Abstract
The severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) is the most recent coronaviruses, which has infected humans, and caused the disease COVID-19. The World Health Organization has declared COVID-19 as a pandemic in March 2020. The SARS-CoV-2 enters human hosts majorly via the respiratory tract, affecting the lungs first. In few critical cases, the infection progresses to failure of the respiratory system known as acute respiratory distress syndrome acute respiratory distress syndrome may be further associated with multi-organ failure and vasoplegic shock. Currently, the treatment of COVID-19 involves use of antiviral and anti-cytokine drugs. However, both the drugs have low efficacy because they cannot inhibit the production of free radicals and cytokines at the same time. Recently, some researchers have reported the use of methylene blue (MB) in COVID-19 management. MB has been used since a long time as a therapeutic agent, and has been approved by the US FDA for the treatment of other diseases. The additional advantage of MB is its low cost. MB is a safe drug when used in the dose of < 2 mg/kg. In this review, the applicability of MB in COVID-19 and its mechanistic aspects have been explored and compiled. The clinical studies have been explained in great detail. Thus, the potential of MB in the management of COVID-19 has been examined.
Keywords: Methylene blue, Leucomethylene blue, COVID-19, SARS-CoV-2, Nitric oxide pathway, Cytokine storm
1. Introduction
The most recent coronaviruses (CoVs) which have infected humans is the severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) that has caused the COVID-19 pandemic. The infections are prevalent and the numbers are ever-increasing, with 169,118,995 confirmed cases and 3,519,175 deaths globally as of 29 May 2021 [1], [2], [3]. The SARS-CoV-2 is a novel beta coronavirus and enters human hosts majorly via the respiratory tract [4], [5]. The virus optimally binds to the human angiotensin converting enzyme 2 (ACE2) receptors, that are overexpressed in the lungs [6]. Additionally, because the lungs are the first organs to be encountered by the virus, they are affected early. The autopsies of many COVID-19 patients reveal inflammation of the endothelium. This is because ACE2 receptors are present on the vascular endothelium lining all the organs [7], [8]. In few critical cases, the infection progresses to respiratory failure known as acute respiratory distress syndrome (ARDS). ARDS may be further associated with multi-organ failure and vasoplegic shock [9], [10], [11]. After the SARS-CoV-2 enters the cell, an inflammatory cascade is initiated by activation of the signal transduction pathways, including NF-κB, that stimulates cytokine production to prevent the replication and spread of the virus [12].
In COVID-19, the local lung infection progresses into systemic pathology that leads to increased fatality. The systemic immune response is also activated following severe tissue injuries. In this case, the systemic immune response is similar to sepsis and is termed as “systemic inflammatory response syndrome” (SIRS). The bone marrow and liver accumulate pro-thrombotic factors producing thrombosis or blood clots [13]. Critically ill COVID-19 patients meet the SIRS criteria (tachypnea, fever, and lymphopenia), and elevated levels of inflammatory markers (eg, ferritin, C-reactive protein), and proinflammatory cytokines. However, distributive shock is rare compared to patients with elevated cytokine levels (non-COVID sepsis, ARDS, CAR T-cell therapy). Further, lactate levels have been rarely reported for large observational studies, which may be attributed to their generally normal values [14]. Currently, COVID-19 has been managed by the administration of antiviral and anti-cytokine drugs. However, they possess low efficacy. The antiviral drugs can be efficacious in ARDS and multi-organ failure only if they are administered before initiation of the inflammatory cascade [15], [16], [17]. Anti-cytokine drugs were also found to be less effective because they inhibit only one or a few of the many cytokines involved, and they do not take free radicals and kinins into account that play a major role in the inflammatory cascade [18], [19]. Thus, the use of only antiviral or anti-cytokine drugs in ARDS is doomed to fail, because of the uncontrollable production of free radicals (ROS, RNS) and cytokines [20], [21], [22]. There is a need for a therapeutic agent which can inhibit the production of both the free radicals and cytokines.
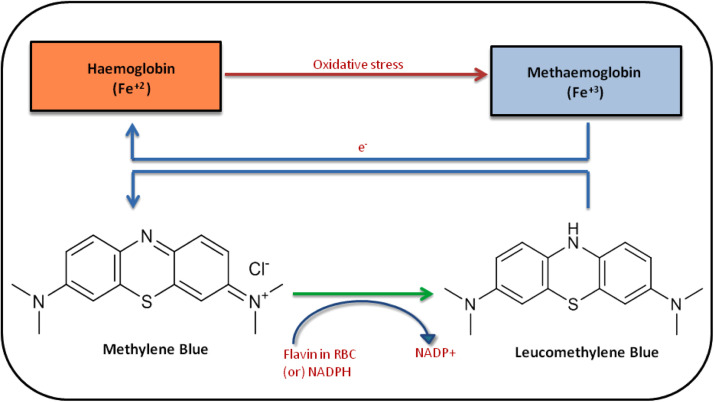
Methylene blue (MB) or methylthionine chloride, chemically (3,7-bis(dimethyl amino) phenothiazine-5-ium chloride), is a tricyclic phenothiazine dye that is deep blue in color [23]. The US FDA has approved MB for other indications and is a low-cost molecule. MB undergoes reduction by nicotinamide adenine dinucleotide phosphate (NADPH) to produce leucomethylene blue (leucoMB), which is a colorless compound. This is a reversible reaction ( Fig. 1). MB undergoes renal excretion via the kidneys as a mixture of MB, leucoMB, and demethylated MB metabolites like azure A and azure B [24]. After oral administration, the maximum concentration of MB is reached at 2 h. Its plasma half-life is 20 h [25].
