Abstract
Lianhua-Qingwen capsule (LQC) is a commonly used Traditional Chinese Medicine (TCM) in China and has 11 herb components. The main active ingredient can target specific molecules and perform many clinic treatment roles. LQC has been authorized by National Medical Products Administration (NMPA) of China to treat severe acute respiratory syndrome (SARS) in 2002–2003, type A influenza virus HIN1 pandemic in 2009, H7N9, H3N2 and coronavirus disease-19 (COVID19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) in 2020. It is also widely used to treat common cold with wind-heat syndrome, chronic rhinosinusitis (CRS), amygdalitis and chronic obstructive pulmonary disease. This article summarizes the advanced research progress of LQC in clinical application, mechanisms and provides new clues in the clinical application of LQC.
Keywords: Lianhua-Qingwen capsule, SARS-COV-2, SARS, Broad-spectrum antiviral
1. Introduction
Traditional Chinese Medicine (TCM) has been widely used to prevent and treat disease for thousands of years in China [1]. Lianhua-Qingwen capsule (LQC) is commonly used to treat SARS in 2003 [1], [2]. In last decades, it is widely used for treating viral influenza, pneumonia caused by coronavirus, common cold and other diseases [3], [4], [5]. LQC comes from two prescriptions Maxing-Shigan-Tang and Yinqiao-San and the main effective components have been identified in recent years [5]. LQC has 11 herb components: Radix Isatidis (Banlangen), Fructus Forsythiae (Lianqiao), Flos Lonicerae Japonicae (Jinyinhua), Rhizoma Dryopteridis Crassirhizomatis (Mianmaguanzhong), Herba Ephedrae (Mahuang), Semen Armeniacae Amarum (Kuxingren), Herba Houttuyniae (Yuxingcao), Herba Pogostemonis (Guanghuoxiang), Radix et Rhizoma Rhodiolae Crenulatae (Hongjingtian), Radix et Rhizoma Rhei (Dahuang) and Radix et Rhizoma Glycyrrhizae (Gancao) and Gypsum Fibrosum (Shigao) [5], [6], [7]. The main active ingredients of Lianhua Qingwen also have been identified: quercetin, kaempferol, luteolin, β-sitosterol, indigo, wogonin, tryptanthrin, [E]-4-phenyl-3-buten-2-one, 1-methyl-2-nonyl-4(1H)-quinolone, stigmasterol, naringenin, 18β-glycyrrhetinic acid [7]. These main active ingredients can target PTGS2, IL6, CASP3, MAPK1, EGFR, ACE2, etc. and involved in T cell activation, viral receptors, and inflammatory responses pathways which are associated with anti-viral and anti-inflammatory responses [7]. LQC can act on multiple targets and pathways to treat the disease.
2. Clinical application and mechanisms
2.1. COVID19
Coronavirus disease −19 (COVID19) is an emerging infectious disease and has spread around the world in 2019–2021 [8]. WHO data shows, up to now COVID19 has infected 186 million people and caused 4035,037 deaths in 224 countries or areas. COVID19 is caused by novel coronavirus SARS-COV-2 which belong to β coronavirus [9]. COVID19 mainly causes fever, cough, myalgia, fatigue and dyspnea [10]. Headache, dizziness, diarrhea, nausea and vomiting also can be found in some patients [10]. China-WHO joint report on COVID-19 indicated the most serious consequence is acute respiratory distress syndrome (ARDS) and multiple organ failure (MOF) which is main reason to induce death [11]. SARS-COV-2 is highly contagious and is spread from human to human mainly caused by air-borne or intimate contact [12]. This characteristic leads to the COVID19 pandemic worldwide. For now, there is still no specific medicine for treating COVID19.
Lianhuaqingwen capsule has been used to treat SARS and influenza pandemic in China and showed broad spectrum antiviral activity. It improved the clinical symptoms which include fever, cough, nasal obstruction, headache and so on [13]. LQC has been widely used to treat patients suffered from COVID19 after outbreak of pandemic. According to recent researches, the antiviral functions and mechanisms of LQC mainly include the following aspects: block binding of virus with receptor, inhibit cytokines storm and improve clinical applications.
2.1.1. Block binding of virus with receptor
After infects in human body, SARS-COV-2 can bind with different receptors to invade cells and Angiotensin-converting enzyme 2 (ACE2) which as an important metallopeptidase involved in Renin-angiotensin system (RAS) is the main functional receptor of SARS-COV-2 [14], [15], [16]. SARS-COV-2 binds to ACE2 receptor on cell surface and invades target cells by endocytosis. It is encased in endosome and uncoated by endosome acidic environment which makes the virus RNA exposed. Then the exposed RNA is released by endosome and goes into nucleus to replicate and reverse transcript. Finally, the newly generated SARS-COV-2 are released from target cells and infect other healthy cells.
LQC may block the binding of SARS-COV-2 with ACE2 to inhibit replication of the virus. In vitro experiments done by Nanshan Zhong et al. showed that in plaque reduction assay, LQC treatment reduce the plaque formation of SARS-COV-2 [17]. The experiment has also shown that under the microscope, LQC reduced virions in infected cells and changed surface virions of infected cells [17]. Meanwhile, the research done by Ming Niu et al. showed components of LQC like Lonicera japonica and Forsythia can block the binding of novel coronavirus with ACE2 in cell surface [18]and other research also found Rheum palmatum can effectively antagonize the binding of spike protein which on the surface of β coronavirus and ACE2 [19].
