Abstract
Background
Anakinra might improve the prognosis of patients with moderate to severe COVID-19 (ie, patients requiring oxygen supplementation but not yet receiving organ support). We aimed to assess the effect of anakinra treatment on mortality in patients admitted to hospital with COVID-19.
Methods
For this systematic review and individual patient-level meta-analysis, a systematic literature search was done on Dec 28, 2020, in Medline (PubMed), Cochrane, medRxiv, bioRxiv, and the ClinicalTrials.gov databases for randomised trials, comparative studies, and observational studies of patients admitted to hospital with COVID-19, comparing administration of anakinra with standard of care, or placebo, or both. The search was repeated on Jan 22, 2021. Individual patient-level data were requested from investigators and corresponding authors of eligible studies; if individual patient-level data were not available, published data were extracted from the original reports. The primary endpoint was mortality after 28 days and the secondary endpoint was safety (eg, the risk of secondary infections). This study is registered on PROSPERO (CRD42020221491).
Findings
209 articles were identified, of which 178 full-text articles fulfilled screening criteria and were assessed. Aggregate data on 1185 patients from nine studies were analysed, and individual patient-level data on 895 patients were provided from six of these studies. Eight studies were observational and one was a randomised controlled trial. Most studies used historical controls. In the individual patient-level meta-analysis, after adjusting for age, comorbidities, baseline ratio of the arterial partial oxygen pressure divided by the fraction of inspired oxygen (PaO2/FiO2), C-reactive protein (CRP) concentrations, and lymphopenia, mortality was significantly lower in patients treated with anakinra (38 [11%] of 342) than in those receiving standard of care with or without placebo (137 [25%] of 553; adjusted odds ratio [OR] 0·32 [95% CI 0·20–0·51]). The mortality benefit was similar across subgroups regardless of comorbidities (ie, diabetes), ferritin concentrations, or the baseline PaO2/FiO2. In a subgroup analysis, anakinra was more effective in lowering mortality in patients with CRP concentrations higher than 100 mg/L (OR 0·28 [95% CI 0·17–0·47]). Anakinra showed a significant survival benefit when given without dexamethasone (OR 0·23 [95% CI 0·12–0·43]), but not with dexamethasone co-administration (0·72 [95% CI 0·37–1·41]). Anakinra was not associated with a significantly increased risk of secondary infections when compared with standard of care (OR 1·35 [95% CI 0·59–3·10]).
Interpretation
Anakinra could be a safe, anti-inflammatory treatment option to reduce the mortality risk in patients admitted to hospital with moderate to severe COVID-19 pneumonia, especially in the presence of signs of hyperinflammation such as CRP concentrations higher than 100 mg/L.
Funding
Sobi.
Introduction
The COVID-19 pandemic, caused by SARS-CoV-2, has affected more than 200 countries, with millions of confirmed cases and deaths worldwide.1 The most severe and lethal forms of COVID-19 have been linked to different and possibly combined pathophysiological processes, including severe acute diffuse alveolar damage and hyaline deposits in the lungs induced by SARS-CoV-2, multiple arterial and venous thromboses resulting from both endotheliitis and hypercoagulability, and a hyperinflammatory response caused by overproduction of pro-inflammatory cytokines. Although systemically a cytokine storm might not always occur in patients,2, 3, 4 there are clear indications of pulmonary compartmentalisation of hyperinflammation.5, 6, 7, 8
To control the hyperinflammatory syndrome induced by SARS-CoV-2, in which the interleukin (IL)-1–IL-6 pathway is involved, various targeted therapies have been tested, predominantly in combination with antibiotics and anticoagulants. Anakinra, a recombinant IL-1 receptor antagonist, was selected as a logical candidate in the early days of the pandemic, since it had previously been tested—with encouraging results—in other hyperinflammatory situations, including the macrophage activation syndrome complicating severe bacterial sepsis and the cytokine release syndrome observed during antitumoral chimeric antigen receptor (CAR) T-cell therapy.9, 10, 11
Research in context.
Evidence before this study
Since the emergence of the COVID-19 pandemic, numerous drugs have been administered to patients with the aim of preventing major detrimental consequences, such as respiratory and multiorgan failure and death. During the early stages of the pandemic, physicians realised that drugs aiming to regulate the host immune response might play an important role in the treatment of COVID-19. Evidence from a small number of patients with moderate or severe COVID-19 treated with anakinra, an interleukin-1 receptor antagonist, has suggested therapeutic efficacy of the drug. We systematically searched the available literature for published studies of anakinra treatment in patients admitted to hospital with COVID-19, to investigate its effect on mortality. Medline (PubMed), Cochrane, medRxiv, bioRxiv, and ClinicalTrials.gov databases were searched on Dec 28, 2020, using the terms “COVID-19” or “SARS-CoV-2” and “anakinra”, “interleukin-1”, and “interleukin blockade”.
Added value of this study
This study is, to our knowledge, the first patient-level meta-analysis to analyse the effects of anakinra treatment in patients admitted to hospital with moderate to severe COVID-19, showing a significant reduction in mortality with anakinra and also demonstrating the safety of the treatment. Most importantly, this study identifies a subgroup of patients who might benefit most from treatment with anakinra: those with C-reactive protein concentrations higher than 100 mg/L. Large, randomised controlled trials are urgently needed to further investigate the effectiveness of anakinra in this setting.
