Abstract
Background:
Patients with left bundle branch block (LBBB) often respond to cardiac resynchronization therapy (CRT) with left ventricular ejection fraction (LVEF) improvement. Guideline directed medical therapy (GDMT) not CRT is first line therapy for patients with reduced LVEF with LBBB. However, there is little data on how patients with reduced LVEF and LBBB respond to GDMT.
Methods:
Using data from the Duke Echocardiography Laboratory Database, we identified patients with a baseline ECG and LVEF≤35% who had a follow-up LVEF 3–6 months later. We excluded patients with severe valve disease, a cardiac device, LVAD, or heart transplant. QRS morphology was classified as: LBBB, QRS<120msec (NQRS), or a wide QRS≥120msec but not LBBB (WQRS). ANOVA testing compared mean change in LVEF between the three groups with adjustment for significant comorbidities and GDMT.
Results:
659 patients met the above criteria: 111 LBBB (17%), 59 WQRS (9%) and 489 NQRS (74%). Adjusted mean increase in LVEF over 3–6 months in the 3 groups was 2.03, 5.28, and 8.00 respectively (p<0.0001). Results were similar when adjusted for interim revascularization and myocardial infarction. Comparison of mean LVEF improvement between patients with LBBB on GDMT and those not on GDMT showed virtually no difference (3.50 vs. 3.44%). The combined end point of heart-failure hospitalization or mortality was highest for patients with LBBB.
Conclusions:
LBBB is associated with a smaller degree of LVEF improvement compared to other QRS morphologies, even with GDMT. Some patients with LBBB may benefit from CRT earlier than guidelines currently recommend.
Keywords: Left bundle branch block, Left ventricular functional recovery, guideline directed medical therapy, heart failure
Condensed Abstract
Patients with left bundle branch block (LBBB) often respond to cardiac resynchronization therapy (CRT) with left ventricular ejection fraction (LVEF) improvement. Guideline directed medical therapy (GDMT) not CRT is first line therapy for patients with reduced LVEF with LBBB. However, there is little data on how patients with reduced LVEF and LBBB respond to GDMT. The present study identified patients with baseline LVEF≤35% and stratified rates of LV functional recovery by QRS morphology over a period of 3–6 months. Patients with LBBB had significantly less rates of LV functional recovery compared to patients with a narrow QRS duration (NQRS).
Introduction:
In patients with cardiomyopathy, traditional interventions to improve left ventricular ejection fraction (LVEF) include medical modulation of the renin-angiotensin-aldosterone axis or direct intervention on a reversible cardiac pathology, such as coronary artery disease, valvular heart disease, or arrhythmia-induced tachycardia (among others).(1–3) More recently, cardiac resynchronization therapy (CRT) has introduced correction of electromechanical dyssynchrony as a powerful new mechanism to induce LV functional recovery (4–8).
Most clinical trials that have studied CRT have found that it is only efficacious in patients with left bundle branch block (LBBB).(5,9–12) This implicitly suggests that LBBB may represent a previously unrecognized cause of LV dysfunction. While LBBB has long been identified as a comorbid factor carrying an adverse prognosis, there is now evidence that LBBB not only leads to adverse patient outcomes in otherwise healthy patients, but as seen in dog models, and small retrospective series, may also be a potential cause of non-ischemic cardiomyopathy itself.(13–17)
While CRT is considered the definitive treatment for patients with LBBB and symptomatic cardiomyopathy, it remains unclear how LBBB affects rates of LV functional recovery in patients without a cardiac device. For example, current guidelines recommend at least 3 months of guideline directed medical therapy (GDMT) prior to implantation of CRT, in the hopes that medical therapy alone will lead to improvement in LVEF.(8) However, it is worth emphasizing that none of the major trials supporting medical therapy stratified outcome analyses by the presence or absence of LBBB or reported QRS morphology as a baseline clinical characteristic.(18–25)
This study sought to examine how LBBB affects rates of LV functional recovery in patients with cardiomyopathy. We made use of the Duke Echocardiography Laboratory Database and Duke ECG database to identify patients with a diagnosis of cardiomyopathy, an ECG, and a follow-up echocardiogram (Echo) in 3 to 6 months. We hypothesized that in the “real world,” LBBB would be a significant predictor for decreased rates of LV functional recovery, and that many patients with LBBB would not improve their LVEF>35%. This would suggest that some patients might benefit from receiving CRT earlier than current guidelines recommend.
Methods:
Data Sources:
The study cohort was selected from the Duke Echocardiography Laboratory Database. It includes all clinical Echos performed at Duke University Health System since 1995, and its set-up has been described previously.(26) Basic demographic information is available, and patient clinical data is imported from the Duke Decision Support Repository and Duke Databank for Cardiovascular Disease. For the purposes of this study, the Duke Echocardiography Laboratory Database was linked to the ECG reporting database to identify patients with both a baseline Echo and ECG.
Direct chart review was conducted to determine medication use. The occurrences of the following non-baseline events were identified from the Duke Databank: (1) intercurrent percutaneous coronary intervention; (2) intercurrent bypass surgery; and (3) intercurrent myocardial infarction. The time period for intercurrent revascularization procedures was pre-specified to include dates from 2 weeks before baseline Echo to the time of follow-up Echo. Intercurrent myocardial infarction was pre-specified to be any myocardial infarction that occurred between baseline and follow-up Echo. Heart failure hospitalizations after follow-up Echo could be obtained for any admission at a Duke-affiliated hospital through the electronic medical record. All-cause mortality was obtained through the medical record and National Death Index.
Definitions
The diagnosis of cardiomyopathy reflected an LVEF≤35%, assessed visually by an attending cardiologist with level 3 training in echocardiography. The designation of LBBB in the Duke ECG database matches the clinical diagnosis of attending cardiologists at Duke University Hospital responsible for reading patient ECGs. Patients without a clinical read of LBBB were stratified by QRS duration. Patients without LBBB who had a wide QRS duration≥120msec were placed into one group (WQRS), while patients with a narrow QRS duration<120 msec were placed into a second comparator group (NQRS). For the purpose of a sensitivity analysis, a physician trained in the Strauss criteria provided an additional over-read of all LBBB ECGs and placed these patients either into a strict LBBB group or back into the WQRS group.(27) Use of GDMT was defined as use of a beta-blocker (BB) plus an angiotensin converting enzyme inhibitor (ACE-I) or angiotensin receptor blocker (ARB). Patients were categorized as being on medication if they had documented drug use between Echos for at least 30 days, and were not stopped more than 2 weeks prior to follow-up Echo. To show changes in medications, the doses prior to baseline Echo and at time of follow-up Echo were also obtained.
Study Population
The study cohort was comprised of patients with an LVEF≤35% on baseline Echo, who had an ECG within 30 days after or 6 months beforehand. Only patients with baseline Echos occurring prior to January 1, 2014 were eligible for inclusion. Patients were excluded if they had severe aortic or mitral valve disease, a history of prior heart transplantation, evidence of any prior cardiac device including pacemaker or defibrillator, a prior left ventricular assist device, or no follow-up Echo. An eligible follow-up Echo was defined as the first Echo occurring between 3 to 6 months after baseline. Patients with a heart transplant, placement of a cardiac device (pacemaker/defibrillator), or left ventricular assist device prior to follow-up Echo were excluded.
Study Outcomes:
The primary outcome of this study was change in LVEF over a period of 3–6 months. This time frame was selected as current guidelines, supported by clinical trials, recommend at least 3 months of GDMT for LV functional recovery.(8,28) This time frame also allowed for an adequate study population. LVEF was selected as it was available in almost all patients in the Echo database and is the primary Echo criterion in current device implantation guidelines. A secondary outcome assessed rates of LV functional recovery to an LVEF>35%. Clinical outcomes included time to heart failure hospitalization and time to mortality, both measured after follow-up Echo.
