Abstract
Objective
To address the lack of information about clinical sequelae of coronavirus disease 2019 (COVID-19).
Patients and Methods
Previously hospitalized COVID-19 patients who were attending the outpatient clinic for post–COVID-19 patients (ASST Ovest Milanese, Magenta, Italy) were included in this retrospective study. They underwent blood draw for complete blood count, C-reactive protein, ferritin, D-dimer, and arterial blood gas analysis and chest high-resolution computed tomography (HRCT) scan. The primary endpoint was the assessment of blood gas exchanges after 3 months. Other endpoints included the assessment of symptoms and chest HRCT scan abnormalities and changes in inflammatory biomarkers after 3 months from hospital admission.
Results
Eighty-eight patients (n = 65 men; 73.9%) were included. Admission arterial blood gas analysis showed hypoxia and hypocapnia and an arterial partial pressure of oxygen/fractional inspired oxygen ratio of 271.4 (interquartile range [IQR]: 238-304.7) mm Hg that greatly improved after 3 months (426.19 [IQR: 395.2-461.9] mm Hg, P<.001). Forty percent of patients were still hypocapnic after 3 months. Inflammatory biomarkers dramatically improved after 3 months from hospitalization. Fever, resting dyspnea, and cough were common at hospital admission and improved after 3 months, when dyspnea on exertion and arthralgias arose. On chest HRCT scan, more than half of individuals still presented with interstitial involvement after 3 months. Positive correlations between the interstitial pattern at 3 months and dyspnea on admission were found. C-reactive protein at admission was positively associated with the presence of interstitial involvement at follow-up. The persistence of cough was associated with presence of bronchiectasis and consolidation on follow-up chest HRCT scan.
Conclusion
Whereas inflammatory biomarker levels normalized after 3 months, signs of lung damage persisted for a longer period. These findings support the need for implementing post–COVID-19 outpatient clinics to closely follow-up COVID-19 patients after hospitalization.
Abbreviations and Acronyms: ABG, arterial blood gas; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CT, computed tomography; DOE, dyspnea on exertion; GGO, ground-glass opacity; HRCT, high-resolution computed tomography; IQR, interquartile range; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; PaO2/FiO2, ratio of arterial partial pressure of oxygen to fractional inspired oxygen; PFT, pulmonary function test; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; V/Q, ventilation/perfusion ratio
Over the past months, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been responsible for the coronavirus disease 2019 (COVID-19) in a huge number of patients all over the world.1, 2, 3 Typical symptoms reported among COVID-19 patients, either hospitalized or not, included fever, dyspnea, cough, weakness, headache, nausea, diarrhea, and vomiting.4 Although most patients had no or few symptoms, a portion of them required hospitalization and eventually developed acute respiratory distress syndrome, which is potentially fatal.5
A wealth of evidence is now available about pathophysiology, clinical characteristics, and complications of the acute phase of COVID-19,4 but less is known about long-term consequences and few reports have been published to date. In the early recovery stage (ie, 30 days), abnormal findings on pulmonary function tests (PFTs) and chest computed tomography (CT) scans were found in more than half of COVID-19 patients.6 Another report described that most patients who recovered from COVID-19 had no signs of lung damage after 4 weeks from hospital discharge.7 Carfi et al8 reported that after a mean of 60 days from the onset of the first COVID-19 symptom, only 13% of patients were symptom-free, whereas more than one-third was still experiencing 1 or 2 symptoms and more than a half at least three symptoms, but none of them had fever or any other symptom of acute illness. In a study that followed patients after 90 days, clinical sequelae were commonly encountered, including fatigue, arthralgia, post-activity polypnea, resting tachycardia, psychosocial symptoms, and alopecia.9
Because no report has been published that assessed blood gas exchanges in patients who have recovered from COVID-19, the aim of our study was to evaluate whether oxygen and carbon dioxide levels were restored in post–COVID-19 patients attending our dedicated outpatient clinic in a COVID-19 hub in Lombardy (Italy).