2.1.2. Inhibition of cytokines/chemokines responses
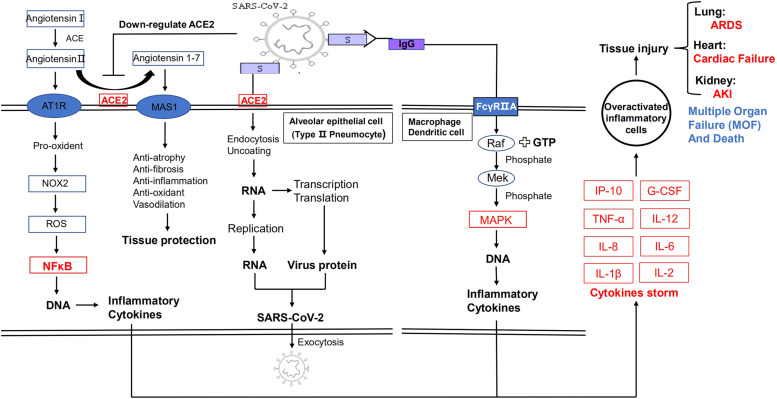
The cytokines storm is an excessive self-defense system in host caused by SARS-COV-2, which can induce lung injury and severe disease [20]. When SARS-COV-2 infects human body, T cell can be activated in the same time. Activated T cells proliferate rapidly and secrete Granulocyte-macrophage Colony Stimulating Factor (GM-CSF) and interleukin-16(IL-16). IL-16 bind to T cell surface glycoprotein CD4 and CD4 can promote CD4 T cells to produce cytokines and activate other immune cells. GM-CSF is an important cytokine which can induce macrophages and neutrophils over-activated. Similarly, B cells, macrophages and natural killer cells (NK cell) are activated by compound of SARS-COV-2 and IgG antibody. The compound bind to low affinity immunoglobulin gamma Fc receptor II-a(FCGR2A) and activate Raf-Mek-MAPK pathway which induce overexpression of cytokines including interleukin, TNFα, interferon, and GM-CSF. Finally, cytokines storm occurs and cause coagulation disorders, systemic inflammatory response and tissue damage. The pathological mechanisms of disease show in Fig. 1.
Fig. 1.
Pathogenesis of SARS-COV-2. S protein of SARS-COV-2 bind to ACE2 and endocytosed by target cells. In target cells, SARS-COV-2 replicated, assembled and released by exocytosis. Besides, SARS-COV-2 can downregulate ACE2 and make angiotensinⅡ bind to AT1R which induce NFκB pathway to regulate expression of DNA of inflammatory cytokines. SARS-COV-2 also can be recognized by IgG and induce immune cells to secrete cytokines. Finally, cytokines storm occurs and induce tissue injury.
Recent clinical studies show that severe COVID19 patients has cytokines storm but patients with mild symptoms do not have [21]. Inhibition of the cytokines storm is the key to improve the treatment. In vitro experiments which Zhong Nanshan did, LQC significantly decreased the mRNA expression levels of TNF-α, IL-6, CCL-2/MCP-1, and CXCL-10/IP-10 in infected cells [17]. These cytokines release in excess in SARS-COV-2 patients. The change of cytokines expression level suggests the LQC has potential effect to inhibit cytokine storm caused by SARS-COV-2 in human. In addition to this, previous experiments have found LQC components such as Rheum palmatum can effectively suppress the excessive release of inflammatory mediators like interleukin-1β, IL-2, IL-4 and IL-13, thus improve the lung injury [22], [23], [24].
2.1.3. Improvement of clinical manifestation
In blood, SARS-COV-2 can down-regulate ACE2. Normally, The ACE2 catalyze angiotensinⅡ to angiotensin 1–7 which bind to MAS1 on cell surface and protect cells from atrophy, fibrosis, inflammation and oxidant. When ACE2 is down-regulated by coronavirus, angiotensinⅡ cannot be catalyzed and cannot bind to cell surface AT1R receptor. The pro-oxidant signals are released and promote NADPH oxidase to form reactive oxygen species (ROS). ROS can promote NFκB to induce DNA expression which is translated to produce inflammatory cytokines such as TNFα, IL-6, IL-12, etc. The binding of angiotensinⅡwith AT1R also can release pro-atrophy, pro-fibrosis, pro-inflammation, vasoconstriction signals directly. By these ways, SARS-COV-2 cause tissue injury and severe clinical symptoms. SARS-COV-2 mainly cause pathological injuries in lung [20]. And the pathological pathway showed in Fig. 1.
Improvement of symptoms could be associated with the antiviral and anti-inflammatory function of LQC. In addition, LQC components can also directly act on tissues. Pogostemon cablin can moderate diarrhea and protect the gastrointestinal tract [25]. Rhodiola rosea can suppress the oxidative stress and apoptosis then improve lung injury [19], [26].
