Abstract
Objectives:
Hypertension, diabetes, depressive symptoms, and smoking are predictors of cognitive decline in late life. It is unknown if these risk factors are associated with cognition during midlife or if the associations between these risk factors and cognition vary by race. This longitudinal study examined (1) risk factors for decline in episodic memory, processing speed, and working memory in midlife women and (2) if the associations between risk factors and cognitive decline were moderated by race.
Method:
Participants (aged 42–52) were European American (n = 1,000), African American (n = 516), and Asian American (n = 437) women from the Study of Women’s Health Across the Nation. Two-level hierarchical linear models tested risk factors, race, and their interactions as predictors of cognitive change over time.
Results:
African Americans had poorer baseline episodic memory, processing speed, and working memory and greater episodic memory decline compared to European Americans. Asian Americans had poorer episodic memory and working memory, but better processing speed than European Americans. Depressive symptoms were associated with poorer episodic memory and processing speed at baseline; further, diabetes was associated with poorer processing speed at baseline. Greater depressive symptoms were associated with poorer episodic memory at baseline for African Americans but not European Americans.
Conclusions:
Our study results highlight racial disparities in cognition during midlife. Depressive symptoms may be particularly detrimental to the cognitive health of African Americans. Clinical and public health interventions for healthy cognitive aging should be tailored to the unique risks of racial groups.
Keywords: racial differences, risk factors, cognitive decline, midlife, women
African Americans are at increased risk for dementia compared to European Americans (Mayeda, et al., 2016; Mehta & Yeo, 2017; Steenland et al., 2015) and there are nearly twice as many women diagnosed with Alzheimer’s disease (AD) than men (Hebert et al., 2013). Indeed, race and sex interact to determine risk for cognitive decline with age. African American women experienced steeper cognitive decline compared to their European Americans counterpart (Avila et al., 2019). Greater attention on the cognitive disparities and cognitive aging of women from racially diverse backgrounds is warranted to better understand how to prevent cognitive decline.
Midlife is an ideal time to study cognitive aging since the neuropathology for dementia may begin at this stage in life (Sperling et al., 2011). In later life, we know that depressive symptoms, diabetes, hypertension, and smoking are risk factors for cognitive decline and/or impairment in older adults (see Supplemental Materials 1; e.g., Anstey et al., 2007; Brewster et al., 2017; Hajjar et al., 2017; Wennberg et al., 2017). However, it is unknown if these risk factors are important in predicting cognitive decline during midlife (see Supplemental Materials 2; e.g., Anstey et al., 2014; Debette et al., 2011; D. Knopman et al., 2001; Singh-Manoux & Marmot, 2005; Tarraf et al., 2017). Even less is known about predictors for cognitive decline during midlife in multi-racial samples (Anstey et al., 2014; Lachman, 2016; Lachman et al., 2015).
The current study - using longitudinal data from the Study of Women’s Health Across the Nation (SWAN; Sowers et al., 2000) - will fill important gaps in knowledge by determining if diabetes, hypertension, smoking, and/or depressive symptoms are associated with cognitive decline in midlife women. We expect these risk factors will be associated with cognitive changes at midlife. Further, based on a health disparity framework, we predict stronger associations between risk factors and cognitive decline for African American women compared to European American women. We will explore risk for cognitive decline in Asian American women relative to our other racial groups. This study will focus on cognitive outcomes that are likely to change with age, namely episodic memory, working memory, and processing speed (Kandiah et al., 2009; Kirova et al., 2015; McGuinness et al., 2010; O’Brien & Thomas, 2015; Stopford et al., 2010). By gaining a better understanding of predictors of poor cognition during midlife in a multiracial sample of women, prevention and treatment efforts can be better directed to persons most at risk for cognitive decline.
Health Disparity Theoretical Framework
This study is guided by the cumulative disadvantage theory, which posits that the adverse effects of multiple stressors accumulate over the lifespan in racial and ethnic minority populations, resulting in ever-growing health disparities relative to the majority population (Dannefer, 1987, 1988, 2003). We apply this framework to cognitive aging in racial minorities. Lifetime chronic stress exposure - including racism and discrimination - are higher in African Americans compared to European Americans, which may increase cognitive vulnerability to additional stresses in midlife, such as depressive symptoms or hypertension (Boardman & Alexander, 2011; Brown et al., 2020; Cohen & Janicki-Deverts, 2012; Sternthal et al., 2011; Thoits, 2010; Turner & Avison, 2003).
Our study of differential risk for poor cognitive outcomes in minority versus majority samples occurs in the context of overall poorer cognitive performances in racial minorities in the U.S. There are cross-sectional (Carvalho et al., 2015; Castora-Binkley et al., 2013; Marsiske et al., 2013) and longitudinal (Gupta et al., 2016; Wolinsky et al., 2011) differences in cognitive outcomes between African Americans and European Americans older adults. African American scores are lower on cognitive measures and/or decline more over time than for European Americans.
In the context of these group differences, we predict that our risk factors will be more strongly associated with poor cognitive outcomes for African Americans than European Americans (D. Knopman et al., 2001; Dore et al., 2015; Mayeda et al., 2014; Obidi et al., 2008; Rajan et al., 2016; Zahodne et al., 2014). There are some preliminary data to support our prediction. For example, African-Americans demonstrate a stronger association between diabetes and poorer episodic memory, processing speed, and working memory relative to European Americans (Dore et al., 2015; Mayeda et al., 2014; Obidi et al., 2008; Rajan et al., 2016). Further, smoking was significantly associated with poorer processing speed for African American but not European Americans in midlife (D. Knopman et al., 2001).
However, the literature is not entirely consistent as to whether risk factors are more strongly associated with adverse cognitive outcomes for African Americans than European Americans (Supplemental Materials 3; Gottesman et al., 2014; Knopman et al., 2001; Schneider et al., 2014; Sol et al., 2020; Zahodne et al., 2014). For example, European American midlife adults with hypertension exhibited greater cognitive decline in processing speed compared to African Americans with hypertension (e.g., Gottesman et al., 2014). In another study, there were no significant differences in the association between depressive symptoms and memory decline in African American and European American older adults (e.g., Sol et al., 2020). Due to the mixed evidence that risk factors for cognitive decline have a stronger association with cognitive outcomes in African Americans than European Americans, more research is needed and we will address this issue.
Cognitive Aging in Asian Americans
In contrast to African Americans and European Americans, little is known about cognitive aging in Asian Americans. Preliminary evidence suggests that Asian Americans have lower rates of dementia than African Americans and European Americans (Mayeda et al., 2017; Mayeda et al., 2016; Mehta & Yeo, 2017). Hypertension is a risk factor for dementia in older Japanese men (Launer et al., 2000) and greater depressive symptoms are more common in cognitively impaired Chinese American older adults than in their European American counterparts (Chao et al., 2014).
By including Asian Americans in the present study, we will fill gaps in knowledge about cognition in Asian Americans during midlife and determine how changes in cognition over time are different from and similar to a racial minority and majority group. Indeed, in exploratory hypotheses, we expect that Asian American outcomes will be more similar to European Americans than African Americans. African Americans - relative to European and Asian Americans - are disproportionately affected by lower socioeconomic status (SES), racial discrimination, stressful life events, and poor healthcare access (Assari, 2018; Lewis & Van Dyke, 2018; Mays et al.,2014; Stevens-Watkins et al., 2015; Williams et al., 2016), which might account for poorer cognitive outcomes over time relative to Asian Americans and European Americans.
The Current Study
The current study will determine if risk for cognitive decline earlier in midlife “sets the stage” for cognitive outcomes later in midlife. In a multiracial sample of midlife women, we test if diabetes, hypertension, smoking, and depressive symptoms – measured at the beginning of the longitudinal study – predicted cognition and cognitive decline in episodic memory, working memory, and processing speed later in midlife. Although there is some evidence that these risk factors are associated with cognitive decline in midlife, the data are not entirely consistent (e.g., Mayeda et al., 2014; Schneider et al., 2014; Vásquez et al., 2016). We predict that diabetes, hypertension, smoking, and depressive symptoms will be associated with longitudinal decline in all cognitive outcomes.
Next, we will determine if race moderates the associations between risk factors (i.e., diabetes, hypertension, depressive symptoms, smoking) and cognitive decline in processing speed, working memory, and episodic memory. We hypothesize that, due to greater adversities that accumulate over the life course (Assari, 2018; Glymour & Manly, 2008; Lewis & Van Dyke, 2018; Mays et al., 2014; Stevens-Watkins et al., 2015; Williams et al., 2016) the associations between risk factors and cognitive decline in processing speed, working memory, and episodic memory will be stronger for African American women compared to European American and – in an exploratory hypothesis – to Asian American women. Based on limited evidence (i.e., Chao et al., 2014; Launer et al., 2000), an exploratory hypothesis is that the associations between risk factors and cognitive decline in Asian Americans will be more similar to those of European Americans than African Americans.
Method
Participants
Participant (N = 1,953) data are from the Study of Women’s Health Across the Nation (SWAN; Sowers et al., 2000). SWAN is a longitudinal epidemiological study assessing psychological, biological, cultural, and social factors that contribute to the health of American women during midlife. SWAN inclusion criteria were aged 42–52 years old, not taking hormone medications, having a uterus and at least one ovary, and having a menstrual period within the past three months. Participants identified as European American (n = 1,000), African American (n = 516), and Asian American (n = 437; including Chinese Americans and Japanese Americans)4. Study sites included University of Pittsburgh, University of California-Los Angeles, University of California-Davis/Kaiser Permanente, Rush Presbyterian-St. Luke’s Medical Center, Massachusetts General Hospital, and University of Michigan.
Procedures
Race and educational attainment were collected at the screening visit in 1996–1997. After the baseline visit, participants completed follow-up visits approximately once a year. Visit 10 occurred between 2006–2008 and is the endpoint for the current study. At the yearly visits, questionnaires were administered to assess depressive symptoms, family income, age, and physical health outcomes. Measures of hypertension, diabetes, smoking, and depressive symptoms were collected at baseline, visits one, two (diabetes was not collected at this visit), and three (Table 1). Cognitive measures were collected at visits four, six, seven, eight, nine, and 10. Participants were administered cognitive measures at either visit eight or visit nine but not both. Cognitive outcomes at visits four and six were used to control for practice effects as described below and were not included in longitudinal analyses. Cognitive trajectory was measured from visit seven through visit 10. All questionnaires were translated into Japanese and Cantonese for some of the Asian American participants, then back translated and verified by an adjunct committee for accuracy. The use of this data was approved by the University of Massachusetts Amherst Institutional Review Board.