Patients and Methods
Patient Enrollment
Because of the large spread of SARS-CoV-2 and its potential long-term impact on lungs, an outpatient clinic for post–COVID-19 patients was implemented at Magenta Hospital (Azienda Socio Sanitaria Territoriale Ovest Milanese; Magenta, Milan, Lombardy, Italy). Admission to this outpatient clinic was primarily dedicated to all adult patients (≥ 18 years) who had been hospitalized for SARS-CoV-2 pneumonia (laboratory real-time-polymerase chain reaction SARS-CoV-2 positivity and chest X-ray or CT scan suggestive for interstitial pneumonia along with typical symptoms) and with the following characteristics during hospital admission: (1) respiratory failure defined by arterial partial pressure of oxygen (PaO2) < 60 mm Hg in ambient air and/or resting peripheral capillary oxygen saturation < 93% in ambient air and/or ratio of PaO2 to fractional inspired oxygen (PaO2/FiO2), (ie, Horowitz Index) < 300 mm Hg10; (2) continuous positive airway pressure for at least 72 hours; (3) interstitial involvement >40% on chest X-ray or CT scan; (4) length of hospital stay > 7 days; and (5) evidence of venous thromboembolism, either pulmonary or peripheral, during hospital stay. All patients were treated with antibiotics, glucocorticoids (methylprednisolone 0.1-1.5 mg/kg for at least 7 days, then tapered) and oxygen therapy, while a portion of them were also treated with darunavir/ritonavir, lopinavir/ritonavir, hydroxychloroquine, and tocilizumab according to contemporary evidence about therapeutics.
After 3 months from hospital discharge, patients were admitted to the outpatient clinic and underwent the following evaluations to comprehensively assess the clinical status and any residual lung damage following COVID-19: blood draw for complete blood count, C-reactive protein (CRP), ferritin, and D-dimer, among others; arterial blood gas (ABG) analysis; chest high-resolution CT (HRCT) scan. HRCT scans were then reviewed by a trained radiologist to assess for interstitial involvement (such as ground-glass opacities [GGOs] and fibrosis) and consolidation.
From May 25 to August 31, 2020, 88 patients were consecutively considered for the present analysis. All clinical, laboratory, and radiologic information was included in a locked database that was accessible only to researchers involved in the study.
Ethical approval for this study and the need for written informed consent were not required because of the retrospective nature of the study; the ethical considerations of this research conformed to the Declaration of Helsinki. The study was performed and reported according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for observational studies.11
Comorbidities and Symptom Definition
Data on main comorbidities were collected, such as hypertension, diabetes mellitus, obesity, cardiovascular disease, respiratory and metabolic diseases, and cancer. Cardiovascular disease included coronary artery disease, atrial fibrillation, heart failure, and venous thromboembolism. Respiratory disease included chronic obstructive pulmonary disease and asthma. Metabolic disease included hyperlipidemia and hypothyroidism.
Fever was defined for a body temperature >37.5 °C. Dyspnea on exertion (DOE) was defined based on patient’s description of dyspnea for usually well-tolerated activities, such as climbing stairs.
Study Endpoints
The primary endpoint of the study was the evaluation of persistently abnormal blood gas exchanges (PaO2 and arterial partial pressure of carbon dioxide [PaCO2]) between the time of hospital admission and after 3 months. Secondary endpoints included (1) the assessment of symptoms at 3 months compared with symptoms at hospital admission; (2) the assessment of persisting chest HRCT scan abnormalities, if any; and (3) variation of inflammatory biomarkers between the time of hospital admission and after 3 months.
Statistical Analysis
The distribution of continuous data was examined using the Kolmogorov-Smirnov test. Non-normally distributed variables are expressed as median and interquartile range (IQR), whereas normally distributed variables are presented as mean ± SD. Variations of continuous variables between two time points were tested with Wilcoxon signed rank test or paired sample Student t test, as appropriate, whereas for categorical variables the McNemar’s test was used. Comparisons between categorical variables were tested with the Fisher exact test. Ranked Spearman’s correlation coefficients were used to evaluate correlations between symptoms at the time of admission and chest HRCT findings after 3 months. For all statistical analyses, a two-sided P<.05 was considered statistically significant. Analyses were performed using IBM SPSS Statistics for Mac, version 26.0 (IBM CO., Armonk, NY, USA) and GraphPad Prism, version 8.2 for Windows (GraphPad Software, La Jolla, CA, USA).
Results
General Characteristics of the Cohort
Eighty-eight patients were included in this study, with a large prevalence of males (n=65, 73.9%) and with a mean age of 62.7 years (Table 1). At baseline, all patients met criteria for respiratory failure (mean PaO2 57.4±12.4 mm Hg). Mean values of PaCO2 were 32.8±4.5 mm Hg.