In clinical, the randomized controlled trial led by Zhong Nanshan which include 284 patients showed that patients receive combination of LQC with usual treatment (which based on The Protocol for Diagnosis and Treatment of Novel Coronavirus Pneumonia 4th edition) for 14 days has improved the rate of symptom recovery markedly (58% at day 5, 80% at day 10% and 91% at day 14) [27]. The symptom recovery time was also shorter than patients treated with usual treatment. LQC shortened the duration of different symptoms (fever-1 day, fatigue and cough-3 days) [27]. The clinical cure rate and CT manifestation recovery rate also become higher in LQC group [27]. The study led by Zhang Junhua also found that combined conventional treatment such as oxygen therapy, antiviral, antimicrobial with LQC can improve the clinical efficacy of COVID-19 patients [28].
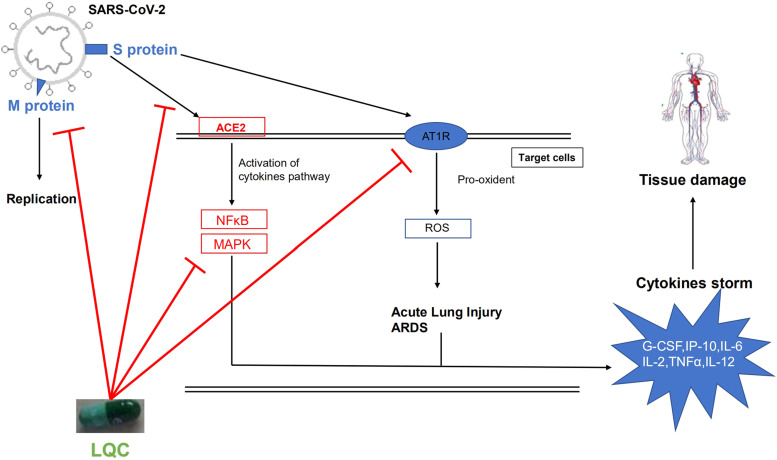
In summary, LQC can be used to treat COVID-19 by improving the clinical manifestations, improving recovery of symptoms, shortening duration of symptoms and the mechanisms include blocking binding of virus with receptor and inhibiting the cytokines storms [29]. The mechanisms of LQC show in Fig. 2. It shows great potentials to treat COVID-19 in clinical, LQC could be considered for COVID-19 treatment around the world.
Fig. 2.
Mechanism of Lianhuaqingwen capsule (LQC). LQC can inhibit M protein of SARS-COV-2 which help virus replication. LQC also inhibit binding of S protein with ACE2 and activation of cytokines. By blocking virus binding with cells, LQC prevent cytokines storm from occurring and protect the tissue.
2.2. Severe acute respiratory syndrome (SARS)
Severe acute respiratory syndrome (SARS) is caused by coronavirus SARS-COV, outbreak in 2003 in Guangdong province, China [30]. SARS is highly contagious and fatal. The genetic characteristics of SARS-COV-2 (COVID19) has 79.5% homology with SARS-COV (SARS) [31]. Similarly, SARS-COV can entry into target cells by membrane fusion and ACE2 is the important functional receptor [30]. SARS-COV also can enter the cells by pH-dependent endocytosis and the spike proteins of the virus is the key molecules [32]. The patients with SARS suffered from persistent fever, chills, myalgia, malaise, headache and more serious symptom is dyspnea. In addition to this, patients also have sputum, sore throat, dizziness, vomiting and diarrhea [33], [34], [35]. Clinically, 20–36% patients may require ICU admission, and 13–26% may progress into acute respiratory distress syndrome (ARDS) [34], [36]. After outbreak of SARS, LQC has been used clinically and has played a significant role. The function and mechanisms of LQC also has been investigated clearly.
2.2.1. Block binding of SARS-COV with ACE2
SARS-COV can enter into cells by ACE2 on cell surface. ACE2 can activate Mitogen Activated Protein Kinases (MAPK) and induce proinflammatory factor over-expression. Meanwhile, ACE2 helps SARS-COV infect cells and replicate. Previous study also found that ACE2 could activate Alveolar Epithelial Type 1 (AT1) receptor which increase pulmonary capillary permeability and lead to acute lung injury and acute respiratory distress syndrome (ARDS) [37].
LQC block the binding of SARS-COV with ACE2. For active ingredients of LQC, stigmasterol, naringenin, 18β-glycyrrhetinic acid and quercetin can bind with ACE2 and inhibit the SARS-COV binding [7]. For components of LQC, studies led by Xiaoying et al. has found Radix et Rhizoma Rhei (Dahuang) and Radix et Rhizoma Glycyrrhizae (Gancao) can inhibit the absorption and membrane penetration of SAR-COV and inhibit the replication of SARS-COV [7].
2.2.2. Enhance immune system
After SARS-COV get into host cells, the virus can suppress the interferon and its signal pathways to evade immune responses [38]. Interferon is product of congenital immunity which can regulate anti-viral proteins and inhibit virus replication. By suppressing interferons, SARS-COV invade host cells and evade immune responses. Finally, the virus causes severe symptoms and death.