Measures
Hypertension, Diabetes, and Smoking.
Hypertension risk was measured by average systolic blood pressure. An average blood pressure value was created based on an average of the measures at baseline and visits one to three. Diabetes risk was measured by fasting blood glucose and baseline and visit one and three scores were averaged. Smoking was measured at baseline by a self-report of current smoking, answered by “yes” or “no.”
Depressive Symptoms.
Participants were administered the Center for Epidemiological Studies-Depression Scale (CES-D), a 20-item measure assessing depressive symptoms (Radloff, 1977). Depressive symptom totals at baseline and visits one to three were averaged to create a depressive symptom score. The CES-D has high internal consistency in general and clinical populations (coefficient α= .80 and α = .90, respectively; Radloff, 1977).
East Boston Memory Test.
The East Boston Memory Test (EBMT) is a measure of verbal episodic memory (Albert et al., 1991). Participants are read a short story and then engage in immediate and 10-minute delay recall; recall scores range between 0 −12 points. We used the delayed recall score because it is a measure of episodic memory and a clinical hallmark of Alzheimer’s disease (Weintraub et al., 2012). The EBMT correlates significantly with the Wechsler Memory Scale-Revised Logical Memory scores (WMS-R; Wechsler, 1987).
Symbol Digit Modalities Test.
The Symbol Digit Modalities Test (SDMT) measured processing speed (Smith, 1982). Participants were timed on how quickly they could match numbers with symbols; scores range from 0–110 points. The SDMT correlates significantly with Wechsler Adult Intelligence Scale-Fourth Edition Coding (Sheridan et al., 2006; Wechsler, 2008).
Digit Span Backwards.
Digit Span Backwards (DSB) from the Wechsler Adult Intelligence Scale-Third Edition measured working memory (Tulsky et al., 1997; Wechsler,1997). Participants were asked to repeat a series of numbers backwards; scores range from 0–12 points.
Covariates.
Covariates were baseline age, income, education, menopause status, and practice effects (to correct for effects of repeat cognitive testing) because these factors are known to be associated with our risk factors and/or cognitive outcomes. Menopause status, collected at visit seven (e.g., the first cognitive assessment) was operationalized as seven categories: post bilateral salpingo oophorectomy, natural post-menopause, late peri-menopause, early peri-menopause, pre-menopause, unknown due to hormone therapy use, and unknown due to hysterectomy. Practice effect calculation are described below.
Measurement Invariance
Barnes and colleagues (2016) used multigroup confirmatory factor analyses to demonstrate measurement invariance of a battery of tests that included our cognitive measures in African Americans and European Americans older adults. To date, there are no studies of measurement invariance of our cognitive measures in Asian American samples.
Data Analyses
Descriptive statistics were run to characterize the sample and to evaluate the data for normal distribution and outliers (IBM SPSS Statistics for Mac, Version 23.0. Armonk, NY: IBM Corp). We determined baseline differences on our predictors and cognitive outcomes between racial groups using one-way ANOVA and chi-square analyses. Multilevel modeling (MLM) within the Mplus software tested all hypotheses given its ability to account for dependency in the data due to repeated measures over time (Version 8, Muthén & Muthén, 2017). Specifically, we fit a two-level growth curve to model within-person change over time at level 1 and between-person differences at level 2. We also employed full information maximum likelihood estimation, which provides model-based estimates for participants with some missing data. Thus, analyses included all participants who completed at least one measurement occasion of a study variable. Effect size was calculated by evaluating the additional percent variance explained with the addition of predictors (pseudo r2) for significant associations. Given the number of model comparisons (i.e., 15), p value was set to 0.01 for all primary analyses.
All covariates (age, income, education, menopause status) with the exception of practice effects, described below - which already have a mean of zero - were grand mean centered and included in all analyses. Primary continuous predictor variables (i.e., average depressive symptoms, average systolic blood pressure, and average fasting blood glucose) were also centered around the grand mean. Smoking at baseline, a categorical variable, remained uncentered. Race was dummy coded, such that European Americans served as the reference group for all analyses. Interaction terms were created using the centered continuous variables and the un-centered dummy coded racial groups.
Practice effects can occur with repeated administration of the same or similar tests and can skew the measurement of change over time in cognitive abilities (Lamar et al., 2003; Rabbitt et al., 2008; Salthouse, 2016). We controlled for practice effects in our MLM analyses, similar to other studies with the SWAN dataset (i.e., Karlamangla et al., 2017). Recent research suggests that practice effects in cognitive test scores provide valuable information about the diagnosis, prognosis, and brain pathology in late life cognitive disorders (Duff et al., 2014; Duff et al., 2018; Duff et al., 2015; Duff et al., 2011; Duff et al., 2010).We measured practice effects – from the first to second cognitive outcome measurement (i.e., visit four to visit six) – by calculating reliable change scores for the three cognitive outcomes (Duff, 2012; McSweeny et al., 1993) using a standardized regression-based change formula. First, we ran a stepwise linear regression, where the dependent variable was the visit six score and the independent/predictor variables were visit four score, age, education, and income. The regression model indicates which variables significantly predict the visit six score and results (e.g., constant, beta weights of included variables) were used to create formulas to calculate a predicted visit six score.5 The reliable change score is calculated by the difference between the actual visit six score and the predicted visit six score divided by the standard error of estimate, which also is derived from the regression model. These practice effects are z-scores, with positive values indicating larger-than-expected cognitive change and negative values indicating smaller-than-expected change from first to second cognitive assessment. Since practice effects are defined as the difference between scores at visit four and six, cognitive change over time was measured using data from visits seven to 10.
Practice effects are an individual difference variable that are unique to each participant. Like other covariates - such as age, income, or education - practice effects may influence each participant’s cognitive performance and cognitive trajectory and can mask change over time if not properly accounted (Rabbitt et al., 2008; Salthouse, 2009, 2016). Thus, practice effects were included as predictors for their respective cognitive outcome in the multi-level models (e.g., the practice effect for working memory was included in analyses where working memory was the outcome). Practice effects differ based on sex, age, education, and neuropsychiatric status (Calamia et al., 2012). We examined whether there are racial group differences in practice effects.
Results
Participants
Participants (N = 1,953) were European Americans (n = 1,000), African Americans (n = 516), and Asian Americans (n = 437; Table 2). At baseline, European Americans had significantly higher education than African Americans and Asian Americans, as well as significantly greater income than African Americans. More European Americans were in the later stages of menopause than African Americans and Asian Americans American (ps < 0.001) African Americans had significantly higher depressive symptoms, fasting blood glucose (diabetes) and systolic blood pressure (hypertension) compared to Asian Americans and European Americans at baseline (Table 3). At baseline, African Americans had significantly lower scores on all three cognitive outcomes than European Americans and Asian Americans.
Group Differences in Practice Effects.
One-way ANOVAs indicated that EBMT, SDMT, and DSB practice effects were significantly different between our groups (Table 3). Tukey post hoc analyses indicated that for EBMT and DSB scores, European Americans had significantly greater reliable change than Asian Americans and African Americans. For SDMT scores, African Americans had significantly less reliable change than European Americans and Asian Americans. Thus, African Americans showed the least gains in repeat administration of episodic memory, working memory, and processing speed tasks.
Attrition Across the Study Period.
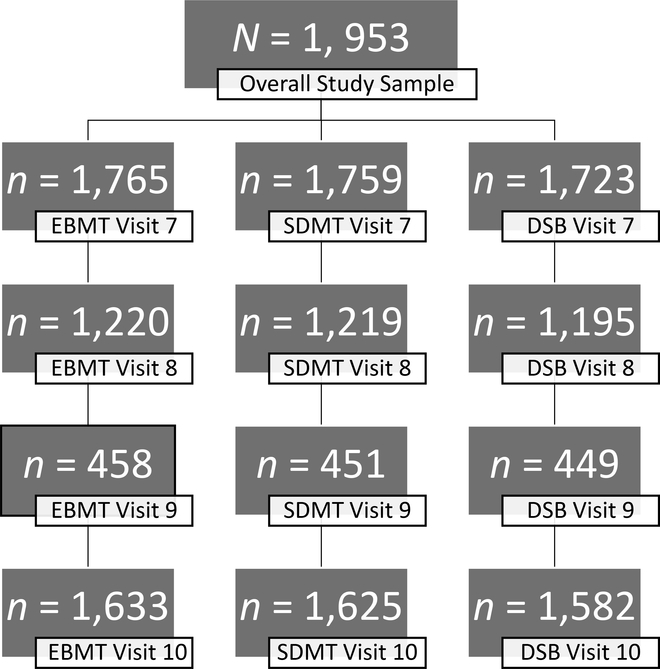
To determine if there were differences between those who were lost to follow up, demographics characteristics were compared between participants who completed and did not complete the last cognitive assessment, separately by measure (i.e., EBMT, SDMT, and DSB; Table 4; Figure 1). Participants who did not complete the last cognitive assessments tended to have lower income, higher diabetes, greater depressive symptoms, higher hypertension, more smokers, and more African Americans than completers.
Preliminary Analyses
Unconditional Models.
We ran unconditional models to characterize the outcome and rate of change for the overall sample for each cognitive variable. On average, there was no significant decrease in yearly rate of change in EBMT (b = − 0.001, p = 0.959) or DSB scores (b = − 0.023, p = 0.120). However, there was significant variability between participants in EBMT scores at baseline (τ00 = 1.104, p < 0.001). There was significant variability between participants in DSB scores at baseline (τ00 = 3.811, p < 0.001) and in the rate of change in DSB scores (τ11 = 0.058, p = 0.001). There was significant decrease in yearly rate of change in SDMT scores on average (b = − 0.119, p = 0.045) as well as variability between participants in rate of change (τ11 = 0.707, p = 0.020). Thus, processing speed – but not episodic memory or working memory – scores decreased over time in our midlife sample.