Table 1.
| Characteristics | Overall cohort (N=88) |
|---|---|
| Age, years | 62.7±9.5 |
| Males/females | 65/23 (73.9/26.1) |
| BMI, kg/m2 | 26.9 (24.6-31.7) |
| Race | |
| Caucasian | 83 (94.3) |
| Otherc | 5 (5.7) |
| Medical history | |
| Hypertension | 40 (45.5) |
| Diabetes mellitus | 11 (12.5) |
| CV disease | 14 (15.9) |
| Obesity | 8 (9.1) |
| Respiratory diseases | 11 (12.5) |
| Metabolic diseases | 4 (4.5) |
| Cancer | 6 (6.8) |
| Vitals at admission | |
| SBP, mm Hg | 125 (115-131.2) |
| DBP, mm Hg | 75 (70-85) |
| HR, beats/min | 87.6±14 |
| SpO2, % | 93 (89-95) |
| Laboratory findings at admission | |
| WBC, n × 109/L | 6.7 (5.1-8.4) |
| Neutrophils, n × 109/L | 4.9 (3.5-6.7) |
| Lymphocytes, n x 109/L | 1.0 (0.7-1.3) |
| Hemoglobin, g/dL | 14.1±2.7 |
| Platelets, n × 109/L | 212.2±97.3 |
| CRP, mg/L | 83.3 (52-124) |
| Ferritin, ng/L | 1,265.8±1,376.9 |
| LDH, U/L | 381.9±140.4 |
| D-dimer, ng/mL | 362.5 (260-529.2) |
| Procalcitonin, μg/L | 0.15 (0.05-0.3) |
| Arterial blood gas analysis at admission | |
| PaO2, mm Hg | 57.4±12.4 |
| PaCO2, mm Hg | 32.8±4.5 |
| HCO3- , mEq/L | 25±3.6 |
| Lactate, mmol/L | 1.1 (0.9-1.5) |
| PaO2/FiO2, mm Hg | 271.4 (238.0-304.7) |
| Chest X-ray at admission | |
| No abnormal finding | 10 (11.4) |
| Involvement of 1 lobe | 3 (3.4) |
| Involvement of more than 1 lobe | 8 (9.1) |
| Bilateral involvement | 67 (76.1) |
| Outcomes | |
| Length of hospital stay, days | 18.8±11.5 |
| Need for intubation and MV | 11 (12.9) |
| Death | 2 (2.3) |
| Thrombotic complications | 7 (8.0) |
| Need for O2-therapy at discharge | 4 (4.5) |
BMI, body mass index; CRP, C-reactive protein; CV, cardiovascular; DBP, diastolic blood pressure; HCO3-, bicarbonate; HR, heart rate; LDH, lactate dehydrogenase; MV, mechanical ventilation; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; PaO2/FiO2, ratio of arterial partial pressure of oxygen to fractional inspired oxygen; SBP, systolic blood pressure; SpO2, peripheral capillary oxygen saturation; WBC, white blood cells.
Values shown are n (%), mean ± SD, or median (IQR), as appropriate.
For Race, “Other” includes Hispanic (n=4) and Asian (n=1).
The most common comorbidity was hypertension (n=40, 45.5%). Laboratory findings at admission showed increased levels of inflammatory biomarkers, especially CRP and ferritin (Table 1). The vast majority of patients (n=67, 76.1%) showed bilateral involvement on chest X-ray at admission (Table 1). Admission arterial ABG analysis highlighted hypoxia and hypocapnia (Table 2).
Table 2.
Changes of Laboratory Findings and Arterial Blood Gas Analysis Between Time of Hospital Admission and 3 Monthsa,b
| Tests | Time of hospital admission | 3 months | P |
|---|---|---|---|
| WBC, n × 109/L | 6.7 (5.1-8.4) | 6.45 (5.10-7.60) | .19 |
| Lymphocytes, n × 109/L | 1.0 (0.7-1.3) | 0.32 (0.26-0.40) | <.001 |
| CRP, mg/L | 83.3 (52-124) | 2.9 (2.9-2.9) | <.001 |
| Ferritin, ng/L | 1,265.8±1376.9 | 260.35±292.18 | <.001 |
| D-dimer, ng/mL | 362.5 (260-529.2) | 179.0 (113.7-265.5) | <.001 |
| PaO2, mm Hg | 57.4±12.4 | 90.5±15.6 | <.001 |
| PaCO2, mm Hg | 32.8±4.5 | 34.9±4.8 | .001 |
| PaO2/FiO2, mm Hg | 271.4 (238.0-304.7) | 426.2 (395.2-461.9) | <.001 |
CRP, C-reactive protein; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; PaO2/FiO2, ratio of arterial partial pressure of oxygen to fractional inspired oxygen; WBC, white blood cells.