Fig. 1.
Schematic representation of the Methylene blue accompanied by supplemental oxygen. The methylene blue undergoes reduction by nicotinamide adenine dinucleotide phosphate (NADPH) to produce leucomethylene blue (leucoMB). Note: Fe+2: Ferrous; Fe+3: Ferric; RBC: Red blood cells; NADPH: Nicotinamide adenine dinucleotide phosphate; NADP+: Nicotinamide adenine dinucleotide.
MB has a long history of more than 140 years, but it has managed to revive itself because of its wide range of applications. It is one of the most famous drugs to be repurposed for different clinical applications several times. MB was prepared as a dye for textiles by Caro in 1876 [26], [27]. Further, Paul Ehrlich developed the staining activity of MB and formed the groundwork of modern chemotherapy in 1891 [28]. Then, in the late 19th century it was used in the treatment of malaria, however, its use was discontinued because of the inevitable adverse effects; blue sclera and green urine [29], [30]. In the 1920s, MB was used as an antidote for cyanide poisoning because the reduction potential of MB is equal to the reduction potential of oxygen, and MB is readily reduced by the elements of the electron transport chain [31], [32]. Further, MB was found to reverse toxic methemoglobinemia [33]. MB has been found to reverse hypotension in sepsis and is useful in cases of vasoplegia [34]. Due to its unique physicochemical characteristics including its ionic charges, redox chemistry, light spectrum properties, and planar structure, MB exerts a wide range of clinical applications on the nervous system [35], [36]. MB has been used in photodynamic therapy for excisional wounds, hepatitis C, HIV, and psoriasis [37], [38], [39]. MB has also been established as a diagnostic marker for oral cancer [40], [41] and breast cancer [42].
Thus, MB is a versatile molecule. Recently, there have been various reports on the use of MB for COVID-19 management. This is because MB inhibits superoxide anion (ROS precursor) formation by blocking the xanthine oxidase pathway, nitric oxide (RNS precursor) formation by directly inhibiting nitric oxide synthase, and cytokine production by attenuating NF-κB pathway [43], [44], [45], [46], and ultimately inhibits the production of both free radicals and cytokines. This is evident from the various ongoing clinical studies reported in this regard. In this review, the applicability of methylene blue in COVID-19 and its mechanistic aspects have been explored and compiled. The clinical studies have been explained in great detail. Thus, the potential of methylene blue in the management of COVID-19 has been examined.
2. Possible mechanisms of methylene blue in reducing COVID-19 pathogenesis
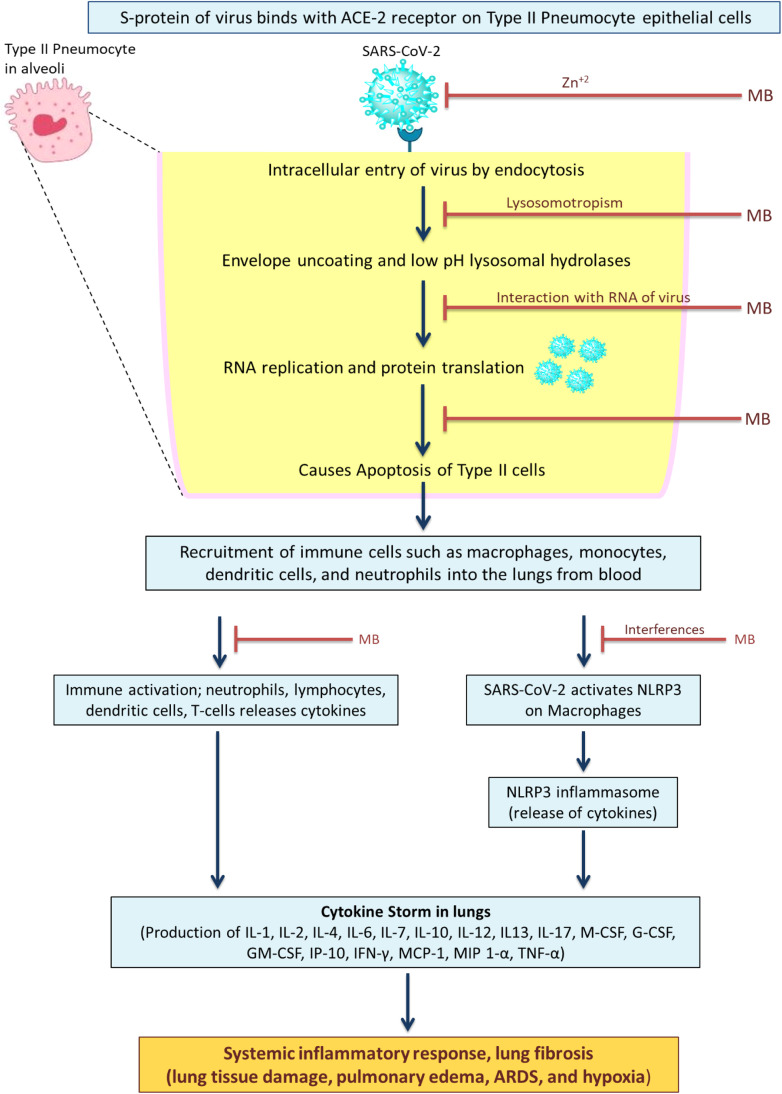
The possible features that could support the antiviral effect against SARS-COV-2 are discussed ( Fig. 2). In the early 1930s, the first observations of MB ability to inactivate bacteriophages and viruses were reported [47]. Antiviral effectiveness and action are influenced by the target's vicinity to the singlet oxygen generator. The possible feature that could support the antiviral effect against SARS-COV-2 is the positive charge of MB. This charge improves its propensity for binding to negatively charged RNA of viruses and causes viral RNA protein cross-linkage [48]. In addition, methylene blue is Zn+2 ionophore. As the Zn+2 metal inhibits the elongation of RNA-dependent RNA polymerase, a component of the RNA viruses that are not found in the human body. So, this suggests that this might potentially inhibit the SARS-COV-2 [49].