Statistical Analysis
Normality of continuous change in LVEF between baseline and follow-up Echo was assessed graphically and found to be valid. ANOVA (analysis of variance) modeling was used to test for unadjusted mean differences in LVEF change across study groups. Multiple comparison procedures using Tukey’s adjustment were conducted to determine significant differences in mean LVEF change across LBBB vs. WQRS vs. NQRS patient populations.(29)
Regression analyses were used to examine adjusted differences in continuous changes in LVEF over time between the 3 groups. The following clinical characteristics were pre-specified to be included: age, gender, use of GDMT, history of atrial arrhythmias, and LVEF. In addition, backward selection of baseline characteristics (Table 1) found significant (p<0.05) between groups created a parsimonious model of factors associated with LVEF change, using p<0.10 for inclusion in the final model. These included: baseline left atrial size, prior myocardial infarction, heart rate, cerebrovascular disease, and history of congestive heart failure stratified by time of diagnosis (no congestive heart failure, diagnosis within 1 month of baseline Echo, ≥1month, and ≥1year). Though significantly different, LV end systolic diameter was not included in the model as the median difference between the LBBB and NQRS groups was only 2 mm and LVEF was already a covariate. Linearity of all continuous confounders was assessed and linear spline transformations were applied to age and heart rate to approximate nonlinearity. Least Squares methods were used to provide the adjusted mean change in LVEF overtime in the 3 groups and slope estimates were reported with 95% CI and p-values for a significant change in LVEF overtime. Multiple comparison procedures using Tukey’s adjustment were conducted to determine specific group differences in adjusted mean LVEF change.(29)
Table 1:
Baseline Clinical Characteristics
| Overall n=659 | LBBB n=111 | WQRS n=59 | NQRS n=489 | P-Value | |
|---|---|---|---|---|---|
|
| |||||
| Demographics | |||||
| Age, median (IQR) | 59 (48–69) | 65 (56–75) | 62 (50–72) | 57 (46–67) | <.001 |
| Female gender | 270 (41.0%) | 61 (55.0%) | 14 (23.7%) | 195 (39.9%) | <.001 |
| Congestive Heart Failure | 145 (22.0%) | 32 (28.8%) | 16 (27.1%) | 97 (19.8%) | 0.072 |
| Duration in Months (IQR) | 1 (0–13) | 7 (0–46) | 2 (0–11) | 1 (0–7) | 0.012 |
| Hypertension | 448 (68.0%) | 84 (75.7%) | 40 (67.8%) | 324 (66.3%) | 0.158 |
| Diabetes | 225 (34.1%) | 42 (37.8%) | 20 (33.9%) | 163 (33.3%) | 0.664 |
| Peripheral Artery Disease | 56 (8.5%) | 13 (11.7%) | 4 (6.8%) | 39 (8.0%) | 0.393 |
| Cerebrovascular Disease | 78 (11.8%) | 22 (19.8%) | 6 (10.2%) | 50 (10.2%) | 0.017 |
| Chronic Kidney Disease | 88 (13.4%) | 16 (14.4%) | 5 (8.5%) | 67 (13.7%) | 0.503 |
| History of smoking | 259 (39.3%) | 44 (39.6%) | 30 (50.8%) | 185 (37.8%) | 0.154 |
| Hyperlipidemia | 297 (45.1%) | 56 (50.5%) | 29 (49.2%) | 212 (43.4%) | 0.320 |
| Coronary Artery Disease | 387 (58.7%) | 67 (60.4%) | 41 (69.5%) | 279 (57.1%) | 0.173 |
| Prior MI | 215 (32.6%) | 28 (25.2%) | 27 (45.8%) | 160 (32.7%) | 0.025 |
| Atrial Fibrillation/Flutter | 144 (21.9%) | 21 (18.9%) | 11 (18.6%) | 112 (22.9%) | 0.540 |
| Medications | |||||
| BB | 533 (80.9%) | 90 (81.1%) | 45 (76.3%) | 398 (81.4%) | 0.639 |
| ACE-I or ARB | 529 (80.3%) | 89 (80.2%) | 51 (86.4%) | 389 (79.6%) | 0.454 |
| Statin | 289 (43.9%) | 51 (45.9%) | 35 (59.3%) | 203 (41.5%) | 0.030 |
| Aldosterone Antagonist | 153 (23.2%) | 26 (23.4%) | 13 (22.0%) | 114 (23.3%) | 0.975 |
| Loop Diuretic | 359 (54.5%) | 58 (52.3%) | 35 (59.3%) | 266 (54.4%) | 0.677 |
| ECG Characteristics | |||||
| QRSd, median (IQR) | 96 (85–121) | 147 (135–157) | 136 (127–148) | 90 (82–100) | n/a |
| HR, median (IQR) | 90 (76–106) | 86 (72–102) | 88 (77–103) | 91 (76–106) | 0.068 |
| HR > 120 | 61 (9.6%) | 5 (4.5%) | 6 (11.3%) | 50 (11.4%) | 0.166 |
| Baseline ECHO | |||||
| LVEF, median (IQR) | 25 (20–30) | 25 (20–30) | 25 (20–35) | 25 (20–30) | 0.162 |
| LVEDD, median (IQR) | 5.5 (4.9–6.2) | 5.6 (4.9–6.2) | 5.7 (5.2–6.3) | 5.5 (4.9–6.1) | 0.144 |
| LVESD, median (IQR) | 4.7 (4.0–5.4) | 4.8 (4.3–5.6) | 4.9 (4.2–5.5) | 4.6 (3.9–5.4) | 0.047 |
| Left Atrial Size | 0.060 | ||||
| Normal | 237 (36.0%) | 44 (39.6%) | 12 (20.3%) | 181 (37.0%) | |
| Mildly Enlarged | 271 (41.1%) | 41 (36.9%) | 35 (59.3%) | 195 (39.9%) | |
| Moderately Enlarged | 133 (20.2%) | 23 (20.7%) | 9 (15.3%) | 101 (20.7%) | |
| Severely Enlarged | 18 (2.7%) | 3 (2.7%) | 3 (5.1%) | 12 (2.5%) | |
| LVH (Echo read) | 0.527 | ||||
| None | 328 (50.6%) | 54 (49.1%) | 25 (43.9%) | 249 (51.8%) | |
| Mild | 234 (36.1%) | 46 (41.8%) | 24 (42.1%) | 164 (34.1%) | |
| Moderate | 76 (11.7%) | 8 (7.3%) | 7 (12.3%) | 61 (12.7%) | |
| Severe | 10 (1.5%) | 2 (1.8%) | 1 (1.8%) | 7 (1.5%) | |
| Inter-current Procedures/Events | |||||
| Revascularization | |||||
| PCI | 129 (19.6%) | 22 (19.8%) | 14 (23.7%) | 93 (19.0%) | NA |
| CABG | 93 (14.1%) | 12 (10.8%) | 8 (13.6%) | 73 (14.9%) | NA |
| Intercurrent-MI | 186 (28.2%) | 17 (15.3%) | 18 (30.5%) | 151 (30.9%) | NA |
MI – Myocardial Infarction, HR – Heart Rate, LVEDD – LV End Diastolic Diameter, LVESD – LV End Systolic Diameter, LVH – LV Hypertrophy, PCI – Percutaneous Coronary Intervention, CABG – Coronary Artery Bypass Graft
A second analysis conducted unadjusted and adjusted binary logistic regression analyses to determine the incidence of LVEF increase to >35% at follow-up for NQRS and WQRS patient populations in reference to LBBB patient population. Adjusted analyses contained the same list of covariates included in the final multivariate model used for continuous change in LVEF. Last, time to heart failure hospitalization, time to mortality, and time to the combined end point of heart failure hospitalization or mortality was estimated and displayed graphically for the three QRS groups using the Kaplan-Meier technique.