The components and active ingredients of LQC can inhibit SARS-COV by multiple targets and pathways [7]. Herba Houttuyniae (Yuxingcao) could inhibit activation of 3 C-like protein (3CLpro) and improve the lymphocytes proliferation in spleen of mice [7]. Forsythoside which is active ingredient of Fructus Forsythiae (Lianqiao) could up-regulate the expression of IFN-α and regulate immune responses to inhibit SARS-COV [39]. In vitro experiment led by Zhu Shunya et al. has also found LQC could inhibit the SARS-COV function in Vro-E6 cells [40].
2.3. Influenza
Influenza is caused by influenza virus which belongs to orthomyxovirus. Hemagglutinin (HA) and neuraminidase (NA) are envelope proteins [41]. NA can provide virus access to release virus particles from infected cells by budding and HA helps virus attaches to epithelial cells, trigger endocytosis and cluster red blood cells together [41], [42]. The genomes of influenza virus are highly variable. The influenza virus can escape from the host immune responses by changing HA and NA antigens [43]. The genome variable of influenza virus reduces the effectiveness of drugs and make viruses more easily to spread. Influenza can be divided to type A, B and C influenza. H1N1 and H7N9 are infamous subtypes in type A influenza. H1N1 has caused a pandemic with high mortality in1918 which also has been called Spanish flu. In Spanish flu, 500 million people all over the world were infected and 50–100 million people dead. It is one of the worst pandemics in history. In 2009, a new strain H1N1 virus spread worldwide again and it transferred from human to human by airborne droplets and close encounter instead of transferring from pigs to human of traditional zoonotic swine flu [41], [44]. Influenza can induce sore throat, muscular soreness, fever, headache, nasal obstruction and rhinorrhea in clinical [45]. LQC has shown anti-influenza activity clinically in 2009 H1N1 pandemic of China.
2.3.1. Inhibit influenza replication
The experiments led by Ding Yuewen et.al found that LQC could be used to treat different strains of influenza viruses including H1N1 and H7N9 [46]. In the study, LQC inhibited virus proliferation in plaque reduction assay and significant reduction of virus titers mostly occurred between 0 and 2 h [46]. This suggested LQC has function to inhibit early-stage replication of influenza virus. In vivo experiment done by Yuewen Ding has also found high dose of LQC could efficiently inhibit influenza virus replication in mice and mice treated with LQC has significant reduction of alveolar inflammatory exudate [47].
2.3.2. Inhibit inflammatory cytokines
The study found LQC could inhibits cytokines/chemokine expression caused by influenza A virus [46]. Previous study showed inhibition of NF-κB can induce retention of virus nucleocapsid protein (RNP) in infected cell nucleus [48]. LQC can inhibit NF-κB activation which could be induced by influenza A virus to help virus replication. So LQC can block RNP releasing from host cells by inhibiting NF-κB activation. Besides these functions, another study led by Ding Yuewen in 2016 has shown that LQC inhibited the production of Caspase-3 degradation fragments [47]. Caspase-3 is an important protease included in cell apoptosis and influenza virus could induce expression of Caspase-3 and cause cell apoptosis. Cell apoptosis will release Caspase-3 fragments and RNP. The decrease of Caspase-3 degradation fragments production suggested LQC can suppress cell apoptosis and inhibit the RNP release from cells.
2.3.3. Improve clinical symptoms and prevention
LQC can improve clinical symptoms (headache, sore throat, fever, etc.) caused by influenza [45]. The clinical trial done by Zhongping Duan et al. randomized 244 patients to two groups and each group take either LHC or Oseltamivir. After observation for 7 days, the results showed severity of illness and the duration of symptoms of LQC-treated patients shortened significantly (P < 0.05) [3]. Besides excellent treatment effect, the study carried in Hebei province, China has found only 1.2% of people who used LQC in advance has influenza symptoms in pandemic which is prominently lower than 8.8% who do not use LQC in advance [49]. This study suggested LQC also can be used to prevent the influenza pandemic.
2.4. Chronic rhinosinusitis (CRS)
Chronic rhinosinusitis (CRS) refers to a chronic inflammation condition in nasal cavity and paranasal sinuses and is mostly secondary to acute rhinosinusitis. The incidence of CRS account for 1–9% population all over the world and in China 8% people are suffered from CRS [50], [51]. Usually, CRS causes nasal obstruction, nasal discharge, facial pain and even smell reduction or loss [50]. The course of CRS usually exceeds 12 weeks. According to clinical characteristics, CRS are divided to chronic rhinosinusitis without nasal polyps (CRSsNP) and chronic rhinosinusitis with nasal polyps (CRSwNP) [52]. The treatment strategy for CRS is limited. Surgery usually has been used to treat CRSwNP and hormones are used to treat both CRSwNP and CRSsNP. Because pathogenesis of CRS is still not clear, many patients cannot recover completely, especially patients with CRSsNP. LQC is considered to be an emerging treatment method.
2.4.1. Inhibit inflammation
LQC increase production of nasal nitric oxide (NO) which has function to protect cells from inflammation and virus infection [52]. LQC also can regenerate and rearrange nasal cilia to make them return to work. The study also found LQC reduce the percentage of T cells (include CD4+ and CD8+ T cells) in nasal lavage fluid which indicates LQC decreased infiltration of inflammatory cells and alleviated inflammatory reactions [52]. This research also demonstrated LQC reduce the production of cytokines/chemokines including IL-4, IL-5, eosinophil cation protein (ECP), IFN-γ, neutrophil myeloperoxidase (MPO) and tumor necrosis factor (TNF)-α. LQC is a promising treatment method to manage CRS.