Implications of all the available evidence
Anakinra could be an effective and safe immunomodulatory treatment to prevent unfavourable outcomes in moderate-to-severe cases of pneumonia due to COVID-19. Additionally, anakinra might be helpful in avoiding adverse events, such as the secondary infections observed frequently with dexamethasone use, and could be considered as an alternative treatment option in specific subgroups (eg, patients with diabetes). Larger trials are ongoing, and their results are urgently needed to further investigate the most effective use of anakinra in the treatment of COVID-19 (ie, to establish in which patient population it is most suitable, which is the ideal biomarker to define patient status, which dose should be administered, and which is the most appropriate timepoint to administer the drug during the course of the disease).
Case series of a small number of patients with moderate or severe COVID-19 treated with anakinra have been published during the early period of the pandemic, suggesting promising results and paving the way for additional larger studies.12, 13, 14, 15 In most studies, anakinra was administered on an off-label basis in patients with clinical or laboratory signs of hyperinflammation, or both, or in the context of clinical trials, in an attempt to dampen the IL-1-driven inflammation that is believed to play an important role in COVID-19 pathophysiology. We aimed to investigate the effect of anakinra treatment on mortality in patients admitted to hospital with COVID-19, and to better characterise subgroups of patients most likely to benefit from such a tailored immunotherapy.
Methods
Search strategy and selection criteria
This systematic review and patient-level meta-analysis was done according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement,16 based on a pre-specified protocol (PROSPERO: CRD42020221491). Eligibility criteria were defined with the PICO statement as follows: P=patients admitted to hospital with laboratory-confirmed SARS-CoV-2 infection; I=treatment with anakinra; C=patients receiving standard of care or placebo comparator on top of standard of care; O=mortality rate, and rate of common adverse events such as elevated liver function tests, leukopenia, and secondary infections. All randomised trials, comparative studies, and observational studies, published as full text in English were included, whereas editorials, conference abstracts, studies done in animals, case reports, and articles not written in the English language or not providing the full text were excluded. Systematic reviews were consulted for additional information, but were excluded to avoid duplication.
The search was done on Dec 28, 2020, and repeated on Jan 22, 2021, by two independent authors (EKy and EJG-B), across Medline (PubMed), Cochrane, medRxiv, bioRxiv, and ClinicalTrials.gov databases using the following terms: “COVID-19” or “SARS-CoV-2” and “anakinra”, “interleukin-1”, and “interleukin blockade”. Details of the search strategy are provided in the appendix (pp 1–2). Both reviewers assessed all articles first on the basis of the title, then on the basis of the abstract, and finally the full text to identify eligible studies. Any between-reviewer disagreements were resolved by discussion between them. Individual patient-level data were requested from investigators and corresponding authors of all eligible articles. Data from each study were first checked against reported results and queries were resolved with the investigators. Data were assessed in a consistent manner across all studies with standard definitions and variables. Data on the following variables were collected: first author name, country of origin, publication date, study design, total number of patients, criteria for enrolment, number of patients treated with anakinra, number of controls, age and comorbidities of participants, serum ferritin and C-reactive protein (CRP) concentrations at baseline, respiratory ratio at baseline, route of anakinra administration, intake of steroids, mortality, onset of respiratory failure necessitating invasive mechanical ventilation, and onset of adverse events (elevated liver function tests, leukopenia, and secondary infections). If individual patient-level data were not available, all above available data were extracted independently by EKy and EJG-B.
The quality of each study was evaluated by EKy and EJG-B with the Newcastle-Ottawa scale.17 Across the entire process, between-reviewer disagreements were resolved by discussion between them.
Data analysis
The primary endpoint was mortality after 28 days. For studies with a shorter follow-up, data were censored at the last observation. The secondary endpoint was safety, assessed by the rate of adverse events. Odds ratios and their respective 95% CIs were used to compare anakinra-treated patients to control groups for each endpoint. Pre-defined subgroup analyses were done for the primary endpoint according to the following variables: the baseline ratio of the arterial partial oxygen pressure divided by the fraction of inspired oxygen (PaO2/FiO2), baseline CRP and serum ferritin concentrations, and the Charlson Comorbidity Index. A logistic regression analysis was done: first, to detect any interaction effect of anakinra treatment with the different studies; and second, to detect whether the effect of anakinra on the primary outcome remained independent of factors interfering with mortality (ie, age, Charlson comorbidity index, CRP, and PaO2/FiO2). Predefined cutoffs were used for characterisation of the above subgroups.18, 19, 20, 21 Sensitivity analyses were planned for time period (ie, before and after implementation of dexamethasone) and for randomised controlled trials. Meta-analysis of aggregate data was done by the Mantel-Haenszel method. The Sidik-Jonkman estimator was used for τ2 and the Q-profile method used for the confidence interval of τ2 and τ. A continuity correction of 0·1 was applied in studies with zero cell frequencies. Finally, heterogeneity and inconsistency were assessed in a meta-analysis of aggregate data from all eligible studies using the I 2 criterion. The fixed-effects model was used for I 2 less than 50%, and the random-effects model used for I 2 50% or greater. The corresponding forest plot was produced, and publication bias was assessed with the funnel plot. Studies without available individual patient-level data were only included in the aggregate data meta-analysis. No imputation was made for missing data within individual patient-level data. All statistical analyses were done with SPSS (version 23) and Review Manager (version 5.3).
Role of the funding source
Sobi supported this study by providing a small research grant to cover the cost of the statistical analysis. The funder had no role in study design or conduct, data analysis, data interpretation, or in preparation of the manuscript.