Sensitivity analyses:
Three sensitivity analyses repeated the main analysis for continuous change in LVEF over time. The first focused only on the subset of patients who were on GDMT. The second excluded patients with any inter-current revascularization or myocardial infarction. The third reclassified patients in the LBBB group to either a Strict LBBB group or back into the WQRS group. A final analysis compared patients on GDMT with those not on GDMT by testing for an interaction between GDMT and the 3 QRS groups.
Results:
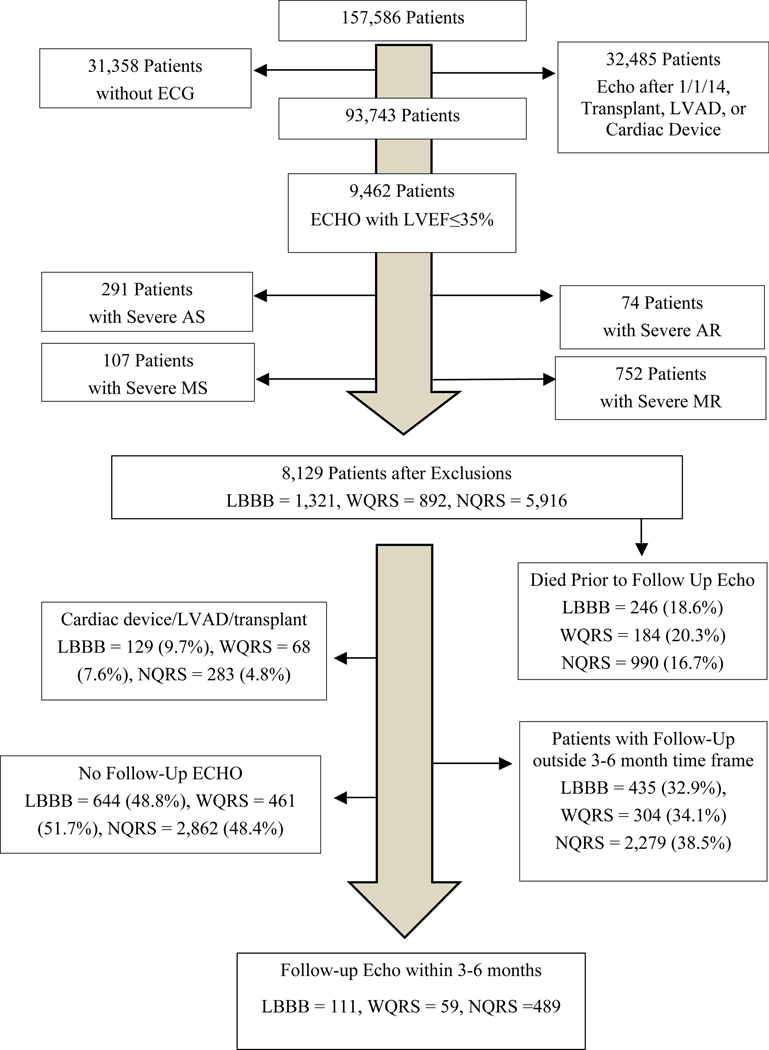
The study population consort diagram is presented in Figure 1. Less NQRS patients died before potential follow-up Echo. More LBBB patients were excluded for receiving a cardiac device, left ventricular assist device, or heart transplant. Baseline clinical characteristics are presented in Table 1. Patients with LBBB were more likely to be older, female, and have cerebrovascular disease. Patients with WQRS were more likely to have had a prior myocardial infarction, be on statin medications, and have an enlarged left atrium. Patients with NQRS tended to be younger, have faster heart rates, and have less baseline heart failure. Patients with NQRS also had a higher percentage of patients with baseline heart rates >120bpm, though this difference was not statistically significant. All 3 groups were prescribed similar rates of BBs, ACE-Is, ARBs, and aldosterone antagonists. Medications at baseline and follow-up are presented in Appendix 1 and show remarkable similarity in mean dose and use rates. More LBBB and WQRS patients used ARBs at baseline, though use rates were low (between 7–14%). Roughly two thirds of patients in each group were on GDMT (76/111 patients [69%] for LBBB; 39/59 patients [66%] for WQRS; 330/489 patients [67%] for NQRS). There were no significant differences in valvular heart disease between the three groups.
Figure 1 –
Study population consort diagram. The Echo database contains 157,586 unique patients. Black arrows show the number of patients removed for exclusionary criteria. Only 8,129 qualified for a baseline Echo. More patients with LBBB were subsequently excluded for receiving a cardiac device, LVAD, or transplant. Less NQRS patients died prior to a potential follow-up Echo. Only 659 patients were ultimately included in the study.
Table 2 displays descriptive data for change in LVEF from baseline to follow-up Echo between the 3 groups. Follow-up between the 3 groups was very similar, with a mean (SD) of 133 (26) days after baseline echo. Table 3 shows ANOVA results from changes in LVEF over time. When adjusted for pre-specified comorbidities and significant differences in baseline characteristics, the mean changes in LVEF were 2.03, 5.28, and 8.00% for LBBB, WQRS, and NQRS respectively. Differences between LBBB and NQRS were highly significant (p<0.0001), though not significant between LBBB and WQRS.
Table 2:
Descriptive Changes in LVEF From Baseline to Follow-up
| Variable of Interest | Overall (N=659) | LBBB (N=111) | WQRS (N=59) | NQRS (N=489) |
|---|---|---|---|---|
| Baseline LVEF, mean ± SD | 25.6 ± 7.02 | 24.7 ± 6.34 | 26.4 ± 6.90 | 25.8 ± 7.17 |
|
| ||||
| Follow-up LVEF, mean ± SD | 33.7 ± 12.42 | 28.1 ± 11.46 | 31.3 ± 11.26 | 35.3 ± 12.36 |
|
| ||||
| Change in LVEF, mean ± SD | 8.06 ± 11.76 | 3.41 ± 9.43 | 4.86 ± 9.22 | 9.50 ± 12.17 |
|
| ||||
| Follow-up LVEF>35% | 250 (38%) | 25 (23%) | 16 (27%) | 209 (43%) |
|
| ||||
| LVEF Change Categories, % | ||||
| Any LVEF worsening | 111 (17%) | 27 (24%) | 13 (22%) | 71 (15%) |
| Unchanged | 154 (23%) | 39 (35%) | 14 (24%) | 101 (21%) |
| + 1–14% | 193 (29%) | 29 (26%) | 22 (37%) | 142 (29%) |
| + > 15% | 201 (31%) | 16 (14%) | 10 (17%) | 175 (36%) |
|
| ||||
| Interval (in days) to Follow-up ECHO, median (IQR) | 131 (112–154) | 134 (116–156) | 127 (106–159) | 131 (111–153) |
|
| ||||
| Changes in LVEF from Baseline to Follow-up for Patients on GDMT. | ||||
|
| ||||
| N=445 | N=76 | N=39 | N=330 | |
|
| ||||
| Baseline LVEF, mean ± SD | 25.2 ± 7.10 | 24.2 ± 6.50 | 25.8 ± 7.14 | 25.4 ± 7.22 |
|
| ||||
| Follow-up LVEF, mean ± SD | 33.9 ± 12.54 | 27.8 ± 11.38 | 30.9 ± 11.02 | 35.6 ± 12.47 |
|
| ||||
| Change in LVEF, mean ± SD | 8.61 ± 11.74 | 3.59 ± 9.32 | 5.05 ± 9.24 | 10.19 ± 12.11 |
|
| ||||
| Interval (in days) to Follow-up ECHO, median (IQR) | 132 (111–155) | 133 (116–152) | 132 (111–155) | 127 (106–155) |
Table 3:
ANOVA results from Changes in LVEF overtime by QRS group
| Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|
| Mean Change | Effect Estimate (95% CI) | ANOVA P-Value | LS Mean Change | Effect Estimate (95% CI) | ANOVA P-Value | |
| LBBB | 3.41 (±9.43) | - | <0.0001 | 2.03 (±13.59) | - | <0.0001 |
| WQRS | 4.86 (±9.22) | 1.45 (2.19 to 5.09) | 5.28 (±12.62) | 3.25 (−0.26 to 6.75) | ||
| NQRS | 9.50 (±12.17) | 6.08 (3.71 to 8.46) | 8.00 (±21.55) | 5.97 (3.62 to 8.31) | ||
| Tukey Multiple Comparison Adjusted Analyses | ||
|---|---|---|
| Comparison | Unadjusted Tukey P-Value | Adjusted Tukey P-Value |
| LBBB vs. NQRS | <0.0001 | <0.0001 |
| LBBB vs. WQRS | 0.7145 | 0.1636 |
| NQRS vs. WQRS | 0.0101 | 0.1672 |
Adjusted for: Baseline LVEF, GDMT, History of Atrial Fibrillation/Flutter, Age, Gender, History of Cerebrovascular Disease, Prior Myocardial Infarction, Baseline Heart Rate on ECG, History and Duration of Heart Failure, and Left Atrium Size; One patient excluded due to missing Heart Rate.