2.4.2. Improve clinical symptoms
LQC can improve the quality of patients’ life [52]. Recent research found LQC can improve the CRSsNP by controlling inflammatory reactions in nasal mucosa [52]. Visual Analog Scale (VAS) and Sino-Nasal Outcome Test (SNOT)-22 has been used to evaluate life quality of CRSsNP patients. LQC can decrease VAS and SNOT-22 scores of patients which means LQC improved symptoms caused by CRSsNP and improved life quality of patients [52].
2.5. Adjuvant drug
LQC can be used as adjuvant drugs to improve the efficacy of major drugs. Tonsillitis and hand-foot-and-mouth disease (HFMD) is common disease in China. LQC has been used widely to treat these diseases.
2.5.1. Tonsillitis
Tonsillitis is due to bacterium infection which mainly caused by streptococcus and staphylococcus. In study led by Qiu Wen, combination of Phenoxymethylpenicillin Potassium Tablets with LQC can significantly improve recovery of temperature, alleviate tonsil enlargement and throat pain [53]. LQC can effectively treat acute tonsillitis, improve inflammation recovery, improve cure rate and have minor adverse effects. LQC can be used as adjuvant drugs to increase cure rate and treatment efficiency.
2.5.2. Hand-foot-and-mouth disease (HFMD)
HFMD mainly occurs under the age of 5 and mostly caused by Coxsachie (Cox A16) virus and human enterovirus 71 (EV 71). LQC has broad-spectrum antiviral, antibacterial and anti-inflammatory functions. Combination of LQC with conventional therapy can decrease the recovery time of temperature, dental ulcer, rash and herpes [54]. As adjuvant drugs, LQC significantly improve the treatment efficacy and has no adverse effect.
3. Adverse effect
With the widely use of LQC in clinical, the adverse effects also have been found. The adverse effects mainly occur after first administration of LQC and include discomfort in gastrointestinal (73.9%) and skin (9.6%) which mainly include nausea, vomiting, diarrhea and rash [55]. Most adverse effects are mild and patients are not extremely discomfortable. Monitoring of LQC and standardizing of drug instructions should be strengthened to promote rational application of LQC and ensure the safety of patients.
4. Discussion
Lianhua Qingwen Capsule (LQC) has broad-spectrum antiviral, antibacterial and anti-inflammatory functions. It also has been approved for clinical use and play a crucial role in treating SARS, COVID19, HIN1 and H7N9 pandemic in China ( Table 1). According to research carried out by Zhang Boli, LQC has been widely recommended throughout China for the treatment of novel coronavirus [56]. The main functions of LQC include inhibition of binding between virus and host cells, inhibition of viral replication and release, reducing expression level of chemokines/cytokines, enhancing immune system and improving symptoms caused by different diseases. LQC has good effectiveness, high safety and few adverse effects. In past decades, LQC has been included in Chinese guidelines for the prevention and treatment for infectious disease for many times and approved by 15 countries as Chinese patent medicine.
Table 1.
List of disease that treated by LQC.
| Disease | Pathogen | Symptoms | Inflammatory cytokines | Symptom improvement | Adverse effect | Ref |
|---|---|---|---|---|---|---|
| COVID-19 | SARS-COV-2 | Fever, cough, fatigue, headache, dizziness, vomiting, ARDS, MOF | Decrease | Yes | Mild | [10], [11], [12], [13], [17], [20], [21], [22], [27], [28] |
| SARS | SARS-COV | Fever, headache, dyspnea, sore throat,ARDS | Decrease | Yes | Mild | [33], [34], [35], [39], [40] |
| H1N1 | Type A Influenza virus | Fever, headache, dizziness, fatigue, rhinorrhea | Decrease | Yes | Mild | [47], [48], [49] |
| H7N9 | Type A Influenza virus | Fever, headache, dizziness, fatigue, rhinorrhea | Decrease | Yes | Mild | [47], [48], [49] |
| Chronic rhinosinusitis | Acute rhinosinusitis | nasal obstruction, nasal discharge, facial pain, smell reduction or loss | Decrease | Yes | Minor | [50], [51], [52] |
| Tonsillitis | Streptococcus/ staphylococcus | Fever, alleviate tonsil enlargement, throat pain | Decrease | Yes | NO | [53] |
| Hand-foot-and-mouth disease | Coxsachie (Cox A16) virus /human enterovirus 71 (EV 71) | Fever, dental ulcer, rash, herpes | Decrease | Yes | Mild | [54] |
LQC has become an emerging research object and with development of cytomics, proteomics, genomics and other technologies, the components and mechanisms of LQC will be revealed. LQC is a promising drug which can be approved by more countries and used clinically worldwide in the future.
CRediT authorship contribution statement
Shen Xuhui: Document retrieval, Writing – original draft, Writing – review & editing. Yin Fugen: Conception, communication and revision.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgements
We are grateful for critical revision of the manuscript by Anamika Dinesh (MD), India.