Fig. 2.
Methylene blue as potential repurposed drug candidate for COVID-19. The diagram depicts the potential interactions of MB with SARS-CoV-2. By protonation, MB accumulates in the lysosome, raising its pH and blocking low pH dependent hydrolases that are required for the virus's uncoating and membrane fusion. MB inhibits the binding of the viral spike (S) protein (glycoprotein) with angiotensin converting enzyme 2 (ACE2) receptors initiates SARS-CoV infection of host cells then being proteolytically activated by human proteases. MB has the ability to transport Zn2+ across the viral envelope by endo-lysosomes. As the Zn+2 metal inhibits the elongation of RNA dependent RNA polymerase. The methylene interferes with the NLRP3 on macrophages and prevents the cytokine storm in lungs.
Lungs, the first organ to be infected with SARS-CoV2 with noticeable findings such as dry cough and oxygen saturation drop (hypoxia). It is well reported that nitric oxide is majorly involved in viral-induced pneumonia [50]. Nitric oxide synthesis is activated by the cytokines IFN-α, IL-1, IL-2, IL-6, and TNF- α, which are released during COVID-19-associated hyper-inflammation [51]. In COVID-19 patients due to the elevated blood nitrites and nitrates levels are proof of Nitric oxide imbalance [52]. Nitric oxide suppresses mitochondrial respiration through cytochrome oxidase and NADH ubiquinone oxidoreductase targeting, and potentially amplifying the hypoxic state caused by SARS-CoV-2 infection. The nitric oxide synthase inhibitors (eg: MB) may appear to be operative in slowing the disease progression and increasing hemoglobin recruitment and oxygen saturation. Hemoglobin is the molecule that allows erythrocytes to carry oxygen. Hemoglobin binds with 4 molecules of oxygen as it is made up of four globins, each of which is connected to a heme molecule. Methaemoglobin (Fe+3) is an oxidized form of hemoglobin (Fe+2). Methaemoglobin is unable to transport oxygen, and excessive amounts (more than 20%) can result in cell ischemia and shock. MB could show protection against hypoxic/ischemic tissues damage. In the non-enzymatic reduction of methemoglobin, methylene blue works as an electron donor. The MB is reduced to leuco-MB by reacting within red blood cells (RBC) or by a NADPH methemoglobin reductase. The leuco-MB acts as a reducing agent of oxidized hemoglobin, ferric ion (Fe+3) back to its oxygen-carrying ferrous state (Fe+2) within the RBC [53]. Nitric oxide and cyclic guanosine-3′,5′-monophosphate (cGMP) together form broad signal transduction system which involves the multiple roles of cGMP in physiological regulation, such as smooth muscle relaxation, visual transduction, intestinal ion transport, and platelet function [54].
NO is produced by nerve terminals and endothelial cells, which promotes the activity of soluble guanylate cyclase, resulting in an increase in cGMP, calcium shortage in the cytosolic region, and cavernous smooth muscle relaxation [54]. Soluble GC is highly present in the lungs and brain and is a heme-containing protein present in the cytosol of almost all mammalian cells [54]. However, the use of NO inhibitors is limited because of their lack of specificity in blocking the various NOS isoforms (L - NMMA, L - NAME). Thus nitric oxide synthase inhibitors are not currently in clinical usage, posing a risk of broad tissue necrosis and a greater death rate [55]. Whereas, MB acts as an effective guanylyl cyclase inhibitor, and prevents vascular smooth muscle relaxation by blocking the release of cGMP without impacting NO production directly [55].