At follow-up, 23% of patients with LBBB improved their LVEF to >35%, compared to 27% with WQRS, and 43% with NQRS. On the whole, patients with LBBB were more likely to stay the same or worsen their LVEF on follow-up and the least likely to improve their LVEF >35% (Table 2).
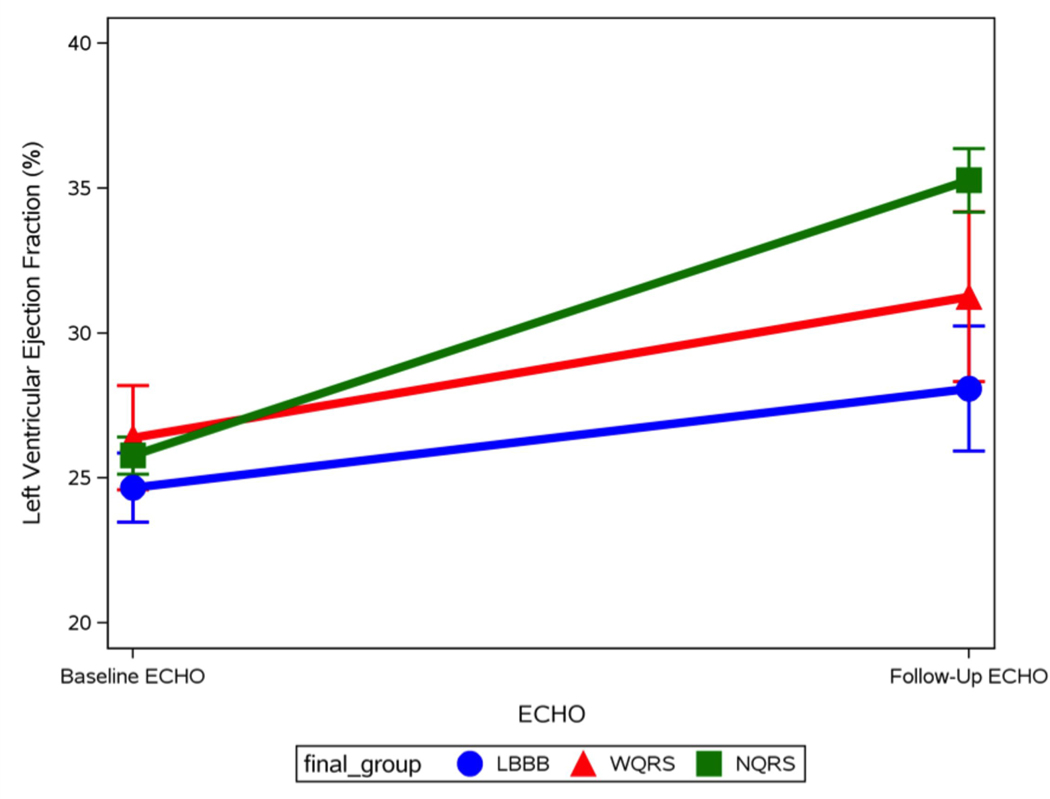
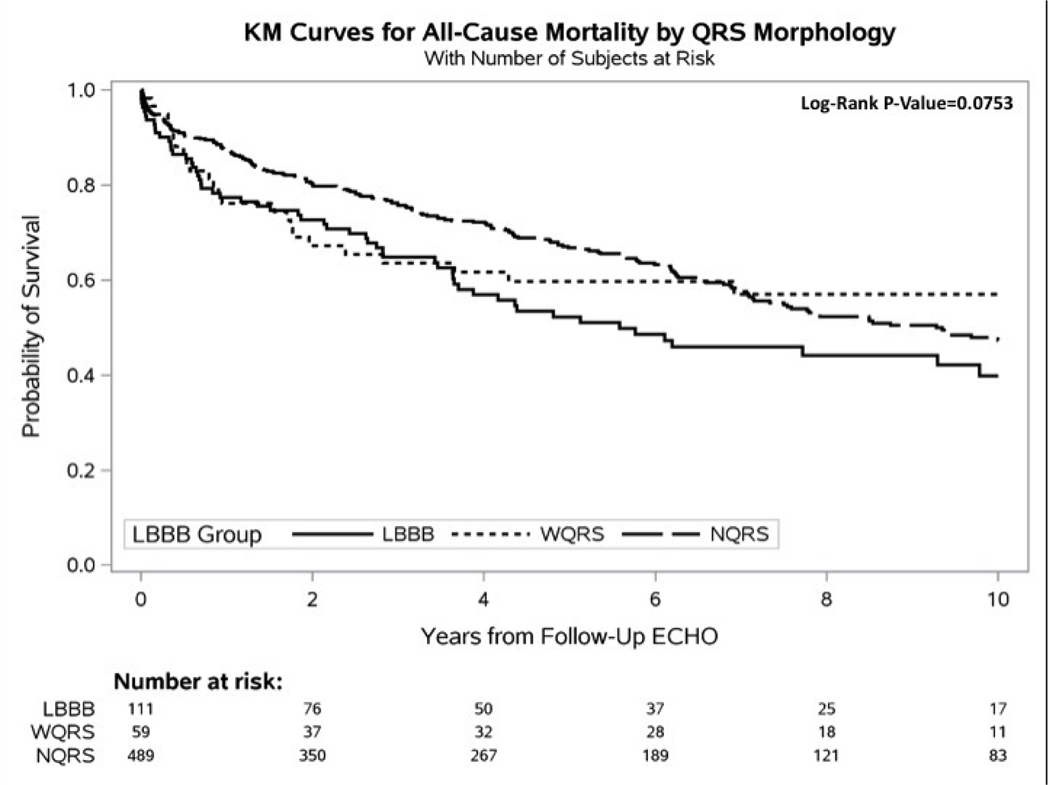
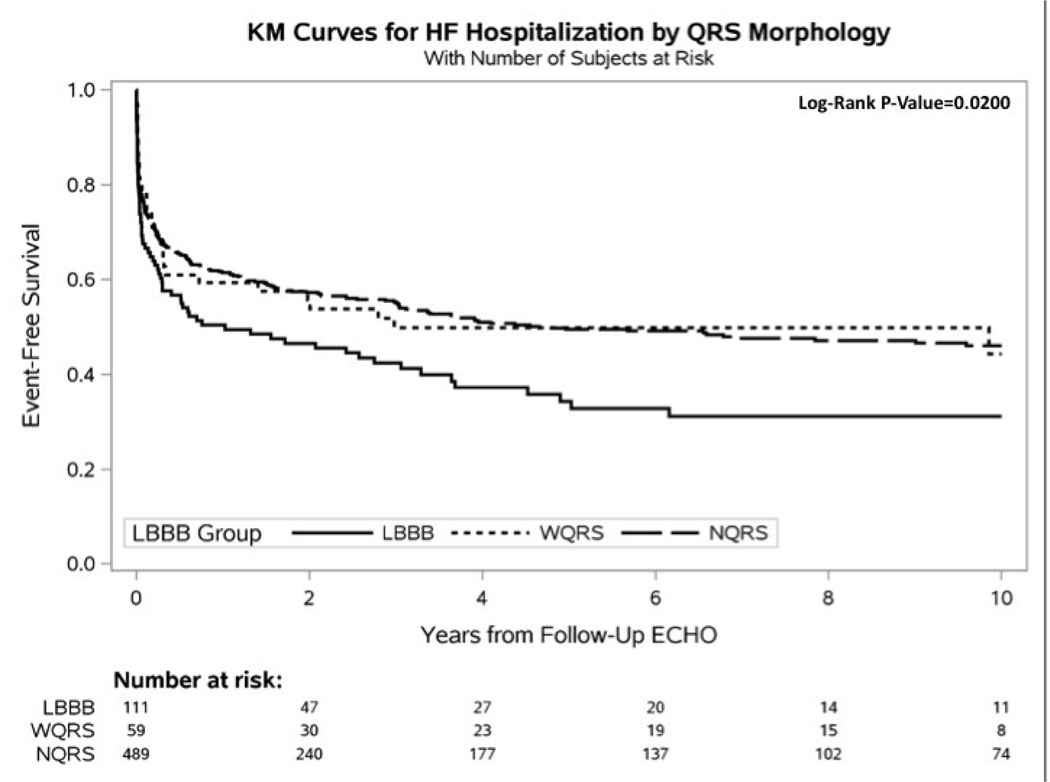
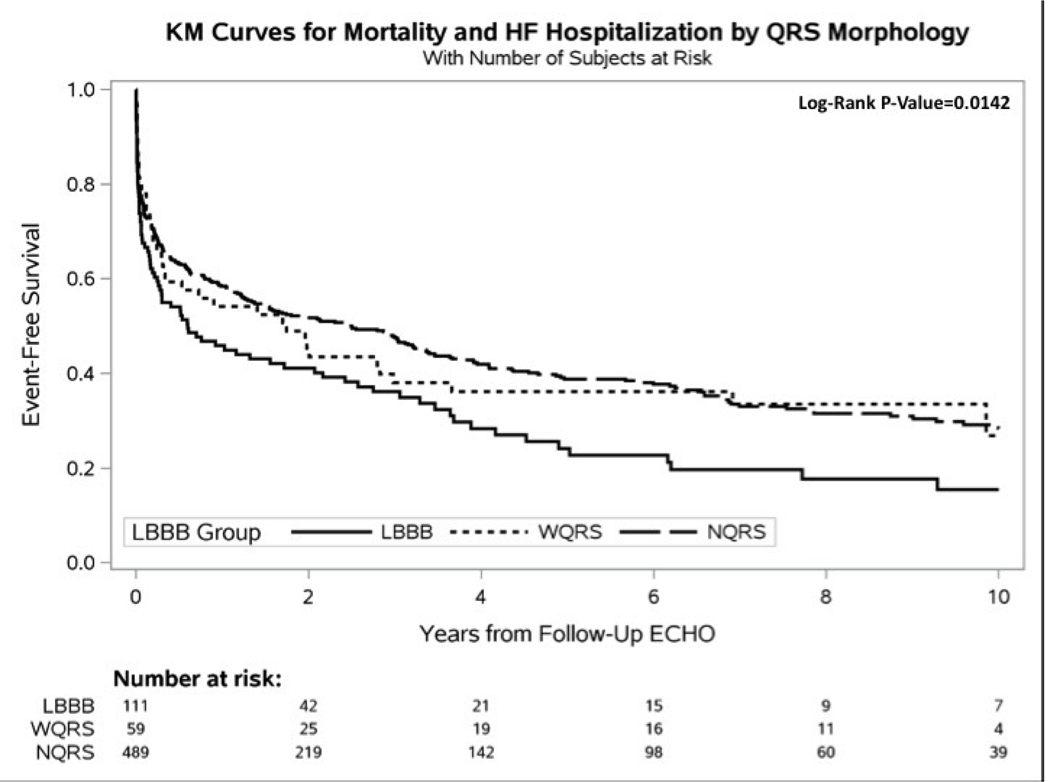
Figure 2 shows mean plots for LVEF change between the 3 groups for baseline and follow-up echo. Figure 3 shows Kaplan-Meier curves for time to heart failure hospitalization, all-cause mortality, and heart failure hospitalization or all-cause mortality. Patients with LBBB were significantly more likely to experience a heart failure event and a combined end point of heart failure hospitalization or mortality. Median follow-up was 4.3 years.
Figure 2/.
Central Illustration –Mean LVEF with standard deviation shown for baseline and follow-up Echo, graphed for the 3 QRS groups. Patients from the 3 QRS groups have similar mean LVEF at baseline. NQRS patients have the highest rates of LV functional recovery, while LBBB patients have the lowest.
Figure 3 –