Racial Differences at Baseline and Cognitive Change.
African Americans experienced significantly poorer EBMT scores at baseline than European Americans as well as a faster decrease in EBMT scores over time compared to European Americans (Table 5). Race accounted for 6.67% of the variance in yearly rate of change and 2.16% in the variance of EBMT scores at baseline.
African Americans had significantly poorer SDMT scores at baseline compared to European Americans. In contrast, Asian Americans had significantly better processing speed at baseline compared to European Americans. Race accounted for 12.21% of the variance in SDMT scores at baseline.
African Americans and Asian Americans had significantly poorer DSB scores at baseline compared to European Americans. Race accounted for 5.91% of the variance in DSB scores at baseline.
Thus, African American women experienced more episodic memory decline compared to European American women. African American women experienced poorer episodic memory, processing speed, and working memory scores at baseline compared to European American women. Asian Americans had poorer DSB scores and better processing speed scores at baseline compared to European Americans.
Primary Analyses
Risk factors predicting cognitive outcomes.
Three MLMs – one each predicting a cognitive outcome – from risk factors indicated that none of the risks (i.e., hypertension, diabetes, smoking, and depressive symptoms) were associated with cognitive decline over time. Depressive symptoms were associated with poorer EBMT scores after controlling for diabetes, hypertension, and smoking (Table 6). Depressive symptoms accounted for 1.16% of the variance in EBMT scores. Including depressive symptoms in the model significantly improved overall model fit compared to the model without depressive symptoms, χ2(2) = 7.326, p = 0.03.
Diabetes and depressive symptoms were associated with poorer SDMT scores at the cognitive baseline and accounted for 0.60% and 0.79%, respectively (Table 7). Including depressive symptoms significantly improved overall model fit compared to the model without depressive symptoms, χ2(2) = 15.884, p < 0.001; likewise, including diabetes significantly improved overall model fit compared to the model without diabetes, χ2(2) = 36.284, p < 0.001.
Race as a moderator in the associations between risk factors and cognitive outcomes.
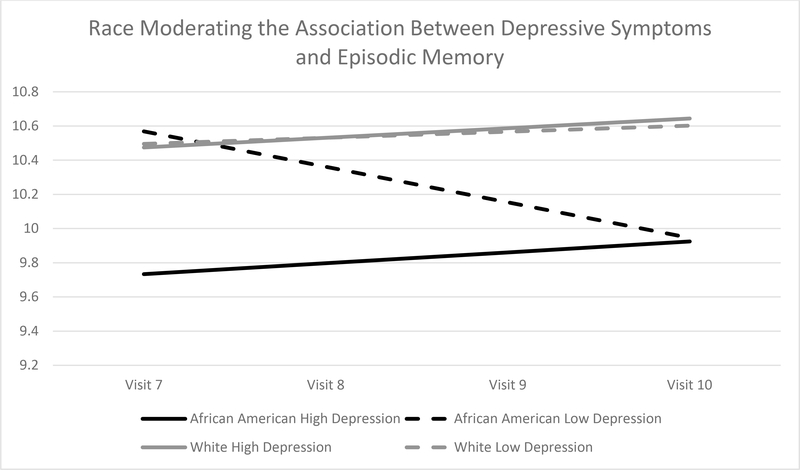
As hypothesized, African Americans with high depressive symptoms experienced poorer EBMT scores at the cognitive baseline relative to African Americans with lower depressive symptoms, whereas European Americans with high depressive symptoms did not experience poorer EBMT scores relative to European American with lower depressive symptoms (Table 8; Figure 2). Simple slope comparisons indicated that depressive symptoms were significantly associated with EBMT scores at baseline for African Americans, but not for European Americans (b = − 0.40, p < 0.001). The interaction term of race and depressive symptoms (i.e., African American-depressive symptoms and Asian American-depressive symptoms) accounted for 1.77% of the variance in EBMT scores at cognitive baseline. Adding the interaction terms significantly improved overall model fit compared to the main effects model, χ2(4) = 15.684, p = 0.003.
Discussion
Our study results highlight racial disparities in cognition during midlife. We found that African American women had lower cognitive scores, benefitted less from repeat testing compared to European Americans, and evidenced significantly faster decrease in episodic memory over time compared to European Americans. A novel finding was that Asian Americans had poorer episodic memory and working memory but greater processing speed than European Americans. Depression and diabetes were significantly associated with poorer cognition during midlife. Consistent with the cumulative disadvantage theory – that multiple stressors on health outcomes accumulate over the lifespan in racial minorities – African Americans may be more vulnerable to the adverse effects of depressive symptoms on cognition at midlife than European Americans. We address these themes and other key findings below.
Racial Group Differences in Cognition
African American women had poorer episodic memory, working memory, and processing speed scores at baseline compared to European American women. African American women also showed the least gain in repeat administration of episodic memory, working memory, and processing speed tasks and exhibited greater episodic memory decline than European American women. Our findings are consistent with robust literature illustrating poorer cognition in African Americans compared to European Americans across the lifespan (Mehta et al., 2004; Sol et al., 2020; Vásquez et al., 2016; Weuve et al., 2018; Zahodne et al., 2017; Zahodne et al., 2016). The current study is one of the first that examined cognition, practice effects, and decline over time between women of different racial groups in midlife women (e.g., Karlamangla et al., 2017; Kazlauskaite et al., 2020).
Midlife African American women exhibited greater prevalence or severity of all four risk factors for poor cognition (i.e., depressive symptoms, diabetes, hypertension, and smoking) compared to European American and Asian American women. These findings are consistent with larger epidemiologic studies. For example, 54% of African Americans have high blood pressure or hypertension compared to 46% European Americans (Centers for Disease Control, 2019). Midlife is a period of heightened risk for adverse health and cognitive outcomes in African American women.
Asian American’s lower risk for dementia (e.g., Mayeda et al., 2017) suggests that they may fare better than other underrepresented populations in the U.S. with regard to cognitive aging at midlife. Indeed, cognition in midlife for Asian American women was less disadvantaged than for African American women. For example, Asian Americans show better practice effects on processing speed than African Americans, suggested greater benefit from repeat testing, and had greater processing speed scores at baseline than European Americans. However, Asian Americans had some cognitive vulnerabilities. Asian American women had poorer episodic memory and working memory at baseline compared to European American women.
This is one of the first studies to illustrate domain-specific differences in cognition between Asian Americans and European Americans; other studies focus more on differences in global cognition (i.e., Shadlen et al., 2001). Our data suggest that it might be wise to focus on memory and executive dysfunction as potential harbingers of cognitive decline in Asian American women, since these were a relative group weakness. The relatively stronger performance of Asian Americans on processing speed suggests better white matter integrity at midlife than for African American and European American women and thus fewer cerebrovascular risks. Whereas these hypotheses are highly speculative at the moment, they might help to direct future studies on cognitive aging in Asian Americans, who are the fastest growing minority population in the US, behind people who identify as biracial or multiracial (Vespa, et al., 2018).
When clinical neuropsychologists better understand the origins of racial group differences on cognitive tests, the measures can be used in ethical, reliable, and valid manners with racially diverse populations. Indeed, many potential causes for racial group differences on cognitive measures have been studied, including acculturation (e.g., Martinez-Miller et al., 2020), early-life adversity (e.g., Zhang, Hayward, & Yu, 2016), and SES (e.g., Jean et al., 2019). The most attention has been devoted to understanding if and how cognitive differences observed between racial groups are due to SES (Kawachi et al., 2005; Manly, 2005). In our study, racial group differences in cognition remained after controlling for income and education, suggesting that SES does not fully explain why there are racial group differences in cognition during midlife.
Predictors of Poor Cognition and Cognitive Decline
A primary goal of our project was to determine if diabetes, hypertension, depressive symptoms, and smoking were predictive of cognitive decline for women at midlife and – more important – if these risk factors were differentially associated with cognitive outcomes across different racial groups. Overall, there was little evidence of cognitive decline in our sample. Thus, our aim to study predictors of cognitive decline in midlife women needs to be viewed from the lens of general cognitive stability in our sample. Indeed, diabetes, hypertension, depressive symptoms, and smoking were not significantly predictive of cognitive decline over time.
We found that diabetes and depressive symptoms were proximally associated with poorer processing speed and episodic memory. In other studies, depressive symptoms also were associated with poorer processing speed in older adults (e.g., Brewster et al., 2017) and midlife adults (e.g., Anstey et al., 2014). We suspect that if our midlife sample is followed further into older adulthood – when there will be more variance in changes in cognition – we will be better able to identify predictors of cognitive decline at midlife. Given the associations between diabetes and depressive symptoms with processing speed and episodic memory, these two risk factors will be important to study in the context of later life cognitive changes.
Findings were partially consistent with the hypothesis that risk factors would be more detrimental to cognition for African Americans than European Americans. Greater depressive symptoms were associated with significantly poorer episodic memory at baseline for African Americans but not for European Americans. These data are consistent with findings in cross-sectional studies of midlife and older adults (Hamilton et al., 2014; Wright et al., 2019, Zahodne et al., 2014). The detrimental effect of depressive symptoms is a major public health concern because African Americans report more chronic and severe depression than European Americans (Williams et al., 2007), which we also found in our data.
We interpret these findings in light of the cumulative disadvantage theory (Dannefer, 1987, 1988, 2003). African Americans suffer more chronic adversity – over their lifetimes – than European Americans due to racism and discrimination. This systemic adversity across the life course might cause African Americans to be more vulnerable to the adverse effects of a psychological stressor (i.e., depressive symptoms).
Limitations
The sample was comprised of women and results may not generalize to men. We do not have the immigration status for our sample, which is important because the majority of the Asian American population are foreign-born (Vespa et al., 2018). Our Asian American sample consisted of persons of Japanese and Chinese descent and results may not generalize to other Asian ethnic groups. Risk factors were assessed at the beginning of the study and not throughout the study period. As far as we know, no research studies have conducted measurement invariance studies comparing psychometric properties of our cognitive measures for Asian Americans samples. Those who did not complete the last cognitive assessment had greatest risk for poor cognitive outcomes than their counterparts who did complete the last assessments. Thus, attrition may have impeded our ability to find cognitive decline over time, as well as significant associations between risk factors and cognitive decline in our study due to restricted variance in the final cognitive assessments.