Results
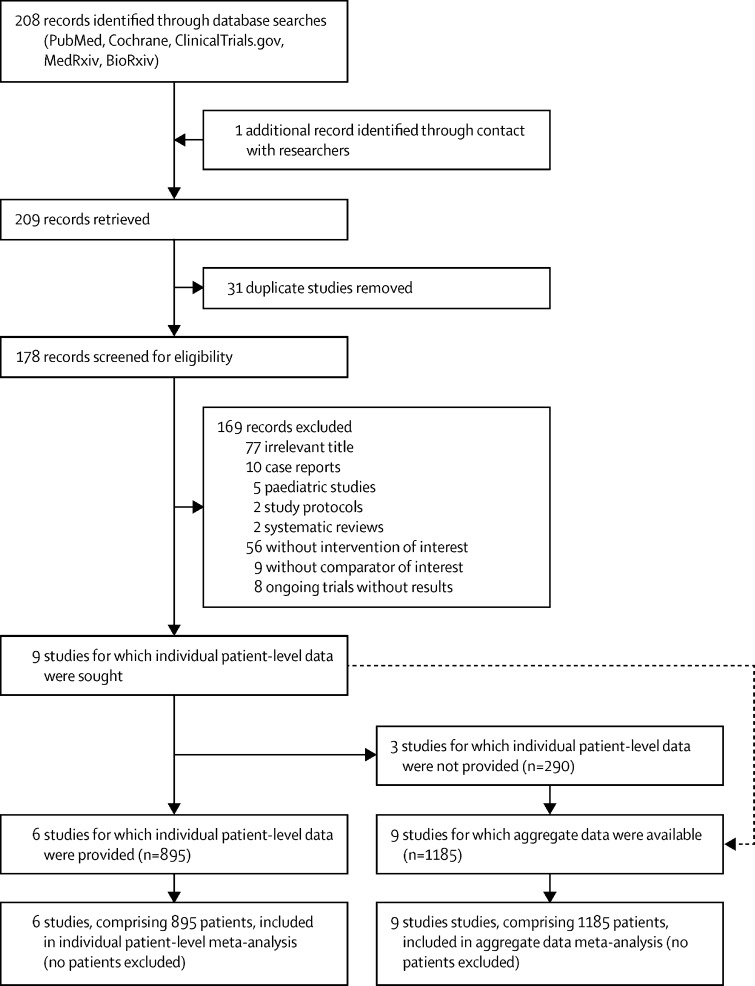
209 articles were identified: 208 from the initial search and one from contact with researchers. After removal of duplicates, 178 full-text articles were screened. After excluding 169 records, there were nine eligible studies for which individual patient-level data were sought.22, 23, 24, 25, 26, 27, 28, 29, 30 Aggregate data from these nine studies, comprising 1185 patients, were analysed, and individual patient-level data were provided for six of these studies, comprising 895 patients (figure 1 ). Eight studies were observational (either prospective or retrospective) and only one was a randomised controlled trial.24 103 (12%) of 895 patients were on mechanical ventilation at baseline. Among the three studies for which individual patient-level data were not obtained,24, 27, 30 two favoured anakinra treatment27, 30 and one did not.24 Characteristics of the included studies are summarised in table 1 . Three studies were done in France,22, 23, 24 three in Italy,25, 26, 27 one in the Netherlands,28 one in Greece,29 and one in Oman.30 Data published during the early period of the pandemic in an Italian study31 were integrally included in a larger study of the same group;26 thus, to avoid duplication, the first cohort was not included. Seven studies were done before implementation of dexamethasone in standard-of-care treatment of patients with COVID-19; one study recruited anakinra-treated patients after dexamethasone implementation, whereas patients in the control group were recruited before its implementation and all patients in the control group received methylprednisolone;30 and only one study prospectively recruited both anakinra-treated and control patients after implementation of dexamethasone into standard of care.29 In another study, methylprednisolone was part of the intervention as it was co-administered with anakinra.25 Mortality data after 28 days were available from seven studies, whereas data were censored at the last observation in two studies23, 30 with a shorter follow-up. An assessment of the individual risk of bias according to the Newcastle-Ottawa scale is provided in the appendix (p 5). The overall quality of included studies was high.
Figure 1.
Study selection
Table 1.
Characteristics of included studies
| Study type; individual patient data available? | Study setting and period | Inflammation criteria for inclusion |
Number of patients |
Route of administration | Steroid intake | ||
|---|---|---|---|---|---|---|---|
| Anakinra group | Control group | ||||||
| Cauchois et al (2020)22 | Observational; yes | France: not mentioned | CRP >110 mg/L | 12 | 10 | Intravenous | No dexamethasone as standard of care; no other steroids |
| Huet et al (2020)23 | Observational; yes | France: March, 2020 (historical controls); March 24–April 6, 2020 (anakinra group) | .. | 52 | 44 | Subcutaneous | No dexamethasone as standard of care; steroid pulse in 2 of 52 patients in anakinra group |
| The CORIMUNO-19 Collaborative group (2021)24 | Randomised controlled trial; no | France: April 8–26, 2020 | CRP >25 mg/L | 59 | 55 | Intravenous | Dexamethasone in 1 of 59 in anakinra group; other glucocorticoids in 6 of 59 in anakinra group, and 8 of 55 in control group |
| Bozzi et al (2021)25 | Observational; yes | Italy: Feb 25–March 30, 2020 | CRP > 100 mg/L or ferritin >1000 μg/L, or both | 65 | 55 | Subcutaneous; intravenous if on invasive mechanical ventilation | No dexamethasone as standard of care; methylprednisolone co-administered with anakinra |
| Cavalli et al (2021)26 | Observational; yes | Italy: March 10–17, 2020 (historical controls); March–May, 2020 (anakinra group) | CRP >100 mg/L or ferritin >900 μg/L | 62 | 275 | Intravenous | Dexamethasone in 54 of 275 controls and in 7 of 62 in anakinra group |
| Pontali et al (2021)27 | Observational; no | Italy: Feb 26–April 29, 2020 | CRP or ferritin >3 times the normal limits | 63 | 44 | Intravenous | No dexamethasone as standard of care; methylprednisolone in 33 of 63 patients in anakinra group |
| Kooistra et al (2020)28 | Observational; yes | Netherlands: March 11–April 27, 2020 | Ferritin >1800 μg/L; clinical hyperinflammation signs (persistent fever, unexplained progression of multiorgan failure) | 21 | 39 | Intravenous | Dexamethasone in 14 of 39 patients on standard of care and in 3 of 21 in anakinra group |
| Kyriazopoulou et al (2021)29 | Observational; yes | Greece: April 16–Sept 12, 2020 | suPAR >6 μg/L | 130 | 130 | Subcutaneous | Dexamethasone as standard of care in 47 of 130 controls and in 52 of 130 in anakinra group |
| Balkhair et al (2021)30 | Observational; no | Oman: April 1–June 14, 2020 (historical controls); June 15–July 25, 2020 (anakinra group) | .. | 45 | 24 | Subcutaneous | Dexamethasone in 24 of 45 in anakinra group, and 3 of 24 controls; methylprednisolone in 1 of 45 in anakinra group, and in 13 of 24 controls |
CRP=C-reactive protein. suPAR=soluble urokinase-type plasminogen activator receptor.