Kaplan-Meier Curves. A. Time-to-Mortality, B. Time-to-Heart Failure Hospitalization, and C. Time-to-Heart Failure Hospitalization or Mortality. Mortality data was acquired through the National Death Index (NDI). Heart failure hospitalization data was acquired through the medical record at Duke-affiliated hospitals. Because all Echos were performed prior to 2015, events were censored at December 31, 2014, matching the end date of the NDI query. All curves were censored at 10 years post follow-up Echo.
Table 4 compares the odds ratio of NQRS and WQRS vs. LBBB for improving LVEF>35%. In patients with NQRS, the odds of LV functional recovery past an LVEF>35% were 2.57 (95% CI 1.59–4.15) times higher than in patients with LBBB (p=0.001). Patients with WQRS had a non-significant 1.28 times greater odds than LBBB patients (p=0.51). This relationship was essentially unchanged when adjusted for comorbidities.
Table 4:
Logistic Regression Results for LVEF @ follow-up > 35% by QRS group
| Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-Value | Overall 3 Group P-Value | OR (95% CI) | P-Value | Overall 3 Group P-Value | |
| NQRS vs. LBBB | 2.57 (1.59–4.15) | 0.0001 | 0.0001 | 2.59 (1.51–4.47) | 0.0006 | 0.0011 |
| WQRS vs. LBBB | 1.28 (0.62–2.65) | 0.5054 | 1.47 (0.66–3.28) | 0.3476 | ||
Adjusted for: Baseline LVEF, GDMT, History of Atrial Fibrillation/Flutter, Age, Gender, History of Cerebrovascular Disease, Prior Myocardial Infarction, Baseline Heart Rate on ECG, History and Duration of Heart Failure, and Left Atrium Size; One patient excluded due to missing Heart Rate.