The binding of the viral spike (S) protein (glycoprotein) with ACE2 receptors initiates SARS-CoV infection of host cells then being proteolytically activated by human proteases. This is a necessary step for SARS-CoV-2 replication [56]. The blocking of receptor-binding domain ACE2 protein-protein interaction prevents infection efficiency. Erythrosine B is proved to be the selective inhibitor of protein-protein interactions. Bojadzic research group evaluated MB inhibitory activity against the interaction between SARS-CoV- 2 spike protein and its associated receptor ACE2. MB had a similar IC50 (3.5 µM) when it came to inhibiting the entry of a SARS-CoV-2 spike carrying pseudovirus into ACE2-expressing cells [57]. Additionally, the endosome and lysosomes are involved in getting the SARS-CoV-2 into the cells. Methylene blue increases endosomes and lysosomes' intracellular pH may further aid viral detoxification. Also, the reduced product of MB, leuco-MB, might easily permeate and protonate lysosomal membranes, favoring pH increase [58]. As a result, it might be possible that endosome differentiation is inhibited at intermediate stages of endocytosis. Therefore, it prevents the endocytosis of virions into the cells. The critical points in SARS-CoV-2 infection could be the exhaustion of antiviral defenses associated with the innate immune responses, as well as an increased generation of pro-inflammatory cytokines. As per the recent reports of the COVID-19 patients, cytokine storm was observed. The patients showed abnormal levels of the cytokines and chemokines that is, IL-1, IL-2, IL-4, IL-6, IL-7, IL-10, IL-12, IL13, IL-17, M-CSF, G-CSF, GM-CSF, IP-10, IFN-γ, MCP-1, MIP 1-α, hepatocyte growth factor (HGF), TNF-α, and vascular endothelial growth factor (VEGF) [59], [60]. This condition results in tissue and organ damage. The anti-inflammatory properties of MB may protect against COVID-19-induced hyper inflammation. NLRP3 (NOD-like receptor protein 3 inflammasome formation and activation is required for viral infection. In SARS-COV-2 patients, viroporin 3a activates the NLRP3 inflammasome and causes macrophages to secrete IL-1 [61]. As a result of uncontrolled SARS-CoV-2 replication, a persistent and abnormal NLRP3 inflammasome signaling could cause hyper-inflammatory response (cytokine storm) in severe cases of COVID-19. So, instead of blocking individual cytokines, blocking this NLRP3 complex might prevent the cytokine storm. The MB inhibits the formation of NLRP3 complex [62].
Recently, dysregulated signaling of the peptide bradykinin, known as the ‘bradykinin storm’, has emerged as an explanation for the complications associated with COVID-19. Along with bradykinin, two other vasoactive peptides – substance P and neurotensin – are likely to cause microvascular permeability and inflammation, and are responsible for the pathology involved in COVID-19. Thus, ‘bradykinin storm’ is better referred to as ‘vasoactive peptide storm’. If these three peptides are inhibited, it can terminate the ‘vasoactive peptide storm’ and can better modulate the effects of COVID-19 [63]. MB may terminate the effects of bradykinin by inhibiting nitric oxide synthase inhibitor and promoting saturation of oxygen [64], [65].
3. Dosage of methylene blue in humans
MB has been used in the treatment of various diseases since time immemorial and has been administered mainly through oral and intravenous routes. MB is a safe drug when used in doses of < 2 mg/kg [53]. However, it may produce toxic manifestations at high doses. At 2–4 mg/kg dose, it can cause skin desquamation in the infants, and at 7 mg/kg it may cause nausea, vomiting, fever, abdominal pain, hemolysis, and chest pain. As the dose increases to 7.5 mg/kg, it may cause confusion and hyperpyrexia. At a dose of 20 mg/kg, it may lead to hypotension. At a very high dose of 80 mg/kg, it may cause a bluish skin discoloration like cyanosis [23], [66], [67].
MB is administered at a dose of 1–2 mg/kg as an intravenous bolus for vasodilation with hypotension [68], [69], and is followed by intravenous continuous infusion for a duration of 48–72 h in the case of vasoplegic syndrome [70]. A continuous infusion of MB at 0.25–1 mg/kg.h is indicated for sepsis treatment [34]. For anaphylactic shock, MB is given as intravenous bolus injection at a dose of 1.5–2 mg/kg [71]. In the case of hereditary methemoglobinemia, a dose of up to 300 mg is administered orally for a lifetime [72]. In acute methemoglobinemia, a dose of 1.3 mg/kg is administered by intravenous injection for 20 min once or twice per day [73]. For the prevention of urinary tract infection in the elderly, MB is administered orally at a dose of 65 mg/day. In pediatric malaria, 12 mg/kg of MB is administered orally twice a day for 3 days [24], [30], [74]. Thus, the selection of proper dosage and the route of administration is quintessential for the safe use of MB as a therapeutic agent and prevent adverse effects associated with its use. It has been suggested that MB should be administered orally at a dose of 2–3 mg/kg body weight/day divided into three daily doses for 7–10 days in patients newly infected with SARS-CoV-2 [43].