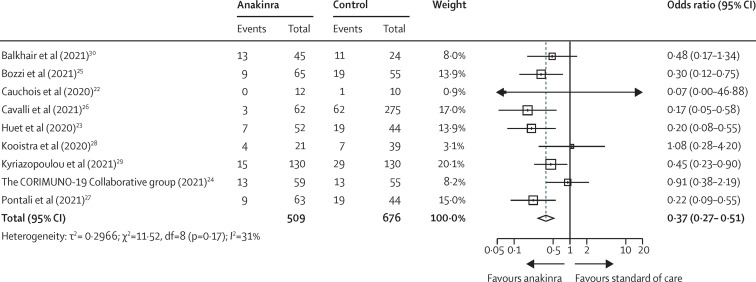
Aggregate data from nine studies, comprising 1185 patients (509 on anakinra and 676 controls), showed a significant reduction in mortality among anakinra-treated patients compared with patients receiving standard of care with or without placebo; the pooled odds ratio (OR) for mortality was 0·37 (95% CI 0·27–0·51; I 2 31%), as shown in figure 2 . There was no evidence of publication bias (test for funnel plot asymmetry: t=–0·9137, p=0·39; appendix p 6). In the individual patient-level meta-analysis, comprising 895 patients, 38 (11%) of 342 anakinra-treated patients died versus 137 (25%) of 553 patients receiving standard of care with or without placebo (OR 0·38 [95% CI 0·26–0·56]; p<0·0001; table 2 ). No interaction effect was observed among the six studies and anakinra treatment for the primary outcome (p=0·15; table 2). After adjusting for age, comorbidities, baseline PaO2/FiO2, lymphopenia and CRP concentrations, anakinra was shown to independently protect against mortality (adjusted OR 0·32 [95% CI 0·20–0·51]; p<0·0001; table 2). Similar ORs were estimated for anakinra treatment after adjustment for ferritin (data available for 486 patients; adjusted OR 0·35 [95% CI 0·23–0·52]; p<0·0001) and IL-6 concentrations (data available for 530 patients; 0·54 [0·33–0·87]; p=0·01). The predefined sensitivity analyses for randomised controlled trials were not possible, since only one randomised controlled trial was included in this meta-analysis.
Figure 2.
Forest plot showing mortality from aggregate data meta-analysis
Odds ratios calculated with a fixed-effects Mantel-Haenszel test.
Table 2.
Univariate and multivariate logistic regression analysis of variables associated with mortality in the individual patient-level data analysis of 895 patients
|
Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | |
| Anakinra treatment | 0·38 (0·26–0·56) | <0·0001 | 0·32 (0·20–0·51) | <0·0001 |
| Age >72 years* | 4·97 (3·5–7·06) | <0·0001 | 1·89 (1·12–3·20) | 0·018 |
| Charlson comorbidity index >2* | 6·35 (4·01–10·06) | <0·0001 | 3·75 (1·99–7·07) | <0·0001 |
| PaO2/FiO2 <100 | 2·18 (1·50–3·17) | <0·0001 | 2·89 (1·80–4·64) | <0·0001 |
| CRP >100 mg/L | 1·76 (1·21–2·55) | 0·003 | 1·21 (0·76–1·92) | 0·42 |
| Lymphopenia (<580 lymphocytes per mm3)* | 3·08 (2·12–4·49) | <0·0001 | 3·05 (1·90–4·89) | <0·0001 |
| Study | .. | 0·15 | .. | .. |
CRP=C-reactive protein. PaO2/FiO2=ratio of the arterial partial oxygen pressure divided by the fraction of inspired oxygen.
For continuous variables, the best cutoff was estimated from the receiver operating characteristic using the Youden Index.