References
- 1.Yi X. The antiviral report of Lianhua Qingwen capsule. Chin. Health Care. 2012;(9):13–14. [Google Scholar]
- 2.Liu S.H., Chuang W.C., Lam W., Jiang Z., Cheng Y.C. Safety surveillance of traditional Chinese medicine: current and future. Drug Saf. 2015;38(2):117–218. doi: 10.1007/s40264-014-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duan Z.P., Jia Z.H., Zhang J., Liu S., Chen Y., Liang L.C., Zhang C.Q., Zhang Z., Sun Y., Zhang S.Q., Wang Y.Y., Wu Y.L. Natural herbal medicine Lianhuaqingwen capsule anti-influenza A (H1N1) trial: a randomized, double blind, positive controlled clinical trial. Chin. Med. J. 2011;124(18):2925–2933. [PubMed] [Google Scholar]
- 4.Liu C.Y., Li X.Q., Cai S.Q. Research progress on the pharmacological and clinical study of Lianhua Qingwen capsule. Chin. Med. Pharmacol. Clin. 2010;26(06):84–85. [Google Scholar]
- 5.Wang C.H., Zhong Y., Zhang Y., Liu J.P., Wang Y.F., Jia W.N., Wang G.C., Li Z., Zhu Y., Gao X.M. A network analysis of the Chinese medicine Lianhua-Qingwen formula to identify its main effective components. Mol. Biosyst. 2016;12(2):606–613. doi: 10.1039/c5mb00448a. [DOI] [PubMed] [Google Scholar]
- 6.Jia W., Wang C., Wang Y., Pan G., Jiang M., Li Z., Zhu Y. Qualitative and quantitative analysis of the major constituents in Chinese medical preparation Lianhua-Qingwen capsule by UPLC-DAD-QTOF-MS. Sci. World J. 2015;2015:731–765. doi: 10.1155/2015/731765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ling X.Y., Tao J.L., Sun X., Yua B. Exploring material basis and mechanism of Lianhua Qingwen prescription against coronavirus based on network pharmacology. Chin. Tradit. Herb. Drugs. 2020;51(07):1723–1730. [Google Scholar]
- 8.Lu R.G., Wu J., Bai X., Liu W.Q. Research progress on the infection mechanism of coronavirus SARS-COV-2. Chin. J. Virol. 2020;36(05):927–935. [Google Scholar]
- 9.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L.Q., Huang T., Wang Y.Q., Wang Z.P., Liang Y., Huang T.B., Zhang H.Y., Sun W., Wang Y. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J. Med. Virol. 2020;92(6):577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li T.F., Deng H.W., Li L., Zhang Y., Feng D.D., Hu W.X. The Latest Treatment Progress of COVID-2019 by Chinese and Western Medicine. Clin. J. Tradit. Chin. Med. 2020;32(08):1419–1423. [Google Scholar]
- 12.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., Tan K.S., Wang D.Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil. Med. Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D., Li Z., Liu Y. An overview of the safety, clinical application and antiviral research of the COVID-19 therapeutics. J. Infect. Public Health. 2020;13(10):1405–1414. doi: 10.1016/j.jiph.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-COV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bao L., Deng W., Huang B., Gao H., Liu J., Ren L., Wei Q., Yu P., Xu Y., Qi F., Qu Y., Li F., Lv Q., Wang W., Wang S., Song Z., Zhao L., Liu P., Zhao L., Ye F., Wang H., Zhou W., Zhu N., Zhen W., Yu H., Zhang X., Guo L., Chen L., Wang C., Wang Y., Wang X., Xiao Y., Sun Q., Liu H., Zhu F., Ma C., Yan L., Yang M., Han J., Xu W., Tan W., Peng X., Jin Q., Wu G., Qin C. The pathogenicity of SARS-COV-2 in hACE2 transgenic mice. Nature. 2020;583(7818):830–833. doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- 17.Li R.F., Hou Y.L., Huang J.C., Pan W.Q., Ma Q.H., Shi Y.X., Li C.F., Zhao J., Jia Z.H., Jiang H.M., Zheng K., Huang S.X., Dai J., Li X.B., Hou X.T., Wang L., Zhong N.S., Yang Z.F. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-COV-2) Pharmacol. Res. 2020;156 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niu M., Wang R.L., Wang Z.X., Zhang P., Bai Z.F., Jing J., Guo Y.M., Zhao X., Zhan X.Y., Zhang Z.T., Song X.A., Qin E.Q., Wang J.B., Xiao X.H. Rapid extablishment of traditional Chinese medicine prevention and treatment of 2019-nCoV based on clinical experience and molecular docking. Chin. J. Chin. Mater. Med. 2020;45(06):1213–1218. doi: 10.19540/j.cnki.cjcmm.20200206.501. [DOI] [PubMed] [Google Scholar]