Implications
Racial disparities in cognitive aging are a major public health concern, particularly because the U.S. population is growing older and more racially diverse. The midlife developmental period presents an opportunity for preventative interventions to guard against racial disparities in cognitive decline in late life. Clinicians must understand that structural and system level barriers (i.e., racism and discrimination) create and perpetuate health inequities across the life course for African Americans, which might ultimately lead to the cognitive disparities that we found. A better understanding of the causes of the cognitive disparities that exist will allow for development of more effective clinical interventions. Research and clinical focus on African American women is warranted because they live at the intersection of greater risk for dementia because of their race (i.e. African American) and gender (i.e., women) compared to European Americans and men (Avila et al., 2019; Hebert et al., 2013).
Our results are a significant step in this direction. Future research should examine whether the mechanisms linking depressive symptoms to poor cognition operate differently in African American women, thus positioning this population for cognitive vulnerability compared to women from other racial backgrounds. Clinical interventions tailored to racial groups are needed to maintain cognitive health in midlife. Targeted public health prevention efforts should be designed and implemented that can educate both practitioners and the African American community about the detrimental effects of depression on African American women’s cognitive health.
Supplementary Material
Key Points.
Question:
Are there racial group differences in the associations between risk factors and cognitive decline during midlife?
Findings:
Depressive symptoms are a significant predictor of poor cognition for African American women but not for Asian American and European American women.
Importance:
Clinical interventions to promote healthy cognitive aging in African Americans should focus on depressive symptoms.
Next Steps:
Future research should determine why depressive symptoms are more strongly linked to poorer cognition in African Americans compared to other racial groups.
Acknowledgments
Access to the publicly available datasets from this study were obtained from the National Archive of Computerized Data on Aging (NACDA), the aging program within the Inter-university Consortium for Political and Social Research (ICPSR). NACDA is sponsored by the National Institute on Aging (NIA) at the National Institutes of Health (NIH).
The Study of Women’s Health Across the Nation (SWAN) has grant support from United States Department of Health and Human Services. National Institutes of Health (NR004061), United States Department of Health and Human Services. National Institutes of Health. National Institute on Aging (AG012495, AG012505, AG012539, AG012546, AG012553, AG012554), United States Department of Health and Human Services. National Institutes of Health. National Institute of Nursing Research (AG012535), United States Department of Health and Human Services. National Institutes of Health. Office of Research on Women’s Health (AG012531).
APPENDIX A
Table 1.
Study Visits and Measures: When Data Were Collected That Are Used to Test Hypotheses
| Variables | Baseline | Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 6 | Visit 7 | Visit 8 | Visit 9 | Visit 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Diabetes | X | X | X | |||||||
| Hypertension | X | X | X | X | ||||||
| Depressive Symptoms | X | X | X | X | ||||||
| Smoking | X | X | X | X | ||||||
| EBMT | X | X | X | X | X | X | ||||
| SDMT | X | X | X | X | X | X | ||||
| DSB | X | X | X | X | X | X |
Note: EBMT= East Boston Memory Test; SDMT = Symbol Digit Modalities Test; DSB = Digit Span Backwards; Diabetes metrics were not collected at visit 2; Cognitive measures (EBMT, SDMT, DSB) at visit 4 and visit 6 were used to create the practice effect variable and excluded from longitudinal analysis. Cognitive trajectory was measured from visit 7 to visit 10, thus visit 7 is considered cognitive baseline.
Table 2.
Baseline Participant Characteristics by Racial Group
| Characteristics | European American (n = 1,000) | African American (n = 516) | Asian American (n = 437) | χ2 |
|---|---|---|---|---|
| Mean Age (SD) (Minimum-Maximum) |
45.95 (2.73) (42 – 53) |
45.88 (2.61) (42 – 52) |
46.11 (2.58) (42 – 52) |
|
| Family Income (%)*** | 176.81a | |||
| Less than $19,999 | 5.7 | 19.4 | 3.7 | |
| $20,000 to $49,999 | 30.6 | 40.7 | 27.5 | |
| $50,000 to $99,999 | 43.2 | 30.2 | 41.0 | |
| $100,000 or more | 19.3 | 5.6 | 24.0 | |
| Education Attainment (%)*** | 115.22a,b | |||
| Less than High School | 1.2 | 4.5 | 5.3 | |
| High School Graduate | 11.9 | 19.8 | 15.1 | |
| Some College/Technical School | 29.0 | 38.8 | 28.8 | |
| College Graduate | 22.4 | 15.9 | 31.1 | |
| Post Graduate Education | 35.3 | 19.8 | 19.7 | |
| Menopause Status (%)*** | 41.00a,c | |||
| Post Bilateral Salpingo Oophorectomy | 4.1 | 6.2 | 3.0 | |
| Natural Post-menopause | 51.3 | 48.1 | 52.2 | |
| Late Peri-menopause | 9.4 | 13.8 | 8.0 | |
| Early Peri-menopause | 24.2 | 23.8 | 27.0 | |
| Pre-menopause | 1.9 | 1.2 | 3.2 | |
| Unknown Due to Hormone Therapy Use | 6.8 | 2.9 | 5.7 | |
| Unknown Due to Hysterectomy | 2.1 | 3.9 | 0.9 |
Note. Missingness for income ranged from 1.2 to 4.1%. Missingness for education ranged from 0.2 to 1.4%. Missingness for menopause status ranged from 0.2 to 0.9%.
European Americans greater than African Americans.
European Americans greater than Asian Americans.
African Americans greater than Asian Americans.
p < = 0.05
p < 0.01
p < 0.001.
Table 3.
Risk Factors and Cognitive Outcomes at Baseline by Racial Group
| Variables | European American (n = 1,000) | African American (n = 516) | Asian American (n = 437) | Significant Group Differences a | F | χ2 |
|---|---|---|---|---|---|---|
| Risk Factors, M (SD) (Minimum-Maximum) |
||||||
| Depressive Symptoms | 8.43 (6.73) (0.00 – 37.50) |
9.89 (7.57) (0.00 – 42.75) |
8.08 (6.58) (0.25– 50.00) |
AFAM > EA = ASAM | 10.04 | -- |
| Hypertension | 112.48 (12.27) (82.92 – 183.83) |
124.14 (15.84) (93.42 – 199.75) |
110.68 (11.90)*
(87.17 – 147.58) |
AFAM > EA > ASAM | 164.34 | -- |
| Diabetes | 93.69 (23.04) (69.67 – 322.00) |
102.08 (33.87) (73.00 – 325.33) |
93.41 (12.35) (72.00 – 246.33) |
AFAM > EA = ASAM | 22.36 | -- |
| Smoking (% of smokers) | 11.5 | 20.2*** | 7.8* | AFAM> EA> ASAM | -- | 36.37 |
| Cognitive Outcomes, M (SD) | ||||||
| Processing Speed (SDMT) | 59.95 (9.55) (19 – 96) |
51.83 (11.18)*** (7 – 85) |
61.58 (8.80)*
(33 – 87) |
ASAM > EA > AFAM | 131.32 | -- |
| Working Memory (DSB) | 7.55 (2.32) (2 – 12) |
6.02 (2.27)***
(2 – 12) |
6.77 (1.91)***
(2 – 12) |
EA > ASAM > AFAM | 71.48 | -- |
| Episodic Memory (EBMT) | 10.54 (1.58) (2 – 12) |
9.74 (1.97)***
(4 – 12) |
10.21 (1.58)**
(0 −12) |
EA > ASAM > AFAM | 33.11 | -- |
| Practice Effects/Z-scores, M (SD) | ||||||
| Processing Speed (SDMT) | −0.31 (1.00) (−5.34 – 7.19) |
−0.44 (1.13)***
(−7.73 – 3.66) |
0.08 (0.90) (−5.84 – 3.51) |
EA = ASAM > AFAM | 36.76 | -- |
| Working Memory (DSB) | 0.001 (1.00) (−3.45 – 4.02) |
−0.30 (0.98)***
(−3.74 – 3.17) |
−0.15 (0.87)*
(−3.45– 2.45) |
EA > ASAM > AFAM | 14.80 | -- |
| Episodic Memory (EBMT) | 0.007 (1.00) (−6.06 – 1.98) |
−0.37 (1.25)***
(−6.24 – 1.98) |
−0.30 (0.92)***
(−2.97 – 1.82) |
EA > ASAM = AFAM | 23.74 | -- |
Note. EA= European American; AFAM= African American; ASAM= Asian American. Depressive symptom and hypertension values were averaged across baseline, visit 1, visit 2, and visit 3. Diabetes values were averaged across baseline, visit 1, and visit 3. Missingness on EBMT at baseline ranged from 4.8 to 14%. Missingness on SDMT at baseline ranged from 4.8 to 14.9%. Missingness on DSB at baseline ranged from 5.5 to 16..9%.
Significant group differences in risk factors, cognitive outcomes, and practice effects.
p < = 0.05
p < 0.01
p < 0.001 compared to European Americans (reference group).
Table 4.