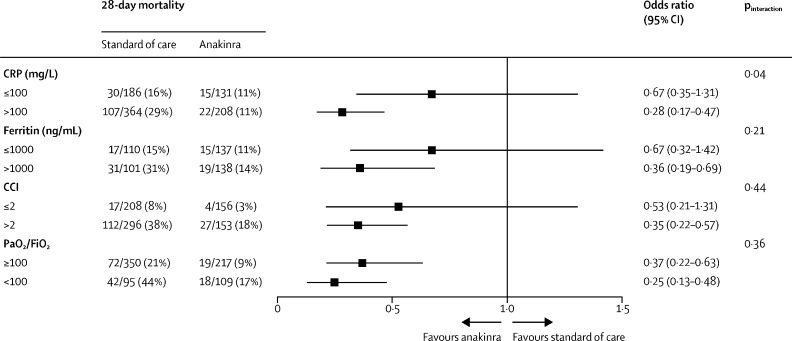
The mortality reduction associated with anakinra treatment was significant in the absence of dexamethasone co-administration (559 patients; OR 0·23 [95% CI 0·12–0·43]), but not with co-administration of dexamethasone (239 patients; 0·72 [0·37–1·41]; pBreslow=0·012). Similarly, this beneficial effect of anakinra was significant in patients breathing spontaneously at baseline (792 patients; OR 0·30 [95% CI 0·19–0·48]) but not in the smaller subgroup of those who were mechanically ventilated at baseline (103 patients; 0·52 [0·20–1·36]; pBreslow=0·45). Subgroup analyses adjusting for CRP and ferritin concentrations and baseline PaO2/FiO2 showed that anakinra was more effective in lowering mortality in patients presenting with CRP concentrations higher than 100 mg/L (OR 0·28 [95% CI 0·17–0·47]), but the therapeutic efficacy of anakinra did not appear to be related to baseline ferritin concentrations or baseline PaO2/FiO2 (figure 3 ). In a subgroup analysis of 116 patients with diabetes and 299 patients without diabetes, the effect of anakinra on mortality was similar in both groups (OR 0·40 [95% CI 0·17–0·91] vs 0·37 [0·19–0·74]; pBreslow=0·90).
Figure 3.
Subgroup analysis of mortality in patients treated with anakinra versus those treated with standard of care
p values of the interaction effect of the treatment on mortality, in each subgroup and among the studies are provided. CRP=C-reactive protein. CCI=Charlson comorbidity index. PaO2/FiO2=ratio of the arterial partial oxygen pressure divided by the fraction of inspired oxygen.
The safety of anakinra was investigated as a secondary endpoint. Anakinra treatment was associated with elevation of liver function tests (pooled OR 3·00 [95% CI 0·26–34·66]; I 2 85%), as well as onset of leukopaenia (3·71 [0·49–27·84]; I 2 51%) and secondary infection (1·35 [0·59–3·10]; I 2 79%; appendix p 7). Thromboembolic events were reported in only two studies;23, 28 thus, no meta-analysis was done for this endpoint. Nevertheless, in both studies, anakinra did not increase the thromboembolic risk compared to standard-of-care treatment or placebo, or both.
Discussion
The results of this systematic review and meta-analysis indicate that, in patients admitted to hospital with pneumonia due to COVID-19, treatment with anakinra reduces mortality when compared with standard of care, with or without placebo. This survival benefit was most profound in patients with hyperinflammation and CRP concentrations higher than 100 mg/L. We also observed a non-significant increase in the risk of adverse events with anakinra.
Identifying and preventing unfavourable outcomes in patients with COVID-19 is a real challenge. Algorithms have been constructed and validated, taking into account various clinical variables, including older age, male sex, high body-mass index, and comorbidities.32, 33, 34, 35 Marked lymphopenia and elevated inflammatory biomarkers such as ferritin, IL-6, and CRP were first shown to be associated with severe disease in early reports of patients with COVID-19. IL-6 is a potent stimulator of CRP production by the liver.36 Therefore, CRP concentrations probably represent the simplest biological test to indirectly evaluate the intensity of ongoing inflammation and a signal indicating a window of opportunity for targeted immunotherapy. Our results show a profound benefit of anakinra in patients with CRP concentrations higher than 100 mg/L.
Some patients with severe COVID-19 develop a hyperinflammatory phenotype known as a cytokine storm. Cytokine storm syndrome is a life-threatening condition requiring intensive care and with a high mortality rate, characterised by overwhelming systemic inflammation, with hyper-ferritinaemia, haemodynamic instability, and multiorgan failure. The trigger for cytokine storm syndrome might be extensive cell death, resulting in massive release of pro-inflammatory intracellular mediators and danger signals, notably including IL-1α.37 These intracellular mediators in turn induce an uncontrolled immune response with continuous activation and expansion of immune cells, which produce large amounts of other pro-inflammatory cytokines such as IL-1β, IL-6, and IL-18, as well as interferon-γ (IFNγ) and tumour necrosis factor (TNF).38 Not all patients experience a cytokine storm,7 but even in those who do not, the pulmonary inflammatory response can be exacerbated and these patients might benefit from immunomodulating therapies.5, 6, 8
IL-1 is located upstream of IL-6 in the inflammation cascade,39 and such a hierarchical process could explain why targeting IL-1 appears to be more efficient in hyperinflammatory forms of COVID-19 than IL-6 inhibition, which has already provided encouraging results in randomised controlled trials of patients with severe COVID-19 admitted to the intensive care unit.40, 41, 42 Dexamethasone was the first immune modulator to show a clear survival benefit when administered to patients admitted to hospital with COVID-19 in need of oxygen supplementation in the RECOVERY trial; thus, after July, 2020, dexamethasone was incorporated into the standard of care for patients admitted to hospital with COVID-19.43
The cohort studies included in the current analysis of anakinra in patients with COVID-19 were not randomised and included patients receiving usual care as historical controls, mainly before implementation of dexamethasone as standard of care. Although the cohort design clearly has its limitations, the observed magnitude of the protective effect of anakinra was convincing enough to continue with additional studies, such as the open-label single-arm prospective SAVE study, which concluded that soluble urokinase-type plasminogen activator receptor (suPAR)-guided anakinra treatment prevented onset of respiratory failure necessitating mechanical ventilation and reduced mortality.29 The only randomised controlled trial completed so far was initiated by the French consortium CORIMUNO. It did not replicate the results observed in observational studies, and was prematurely interrupted because of assumed futility,24 suggesting that anakinra was not effective in reducing the need for non-invasive or mechanical ventilation or in preventing deaths in patients with COVID-19 and mild to moderate pneumonia. However, the 14-day mortality rate was 15% in the anakinra group compared to 24% in the usual care group, and the WHO Clinical Progression Scale was also suggestive of a beneficial effect of anakinra at day 14. Unfortunately, stopping for futility increases the risk of imbalance in prognostic factors and leaves the primary research question unanswered, especially when the decision to stop early does not follow a pre-specified stopping boundary. Additionally, the cutoff criterion chosen for CRP in the CORIMUNO study was relatively low (>25 mg/L), which suggests that a proportion of patients were not in the hyperinflammatory phase of COVID-19, during which anakinra is probably the most effective.36
