 81.Aickin M. The importance of early phase research. J Altern Complement Med. 2007;13(4):447-450. [DOI] [PubMed] [Google Scholar]
- 82.Schwartz GE. Early phase research and the process of scientific discovery. J Altern Complement Med. 2007;13(4):399. [DOI] [PubMed] [Google Scholar]