Demographic Comparison Between Participants that did and did not have Visit 10 Cognitive Data
| Demographics | EBMT Visit 10 Not Completed (320) | EBMT Completed Visit 10 (1,633) | SDMT Visit 10 Not Completed (328) | SDMT Completed Visit 10 (1,625) | DSB Visit 10 Not Completed (371) | DSB Completed Visit 10 (1,582) |
|---|---|---|---|---|---|---|
| Mean Age (SD) | 45.86 (2.74) | 45.99 (2.65) | 45.86 (2.74) | 45.99 (2.65) | 45.95 (2.76) | 45.97 (2.64) |
| (Minimum – Maximum) | (42 – 52) | (42 – 53) | (42 – 52) | (42 – 53) | (42 – 52) | ( 42 – 53) |
| Family Income (%)*** | ||||||
| Less than $19,999 | 15.1 | 7.9 | 15.0 | 7.9 | 14.4 | 7.8 |
| $20,000 to $49,999 | 30.9 | 33.9 | 30.7 | 34.0 | 32.2 | 33.7 |
| $50,000 to $99,999 | 37.9 | 40.8 | 38.2 | 40.7 | 37.2 | 41.0 |
| $100,000 or more | 16.1 | 17.4 | 16.0 | 17.4 | 16.1 | 17.4 |
| Education Attainment (%) | ||||||
| Less than High School | 3.4 | 2.9 | 3.4 | 2.9 | 3.0 | 3.0 |
| High School Graduate | 16.3 | 14.5 | 16.5 | 14.4 | 16.8 | 14.3 |
| Some College/Technical School | 34.1 | 31.2 | 34.1 | 31.2 | 34.3 | 31.1 |
| College Graduate | 21.6 | 23.0 | 21.3 | 23.0 | 21.9 | 22.9 |
| Post Graduate Education | 24.7 | 28.4 | 24.7 | 28.5 | 24.1 | 28.7 |
| Menopause Status (%) | ||||||
| Post Bilateral Salpingo Oophorectomy | 3.1 | 4.7 | 3.4 | 4.6 | 3.8 | 4.6 |
| Natural Post-menopause | 50.9 | 50.7 | 51.5 | 50.6 | 50.4 | 50.8 |
| Late Peri-menopause | 11.3 | 10.0 | 11.0 | 10.1 | 11.7 | 9.9 |
| Early Peri-menopause | 28.0 | 24.1 | 27.6 | 24.2 | 27.6 | 24.1 |
| Pre-menopause | 1.3 | 2.1 | 1.2 | 2.2 | 1.4 | 2.2 |
| Unknown Due to Hormone Therapy Use | 3.1 | 6.0 | 3.1 | 6.0 | 3.0 | 6.1 |
| Unknown Due to Hysterectomy | 2.2 | 2.3 | 2.1 | 2.3 | 2.2 | 2.3 |
| Risk Factors, M (SD) (Minimum – Maximum) | ||||||
| Depressive Symptoms | 9.67 (8.01)* | 8.56 (6.72) | 9.63 (7.93)* | 8.56 (6.74) | 9.58 (7.68)* | 8.54 (6.77) |
| (0.00 – 42.25) | (0.00 – 50.00) | (0.00 – 42.25) | (0.00 – 50.00) | (0.00 – 42.25) | (0.00 – 50.00) | |
| Hypertension | 117.44 (14.50)** | 114.71 (14.22) | 117.69 (14.57)** | 114.65 (14.19) | 118.19 (14.64)*** | 114.45 (14.13) |
| (91.75 – 167.42) | (82.92 – 199.75) | (91.75 – 167.42) | (82.92 – 199.75) | (91.75 – 167.42) | (82.92 – 199.75) | |
| Diabetes | 101.15 (36.47)** | 94.81 (21.88) | 101.22 (36.60)** | 94.76 (21.74) | 100.65 (35.06)** | 94.72 (21.80) |
| (69.67 – 322.00) | (72.00 – 325.33) | (69.67 – 322.00) | (72.00 – 325.53) | (69.67 – 322.00) | (72.00 – 325.33) | |
| Smoking (% of smokers) | 17.6** | 12.1 | 17.4** | 12.1 | 16.8* | 12.1 |
| Race (%)*** | ||||||
| African American | 37.2 | 24.3 | 37.8 | 24.1 | 38.3 | 23.6 |
| Asian American | 12.2 | 24.4 | 11.9 | 24.5 | 11.3 | 25.0 |
| European American | 50.6 | 51.3 | 50.3 | 51.4 | 50.4 | 51.4 |
Note.
p < = 0.05
p < 0.01
p < 0.001.
Table 5.
Change in Episodic Memory, Processing Speed, and Working Memory Predicted by Race
| Measure | Episodic Memory | Processing Speed | Working Memory |
|---|---|---|---|
|
| |||
| Coefficient (SE) | Coefficient (SE) | Coefficient (SE) | |
| Fixed effects | |||
| Intercept, γ00 | 10.480 (0.051)** | 59.601 (0.315)** | 7.418 (0.073)** |
| Age, γ01 | −0.006 (0.015) | −0.487 (0.091)** | −0.020 (0.021) |
| African American, γ02 | −0.348 (0.093)** | −6.08 (0.572)** | −1.103 (0.132)** |
| Asian American, γ03 | −0.180 (0.093) | 2.361 (0.574)** | −0.533 (0.132)** |
| Education, γ04 | 1.64 (0.035)** | 1.563 (0.217)** | 0.406 (0.050)** |
| Family Income, γ05 | 0.180 (0.048)** | 1.829 (0.293)** | 0.162 (0.067) |
| Menopause Status,γ06 | 0.012 (0.025) | 0.262 (0.153) | −0.007 (0.035) |
| Practice Effect, γ07 | 0.348 (0.035)** | 1.093 (0.216)** | 0.179 (0.050)** |
| Linear slope, γ10 | 0.047 (0.22) | −0.074 (0.089) | −0.037 (0.023) |
| Age, γ11 | 0.000 (0.006) | −0.053 (0.026) | −0.004 (0.007) |
| African American, γ12 | −0.111 (0.040)* | 0.077 (0.164) | 0.011 (0.042) |
| Asian American, γ13 | −0.014 (0.039) | −0.147 (0.159) | −0.045 (0.040) |
| Education, γ14 | −0.001 (0.015) | −0.010 (0.061) | 0.006 (0.016) |
| Family Income, γ15 | −0.025 (0.020) | −0.060 (0.083) | −0.039 (0.021) |
| Menopause Status,γ16 | 0.003 (0.011) | −0.062 (0.042) | −0.011 (0.011) |
| Practice Effect, γ17 | −0.037 (0.016) | 0.043 (0.063) | −0.001 (0.016) |
| Random effects | Variance Components | ||
| Intercept, τ00 | 0.861** | 63.112** | 3.168** |
| Linear slope, τ11 | 0.014 | 0.287 | 0.052* |
| Level 1, σ2 | 1.709** | 26.858** | 1.569** |
| Model Fit: H0 Value (df) | −8622.450 (20) | −16092.051 (20) | −9184.985 (20) |
Note.
p < 0.01
p < 0.001 compared to European Americans (reference group).
Table 6.
Change in Episodic Memory Predicted by Smoking, Depressive Symptoms, Diabetes, and Hypertension
| Measure | Covariates Only | Risk Factors |
|---|---|---|
|
| ||
| Coefficient (SE) | Coefficient (SE) | |
| Fixed effects | ||
| Intercept, γ00 | 10.353 (0.037)** | 10.379 (0.039)** |
| Age, γ01 | −0.006 (0.015) | −0.004 (0.015) |
| Smoking, γ02 | -- | −0.273 (0.115) |
| Depressive Symptoms γ03 | -- | −0.015 (0.006)* |
| Hypertension, γ04 | -- | −0.005 (0.003) |
| Diabetes, γ05 | -- | −0.002 (0.002) |
| Education,γ06 | 0.179 (0.035)** | 0.159 (0.035)** |
| Family Income, γ07 | 0.212 (0.046)** | 0.155 (0.047)** |
| Menopause Status, γ08 | 0.012 (0.025) | 0.007 (0.025) |
| Practice Effect, γ09 | 0.365 (0.035)** | 0.351 (0.035)** |
| Linear slope, γ10 | 0.017 (0.016) | −0.016(0.034) |
| Age, γ11 | 0.000 (0.006) | 0.000 (0.007) |
| Smoking, γ12 | -- | 0.010 (0.050) |
| Depressive Symptoms γ13 | -- | 0.003 (0.002) |
| Hypertension, γ14 | -- | 0.000 (0.001) |
| Diabetes, γ15 | -- | 0.000 (0.001) |
| Education,γ16 | 0.003 (0.015) | 0.004 (0.015) |
| Family Income, γ17 | −0.012 (0.020) | −0.005 (0.020) |
| Menopause Status, γ18 | 0.003 (0.011) | 0.003 (0.011) |
| Practice Effect, γ19 | −0.034 (0.015) | −0.032 (0.015) |
| Random effects | Variance Components | |
| Intercept, τ00 | 0.880** | 0.853** |
| Linear slope, τ11 | 0.015 | 0.015 |
| Level 1, σ2 | 1.711** | 1.712** |
| Model Fit: H0 Value (df) | −8649.516 (16) | −8625.650(24) |
Note. Covariates are age, education, family income, menopause status, and practice effect.
p < 0.01
p < 0.001
Table 7.
Change in Processing Speed Predicted by Smoking, Depressive Symptoms, Diabetes, and Hypertension
| Measure | Covariates Only | Risk Factors |
|---|---|---|
|
| ||
| Coefficient (SE) | Coefficient (SE) | |
| Fixed effects | ||
| Intercept, γ00 | 58.605 (0.238)** | 58.881 (0.229)** |
| Age, γ01 | −0.481 (0.096)** | −0.516 (0.089)** |
| Smoking, γ02 | -- | −0.793 (0.669) |
| Depressive Symptoms γ03 | -- | −0.100 (0.032)* |
| Hypertension, γ04 | -- | −0.032 (0.016) |
| Diabetes, γ05 | -- | −0.027 (0.009)* |
| Education,γ06 | 1.577 (0.225)** | 1.208 (0.205)** |
| Family Income, γ07 | 2.875 (0.296)** | 2.287 (0.227)** |
| Menopause Status, γ08 | 0.269 (0.161) | 0.164 (0.146) |
| Practice Effect, γ09 | 1.218 (0.225)** | 3.944 (0.211)** |
| Linear slope, γ10 | −0.087 (0.064) | −0.109 (0.068) |
| Age, γ11 | −0.052 (0.026) | −0.047 (0.026) |
| Smoking, γ12 | -- | 0.183 (0.200) |
| Depressive Symptoms γ13 | -- | −0.014 (0.010) |
| Hypertension, γ14 | -- | −0.004 (0.005) |
| Diabetes, γ15 | -- | −0.003 (0.003) |
| Education,γ16 | −0.005 (0.060) | −0.013 (0.061) |
| Family Income, γ17 | −0.088 (0.080) | −0.117 (0.082) |
| Menopause Status, γ18 | −0.062 (0.042) | 0.064 (0.042) |
| Practice Effect, γ19 | −0.038 (0.063) | 0.013 (0.064) |
| Random effects | Variance Components | |
| Intercept, τ00 | 71.888** | 54.689** |
| Linear slope, τ11 | 0.289 | 0.277 |
| Level 1, σ2 | 26.868** | 26.729** |
| Model Fit: H0 Value (df) | −16179.333 (16) | −8625.650(24) |
Note. Covariates are age, education, family income, menopause status, and practice effect.
p < 0.01
p < 0.001
Table 8.