4. Clinical studies of MB in COVID-19 management
In a medical center, 80 patients with severe COVID-19 were assigned in a random manner to receive oral MB with standard care (MB; n = 40) or standard care only (SC; n = 40). The primary outcome measures were improvement of oxygen saturation and respiratory rate on day 3 and day 5. The secondary outcome measures were the length of hospital stay and mortality within 28 days. It was observed that the MB group demonstrated a significant (p < 0.0001) improvement in both oxygen saturation and respiratory rate on day 3 and day 5. In the SC group, no significant improvement was observed on day 3. However, on day 5, there was a significant improvement in oxygen saturation (p = 0.002) and respiratory rate (p = 0.01). The increase in oxygen saturation rate in MB group was 13.5 folds and 2.1 folds greater on day 3 and day 5, respectively, compared to SC group. Similarly, the increase in the respiratory rate in MB group was 10.1 folds and 3.7 folds greater on day 3 and day 5, respectively, compared to SC group. In the MB group, the hospital stay was shortened significantly (p = 0.004), and the mortality rate was 12.5% compared to 22.5% in the SC group. Thus, the addition of MB in the protocol of COVID-19 treatment enhanced the oxygen saturation levels and improved respiratory distress in severe cases of COVID-19, shortened hospital stay, and reduced mortality [75].
A randomized, parallel, single masking interventional clinical trial (ClinicalTrials.gov Identifier: NCT04370288) was started on 19th April 2020 for the application of MB in the COVID-19 treatment. The study completion date was estimated to be 21st September 2020. Currently, the study is in Phase I recruiting stage. The intervention consisted of an injectable mixture MB, vitamin C, N-acetyl cysteine (MCN). The control group was not given any intervention and was treated with standard medical therapy. The experimental group was administered the mixture of MCN. In a study conducted at Mashhad University of Medical Sciences, Mashhad, Iran in 2020, it was estimated that the metabolites of nitric oxide (nitrite and nitrate), methemoglobin, and prooxidant-antioxidant balance (PAB) are involved in the intensification of hypoxia in hospitalized COVID-19 patients. The study participants were 25 healthy individuals and 25 patients with COVID-19 pneumonia admitted to ICU with Pa02/Fi02 < 200. For all the patients, blood gas, CBC, CRP, LDH measurements were done, and high-resolution computed tomography (HRCT) was done. Five COVID-19 patients in the final stage were administered MCN as a compassionate therapy after standard care (1 mg/kg MB, 1500 mg/kg vitamin C, and 1500 mg/kg N-acetyl cysteine by the oral or intravenous route), and included in the clinical trial that was already running (ClinicalTrials.gov Identifier: NCT04370288). It was observed that nitrite, nitrate, methemoglobin, and oxidative stress were significantly increased in COVID-19 patients compared to healthy individuals. 4 out of 5 patients responded well to the MCN treatment, and increased the survival rate of the patients. The therapeutic effect of MCN can be attributed to the macrophage activation cycle leading to oxidative stress and “cytokine storm”. The additional benefit of this protocol is the use of economical drugs approved by the FDA for other diseases [76].
An open-label, randomized, parallel clinical study was started on 29th April 2020 by Grifol Therapeutics LLC (Instituto Grifols, S.A.) to evaluate the safety and efficacy of convalescent MB treated plasma from donors recovered from COVID-19 disease. The intervention included intravenous infusion of convalescent anti-SARS-CoV-2 MB treated plasma (CAP) and SC. The experimental group received CAP and SC from day 1 to day 29. The active comparator group received only SC from day 1 to day 29. They wanted to determine if CAP and SC can reduce all-cause mortality up to day 29 in COVID-19 hospitalized patients compared to the patients administered with SC alone. Currently, the study has completed phase II recruitment, but no results have been posted yet (ClinicalTrials.gov Identifier: NCT04547127).
A randomized clinical study was conducted to introduce a way to treat patients with severe COVID-19 with respiratory complications. Patients were assigned in a parallel manner to one of the three groups; group 1 received exchange transfusion (500 cc), group 2 received intravenous MB (1 mg/kg) with convalescent plasma (200 cc), and group 3 received exchange transfusion (500 cc) and intravenous MB (1 mg/kg) with convalescent plasma (200 cc). The primary outcome measures were improvement of condition from day 3 to day 5, and general condition of the patient (ventilator parameters, oxygen saturation, serum level of ferritin and D dimer, and CBC). The secondary outcome measures include the change in organ function up to 1 month. The study was initiated on 20th May 2020 and is currently recruiting for phase II (ClinicalTrials.gov Identifier: NCT04376788).
A randomized, open-label clinical study was initiated on 12th October 2020. The COVID-19 outpatients were treated with MB and photodynamic therapy. This is based on the reported studies of the use of MB as a photosensitizer in cancer treatment and protection of serum from viruses. Patients included in the experimental and control groups must have one of the symptoms of nausea, vomiting, headache, dyspnea, and myalgia. The experimental group was administered sublingual MB with low-level light therapy NocUlite, and the control group was administered the conventional therapy which may include anti-inflammatory, steroid, antiplatelet, and anticoagulant. The primary outcome measures are changes in oxygen saturation levels measured by an oximeter (up to 7 days) and days to patient discharge (up to 7 days). Currently, the clinical study is in phase I recruiting stage (ClinicalTrials.gov Identifier: NCT04619290).