- 19.Ho T.Y., Wu S.L., Chen J.C., Li C.C., Hsiang C.Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antivir. Res. 2007;74(2):92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C.L., Wang Y.M., Li X.W., Ren L.L., Zhao J.P., Hu Y., Zhang L., Fan G.H., Xu J.Y., Gu X.Y., Cheng Z.S., Yu T., Xia J.A., Wei Y., W W.J., Xie X.L., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., X J.G., W G.F., Jiang R.M., Gao Z.C., Jin Q., Wang J.W., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui W.W., Jin X., Zhang Y.F., Chang L.P., Wang H.T. Effects of Lianhua Qingwen capsules on IKK/IκB/NF-κB signal pathway in the mouse with LPS-induced acute lung injury. Chin. Tradit. Pat. Med. 2015;37(05):953–958. [Google Scholar]
- 23.Mo H.Y., Ke C.W., Zheng J.P., Zhong N.S. Identification and structural characterization of I84C and I84A mutations that are associated with high-level resistance to human immunodeficiency virus protease inhibitors and impair viral replication. Antimicrob. Agents Chemother. 2007;51(01):732–735. doi: 10.1128/AAC.00690-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong Y., Zhang X.Z., Jing J.Q., Xu W. Influence of rhubarb on the expression of TNF-αand IL-8 in the lungs of rabbits after cardiopulmonary resuscitation. Chin. J. Clin. Emerg. 2017;18(05):366–368. [Google Scholar]
- 25.Zhou T.R. Exploring the mechanism of the active components of Pogostemonis Herba in treatment of IBS-D rat via enteric neurotransmission regulation. Guangzhou Univ. Chin. Med. 2018 [Google Scholar]
- 26.Huangfu Z.M., Xu Q., Wang X., Wang E.P., Feng Y., Zeng J., Zhu R., Zhao C.L. Salidroside can improve lung injury in mouse of chronic intermittent hypoxia. Chin. J. Tissue Eng. Res. 2019;23(31):5036–5040. [Google Scholar]
- 27.Hu K., Guan W.J., Bi Y., Zhang W., Li L., Zhang B., Liu Q., Song Y., Li X., Duan Z., Zheng Q., Yang Z., Liang J., Han M., Ruan L., Wu C., Zhang Y., Jia Z.H., Zhong N.S. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu M., Gao Y., Yuan Y., Yang K.L., Shi S.Z., Tian J.H., Zhang J.H. Efficacy and safety of herbal medicine (Lianhuaqingwen) for treating COVID-19: a systematic review and meta-analysis. Integr. Med Res. 2021;10(1) doi: 10.1016/j.imr.2020.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang F.C., Shen B.X., He C.Y., Zhao W.C., Nie S.L. Clinical efficacy of Lianhua Qingwen granules and its mechanism on COVID-19 based on network pharmacology. Chin. Med. Pharmacol. Clin. 2020;36(02):93–101. [Google Scholar]
- 30.Satija N., Lal S.K. The molecular biology of SARS coronavirus. Ann. N. Y. Acad. Sci. 2007;1102:26–38. doi: 10.1196/annals.1408.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao F., Zhou Y., Liu Y., Gong J., Yuan S.S., Peng H.L., Pang B. The cardiac effect of novel coronavirus comparing to severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus. J. Cardiopulm. Pulm. Dis. 2020;39(09):1032–1036. [Google Scholar]
- 32.Li S.T., Lin L., Wang H., Yin J.N., Ren Y., Zhao Z., Wen J., Zhou C.Q., Zhang X.M., Li X.L., Wang J.Q., Zhou Z.F., Liu J.X., Shao J.M., Lei T.T., Fang J.Q., Xu N.Z., liu S.Q. The epitope study on the SARS-COV nucleocapsid protein. Genom. Prote Bioinform. 2003;1(3):198–206. doi: 10.1016/S1672-0229(03)01025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsang K.W., Ho P.L., Ooi G.C., Yee W.K., Wang T., Chan-Yeung M., Lam W.K., Seto W.H., Yam L.Y., Cheung T.M., Wong P.C., Lam B., Ip M.S., Chan J., Yuen K.Y., Lai K.N. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348(20):1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 34.Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., Szeto C.C., Chung S., Sung J.J. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348(20):1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 35.Hsu L.Y., Lee C.C., Green J.A., Ang B., Paton N.I., Lee L., Villacian J.S., Lin P.L., Earnest A., Leo Y.S. Severe acute respiratory syndrome (SARS) in Singapore: clinical features of index patient and initial contacts. Emerg. Infect. Dis. 2003;9(6):713–717. doi: 10.3201/eid0906.030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsui P.T., Kwok M.L., Yuen H., Lai S.T. Severe acute respiratory syndrome: clinical outcome and prognostic correlates. Emerg. Infect. Dis. 2003;9(9):1064–1069. doi: 10.3201/eid0909.030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ou H.L., Li L.J. Research progress of renin-angiotensin system in newly emerging respiratory infectious diseases. Mod. Pr. Med. 2019;31(01):1–3. [Google Scholar]
- 38.Frieman M., Ratia K., Johnston R.E., Mesecar A.D., Baric R.S. Severe acute respiratory syndrome coronavirus papain-like protease ubiquitin-like domain and catalytic domain regulate antagonism of IRF3 and NF-kappaB signaling. J. Virol. 2009;83(13):6689–6705. doi: 10.1128/JVI.02220-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu P.L., Wang D.Q., Li Z.J. Progress in the pharmacological effects of forsythiaside. J. Chang. Univ. Tradit. Chin. Med. 2011;27(06):1062–1063. [Google Scholar]