Race Moderating the Association Between Depressive Symptoms and Episodic Memory
| Measure | Episodic Memory |
|
|---|---|---|
| Main Effect Coefficient (SE) | Interaction Coefficient (SE) | |
| Fixed Effects | ||
| Intercept, γ00 | 10.478 (0.051)** | 10.486 (0.051)** |
| Age, γ01 | −0.007 (0.015) | −0.009 (0.015) |
| African American, γ02 | −0.347 (0.093)** | −0.334 (0.093)** |
| Asian American, γ03 | −0.192 (0.093) | −0.209 (0.093) |
| Depressive Symptoms, γ04 | −0.017 (0.006)* | 0.000 (0.008) |
| African American-Depressive Symptoms, γ05 | -- | −0.040 (0.012)*a |
| Asian American-Depressive Symptoms, γ06 | -- | −0.025 (0.014) |
| Education, γ07 | 0.157 (0.035)** | 0.153 (0.035)** |
| Family Income, γ08 | 0.152 (0.048)* | 0.153 (0.048)** |
| Menopause Status,γ09 | 0.013 (0.025) | 0.012 (0.025) |
| Practice Effect, γ010 | 0.341 (0.035)** | 0.340 (0.035)** |
| Linear slope, γ10 | 0.047 (0.022) | 0.046 (0.022) |
| Age, γ11 | 0.000 (0.006) | 0.001 (0.006) |
| African American, γ12 | −0.111 (0.040)* | −0.118 (0.040)* |
| Asian American, γ13 | −0.011 (0.039) | −0.015 (0.040) |
| Depressive Symptoms, γ14 | 0.004 (0.002) | 0.001 (0.003) |
| African American- Depressive Symptoms, γ15 | -- | 0.012 (0.005) |
| Asian American- Depressive Symptoms, γ16 | -- | −0.003 (0.006) |
| Education, γ17 | 0.000 (0.015) | 0.001 (0.015) |
| Family Income, γ18 | −0.019 (0.021) | −0.019 (0.021) |
| Menopause Status,γ19 | 0.003 (0.011) | 0.004 (0.011) |
| Practice Effect, γ110 | −0.036 (0.016)* | −0.035 (0.016) |
| Random Effects | Variance Component | |
| Intercept, τ00 | 0.849** | 0.834** |
| Linear slope, τ11 | 0.013 | 0.011 |
| Level 1, σ2 | 1.709** | 1.709*** |
| Model Fit: H0 Value (df) | −8617.927 (22) | 8610.085 (26) |
Note.
Significant simple slope comparison for African Americans.
p < 0.01
p < 0.001 compared to European Americans (reference group).
APPENDIX B
Figure 1: Flow Chart of Study Attrition Across Visit 7 (Baseline) Through Visit 10.
Note. Not all participants completed all three cognitive measures at each time point. Participants were administered the cognitive testing at either visit 8 or visit 9. Full Information Maximum Likelihood (FIML) accounts for missingness in the data by estimating a likelihood of scores for each participant based on existing data available. If participants had at least one data point then they were included in analysis, thus the overall model fit is based on the overall study sample, N = 1,953.
Figure 2.
Interaction Between Depressive Symptoms and Race Predicting Episodic Memory
Footnotes
Table 1 catalogues studies that examined cognitive decline in older adult samples.
Table 2 catalogues studies that examined cognitive decline in midlife adult samples.
Table 3 catalogues studies that examined cognitive decline between African Americans and European Americans.
Researchers also recruited Hispanic participants, but the sample had high attrition during follow up, so these data were not included.
Table 4 describes the standardized regression-based formula to calculate practice effects.
References
- Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, & Funkenstein HH (1991). Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. International journal of Neuroscience, 57(3–4), 167–178. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Sargent-Cox K, Garde E, Cherbuin N, & Butterworth P (2014). Cognitive development over 8 years in midlife and its association with cardiovascular risk factors. Neuropsychology, 28(4), 653–665. 10.1037/neu0000044 [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Von Sanden C, Salim A, & O’Kearney R (2007). Smoking as a risk factor for dementia and cognitive decline: A meta-analysis of prospective studies. American Journal of Epidemiology, 166(4), 367–378. 10.1093/aje/kwm116 [DOI] [PubMed] [Google Scholar]
- Assari S (2018). The Benefits of Higher Income in Protecting against Chronic Medical Conditions Are Smaller for African Americans than Whites. Healthcare, 6(1), 2. 10.3390/healthcare6010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila JF, Vonk JM, Verney SP, Witkiewitz K, Rentería MA, Schupf N, … & Manly JJ (2019). Sex/gender differences in cognitive trajectories vary as a function of race/ethnicity. Alzheimer’s & Dementia, 15(12), 1516–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A (1992). Working Memory. Science, 255(5044), 556–559. [DOI] [PubMed] [Google Scholar]
- Barnes LL, Yumoto F, Capuano A, Wilson RS, Bennett DA, & Tractenberg RE (2016). Examination of the factor structure of a global cognitive function battery across race and time. Journal of the International Neuropsychological Society: JINS, 22(1), 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, & Johns H (2015). Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer’s & Dementia, 11(6), 718–726. 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- Boardman JD, & Alexander KB (2011). Stress trajectories, health behaviors, and the mental health of black and white young adults. Social Science & Medicine, 72(10), 1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone KB, Victor TL, Wen J, Razani J, & Pontón M (2007). The association between neuropsychological scores and ethnicity, language, and acculturation variables in a large patient population. Archives of clinical neuropsychology, 22(3), 355–365. [DOI] [PubMed] [Google Scholar]
- Brewster GS, Peterson L, Roker R, Ellis ML, & Edwards JD (2017). Depressive Symptoms, Cognition, and Everyday Function among Community-Residing Older Adults. Journal of Aging and Health, 29(3), 367–388. 10.1177/0898264316635587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LL, Mitchell UA, & Ailshire JA (2020). Disentangling the stress process: Race/ethnic differences in the exposure and appraisal of chronic stressors among older adults. The Journals of Gerontology: Series B, 75(3), 650–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamia M, Markon K, & Tranel D (2012). Scoring higher the second time around: meta-analyses of practice effects in neuropsychological assessment. The Clinical Neuropsychologist, 26(4), 543–570. [DOI] [PubMed] [Google Scholar]
- Carvalho JO, Tommet D, Crane PK, Thomas ML, Claxton A, Habeck C, … Romero HR (2015). Deconstructing racial differences: The effects of quality of education and cerebrovascular risk factors. Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 70(4), 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castora-Binkley M, Peronto CL, Edwards JD, & Small BJ (2013). A Longitudinal Analysis of the Influence of Race on Cognitive Performance, 70(January), 512–518. 10.1093/geronb/gbt112. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Hypertension Cascade: Hypertension Prevalence, Treatment and Control Estimates Among US Adults Aged 18 Years and Older Applying the Criteria from the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline—NHANES 2013–2016. Atlanta, GA: US Department of Health and Human Services; 2019. [Google Scholar]
- Chao SZ, Matthews BR, Yokoyama JS, Lai NB, Ong H, Tse M, … & Rosen HJ (2014). Depressive symptoms in Chinese-American subjects with cognitive impairment. The American Journal of Geriatric Psychiatry, 22(7), 642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, & Janicki-Deverts D (2012). Who’s stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006, and 2009 1. Journal of Applied Social Psychology, 42(6), 1320–1334. [Google Scholar]
- Dannefer D (1987). Aging as intracohort differentiation: Accentuation, the Matthew effect, and the life course. In Sociological Forum (Vol. 2, pp. 211–236). Springer. [Google Scholar]
- Dannefer D (1988). Differential gerontology and the stratified life course: Conceptual and methodological issues. In Varieties of aging (pp. 3–36). Springer. [PubMed] [Google Scholar]
- Dannefer D (2003). Cumulative Advantage / Disadvantage and the Life Course : Cross-Fertilizing Age and Social Science Theory, 58(6), 327–337. [DOI] [PubMed] [Google Scholar]
- Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, … DeCarli C (2011). Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology, 77(5), 461–468. 10.1212/WNL.0b013e318227b227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A (2014). Executive Functions. Annual Review of Clinical PsychologyPsychol., 64, 135–168. 10.1146/annurev-psych-113011-143750.Executive [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore G, Waldstein S, Evans M, & Zonderman A (2015). Associations between diabetes and cogntive function in socioeconomically diverse African Americans and whites, 18(3), 386–392. 10.1038/nn.3945.Dopaminergic [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K (2012). Evidence-based indicators of neuropsychological change in the individual patient: relevant concepts and methods. Archives of Clinical Neuropsychology, 27(3), 248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Anderson JS, Mallik AK, Suhrie KR, Atkinson TJ, Dalley BC, … & Hoffman JM (2018). Short-term repeat cognitive testing and its relationship to hippocampal volumes in older adults. Journal of Clinical Neuroscience, 57, 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Beglinger LJ, Moser DJ, Schultz SK, & Paulsen JS (2010). Practice effects and outcome of cognitive training: Preliminary evidence from a memory training course. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry, 18(1), 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Foster NL, & Hoffman JM (2014). Practice effects and amyloid deposition: preliminary data on a method for enriching samples in clinical trials. Alzheimer disease and associated disorders, 28(3), 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Horn KP, Foster NL, & Hoffman JM (2015). Short-term practice effects and brain hypometabolism: preliminary data from an FDG PET study. Archives of Clinical Neuropsychology, 30(3), 264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Lyketsos CG, Beglinger LJ, Chelune G, Moser DJ, Arndt S, … & McCaffrey RJ (2011). Practice effects predict cognitive outcome in amnestic mild cognitive impairment. The American Journal of Geriatric Psychiatry, 19(11), 932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J, Charness N, Dijkstra K, & Fitzgibbons JM (2019). Is episodic memory performance more vulnerable to depressive affect in older adulthood?, 5585. 10.1080/13825585.2018.1424314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glymour MM, & Manly JJ (2008). Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychology review, 18(3), 223–254. [DOI] [PubMed] [Google Scholar]