Values shown are mean ± SD or median (IQR), as appropriate.
Variation of ABG Analysis and Laboratory Findings at 3 Months
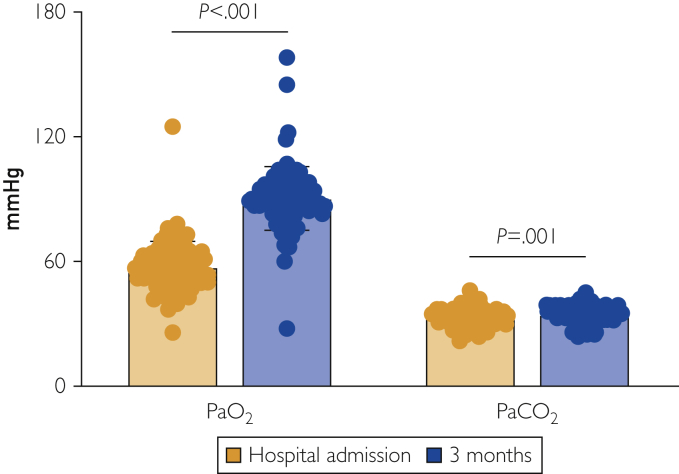
At hospital admission, PaO2/FiO2 was 271.4 (IQR: 238.0-304.7) mm Hg, suggesting a mild acute respiratory distress syndrome. After 3 months, no patient experienced respiratory failure (PaO2 90.5±15.6 mm Hg) and the median PaO2/FiO2 was 426.2 (IQR: 395.2-461.9) mm Hg (P<.001 vs hospital admission). Values for PaCO2 were at the lower limit of normal (34.9±4.7 mm Hg) (Figure 1). A portion of patients (n=35, 39.8%) was persistently hypocapnic (PaCO2 <35 mm Hg), but no significant difference in demographic or clinical characteristics was found when compared with normocapnic patients. Table 2 summarizes all these findings in more detail.
Figure 1.
Arterial blood gas analyses. Arterial partial pressure of oxygen (PaO2) and arterial partial pressure of carbon dioxide (PaCO2) are shown both at the time of hospital admission and 3 months later. While PaO2 normalizes, reduced levels of PaCO2 are found at 3-month follow-up. P for paired sample Student t test.
With regard to inflammatory biomarkers, all patients showed a dramatic decrease of CRP, ferritin, and D-dimer levels after 3 months compared with those assessed at the time of hospitalization (P<.001 for all) (Figure 2A-C). Detailed findings are included in Table 2.
Figure 2.