A phase II randomized, placebo-controlled, single-blind clinical trial was started on 5th November 2020, to study the efficacy of MB in patients diagnosed with COVID-19 infection recently. The experimental group was administered 100 mg MB capsules, and the control group was administered 100 mg placebo capsules, every 12 h for 5 days. The primary outcome measure compared the viral load kinetics in the COVID-19 patients with at least a 25% reduction in the AUC from day 0 to day 21. The secondary outcome measures included the percentage of patients that cleared COVID-19 by days 3, 3, 6, 9, 12, 15, and 21 after diagnosis, had reduced viral load of > 2 log by day 3, and that were alive. Currently, the clinical study is in phase II recruiting stage (ClinicalTrials.gov Identifier: NCT04635605). The summary of undergoing clinical studies of MB in COVID-19 management is presented in Table 1.
Table 1.
Summary of undergoing clinical studies of MB in COVID-19 management.
| Clinical study identifier | Treatment protocol | Study phase and estimated enrollment (n) | Primary outcome/s | Recruitment status | Published results |
|---|---|---|---|---|---|
| NCT04370288 |
|
Phase I n = 20 | Proportion of patients remaining free of need for mechanical ventilation in both groups [Time Frame: Day 7] | Recruiting | 4 out of 5 patients responded well to the MCN treatment, and increased the survival rate of the patients. The therapeutic effect of MCN can be attributed to the macrophage activation cycle leading to oxidative stress and “cytokine storm”[68]. |
| NCT04547127 |
|
Phase II n = 200 | All-Cause Mortality Rate [Time Frame: Up to Day 29] | Completed | Not available yet |
| NCT04376788 |
|
Phase II n = 15 | Improvement of condition (Time frame: 3–5 days) | Recruiting | Not available yet |
| NCT04619290 |
|
Phase I n = 46 | Change form baseline in Arterial oxygen saturation [Time Frame: up to 7 days] | Recruiting | Not available yet |
| NCT04635605 |
|
Phase II n = 64 | To compare the viral load kinetics in the enrolled patients with a SARS-CoV-2 positive nasopharyngeal swab demonstrating a reduction of the area under the curve day 0- day 21 of at least 25% [Time Frame: day 0 - day 21] | Recruiting | Not available yet |
5. Conclusion and future perspectives
The havoc caused by the SARS-CoV-2 virus as the COVID-19 pandemic is evident worldwide from the ever-increasing statistics. In the case of critically ill patients, the current conventional therapies are doomed to fail because they are able to target either the cytokines or free radicals; but not both of them at a time. In this situation, MB has the potential to be utilized in COVID-19 treatment. MB has been approved by the US FDA for the treatment of several other diseases and is an inexpensive ubiquitously available therapeutic agent. Its role in COVID-19 can be understood from the various scientific reports that have been compiled in this review. Further efforts are being made in this direction in the form of clinical trials to study the effect on MB in the clinical setting. The results are satisfactory and provide a ray of hope in this direction. Thus, MB can be termed as a “rescue magic bullet” for COVID-19 treatment. However, for MB administration, meticulous consideration of the dosage is necessary to prevent any untoward effects. MB can be administered thrice orally at a dose of 2–3 mg/kg per day for 7–10 days in newly infected COVID-19 patients. However, this needs to be further studied, and finding the optimal dosage should be the objective of clinical study [43]. The use of MB in novel dosage forms like an anti-COVID mouthwash may also be beneficial [77].
It was reported that there is an increased risk of acute myocarditis in the Covid-19 patients [78]. To treat this condition, plasma consisting of pro-coagulant factors is routinely given to patients with coagulopathies [79]. In this instance, it has been demonstrated that they develop a pro-thrombotic condition with a high risk of pulmonary embolism. However, several procedures were reported to inactivate the pathogens in the plasma [80]. Among these them, the first reported method is the treatment of plasma with MB and light [80]. Treatment with MB and light influences a number of coagulation factors, the most notable of which are fibrinogen and thrombin (factor II). In addition, approximately 30% of anti-hemophilic factor has been lost [80]. The inactivation of active thrombin and stuart factor is catalyzed by the unfractionated heparin. In this MB has also been reported in Heparin monitoring as a contrast agent in photoacoustic 30 (PA) imaging. A study by wang et al. reported that the addition of heparin to MB solution resulted in 3.7-fold higher MB dimerization, indicating that heparin aids MB aggregation [81].
Nowadays, the use of anti-depressants is prevalent among patients. MB is a monoamine oxidase (MAO) inhibitor and can interact with the antidepressants (selective serotonin reuptake inhibitors and MAO inhibitors) to cause severe toxicity of serotonin [82], [83]. It has also been found to interact with dapsone to form hydroxylamine, which oxidizes hemoglobin and may cause hemolysis [84]. Also, MB is contraindicated in patients with severe renal insufficiency. The use of MB in patients with G6PD deficiency can be detrimental, as it may cause severe hemolysis [53]. In such cases, the use of vitamin B12 and ascorbic acid has proven to be beneficial [85], [86], [87]. Additionally, concomitant use of NSAIDs may block the bradykinin activity pathways. Thus, NSAIDs may add benefit to MB therapy in COVID-19 [64], [88].