Sensitivity analyses:
Table 5 displays outcomes of patients on GDMT. Adjusted mean change in LVEF for patients in the LBBB, WQRS, and NQRS groups were 2.02, 4.50, and 8.18%, respectively. Table 6 shows a means table for the interaction analysis between QRS group and GDMT. The interaction was not significant (p=0.53), however there was essentially no difference between patients on GDMT or not on GDMT in the LBBB and WQRS groups (3.50 vs. 3.44 for LBBB; 6.79 vs. 6.44 for WQRS), but there was greater LVEF recovery among patients with NQRS on GDMT (10.50 vs. 8.02).
Table 5:
ANOVA results from Changes in LVEF overtime by QRS group in Patients on GDMT
| Unadjusted (N=445) | Adjusted (N=444) | |||||
|---|---|---|---|---|---|---|
| Mean Change | Effect Estimate (95% CI) | ANOVA P-Value | LS Mean Change | Effect Estimate (95% CI) | ANOVA P-Value | |
| LBBB | 3.59 (±9.32) | - | <0.0001 | 2.02 (±14.12) | - | <0.0001 |
| WQRS | 5.05 (±9.24) | 1.46 (2.98 to 5.89) | 4.50 (±13.13) | 2.48 (−1.90 to 6.86) | ||
| NQRS | 10.19 (±12.11) | 6.60 (3.73 to 9.46) | 8.18 (±21.93) | 6.15 (3.29 to 9.02) | ||
| Tukey Multiple Comparison Adjusted Analyses | ||
|---|---|---|
| Comparison | Unadjusted Tukey P-Value | Adjusted Tukey P-Value |
| LBBB vs. NQRS | <0.0001 | <0.0001 |
| LBBB vs. WQRS | 0.7944 | 0.5068 |
| NQRS vs. WQRS | 0.0228 | 0.1242 |
Adjusted for: Baseline LVEF, Medical Therapy, History of Atrial Fibrillation/Flutter, Age, Gender, History of Cerebrovascular Disease (CVD), Prior Myocardial Infarction (MI), Baseline Heart Rate on ECG, History and Duration of Heart Failure, and Left Atrium Size; One patient excluded due to missing Heart Rate.
Table 6:
Means table for interaction analysis between QRS group and GDMT
| QRS Group | GDMT | Mean EF Change |
|---|---|---|
| NQRS | Yes | 10.50 |
| No | 8.02 | |
| WQRS | Yes | 6.79 |
| No | 6.44 | |
| LBBB | Yes | 3.50 |
| No | 3.44 |
P=0.53
Appendix 2 and 3 display the results of the remaining sensitivity analyses. Outcomes between the 3 groups were very similar when patients with inter-current revascularization or inter-current MI were removed (Appendix 2). The results were also very similar when analyses were rerun with a Strict LBBB group (Appendix 3).
Discussion:
This study shows that patients with a baseline LVEF≤35% and LBBB demonstrate significantly less LV functional recovery than those with NQRS, even after 3–6 months of medical therapy. When adjusted for age, gender, atrial arrhythmias, GDMT, LVEF, and significant co-morbidities, mean LVEF improvement was only 2.03% among patients with LBBB compared to 8.00% for NQRS (effect estimate 5.97%, 95% CI 3.62 to 8.31). These results were very similar when including only patients on GDMT, excluding those with inter-current revascularization or myocardial infarction, or when including only those with strict LBBB.
Patients with WQRS also had significantly less LV Functional recovery than patients with NQRS in unadjusted analyses (4.86% vs. 9.50%; p=0.01). While patients with LBBB trended toward having less LV functional recovery than those with WQRS, the two populations were not significantly different. Reclassifying patients with LBBB to include only those with strict LBBB did not change this relationship, though notably the WQRS group became significantly different than the NQRS group in both unadjusted and adjusted analyses. Taken together, this data suggest that a wide QRS duration, particularly LBBB, is associated with less LV functional recovery than NQRS in patients with a baseline LVEF≤35%.
Evidence for GDMT
In the context of device implantation guidelines, the evidence for GDMT draws from multiple randomized controlled trials that have demonstrated LV functional recovery with the use of BBs, ACE-Is, and ARBs. In 1993, the MDC trial (Metoprolol in Dilated Cardiomyopathy) showed that among patients with LV dysfunction, metoprolol could improve LVEF by 12% over the course of 12 months.(22) Shortly after, MOCHA (Multicenter Oral Carvedilol Heart Failure Assessment) showed that carvedilol could improve EF by 5–8% over a 6-month period.(30) CIBIS-II (Cardiac Insufficiency Bisoprolol Study-II) showed an average of 4% improvement (in fractional shortening) over 6 months with bisoprolol, while REVERT (Reversal of Ventricular Remodeling with Toprol-XL) showed a 6% average improvement over 12 months with metoprolol succinate.(31–33) Additional trials such as CAPRICORN (Carvedilol Post Infarct Survival Control in Left Ventricular Dysfunction), SOLVD (Studies of Left Ventricular Dysfunction), VALHEFT (Valsartan Heart Failure Trial), and VALIANT (Valsartan in Acute Myocardial Infarction Trial) found benefits with ACE-Is or ARBs in conjunction with BB use, further improving average LVEF from 2 to 6 % over periods of 6–20 months.(23–25,34–37) Thus, there is compelling evidence that medications can induce LV functional recovery and should be the foundation of treatment for patients with cardiomyopathy. However, as stated previously, none of these trials included QRS duration in baseline clinical characteristics or reported outcomes in cohorts with conduction abnormalities.
Our “real world” data suggests that both QRS duration and morphology are relevant clinical characteristics. We have not only demonstrated less LV functional recovery with LBBB, but also shown QRS morphology to be significantly associated with increased rates of heart failure hospitalization and the combined end point of heart failure hospitalization or mortality.
Reflecting on what a prolonged QRS duration represents pathophysiologically may help to explain our findings. For patients with right bundle branch block and LBBB, a prolonged QRS primarily reflects delayed activation of the right or left ventricle, respectively. LBBB also implies delayed activation of the LV lateral free wall relative to the LV septal wall, which is the basis for intraventricular dyssynchrony, a cause of worsened LV function.(38) A prolonged QRS duration without bundle branch block represents delayed activation of ventricles due to hemiblock, increased ventricular mass, diseased electrical communication between cardiac myocytes, scar, or a combination of these factors.(39) Notably, this study’s WQRS population had the highest rate of prior myocardial infarction (45.8%), which may have reflected a more significant scar burden and limited potential improvement in LV function. This has been reported in the CRT literature. In a sub-study of MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial-CRT), history of myocardial infarction was associated with a diminished CRT effect on LVEF improvement.(40) Of note, our LBBB population was significantly different than the other QRS groups in that it had the lowest rate of myocardial infarction (25.2%). Thus, ischemic scar in the LBBB population was less likely to be the cause of poor rates of LV functional recovery.