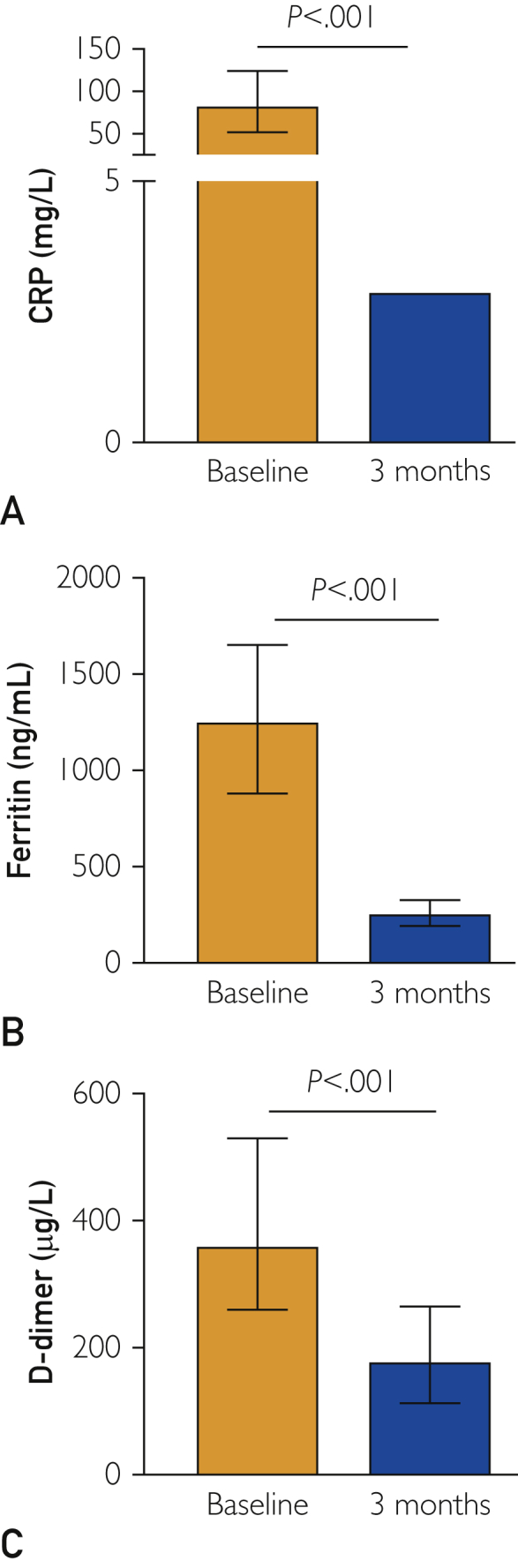
Inflammatory biomarkers. Levels of circulating inflammatory biomarkers at the time of hospital admission and 3 months later are presented. All patients showed a normalization of the inflammatory status at 3 months when compared with the time of hospitalization. P for Wilcoxon signed rank test or paired sample Student t test, as appropriate. CRP, C-reactive protein.
Persistence of Symptoms After 3 Months
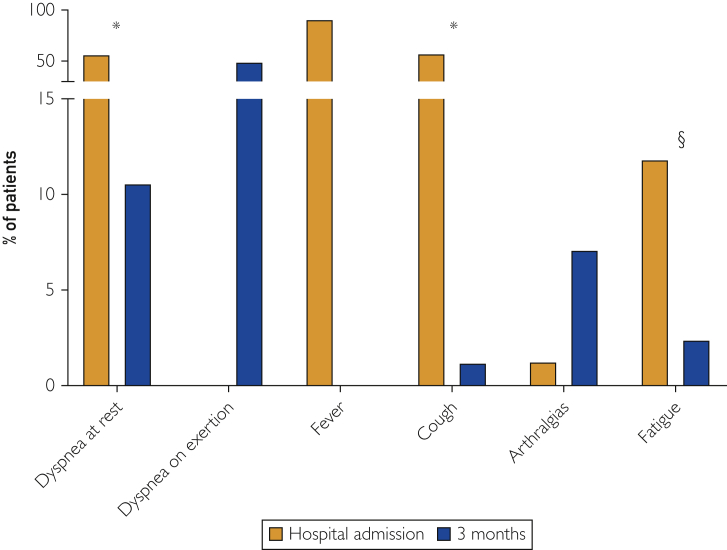
Fever (n=77, 90.6%), dyspnea at rest (n=48, 56.5%), and cough (n=49, 57.6%) were the most common symptoms at the time of hospital admission. Following 3 months from hospital discharge, DOE (n=42, 49.4%) and arthralgias (n=6, 7.1%) were the symptoms that patients complained most frequently about (Figure 3). Dyspnea at rest, cough, and fatigue were persistent in a number of patients, although to a lesser extent compared with the time of hospital admission (Figure 3). No sex-related nor comorbidity-related differences with regard to symptom presentation was found, except for resting dyspnea at the time of hospital admission that was found more frequently in males than in females (63.5% [n=40] vs. 36.5% [n=8], P=.04).
Figure 3.