Lastly, we must understand that we are at the brink of a global medical emergency. Too obsessive methodology and procrastination will not lead us anywhere. Urgent generalized measures must be taken in this regard to save humankind from this pandemic.
CRediT authorship contribution statement
Neha Dabholkar and Srividya Gorantla: Methodology, Investigation, and Writing – original draft; Sunil Kumar Dubey: case studies compilation and review; Amit Alexander: Figures and table; Rajeev Taliyan: review and editing; Gautam Singhvi: Conceptualization, Writing – review & editing.
Conflict of interest statement
Authors declare no conflict of interest.
Acknowledgments
None.
Funding
None.
References
- 1.World Health Organization . WHO Coronavirus Dashboard; 2021. WHO Coronavirus (COVID-19) Dashboard. [Google Scholar]
- 2.Wu Y.C., Chen C.S., Chan Y.J. The outbreak of COVID-19: an overview. J. Chin. Med. Assoc. 2020;83:217–220. doi: 10.1097/JCMA.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pascarella G., Strumia A., Piliego C., Bruno F., Del Buono R., Costa F., Scarlata S., Agrò F.E. COVID-19 diagnosis and management: a comprehensive review. J. Intern. Med. 2020;288:192–206. doi: 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.da Costa V.G., Moreli M.L., Saivish M.V. The emergence of SARS, MERS and novel SARS-2 coronaviruses in the 21st century. Arch. Virol. 2020;165:1517–1526. doi: 10.1007/s00705-020-04628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pal M., Berhanu G., Desalegn C., Kandi V. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): an update. Cureus. 2020 doi: 10.7759/cureus.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Q., Fang P., He R., Li M., Yu H., Zhou L., Yi Y., Wang F., Rong Y., Zhang Y., Chen A., Peng N., Lin Y., Lu M., Zhu Y., Peng G., Rao L., Liu S. O-GlcNAc transferase promotes influenza A virus-induced cytokine storm by targeting interferon regulatory factor-5. Sci. Adv. 2020;6:7086. doi: 10.1126/sciadv.aaz7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dostálová V., Dostál P. Acute respiratory distress syndrome. Vnitr. Lek. 2019;65:193–203. doi: 10.20473/jr.v4-i.2.2018.51-60. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z., Spieth P.M., Chiumello D., Goyal H., Torres A., Laffey J.G., Hong Y. Declining mortality in patients with acute respiratory distress syndrome: an analysis of the acute respiratory distress syndrome network trials. Crit. Care Med. 2019;47:315–323. doi: 10.1097/CCM.0000000000003499. [DOI] [PubMed] [Google Scholar]
- 11.Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E., Dei Poli M., Resta M., Falco M., Albano G., Menicanti L. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemost. 2020;18:1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mogensen T.H., Paludan S.R. Molecular pathways in virus-induced cytokine production. Microbiol. Mol. Biol. Rev. 2001;65:131–150. doi: 10.1128/mmbr.65.1.131-150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polidoro R.B., Hagan R.S., de Santis Santiago R., Schmidt N.W. Overview: systemic inflammatory response derived from lung injury caused by SARS-CoV-2 infection explains severe outcomes in COVID-19. Front. Immunol. 2020;11:1626. doi: 10.3389/FIMMU.2020.01626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanidziar D., Bittner E.A. Hypotension, systemic inflammatory response syndrome, and COVID-19: a clinical conundrum. Anesth. Analg. 2020;131:e175–e176. doi: 10.1213/ANE.0000000000005062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Şimşek Yavuz S., Ünal S. Antiviral treatment of COVID-19. Turk. J. Med. Sci. 2020;50:611–619. doi: 10.3906/sag-2004-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Indari O., Jakhmola S., Manivannan E., Jha H.C. An update on antiviral therapy against SARS-CoV-2: how far have we come? Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.632677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirtaleb M.S., Mirtaleb A.H., Nosrati H., Heshmatnia J., Falak R., Zolfaghari Emameh R. Potential therapeutic agents to COVID-19: an update review on antiviral therapy, immunotherapy, and cell therapy. Biomed. Pharmacother. 2021;138 doi: 10.1016/j.biopha.2021.111518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heimfarth L., Serafini M.R., Martins-Filho P.R., de J., Quintans S.S., Quintans-Júnior L.J. Drug repurposing and cytokine management in response to COVID-19: a review. Int. Immunopharmacol. 2020;88 doi: 10.1016/j.intimp.2020.106947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monteleone G., Sarzi-Puttini P.C., Ardizzone S. Preventing COVID-19-induced pneumonia with anticytokine therapy. Lancet Rheumatol. 2020;2:255. doi: 10.1016/S2665-9913(20)30092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang J.D., McArdle P.J., O’Reilly P.J., Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest. 2002;122:314. doi: 10.1378/chest.122.6_suppl.314S. [DOI] [PubMed] [Google Scholar]
- 21.Fink M.P. Role of reactive oxygen and nitrogen species in acute respiratory distress syndrome. Curr. Opin. Crit. Care. 2002;8:6–11. doi: 10.1097/00075198-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020:1–13. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miclescu A., Wiklund L. Methylene blue, an old drug with new indications? Rom. J. Anestezie Intensive/Rom. J. Anaesth. Intensive Care. 2010;17:35–41. [Google Scholar]
- 24.Schirmer R.H., Adler H., Pickhardt M., Mandelkow E. Lest we forget you - methylene blue. Neurobiol. Aging. 2011;32:2325.e7–2325.e16. doi: 10.1016/j.neurobiolaging.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Walter-Sack I., Rengelshausen J., Oberwittler H., Burhenne J., Mueller O., Meissner P., Mikus G. High absolute bioavailability of methylene blue given as an aqueous oral formulation. Eur. J. Clin. Pharmacol. 2009;65:179–189. doi: 10.1007/s00228-008-0563-x. [DOI] [PubMed] [Google Scholar]
- 26.Mehlhorn H. Caro, Heinrich (1834–1910) Encycl. Parasitol. 2016 doi: 10.1007/978-3-662-43978-4_5031. [DOI] [Google Scholar]
- 27.M. Blue, Cases that would interest you Login to View the image Methylene blue nebulisation in COVID19 patient Login to View the image Login to View the image Login to View the image, (n.d.).