In the present analysis, in contrast to published evidence supporting a synergistic effect of dexamethasone with IL-6 inhibition,41, 42 we did not observe a significant survival benefit of anakinra when co-administered with dexamethasone. For patients already under mechanical ventilation at the start of treatment, anakinra similarly did not show a significant survival benefit. However, decreased statistical power due to the small number of patients included in these subgroups probably contributed to these outcomes. It cannot be ruled out that anakinra has no additional benefit when co-administered with dexamethasone, or if anakinra is given too late, when severe organ failure is already apparent and organ support is needed. However, anakinra might be a safer alternative to dexamethasone when considering the risk of secondary infections, which was low in early reports but might rise after more widespread use of dexamethasone.44, 45 An additional benefit might be seen in specific populations, such as patients with diabetes, who are more susceptible to secondary infections and less likely to tolerate hyperglycaemia.46 Indeed, our study showed a similar risk of secondary infections in the anakinra group compared with the control group, and a survival benefit of anakinra in a subgroup of patients with diabetes.
To the best of our knowledge, this is the first patient-level meta-analysis to investigate the effect of anakinra treatment in patients admitted to hospital with COVID-19, showing a significant reduction in mortality with anakinra, and characterising a subgroup of patients (those with CRP >100 mg/L) as the best candidates for this treatment. The main limitations of this meta-analysis are the retrospective observational design of the majority of studies included, raising concerns about bias and possible confounders among the compared groups, as well as the fact that most of the studies were done before dexamethasone was incorporated into standard-of-care treatment of patients admitted to hospital with COVID-19. Larger randomised trials are urgently needed to clarify the place of anakinra in the anti-COVID19 armamentarium; ongoing trials (appendix pp 3–4) include the REMAP-CAP trial (an international, multifactorial, adaptive platform randomly assigning patients to tocilizumab or sarilumab or anakinra or standard of care and comparing in-hospital mortality and days free of organ support to day 21 among the groups), and the placebo-controlled randomised controlled trial SAVE-MORE (NCT04680949) in Greece and Italy, comparing 28-day outcomes with anakinra versus placebo in patients already receiving standard-of-care treatment for COVID-19.
Data sharing
Data collected for this study, including de-identified individual participant data, will be made available following publication. Requests for data can be made to the corresponding author, outlining specific data needs, analysis plans and dissemination plans. These requests will be reviewed by a steering committee of the International Collaborative Group for Anakinra in COVID-19. Data will be shared after a data access agreement is signed, for any proposed purpose approved by the steering committee and all data will be de-identified and protected health information stripped.
Declaration of interests
EJG-B has received honoraria from AbbVie USA, Abbott CH, Biotest Germany, Brahms, InflaRx, MSD Greece, XBiotech, and Angelini Italy; independent educational grants from AbbVie, Abbott CH, Astellas Pharma Europe, AxisShield, bioMérieux, InflaRx, the Medicines Company and XBiotech; and funding from the FrameWork 7 program HemoSpec (granted to the National and Kapodistrian University of Athens), the Horizon2020 Marie-Curie Project European Sepsis Academy (granted to the National and Kapodistrian University of Athens), and the Horizon 2020 European Grant ImmunoSep (granted to the Hellenic Institute for the Study of Sepsis). MG has received speakers' fees and unrestricted grants from Novartis and Sobi. PP, MKo, and EKo are funded by a COVID-19 grant paid to the Radboud University Medical Center (Radboudumc). JE-O is a co-founder, shareholder, and CSO of ViroGates, Denmark, and named inventor on patents on suPAR owned by Copenhagen University Hospital Hvidovre, Denmark. GK has received from ROCHE-CHUGAI Research Grants (<€20 000), fees from Sobi France for scientific presentations (<€4000) and participated in a SOBI Advisory Board on COVID (unpaid) and in an OLATEC Monitoring Board (unpaid). GCa has received speakers' and consulting fees from Novartis and Sobi. LD has received grants (paid to LD's institution outside the current work) from AbbVie, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Kiniksa, Merk Sharp & Dohme, Mundipharma Pharmaceuticals, Novartis, Pfizer, Roche, Sanofi Genzyme, and Sobi; and consulting fees from AbbVie, Amgen, Biogen, Bristol-Myers Squibb, Celltrion, Galapagos, GlaxoSmithKline, Kiniksa, Novartis, Pfizer, Roche, Sanofi-Genzyme, and Sobi. GH reports consultancy fees from Bristol-Myers Squibb, Lilly, Novartis; speakers' fees from AbbVie, Bristol-Myers-Squibb, Celgene, Lilly, Novartis, Pfizer, Roche, Sanofi-Aventis; support for attending meetings from Bristol-Myers-Squibb, Fresenius-Kabi, Janssen-Cilag, Lilly, Mylan, Roche, UCB; and participation on advisory boards for Bristol-Myers-Squibb and Lilly. FV has received (via the Institut de Recherche pour le Développement) Horizon 2020-EDCTP-European Grants: PANDORA and ITAIL-COVID. All other authors declare no competing interests.