Symptoms at the time of hospital admission and 3 months later are shown. Fever is not present anymore after 3 months. Resting dyspnea, cough, and fatigue are less frequent at follow-up. Dyspnea on exertion is very frequent after 3 months from hospital discharge, while it was not mentioned by patients on admission because of resting dyspnea. P for McNemar’s test: ∗ P<.001; § P=.04
Chest HRCT Scan Findings at 3 Months
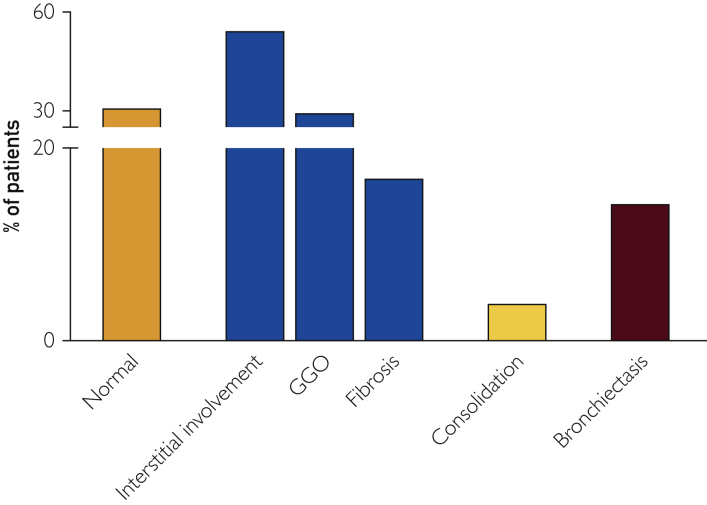
After 3 months, one-third of patients showed a normal CT scan without any abnormalities (Figure 4). More than half of patients still presented interstitial involvement (n=42, 54.5%), including GGOs (n=23, 29.5%) and fibrosis (n=13, 16.9%) (Figure 4). No sex-related difference was observed.
Figure 4.
Chest high-resolution computed tomography (HRCT) scan findings. At 3-month follow-up, all patients underwent HRCT. Interstitial involvement, ground-glass opacities (GGOs), and fibrosis are the most common findings, whereas one-third of patients showed no abnormalities.
Whether chest HRCT findings after 3 months may have any correlation with symptoms and/or inflammatory biomarkers is not known. We found positive correlations between the interstitial pattern, including GGO, at 3 months and dyspnea on admission (Table 3). Moreover, CRP levels at admission were positively associated with the presence of interstitial involvement at follow-up. The presence of bronchiectasis positively correlated with the presence of cough after 3 months from COVID-19 diagnosis. The strongest correlation was found for the persistence of cough after 3 months in those patients with persisting consolidation on follow-up chest HRCT scan (Table 3).
Table 3.
Correlations Among Chest High-Resolution Computed Tomography Scan Findings, Symptoms, and Inflammatory Biomarkers
| Findings | r | P |
|---|---|---|
| Interstitial involvement | ||
| Dyspnea on admission | 0.344 | .003 |
| PaO2 on admission | −0.233 | .046 |
| CRP on admission | 0.312 | .006 |
| GGO | ||
| Dyspnea on admission | 0.254 | .028 |
| Fibrosis | ||
| PaO2 after 3 months | −0.312 | .006 |
| Bronchiectasis | ||
| PaCO2 on admission | −0.242 | .038 |
| Cough after 3 months | 0.280 | .016 |
| Consolidation | ||
| Cough after 3 months | 0.569 | <.001 |
CRP, C-reactive protein; GGO, ground-glass opacity; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen.
Discussion
In the present study, the short-term (3 months) impact of COVID-19 on hospitalized patients has been assessed. We took into consideration different aspects, both subjective (symptoms complained by patients) and objective (chest HRCT findings and circulating inflammatory biomarkers). Apart from the persistence of some symptoms (fatigue, arthralgias, and cough), the presence of DOE, and persistent lung damage still found on 3-month chest CT scan, the most interesting finding is that 40% of patients are still hypocapnic after 3 months, whereas hypoxia completely resolved.