- Gottesman RF, Schneider ALC, Albert M, Alonso A, Bandeen-Roche K, Coker L, … Mosley TH (2014). Midlife hypertension and 20-year cognitive change: The atherosclerosis risk in communities neurocognitive study. JAMA Neurology, 71(10), 1218–1227. 10.1001/jamaneurol.2014.1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta VK, Winter M, Cabral H, Henault L, Waite K, Hanchate A, … Paasche-Orlow MK (2016). Disparities in Age-Associated Cognitive Decline Between African-American and Caucasian Populations: The Roles of Health Literacy and Education. Journal of the American Geriatrics Society, 64(8), 1716–1723. 10.1111/jgs.14257 [DOI] [PubMed] [Google Scholar]
- Hajjar I, Rosenberger KJ, Kulshreshtha A, Ayonayon HN, Yaffe K, & Goldstein FC (2017). Association of JNC-8 and SPRINT systolic blood pressure levels with cognitive function and related racial disparity. JAMA Neurology, 74(10), 1199–1205. 10.1001/jamaneurol.2017.1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JL, Brickman AM, Lang R, Byrd GS, Haines JL, Pericak-Vance MA, & Manly JJ (2014). Relationship Between Depressive Symptoms and Cognition in Older, Non-demented African Americans. Journal of the International Neuropsychological Society, 20(07), 756–763. 10.1017/S1355617714000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y, & Chiriboga DA (2011). Social activity and depressive symptoms in Korean American older adults: The conditioning role of acculturation. Journal of Aging and Health, 23(5), 767–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean KR, Lindbergh CA, Mewborn CM, Robinson TL, Gogniat MA, & Miller LS (2019). Education differentially buffers cognitive performance in black and white older adults. The Journals of Gerontology: Series B, 74(8), 1366–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandiah N, Narasimhalu K, Lee J, & Chen CLPH (2009). Differences exist in the cognitive profile of mild alzheimer’s disease and subcortical ischemic vascular dementia. Dementia and Geriatric Cognitive Disorders, 27(5), 399–403. 10.1159/000210387 [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Lachman ME, Han W, Huang M, & Greendale GA (2017). Evidence for cognitive aging in midlife women: Study of women’s health across the nation. PLoS One, 12(1), e0169008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskaite R, Janssen I, Wilson RS, Appelhans BM, Evans DA, Arvanitakis Z, … & Kravitz HM (2020). Is midlife metabolic syndrome associated with cognitive function change? The study of women’s health across the nation. The Journal of Clinical Endocrinology & Metabolism, 105(4), e1093–e1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi I, Daniels N, & Robinson DE (2005). Health disparities by race and class: why both matter. Health Affairs, 24(2), 343–352. [DOI] [PubMed] [Google Scholar]
- Kirova A-M, Bays RB, & Lagalwar S (2015). Working Memory and Executive Function Decline across Normal Aging, Mild Cognitive Impairment, and Alzheimer’s Disease. BioMed Research International, 2015, 1–9. 10.1155/2015/748212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, … Folsom AR (2001). Cardiovascular risk factors and cognitive decline in middle-aged adults. [DOI] [PubMed]
- Lachman ME (2016). Mind the Gap in the Middle : A Call to Study Midlife, 12, 327–334. 10.1080/15427609.2015.1068048.Mind [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman ME, Teshale S, & Agrigoroaei S (2015). Midlife as a pivotal period in the life course : Balancing growth and decline at the crossroads of youth and old age. 10.1177/0165025414533223 [DOI] [PMC free article] [PubMed]
- Lamar M, Resnick SM, & Zonderman AB (2003). Longitudinal changes in verbal memory in older adults: distinguishing the effects of age from repeat testing. Neurology, 60(1), 82–86. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, & Havlik R (2000). Midlife blood pressure and dementia: the Honolulu Asia Aging Study. Neurobiol Aging, 21(1), 49–55. https://doi.org/S0197-4580(00)00096-8 [pii] [DOI] [PubMed] [Google Scholar]
- Leung P, Cheung M, Kao D, & Gulati AC (2017). Prevalence and predictors of depression in the older Asian Americans in Houston. International Social Work, 60(4), 800–814. [Google Scholar]
- Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, … Wadley VG (2019). Blood pressure and cognitive decline over 8 years in middle-aged and older black and white americans. Hypertension, 73(2), 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, & Van Dyke ME (2018). Discrimination and the Health of African Americans: The Potential Importance of Intersectionalities. Current Directions in Psychological Science. 10.1177/0963721418770442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JJ (2005). Advantages and Disadvantages of Separate Norms for African Americans, 4046. 10.1080/13854040590945346 [DOI] [PubMed] [Google Scholar]
- Marsiske M, Dzierzewski JM, Thomas KR, Kasten L, Jones RN, Johnson KE, … Rebok GW (2013). Race-related disparities in 5-year cognitive level and change in untrained ACTIVE participants. Journal of Aging and Health, 25(8_suppl), 103S–127S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Miller EE, Robinson WR, Avery CL, Yang YC, Haan MN, Prather AA, & Aiello AE (2020). Acculturation, Cognitive Performance and Decline, and Incident Dementia/CIND: The Sacramento Area Latino Study on Aging. American Journal of Epidemiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeda, Elizabeth R, Haan MN, Neuhaus J, Yaffe K, Knopman DS, Sharrett AR, … Mosley TH (2014). Type 2 Diabetes and Cognitive Decline Over 14 Years in Middle-Aged African Americans and Whites: The ARIC Brain MRI Study. Neuroepidemiology, 2549, 220–227. 10.1159/000366506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeda, Elizabeth R, Glymour MM, Quesenberry CP, & Whitmer RA (2017). Heterogeneity in 14-year dementia incidence between Asian American subgroups. Alzheimer Disease & Associated Disorders, 31(3), 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeda, Elizabeth Rose, Glymour MM, Quesenberry CP, & Whitmer RA (2016). Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer’s & Dementia, 12(3), 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays V, Cochran S, & Barnes N (2014). Race, Race-Based Discrimination, and Health Outcomes Among African Americans. Annual Review of Psychology, 58, 201–225 10.1146/annurev.psych.57.102904.190212.Race [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness B, Barrett SL, Craig D, Lawson J, & Passmore AP (2010). Executive functioning in Alzheimer’s disease and vascular dementia. International Journal of Geriatric Psychiatry, 25(6), 562–568. 10.1002/gps.2375 [DOI] [PubMed] [Google Scholar]
- Mehta KM, & Yeo GW (2017). Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimer’s and Dementia, 13(1), 72–83. 10.1016/j.jalz.2016.06.2360 [DOI] [PubMed] [Google Scholar]
- O’Brien JT, & Thomas A (2015). Vascular dementia. The Lancet, 386(10004), 1698–1706. 10.1016/S0140-6736(15)00463-8 [DOI] [PubMed] [Google Scholar]
- Obidi CS, Pugeda JP, Fan X, Dimaculangan CM, Singh SP, Chalisa N, & Perlmuter LC (2008). Race moderates age-related cognitive decline in type 2 diabetes. Experimental Aging Research, 34(2), 114–125. 10.1080/03610730701876938 [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Lunn M, Wong D, & Cobain M (2008). Age and ability affect practice gains in longitudinal studies of cognitive change. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 63(4), P235–P240. [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. [Google Scholar]
- Rajan KB, Arvanitakis Z, Lynch EB, McAninch EA, Wilson RS, Weuve J, … Evans DA (2016). Cognitive decline following incident and preexisting diabetes mellitus in a population sample. Neurology, 87(16), 1681–1687. 10.1212/WNL.0000000000003226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush S, Bryk A, & Congdon R (2013). HLM 7.01 for Windows [Hierarchical linear and nonlinear modeling software] Multivariate Software. Inc, Lincolnwood, IL. [Google Scholar]
- Salthouse TA (2009). When does age-related cognitive decline begin? Neurobiology of Aging, 30(4), 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2016). Aging cognition unconfounded by prior test experience. Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 71(1), 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BC, Gross AL, Bangen KJ, Skinner JC, Benitez A, Glymour MM, … Manly JJ (2015). Association of vascular risk factors with cognition in a multiethnic sample. Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 70(4), 532–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MF, Larson EB, Gibbons LE, Rice MM, McCormick WC, Bowen J, … & Graves AB (2001). Ethnicity and cognitive performance among older African Americans, Japanese Americans, and Caucasians: The role of education. Journal of the American Geriatrics Society, 49(10), 1371–1378. [DOI] [PubMed] [Google Scholar]
- Sheridan LK, Fitzgerald HE, Adams KM, Nigg JT, Martel MM, Puttler LI, … Zucker RA (2006). Normative Symbol Digit Modalities Test performance in a community-based sample. Archives of Clinical Neuropsychology, 21(1), 23–28. 10.1016/j.acn.2005.07.003 [DOI] [PubMed] [Google Scholar]
- Sims RC, Madhere S, Gordon S, Clark E Jr, Abayomi KA, Callender CO, & Campbell AL Jr (2008). Relationships among blood pressure, triglycerides and verbal learning in African Americans. Journal of the National Medical Association, 100(10), 1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A, & Marmot M (2005). High blood pressure was associated with cognitive function in middle-age in the Whitehall II study. Journal of Clinical Epidemiology, 58(12), 1308–1315. [DOI] [PubMed] [Google Scholar]
- Smith A (1982). Symbol Digit Modalities Test. 1982. Western Psychological Services, Los Angeles. [Google Scholar]
- Sol K, Zaheed AB, Kraal AZ, Sharifian N, Arce Rentería M, & Zahodne LB (2020). Psychological predictors of memory decline in a racially and ethnically diverse longitudinal sample of older adults in the United States. International Journal of Geriatric Psychiatry, 35(2), 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers MFR, Crawford SL, Sternfeld B, Morganstein D, Gold EB, Greendale GA, … Sherman S (2000). SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition.