Clinical Implications
A crucial difference between the WQRS and LBBB groups is that a body of evidence suggests only the latter responds to CRT. In MADIT-CRT, those without LBBB received no benefit with CRT, while those with LBBB had a dramatic reduction in the combined end point of heart failure hospitalization or mortality.(4,9) Similar results were found in RAFT (Resynchronization-Defibrillation for Ambulatory Heart Failure Trial).(5) Nevertheless, some controversy exists. A recent meta-analysis of 3 randomized trials argues that a wide QRS duration>150 msec, regardless of QRS morphology, is most important for CRT response.(41) An earlier meta-analysis of 5 randomized trials also showed QRS duration of 160 msec to be most predictive of CRT response.(42) However, both conceded that patients with conduction disease, particularly LBBB, were more likely to have a QRS duration>150msec. In our study, median QRS duration in the WQRS population was 135 msec (IQR 127–148). Patients with LBBB had a median QRS duration of 147 msec (IQR 135–147). Thus, as evidenced by our own data, it is rare for patients to have a QRS duration>150 and not have LBBB.
Overall, our data calls into question current guidelines that mandate at least 3 months of GDMT prior to CRT implantation. This timeline may be reasonable in minimally symptomatic NYHA II patients with LVEF of 35%, however for patients with LBBB, NYHA III or ambulatory NYHA IV symptoms and more severely depressed LVEF, earlier intervention with CRT should be studied. In our data, only 23% of patients with LBBB improved their LVEF to >35%, and 59% experienced no change or even worsened their LVEF. Mean rates of LVEF improvement were modest, and results were essentially unchanged when including only patients on GDMT. Thus, for patients with LBBB, delaying CRT in a heavily symptomatic patient with severely reduced LVEF may be delaying access to a highly effective therapy for little gain. Similarly, given the lesser amount of LV functional recovery seen in patients with WQRS, consideration for a wearable cardioverter defibrillator during the waiting period of 3 months may make sense.
Study Limitations:
This is a retrospective single center study. There was no Echo core lab to review all 1318 LVEFs, and inter-reader variability of LVEF assessment is not insignificant.(43) However, the Duke Echo lab has a history of performing quality improvement, which includes a committee that meets quarterly and whose primary purpose is to reduce variability and promote accuracy. Internal assessment of interclass correlation for LVEF assessment is 0.8872, which is above the threshold recommended for clinical research.(43) Furthermore, all follow-up Echos in this study were performed with baseline Echo images and reports available for review. This may have provided context and limited variability. There was also no reason for bias toward any particular QRS morphology (all were candidates for device implantation). Clinical outcomes also complemented the Echo findings.
Due to selection of patients with low LVEF at baseline, regression to the mean may have accounted for some improvement in LVEF. However, baseline LVEF was controlled for in our statistical models, and there was no reason for this to favor any QRS morphology. Considering the possibility of regression to the mean, it is striking that 59% of patients with LBBB did not improve or worsened their LV function on follow-up.
We also did not have data on NYHA functional class, which may have identified important differences in our study population. Data for heart failure hospitalization was also limited to those patients admitted to a Duke-affiliated hospital. Last, study enrollment required patients to have a follow-up Echo. This may have created selection bias. However, the study consort diagram shows higher rates of patients with LBBB excluded due to need for a cardiac device, left ventricular assist device, or transplant, while exclusions due to mortality were less for the NQRS group. This is in line with extensive data that suggest that LBBB aggravates cardiomyopathy and leads to worse outcomes.(5,9,44) Thus, if need for a follow-up Echo caused bias, it was likely toward weakening our findings.
Conclusion:
Patients with LVEF≤35% and LBBB demonstrate significantly less LV functional recovery than those with a NQRS. Among patients with LBBB, the likelihood of large improvement in LVEF is modest, even when considering revascularization and use of GDMT. These findings suggest that current guidelines that mandate 3 months of GDMT should be more flexible. For some patients with LBBB, recovery of LVEF>35% is unlikely with medicines alone, and these patients may be better served with earlier implantation of CRT.
Supplementary Material
Perspectives:
Competency in medical knowledge: Left bundle branch block (LBBB) is associated with worse outcomes among patients with cardiomyopathy.
Competency in patient care: Cardiac resynchronization therapy (CRT) is a highly effective therapy to treat symptomatic patients with LBBB and cardiomyopathy.
Translational outlook 1: Patients with LBBB are less likely to recover left ventricular function over time, even with guideline directed medical therapy (GDMT).
Translational outlook 2: Highly symptomatic patients with LBBB and very low ejection fraction would likely benefit from direct implantation with CRT.
Acknowledgments:
Prior to his death, Galen S. Wagner, MD contributed significantly to this manuscript.
Funding Source: This study was funded by a Medtronic-Duke Strategic Alliance for Research grant award. Medtronic Inc. was not involved in the study design, the collection, analysis and interpretation of data, the writing of the report, or in the decision to submit the article for publication.
Financial Disclosures:Sze: no relevant disclosures; Samad: salary support through a National Heart, Lung, and Blood Institute (NHLBI) research grant, American Society of Echocardiography research grant, Boston Scientific-Duke Strategic Alliance for Research, sub-award from Duke O’Brien Kidney Research Core Centers Program NIH 1P30DK096493-01, and Medtronic-Duke Strategic Alliance for Research, Honorarium for serving on Advisory Board for Abbott Vascular; Dunning: no relevant disclosures; Atwater: Boston Scientific-Duke Strategic Alliance for Research grant, St Jude-Duke Strategic Alliance Research grant, consulting Honorarium from Boston Scientific, St Jude Medical, Medtronic, Biotronik, and Biosense Webster; Loring: no relevant disclosures; Chiswell: no relevant disclosures; Velazquez: Research grant from NHLBI, Amgen, Pfizer, Novartis, Alnylam, Philips; Consulting Honorarium from Amgen, Novartis, Merck, Expert Exchange; Daubert: Honoraria from: ARCA biopharma, Biosense Webster, Biotronik, Boston Scientific, Gilead, Medtronic, Northwestern Univ., St. Jude, VytronUS, Zoll; Research grants from: ARCA biopharma, Biosense Webster, Boston Scientific, Gilead, Medtronic, St. Jude, NIH; Salary support from American College of Cardiology
Abbreviations list:
- LBBB
Left bundle branch block
- NQRS
Narrow QRS duration
- WQRS
Wide QRS duration, non-left bundle
- Echo
Echocardiographic study
- CRT
Cardiac resynchronization therapy
- LVEF
Left ventricular ejection fraction
- GDMT
Guideline directed medical therapy
- ARB
Angiotensin receptor blockers
- ACE-I
Angiotensin converting enzyme inhibitors
- BB
Beta-blockers
References:
- 1.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura RA, Otto CM, Bonow RO et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg 2014;148:e1–e132. [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Dehmer GJ, Hirshfeld JW et al. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness Criteria for Coronary Revascularization: a report by the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2009;53:530–53. [DOI] [PubMed] [Google Scholar]
- 4.Moss AJ, Hall WJ, Cannom DS et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. The New England journal of medicine 2009;361:1329–38. [DOI] [PubMed] [Google Scholar]