An abnormal lung function measured through PFTs has been recorded in COVID-19 patients. In particular, an impaired diffusion capacity of carbon monoxide is very frequent along with restrictive ventilatory defects at the time of hospital discharge and both findings are likely to be associated with severity disease.12 Although we did not perform any PFT, we assessed ABG after 3 months and found a mild, persisting hypocapnia whereas hypoxia completely normalized. This finding is interesting and may underline the residual, lasting impairment of the alveolar–capillary barrier following SARS-CoV-2 infection causing a ventilation/perfusion ratio (V/Q) mismatch. In fact, in SARS-CoV-2 infection the primary cause of arterial hypoxemia is a V/Q mismatch, meaning that there is pulmonary arterial blood flow in nonventilated alveoli. This concept is also summarized as “happy hypoxemia” — that is, a severe hypoxemia coupled by a relatively mild respiratory discomfort reported by COVID-19 patients.13 Patients with COVID-19 usually present with hypoxemia-driven tachypnea and hyperpnea that determine respiratory alkalosis and hypocapnia followed by a leftward shift of the sigmoid shaped oxyhemoglobin dissociation curve.13,14 In addition, COVID-19 is known to damage the endothelium and is also referred to as an endotheliopathy.15, 16, 17 As a result, the pulmonary capillary endothelium may activate an inflammatory burst through the expression of cytokines and adhesion molecules that sustains the abnormal inflammatory response to the virus and increases the risk for thrombosis, either in small and large vessels.18,19 All these events together might leave some type of scar tissue during the recovery phase and would therefore explain why hypocapnia is still present 3 months after the acute SARS-CoV-2 infection as a result of a persisting V/Q mismatch at the level of damaged alveoli.
The persistence of general and respiratory symptoms has been already described at different times (3 and 6 months) after COVID-19, irrespective of hospitalization. Goërtz et al20 reported that, although the number of symptoms declined during the 3-month follow-up period, some of them were still present, with fatigue and dyspnea being the most frequent ones both during hospitalization and follow-up. Also in our study, some patients still complained about resting dyspnea and fatigue after 3 months. In addition, DOE was described by half of the patients at 3-month follow-up. This aspect is of particular interest as DOE may have an important impact of patient’s quality of life in the recovery process from COVID-19. This may influence the patient’s recovery from COVID-19 due to a reduced mobility or the inability to restart a regular physical activity that is known to be beneficial in patients with respiratory diseases.21,22 Huang et al23 analyzed a longer time frame (ie, 6 months) and found that survivors of COVID-19 complained about fatigue, weakness, sleep disorders, anxiety, and depression. They also analyzed chest CT findings between hospital stay and after 6 months describing GGO as the most common pattern at follow-up, whereas other findings were completely resolved.23 This is in line with what we found on HRCT performed after 3 months, where GGO was frequently encountered along with interstitial involvement. These radiologic findings are often described at the same time patients have a respiratory deterioration and are coupled with lymphocytopenia and increased D-dimer levels.24 Fibrosis is another abnormality that can be observed as early as 7 to 10 days after symptom onset in up to one-third of patients with COVID-19.25,26 Indeed, almost 20% of our patients showed evidence of fibrosis on HRCT scan at the time of follow-up that may arise from the healing process of lung inflammation when scar tissues replace the pulmonary cellular cells. Whether fibrosis in post–COVID-19 patients is prognostically benign or not is still controversial as it might represent either a bridge toward a stabilization of the disease27 or the primum movens toward a frank interstitial disease.27,28 This aspect definitively needs future research to be fully elucidated.
Interestingly, in our cohort CRP levels at admission positively correlated with interstitial involvement. Therefore, patients with higher inflammatory burden during COVID-19 pneumonia may be more at risk to develop pulmonary sequelae. With this regard, a dose-response effect of CRP towards adverse outcomes in COVID-19 patients has been recently demonstrated.29 A median CRP value >108 mg/L was associated with a three-fold increased risk of death and critical illness and a two-fold higher risk of venous thromboembolism and acute kidney injury. The greatest risk for adverse outcomes was found in those patients showing increased levels of both CRP and D-dimer, further supporting the strong relationship between inflammation and thrombosis.16
Study Limitations
Our study has some strengths and some limitations. The major strength deals with the enrollment of a number of patients who have been adequately assessed for respiratory function. In particular, the availability of ABG analysis allows for a prompt evaluation of gas exchanges 3 months after COVID-19. One limitation is the absence of PFTs that might enrich the explanation about the persisting hypocapnia through the demonstration of a decreased lung diffusion capacity for carbon monoxide, as already shown in COVID-19 patients at the time of discharge.12 In addition, the retrospective nature and the limited number of patients recruited at a single center prevent from any definitive conclusions, but support the need for a more in depth follow-up evaluation of COVID-19 patients.