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, … Phelps CH (2011). Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia, 7(3), 280–292. 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Goldstein FC, Levey A, & Wharton W (2015). A Meta-Analysis of Alzheimer’s Disease Incidence and Prevalence Comparing African-Americans and Caucasians. Journal of Alzheimer’s Disease, 50(1), 71–76. 10.3233/JAD-150778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternthal MJ, Slopen N, & Williams DR (2011). Racial disparities in health: how much does stress really matter? Du Bois Review: Social Science Research on Race, 8(1), 95–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens-Watkins D, Perry B, Pullen E, Jewell J, & Oser CB (2014). Examining the associations of racism, sexism, and stressful life events on psychological distress among African-American women. Cultural Diversity and Ethnic Minority Psychology, 20(4), 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopford CL, Thompson JC, Richardson AMT, Neary D, & Snowden JS (2010). Working memory in Alzheimer’s disease and frontotemporal dementia. Behavioural Neurology, 23(4), 177–179. 10.3233/BEN-2010-0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton-Tyrrell Kim, Selzer Faith, Sowers MaryFran, R. (Mary Frances Roy), Neer Robert, Powell Lynda, Gold Ellen B., … McKinlay Sonja. Study of Women’s Health Across the Nation (SWAN): Cross-Sectional Screener Dataset, [United States], 1995–1997. Inter-university Consortium for Political and Social Research [distributor], 2019-March-11. 10.3886/ICPSR04368.v5 [DOI] [Google Scholar]
- Sutton-Tyrrell Kim, Selzer Faith, Sowers MaryFran, R. (Mary Frances Roy), Neer Robert, Powell Lynda, Gold Ellen B., … McKinlay Sonja . Study of Women’s Health Across the Nation (SWAN): Baseline Dataset, [United States], 1996–1997. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2019-May-15. 10.3886/ICPSR28762.v5 [DOI] [Google Scholar]
- Sutton-Tyrrell Kim, Selzer Faith, Sowers MaryFran, R. (Mary Frances Roy), Neer Robert, Powell Lynda, Gold Ellen B., … McKinlay Sonja . Study of Women’s Health Across the Nation (SWAN): Visit 01 Dataset, [United States], 1997–1999. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2019-May-02. 10.3886/ICPSR29221.v3 [DOI] [Google Scholar]
- Sutton-Tyrrell Kim, Selzer Faith, Sowers MaryFran, R. (Mary Frances Roy), Neer Robert, Powell Lynda, Gold Ellen B., … McKinlay Sonja. Study of Women’s Health Across the Nation (SWAN): Visit 02 Dataset, [United States], 1998–2000. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2019-May-02. 10.3886/ICPSR29401.v4 [DOI] [Google Scholar]
- Sutton-Tyrrell Kim, Selzer Faith, Sowers MaryFran, R. (Mary Frances Roy), Neer Robert, Powell Lynda, Gold Ellen B., … McKinlay Sonja. Study of Women’s Health Across the Nation (SWAN): Visit 03 Dataset, [United States], 1999–2001. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2019-May-29. 10.3886/ICPSR29701.v [DOI] [Google Scholar]
- Sutton-Tyrrell Kim, Selzer Faith, Sowers MaryFran, R. (Mary Frances Roy), Neer Robert, Powell Lynda, Gold Ellen B., … McKinlay Sonja . Study of Women’s Health Across the Nation (SWAN): Visit 04 Dataset, [United States], 2000–2002. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2019-May-15. 10.3886/ICPSR30142.v4 [DOI] [Google Scholar]
- Sutton-Tyrell Kim, Selzer Faith, Sowers MaryFran R. (Mary Francis Roy), Finkelstein Joel, Powell Lynda, Gold Ellen B., … Matthews Karen . Study of Women’s Health Across the Nation (SWAN), 2002–2004: Visit 06 Dataset. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2018-November-13. 10.3886/ICPSR31181.v2 [DOI] [Google Scholar]
- Sutton-Tyrell Kim, Selzer Faith, Sowers MaryFran R. (Mary Francis Roy), Finkelstein Joel, Powell Lynda, Gold Ellen B., … Matthews Karen . Study of Women’s Health Across the Nation (SWAN), 2003–2005: Visit 07 Dataset. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2018-November-19. 10.3886/ICPSR31901.v2 [DOI] [Google Scholar]
- Sutton-Tyrell Kim, Selzer Faith, Sowers MaryFran R. (Mary Francis Roy), Finkelstein Joel, Powell Lynda, Gold Ellen, … Brooks Maria Mori. Study of Women’s Health Across the Nation (SWAN), 2004–2006: Visit 08 Dataset. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2018-November-20. 10.3886/ICPSR32122.v2 [DOI] [Google Scholar]
- Sutton-Tyrell Kim, Selzer Faith, Sowers MaryFran R. (Mary Francis Roy), Finkelstein Joel, Powell Lynda, Gold Ellen, … Brooks Maria Mori. Study of Women’s Health Across the Nation (SWAN), 2005–2007: Visit 09 Dataset. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2018-November-20. 10.3886/ICPSR32721.v2 [DOI] [Google Scholar]
- Sutton-Tyrrell Kim, Selzer Faith, Sowers MaryFran, Finkelstein Joel, Powell Lynda, Gold Ellen, … Brooks Maria Mori. Study of Women’s Health Across the Nation (SWAN), 2006–2008: Visit 10 Dataset. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2018-November-15. 10.3886/ICPSR32961.v2 [DOI] [Google Scholar]
- Tarraf W, Rodríguez CJ, Daviglus ML, Lamar M, Schneiderman N, Gallo L, … González HM (2017). Blood Pressure and Hispanic/Latino Cognitive Function: Hispanic Community Health Study/Study of Latinos Results. Journal of Alzheimer’s Disease, 59(1), 31–42. 10.3233/JAD-170017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoits PA (2010). Stress and health: Major findings and policy implications. Journal of Health and Social Behavior, 51(1_suppl), S41–S53. [DOI] [PubMed] [Google Scholar]
- Tulsky D, Zhu J, & Ledbetter MF (1997). WAIS-III/WMS-III Technical Manual. Psychological Corporation: San Antonio, TX. [Google Scholar]
- Turner RJ, & Avison WR (2003). Status variations in stress exposure: Implications for the interpretation of research on race, socioeconomic status, and gender. Journal of Health and Social Behavior, 488–505. [PubMed] [Google Scholar]
- Vásquez E, Botoseneanu A, Bennett JM, & Shaw BA (2016). Racial/Ethnic Differences in Trajectories of Cognitive Function in Older Adults. Journal of Aging and Health, 28(8), 1382–1402. 10.1177/0898264315620589 [DOI] [PubMed] [Google Scholar]
- Vespa J, Armstrong DM, & Medina L (2018). Demographic turning points for the United States: Population projections for 2020 to 2060. Washington, DC: US Department of Commerce, Economics and Statistics Administration, US Census Bureau. [Google Scholar]
- Wechsler D (1997). Wechsler Adult Intelligence Scale - Third Edition. New York: The Psychological Corporation. [Google Scholar]
- Wechsler D (2008). Wechsler adult intelligence scale–Fourth Edition (WAIS–IV). San Antonio, TX: NCS Pearson, 22(498), 1. [Google Scholar]
- Weintraub S, Wicklund AH, & Salmon DP (2012). The neuropsychological profile of Alzheimer disease. Cold Spring Harbor perspectives in medicine, 2(4), a006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennberg AMV, Hagen CE, Gottesman RF, Zipunnikov V, Kaufmann CN, Albert MS, … Spira AP (2017). Longitudinal association between diabetes and cognitive decline: The National Health and Aging Trends Study. Archives of Gerontology and Geriatrics, 72(May), 39–44. 10.1016/j.archger.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, Barnes LL, Mendes De Leon CF, Rajan KB, Beck T, Aggarwal NT, … Evans DA (2018). Cognitive Aging in Black and White Americans: Cognition, Cognitive Decline, and Incidence of Alzheimer Disease Dementia. Epidemiology, 29(1). 10.1097/EDE.0000000000000747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield KE, Allaire JC, Gamaldo A, Aiken-Morgan AT, Sims R, & Edwards C (2008). Blood pressure and memory in older African Americans. Ethnicity and Disease, 18(2), 181. [PubMed] [Google Scholar]
- Williams DR, Gonzalez HM, Neighbors H, Nesse R, Abelson JM, Sweetman J, & Jackson JS (2007). Prevalence and distribution of major depressive disorder in African Americans, Caribbean blacks, and non-Hispanic whites: results from the National Survey of American Life. Archives of General Psychiatry, 64(3), 305–315. [DOI] [PubMed] [Google Scholar]
- Williams DR, Priest N, & Anderson N (2016). Understanding Assocations between Race, Socioeconomic Status and Health: Patterns and Prospects. Health Psychology, 35(4), 407–411. 10.1037/hea0000242.Understanding [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Bentler SE, Hockenberry J, Jones MP, Weigel PA, Kaskie B, & Wallace RB (2011). A prospective cohort study of long-term cognitive changes in older Medicare beneficiaries. BMC Public Health, 11(1), 710. 10.1186/1471-2458-11-710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RS, Waldstein SR, Gerassimakis CS, Sprung MR, Moody DLB, Taylor AD, … Evans MK (2019). Multiple influences on cognitive function among urban-dwelling African Americans. Journal of Racial and Ethnic Health Disparities, 6(4), 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Manly JJ, Azar M, Brickman AM, & Glymour MM (2016). Racial Disparities in Cognitive Performance in Mid- and Late Adulthood : Analyses of Two Cohort Studies, Journal of the American Geriatrics Society, 64(5), 959–964 10.1111/jgs.14113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Manly JJ, Smith J, Seeman T, & Lachman ME (2017). Socioeconomic, health, and psychosocial mediators of racial disparities in cognition in early, middle, and late adulthood. Psychology and Aging, 32(2), 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Nowinski CJ, Gershon RC, & Manly JJ (2014). Depressive symptoms are more strongly related to executive functioning and episodic memory among African American compared with non-hispanic white older adults. Archives of Clinical Neuropsychology, 29(7), 663–669. 10.1093/arclin/acu045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Stern Y, & Manly JJ (2015). Differing effects of education on cognitive decline in diverse elders with low versus high educational attainment. Neuropsychology, 29(4), 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Hayward MD, & Yu Y-L (2016). Life course pathways to racial disparities in cognitive impairment among older Americans. Journal of Health and Social Behavior, 57(2), 184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.