- 40.Zhu S.Y., Li X.Y., Wei Y.L., Yang P.Y., Qin E.D. Inhibitory effects of three prescriptions of traditional Chinese medicine on SARS-associated coronavirus in vitro. Lett. Biotechnol. 2003;05:390–392. [Google Scholar]
- 41.Jilani T.N., Jamil R.T., Siddiqui A.H. H1N1 Influenza. 2020. [PubMed] [Google Scholar]
- 42.Sullivan S.J., Jacobson R.M., Dowdle W.R., Poland G.A. 2009 H1N1 influenza. Mayo Clin. Proc. 2010;85(1):64–76. doi: 10.4065/mcp.2009.0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masci J.R., Wormser G.P. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 6th Edition Edited by Gerald L. Mandell, John E. Bennett, and Raphael Dolin Philadelphia: Elsevier Churchill Livingstone, 2005. 3661 pp., Illustrated. $329 (cloth) Clin. Infect. Dis. 2005;41(2) [Google Scholar]
- 44.Rewar S., Mirdha D., Rewar P. Treatment and prevention of pandemic H1N1 influenza. Ann. Glob. Health. 2015;81(5):645–653. doi: 10.1016/j.aogh.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Niu Q.Q., Chen Y., Liu Y., Mao S.Z., Wang H., Zheng W.K., Zhang J.H. Efficacy and safety of Lianhua Qingwen capsule for influenza: a systematic review. Chin. J. Chin. Mater. Med. 2017;42(08):1474–1481. doi: 10.19540/j.cnki.cjcmm.2017.0044. [DOI] [PubMed] [Google Scholar]
- 46.Ding Y.W., Zeng L.J., Li R.F., Chen Q.Y., Zhou B.X., Chen Q.L., Cheng P.L., Wang Y.T., Zheng J.P., Yang Z.F., Zhang F.X. The Chinese prescription lianhuaqingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function. BMC Complement Alter. Med. 2017;17(1):130. doi: 10.1186/s12906-017-1585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding Y.W. Development and feasibility testing of a critical care EEG Monitoring Database for standardized clinical reporting and multicenter collaborative research. J. Clin. Neurophysiol.: Off. Publ. Am. Electroencephalogr. Soc. 2016;33:133–140. doi: 10.1097/WNP.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wurzer W.J., Planz O., Ehrhardt C., Giner M., Silberzahn T., Pleschka S., Ludwig S. Caspase 3 activation is essential for efficient influenza virus propagation. Embo J. 2003;22(11):2717–2728. doi: 10.1093/emboj/cdg279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dou Y., Yang S.P. Lianhua Qingwen Capsules: natural antibiotics, broad-spectrum antiviral. Chin. Community Physician. 2012;28(35):9. [Google Scholar]
- 50.Fokkens W.J., Lund V.J., Mullol J., Bachert C., Alobid I., Baroody F., Cohen N., Cervin A., Dougles R., Gevaert P., Georgalas C., Goossens H., Harvey R., Hellings P., Hopkins C., Jones N., Joos G., Kalogjera L., Kowalski M., Price D., Riechelmann H., Schlosser R., Senior B., Thomas M., Toskala E., Voegels R., Wang D.Y., Wormald P.J. European position paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol. Suppl. 2012;23:3. [PubMed] [Google Scholar]
- 51.Shi J.B., Fu Q.L., Zhang H., Cheng L., Wang Y.J., Zhu D.D., Lv W., Liu S.X., Li P.Z., Ou C.Q., Xu G. Epidemiology of chronic rhinosinusitis: results from a cross-sectional survey in seven Chinese cities. Allergy. 2015;70(5):533–539. doi: 10.1111/all.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.LIN L., Dai F., Ren G.Q., Wei J.J., Chen Z., Tang X.Y. Efficacy of Lianhuaqingwen granules in the management of chronic rhinosinusitis without nasal polyps. Am. J. Otolaryngol. 2020;41(1) doi: 10.1016/j.amjoto.2019.102311. [DOI] [PubMed] [Google Scholar]
- 53.Qiu W. Observation on curative effects of Lianhua Qingwen capsule on acute suppurative tonsillitis. Med. J. Natl. Def. Forces Southwest China. 2016;26(05):536–539. [Google Scholar]
- 54.Zhao D., Yan J.X. Analysis of clinical efficacy of Lianhua Qingwen Capsule in the treatment of 133 cases of hand-foot-and-mouth disease. Guide Chin. Med. 2011;9(31):388–389. [Google Scholar]
- 55.Peng L.L., Li L., Shen L., Li X.L. Literature analysis of clinical application and adverse drug reaction/event of Lianhua Qingwen Capsule. Chin. Pharmacol. 2015;12(12):753–759. [Google Scholar]
- 56.Zheng W.K., Zhang J.H., Yang F.W., Wang Y.G., Liu Q.Q., Zhang B.L. Comprehensive analysis of diagnosis and treatment schemes for prevention and treatment of novel coronavirus pneumonia by traditional chinese medicine. J. Tradit. Chin. Med. 2020;61(4):277–280. [Google Scholar]