Conclusion
Patients with COVID-19 who recovered from acute SARS-CoV-2 infection present clinical sequelae. In particular, a substantial portion of them still have residual hypocapnia after 3 months. They also show persisting abnormal findings on chest HRCT scans, especially interstitial involvement, GGO, and fibrosis. While inflammatory biomarker levels completely normalized, signs of lung damage (eg, hypocapnia and radiologic abnormalities on CT scan) are still there, suggesting that SARS-CoV-2 stigma persist for a longer period. These findings should push the medical community to carefully evaluate COVID-19 patients, especially those who experienced a moderate-to-severe disease, in the short- and long-term to catch any early sign of respiratory failure that may arise months or years after the acute infection. Future studies on a large number of patients including all aspects of respiratory diagnostics are eagerly needed to know more in depth what COVID-19 patients experience after the initial SARS-CoV-2 infection.
Footnotes
Potential Competing Interests: Drs Bonaventura and Vecchié received a travel grant from Kiniksa Pharmaceuticals, Ltd. to attend the 2019 AHA Scientific Sessions and receive honoraria from Effetti s.r.l. (Milan, Italy) to collaborate on the medical website www.inflammology.org, outside the present work. The remaining authors no potential competing interests.
References
- 1.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrilli C.M., Jones S.A., Yang J. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A Review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 5.Grasselli G., Greco M., Zanella A. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Y., Tan C., Wu J. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21(1):163. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C., Ye L., Xia R. Chest computed tomography and clinical follow-up of discharged patients with COVID-19 in Wenzhou City, Zhejiang, China. Ann Am Thorac Soc. 2020;17(10):1231–1237. doi: 10.1513/AnnalsATS.202004-324OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carfi A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong Q., Xu M., Li J. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27(1):89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Force A.D.T., Ranieri V.M., Rubenfeld G.D. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 11.von Elm E., Altman D.G., Egger M. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 12.Mo X., Jian W., Su Z. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55(6):2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhont S., Derom E., Van Braeckel E., Depuydt P., Lambrecht B.N. The pathophysiology of “happy” hypoxemia in COVID-19. Respir Res. 2020;21(1):198. doi: 10.1186/s12931-020-01462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woyke S., Rauch S., Strohle M., Gatterer H. Modulation of Hb-O2 affinity to improve hypoxemia in COVID-19 patients. Clin Nutr. 2021;40:38–39. doi: 10.1016/j.clnu.2020.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans P.C., Rainger G.E., Mason J.C. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc Res. 2020;116(14):2177–2184. doi: 10.1093/cvr/cvaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu S.X., Tyagi T., Jain K. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat Rev Cardiol. 2021;18(3):194–209. doi: 10.1038/s41569-020-00469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonaventura A., Vecchie A., Dagna L. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21(5):319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagashima S., Mendes M.C., Camargo Martins A.P. Endothelial dysfunction and thrombosis in patients with COVID-19 — brief report. Arterioscler Thromb Vasc Biol. 2020;40(10):2404–2407. doi: 10.1161/ATVBAHA.120.314860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goertz Y.M.J., Van Herck M., Delbressine J.M. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6(4):00542–2020. doi: 10.1183/23120541.00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watz H., Pitta F., Rochester C.L. An official European Respiratory Society statement on physical activity in COPD. Eur Respir J. 2014;44(6):1521–1537. doi: 10.1183/09031936.00046814. [DOI] [PubMed] [Google Scholar]
- 22.Agusti A. Physical activity and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(7):675–676. doi: 10.1164/rccm.200712-1790ED. [DOI] [PubMed] [Google Scholar]
- 23.Huang C., Huang L., Wang Y. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X., Zeng W., Li X. CT imaging changes of corona virus disease 2019(COVID-19): a multi-center study in Southwest China. J Transl Med. 2020;18(1):154. doi: 10.1186/s12967-020-02324-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye Z., Zhang Y., Wang Y., Huang Z., Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020;30(8):4381–4389. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan Y., Guan H., Zhou S. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020;30(6):3306–3309. doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong W., Agarwal P.P. Chest Imaging Appearance of COVID-19 Infection. Radiol Cardiothorac Imaging. 2020;2(1):e200028. doi: 10.1148/ryct.2020200028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smilowitz N.R., Kunichoff D., Garshick M. C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J. 2021;42(23):2270–2279. doi: 10.1093/eurheartj/ehaa1103. [DOI] [PMC free article] [PubMed] [Google Scholar]