- 5.Tang AS, Wells GA, Talajic M et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med 2010;363:2385–95. [DOI] [PubMed] [Google Scholar]
- 6.Cleland JG, Daubert JC, Erdmann E et al. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase]. Eur Heart J 2006;27:1928–32. [DOI] [PubMed] [Google Scholar]
- 7.Linde C, Abraham WT, Gold MR et al. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. Journal of the American College of Cardiology 2008;52:1834–43. [DOI] [PubMed] [Google Scholar]
- 8.Tracy CM, Epstein AE, Darbar D et al. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 2012;60:1297–313. [DOI] [PubMed] [Google Scholar]
- 9.Zareba W, Klein H, Cygankiewicz I et al. Effectiveness of Cardiac Resynchronization Therapy by QRS Morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT). Circulation 2011;123:1061–72. [DOI] [PubMed] [Google Scholar]
- 10.Sweeney MO, van Bommel RJ, Schalij MJ, Borleffs CJ, Hellkamp AS, Bax JJ. Analysis of ventricular activation using surface electrocardiography to predict left ventricular reverse volumetric remodeling during cardiac resynchronization therapy. Circulation 2010;121:626–34. [DOI] [PubMed] [Google Scholar]
- 11.Bilchick KC, Kamath S, DiMarco JP, Stukenborg GJ. Bundle-branch block morphology and other predictors of outcome after cardiac resynchronization therapy in Medicare patients. Circulation 2010;122:2022–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bristow MR, Saxon LA, Boehmer J et al. Cardiac-Resynchronization Therapy with or without an Implantable Defibrillator in Advanced Chronic Heart Failure. N Engl J Med 2004;350:2140–2150. [DOI] [PubMed] [Google Scholar]
- 13.Vaillant C, Martins RP, Donal E et al. Resolution of left bundle branch block-induced cardiomyopathy by cardiac resynchronization therapy. Journal of the American College of Cardiology 2013;61:1089–95. [DOI] [PubMed] [Google Scholar]
- 14.Vernooy K, Verbeek XA, Peschar M et al. Left bundle branch block induces ventricular remodelling and functional septal hypoperfusion. European heart journal 2005;26:91–8. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson P, Wilhelmsen L, Rosengren A. Bundle-branch block in middle-aged men: risk of complications and death over 28 years. The Primary Prevention Study in Goteborg, Sweden. European heart journal 2005;26:2300–6. [DOI] [PubMed] [Google Scholar]
- 16.Imanishi R, Seto S, Ichimaru S, Nakashima E, Yano K, Akahoshi M. Prognostic significance of incident complete left bundle branch block observed over a 40-year period. The American journal of cardiology 2006;98:644–8. [DOI] [PubMed] [Google Scholar]
- 17.Sze E, Dunning A, Loring Z et al. Comparison of Incidence of Left Ventricular Systolic Dysfunction Among Patients With Left Bundle Branch Block Versus Those With Normal QRS Duration. The American journal of cardiology 2017. [DOI] [PubMed] [Google Scholar]
- 18.Drummond GA, Squire IB. The Cardiac Insufficiency Bisoprolol Study II. Lancet 1999;353:1361. [DOI] [PubMed] [Google Scholar]
- 19.Packer M, Bristow MR, Cohn JN et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996;334:1349–55. [DOI] [PubMed] [Google Scholar]
- 20.Packer M, Coats AJ, Fowler MB et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651–8. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein S, Hjalmarson A. The mortality effect of metoprolol CR/XL in patients with heart failure: results of the MERIT-HF Trial. Clin Cardiol 1999;22 Suppl 5:V30–5. [PubMed] [Google Scholar]
- 22.Waagstein F, Bristow MR, Swedberg K et al. Beneficial effects of metoprolol in idiopathic dilated cardiomyopathy. Metoprolol in Dilated Cardiomyopathy (MDC) Trial Study Group. Lancet 1993;342:1441–6. [DOI] [PubMed] [Google Scholar]
- 23.Velazquez EJ, Pfeffer MA, McMurray JV et al. VALsartan In Acute myocardial iNfarcTion (VALIANT) trial: baseline characteristics in context. Eur J Heart Fail 2003;5:537–44. [DOI] [PubMed] [Google Scholar]
- 24.Cohn JN, Tognoni G, Valsartan Heart Failure Trial I. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667–75. [DOI] [PubMed] [Google Scholar]
- 25.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 26.Velazquez EJ, Samad Z, Al-Khalidi HR et al. The MitraClip and survival in patients with mitral regurgitation at high risk for surgery: A propensity-matched comparison. Am Heart J 2015;170:1050–1059 e3. [DOI] [PubMed] [Google Scholar]
- 27.Strauss DG, Selvester RH, Wagner GS. Defining left bundle branch block in the era of cardiac resynchronization therapy. The American journal of cardiology 2011;107:927–34. [DOI] [PubMed] [Google Scholar]
- 28.Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, Udelson JE. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. Journal of the American College of Cardiology 2010;56:392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tukey JW. Comparing individual means in the analysis of variance. Biometrics 1949;5:99–114. [PubMed] [Google Scholar]
- 30.Bristow MR, Gilbert EM, Abraham WT et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation 1996;94:2807–16. [DOI] [PubMed] [Google Scholar]
- 31.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353:9–13. [PubMed] [Google Scholar]
- 32.Lechat P, Escolano S, Golmard JL et al. Prognostic value of bisoprolol-induced hemodynamic effects in heart failure during the Cardiac Insufficiency BIsoprolol Study (CIBIS). Circulation 1997;96:2197–205. [DOI] [PubMed] [Google Scholar]
- 33.Colucci WS, Kolias TJ, Adams KF et al. Metoprolol reverses left ventricular remodeling in patients with asymptomatic systolic dysfunction: the REversal of VEntricular Remodeling with Toprol-XL (REVERT) trial. Circulation 2007;116:49–56. [DOI] [PubMed] [Google Scholar]
- 34.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001;357:1385–90. [DOI] [PubMed] [Google Scholar]
- 35.Doughty RN, Whalley GA, Walsh HA et al. Effects of carvedilol on left ventricular remodeling after acute myocardial infarction: the CAPRICORN Echo Substudy. Circulation 2004;109:201–6. [DOI] [PubMed] [Google Scholar]
- 36.Pouleur H, Rousseau MF, van Eyll C et al. Effects of long-term enalapril therapy on left ventricular diastolic properties in patients with depressed ejection fraction. SOLVD Investigators. Circulation 1993;88:481–91. [DOI] [PubMed] [Google Scholar]
- 37.Solomon SD, Skali H, Anavekar NS et al. Changes in ventricular size and function in patients treated with valsartan, captopril, or both after myocardial infarction. Circulation 2005;111:3411–9. [DOI] [PubMed] [Google Scholar]
- 38.Risum N, Tayal B, Hansen TF et al. Identification of Typical Left Bundle Branch Block Contraction by Strain Echocardiography Is Additive to Electrocardiography in Prediction of Long-Term Outcome After Cardiac Resynchronization Therapy. Journal of the American College of Cardiology 2015;66:631–41. [DOI] [PubMed] [Google Scholar]
- 39.Nada A, Gintant GA, Kleiman R et al. The evaluation and management of drug effects on cardiac conduction (PR and QRS intervals) in clinical development. Am Heart J 2013;165:489–500. [DOI] [PubMed] [Google Scholar]
- 40.Barsheshet A, Goldenberg I, Moss AJ et al. Response to preventive cardiac resynchronization therapy in patients with ischaemic and nonischaemic cardiomyopathy in MADIT-CRT. European heart journal 2011;32:1622–30. [DOI] [PubMed] [Google Scholar]
- 41.Linde C, Abraham WT, Gold MR et al. Predictors of short-term clinical response to cardiac resynchronization therapy. European journal of heart failure 2017;19:1056–1063. [DOI] [PubMed] [Google Scholar]
- 42.Cleland JG, Abraham WT, Linde C et al. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. European heart journal 2013;34:3547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crowley AL, Yow E, Barnhart HX et al. Critical Review of Current Approaches for Echocardiographic Reproducibility and Reliability Assessment in Clinical Research. J Am Soc Echocardiogr 2016;29:1144–1154 e7. [DOI] [PubMed] [Google Scholar]
- 44.Lund LH, Jurga J, Edner M et al. Prevalence, correlates, and prognostic significance of QRS prolongation in heart failure with reduced and preserved ejection fraction. European heart journal 2013;34:529–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.