Contributors
EKy did the literature search and study selection, participated in data analysis, and drafted the manuscript. EJG-B conceptualised the study, and participated in the literature search and study selection. MKy contributed to data analysis. GH drafted the manuscript. TH, GCa, AG, PP, GCh, GK, EP, MG, RC, EKo, MKo, HB, DM, AB, and LD provided clinical data and revised the manuscript for important intellectual content. JE-O, MC, FV, MGN, and JWMvdM revised the manuscript for important intellectual content. All authors gave approval for the version to be published.
Contributor Information
International Collaborative Group for Anakinra in COVID-19:
Evdoxia Kyriazopoulou, Thomas Huet, Giulio Cavalli, Andrea Gori, Miltiades Kyprianou, Peter Pickkers, Jesper Eugen-Olsen, Mario Clerici, Francisco Veas, Gilles Chatellier, Gilles Kaplanski, Mihai G. Netea, Emanuele Pontali, Marco Gattorno, Raphael Cauchois, Emma Kooistra, Matthijs Kox, Alessandra Bandera, Hélène Beaussier, Davide Mangioni, Lorenzo Dagna, Jos W.M. van der Meer, Evangelos J. Giamarellos-Bourboulis, Gilles Hayem, Stefano Volpi, Maria Pia Sormani, Alessio Signori, Giorgio Bozzi, Francesca Minoia, Stefano Aliberti, Giacomo Grasselli, Laura Alagna, Andrea Lombardi, Riccardo Ungaro, Carlo Agostoni, Francesco Blasi, Giorgio Costantino, Anna Ludovica Fracanzani, Nicola Montano, Flora Peyvandi, Marcello Sottocorno, Antonio Muscatello, Giovanni Filocamo, Antonios Papadopoulos, Maria Mouktaroudi, Eleni Karakike, Maria Saridaki, Theologia Gkavogianni, Konstantina Katrini, Nikolaos Vechlidis, Christina Avgoustou, Stamatios Chalvatzis, Theodoros Marantos, Christina Damoulari, Georgia Damoraki, Sofia Ktena, Maria Tsilika, Panagiotis Koufargyris, Athanasios Karageorgos, Dionysia-Irene Droggiti, Aikaterini Koliakou, Garyfallia Poulakou, Konstantinos Tsiakos, Dimitra-Melia Myrodia, Areti Gravvani, Ioannis P. Trontzas, Konstantinos Syrigos, Ioannis Kalomenidis, Eleftheria Kranidioti, Periklis Panagopoulos, Vasileios Petrakis, Simeon Metallidis, Georgia Loli, Olga Tsachouridou, George N. Dalekos, Nikolaos Gatselis, Aggelos Stefos, Sarah Georgiadou, Vassiliki Lygoura, Haralampos Milionis, Maria Kosmidou, Ilias C. Papanikolaou, Karolina Akinosoglou, Efthymia Giannitsioti, Georgios Chrysos, Panagiotis Mavroudis, Chrysanthi Sidiropoulou, Georgios Adamis, Archontoula Fragkou, Aggeliki Rapti, Zoi Alexiou, Styliani Symbardi, Aikaterini Masgala, Konstantina Kostaki, Evangelos Kostis, Michael Samarkos, Petros Bakakos, Vassiliki Tzavara, Katerina Dimakou, Glykeria Tzatzagou, Maria Chini, Vasileios Kotsis, George Tsoukalas, Ioannis Bliziotis, Michael Doumas, Aikaterini Argyraki, Ilias Kainis, Massimo Fantoni, Antonella Cingolani, Andrea Angheben, Chiara Simona Cardellino, Francesco Castelli, Francesco Saverio Serino, Emanuele Nicastri, Giuseppe Ippolito, Matteo Bassetti, and Carlo Selmi
Supplementary Material
References
- 1.WHO Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019?gclid=Cj0KCQiAhs79BRD0ARIsAC6XpaXhxHeN64r7-j5rvv0ZDtNGxNkA0e2EWCAUr8QWWj-qi_PPrXOljroaAjXBEALw_wcB
- 2.Mason RJ. Thoughts on the alveolar phase of COVID-19. Am J Physiol Lung Cell Mol Physiol. 2020;319:L115–L120. doi: 10.1152/ajplung.00126.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jouan Y, Baranek T, Si-Tahar M, Paget C, Guillon A. Lung compartmentalization of inflammatory biomarkers in COVID-19-related ARDS. Crit Care. 2021;25:120. doi: 10.1186/s13054-021-03513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendib I, Beldi-Ferchiou A, Schlemmer F, et al. Alveolar compartmentalization of inflammatory and immune cell biomarkers in pneumonia-related ARDS. Crit Care. 2021;25:23. doi: 10.1186/s13054-020-03427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kox M, Waalders NJB, Kooistra EJ, Gerretsen J, Pickkers P. Cytokine levels in critically ill patients with COVID-19 and other conditions. JAMA. 2020;324:1565–1567. doi: 10.1001/jama.2020.17052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGonagle D, Ramanan AV, Bridgewood C. Immune cartography of macrophage activation syndrome in the COVID-19 era. Nat Rev Rheumatol. 2021;17:145–157. doi: 10.1038/s41584-020-00571-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strati P, Ahmed S, Kebriaei P, et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy-associated toxicity in large B-cell lymphoma. Blood Adv. 2020;4:3123–3127. doi: 10.1182/bloodadvances.2020002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24:731–738. doi: 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navarro-Millán I, Sattui SE, Lakhanpal A, Zisa D, Siegel CH, Crow MK. Use of anakinra to prevent mechanical ventilation in severe COVID-19: a case series. Arthritis Rheumatol. 2020;72:1990–1997. doi: 10.1002/art.41422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pontali E, Volpi S, Antonucci G, et al. Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease. J Allergy Clin Immunol. 2020;146:213–215. doi: 10.1016/j.jaci.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aouba A, Baldolli A, Geffray L, et al. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann Rheum Dis. 2020;79:1381–1382. doi: 10.1136/annrheumdis-2020-217706. [DOI] [PubMed] [Google Scholar]
- 15.Dimopoulos G, de Mast Q, Markou N, et al. Favorable anakinra responses in severe covid-19 patients with secondary hemophagocytic lymphohistiocytosis. Cell Host Microbe. 2020;28:117–123. doi: 10.1016/j.chom.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart LA, Clarke M, Rovers M, et al. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement. JAMA. 2015;313:1657–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 18.Izcovich A, Ragusa MA, Tortosa F, et al. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng L, Li H, Li L, et al. Ferritin in the coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Lab Anal. 2020;34 doi: 10.1002/jcla.23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou W, Qin X, Hu X, Lu Y, Pan J. Prognosis models for severe and critical COVID-19 based on the Charlson and Elixhauser comorbidity indices. Int J Med Sci. 2020;17:2257–2263. doi: 10.7150/ijms.50007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 22.Cauchois R, Koubi M, Delarbre D, et al. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc Natl Acad Sci USA. 2020;117:18951–18953. doi: 10.1073/pnas.2009017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2:e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The CORIMUNO-19 Collaborative group Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet Respir Med. 2021;9:295–304. doi: 10.1016/S2213-2600(20)30556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bozzi G, Mangioni D, Minoia F, et al. Anakinra combined with methylprednisolone in patients with severe COVID-19 pneumonia and hyperinflammation: an observational cohort study. J Allergy Clin Immunol. 2021;147:561–566. doi: 10.1016/j.jaci.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavalli G, Larcher A, Tomelleri A, et al. Interleukin-1 and interleukin-6 inhibition compared with standard management in patients with COVID-19 and hyperinflammation: a cohort study. Lancet Rheumatol. 2021;3:e253–e261. doi: 10.1016/S2665-9913(21)00012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pontali E, Volpi S, Signori A, et al. Efficacy of early anti-inflammatory treatment with high doses of intravenous anakinra with or without glucocorticoids in patients with severe COVID-19 pneumonia. J Allergy Clin Immunol. 2021;147:1217–1225. doi: 10.1016/j.jaci.2021.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kooistra EJ, Waalders NJB, Grondman I, et al. Anakinra treatment in critically ill COVID-19 patients: a prospective cohort study. Crit Care. 2020;24:688. doi: 10.1186/s13054-020-03364-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kyriazopoulou E, Panagopoulos P, Metallidis S, et al. An open label trial of anakinra to prevent respiratory failure in COVID-19. eLife. 2021;10 doi: 10.7554/eLife.66125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balkhair A, Al-Zakwani I, Al Busaidi M, et al. Anakinra in hospitalized patients with severe COVID-19 pneumonia requiring oxygen therapy: results of a prospective, open-label, interventional study. Int J Infect Dis. 2021;103:288–296. doi: 10.1016/j.ijid.2020.11.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavalli G, De Luca G, Campochiaro C, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sperrin M, McMillan B. Prediction models for covid-19 outcomes. BMJ. 2020;371 doi: 10.1136/bmj.m3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jimenez-Solem E, Petersen TS, Hansen C, et al. Developing and validating COVID-19 adverse outcome risk prediction models from a bi-national European cohort of 5594 patients. Sci Rep. 2021;11 doi: 10.1038/s41598-021-81844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cavalli G, Colafrancesco S, Emmi G, et al. Interleukin 1α: a comprehensive review on the role of IL-1α in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun Rev. 2021;20 doi: 10.1016/j.autrev.2021.102763. [DOI] [PubMed] [Google Scholar]
- 38.Caricchio R, Gallucci M, Dass C, et al. Preliminary predictive criteria for COVID-19 cytokine storm. Ann Rheum Dis. 2021;80:88–95. doi: 10.1136/annrheumdis-2020-218323. [DOI] [PubMed] [Google Scholar]
- 39.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campochiaro C, Della-Torre E, Cavalli G, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bassetti M, Kollef MH, Timsit JF. Bacterial and fungal superinfections in critically ill patients with COVID-19. Intensive Care Med. 2020;46:2071–2074. doi: 10.1007/s00134-020-06219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cavalli G, Dagna L. The right place for IL-1 inhibition in COVID-19. Lancet Respir Med. 2021;9:223–224. doi: 10.1016/S2213-2600(21)00035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data collected for this study, including de-identified individual participant data, will be made available following publication. Requests for data can be made to the corresponding author, outlining specific data needs, analysis plans and dissemination plans. These requests will be reviewed by a steering committee of the International Collaborative Group for Anakinra in COVID-19. Data will be shared after a data access agreement is signed, for any proposed purpose approved by the steering committee and all data will be de-identified and protected health information stripped.