- 28.Valent P., Groner B., Schumacher U., Superti-Furga G., Busslinger M., Kralovics R., Zielinski C., Penninger J.M., Kerjaschki D., Stingl G., Smolen J.S., Valenta R., Lassmann H., Kovar H., Jäger U., Kornek G., Müller M., Sörgel F. Paul Ehrlich (1854-1915) and his contributions to the foundation and birth of translational medicine. J. Innate Immun. 2016;8:111–120. doi: 10.1159/000443526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu G., Nagbanshi M., Goldau N., Mendes Jorge M., Meissner P., Jahn A., Mockenhaupt F.P., Müller O. Efficacy and safety of methylene blue in the treatment of malaria: a systematic review. BMC Med. 2018;16:59. doi: 10.1186/s12916-018-1045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schirmer R.H., Coulibaly B., Stich A., Scheiwein M., Merkle H., Eubel J., Becker K., Becher H., Müller O., Zich T., Schiek W., Kouyaté B. Methylene blue as an antimalarial agent. Redox Rep. 2003;8 doi: 10.1179/135100003225002899. 272-5. [DOI] [PubMed] [Google Scholar]
- 31.Hanzlik P.J. Methylene blue as antidote for cyanide poisoning. J. Am. Med. Assoc. 1933;100:357. doi: 10.1001/jama.1933.02740050053028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung J.Y., Wang J., Zhang X.Q., Song J., Tomar D., Madesh M., Judenherc-Haouzi A., Haouzi P. Methylene blue counteracts cyanide cardiotoxicity: cellular mechanisms. J. Appl. Physiol. (1985) 2018;124:1164–1176. doi: 10.1152/japplphysiol.00967.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.David S.R., Sawal N.S., Bin Bin Hamzah M.N.S., Rajabalaya R. The blood blues: a review on methemoglobinemia. J. Pharmacol. Pharmacother. 2018;9 doi: 10.4103/jpp.JPP_79_17. [DOI] [Google Scholar]
- 34.Kwok E.S.H., Howes D.W. Use of methylene blue in sepsis: a systematic review. J. Intensive Care Med. 2006;21 doi: 10.1177/0885066606290671. 359-63. [DOI] [PubMed] [Google Scholar]
- 35.Oz M., Lorke D.E., Hasan M., Petroianu G.A. Cellular and molecular actions of Methylene Blue in the nervous system. Med. Res. Rev. 2011;31:93–117. doi: 10.1002/med.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ginimuge P.R., Jyothi S.D., Resident S. Methylene blue: revisited. J. Anaesthesiol. Clin. Pharmacol. 2010;26:517–520. [PMC free article] [PubMed] [Google Scholar]
- 37.Zolfaghari P.S., Packer S., Singer M., Nair S.P., Bennett J., Street C., Wilson M. In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent. BMC Microbiol. 2009;9:27. doi: 10.1186/1471-2180-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Müller-Breitkreutz K., Mohr H. Hepatitis C and human immunodeficiency virus RNA degradation by methylene blue/light treatment of human plasma. J. Med. Virol. 1998;56 doi: 10.1002/(SICI)1096-9071(199811)56:3<239::AID-JMV11>3.0.CO;2-9. 239-45. [DOI] [PubMed] [Google Scholar]
- 39.Salah M., Samy N., Fadel M. Methylene blue mediated photodynamic therapy for resistant plaque psoriasis. J. Drugs Dermatol. 2009;8 [PubMed] [Google Scholar]
- 40.Hwang S.H., Kim S.W., Song E.A., Lee J., Kim D.H. Methylene blue as a diagnosis and screening tool for oral cancer and precancer. Otolaryngol. Head. Neck Surg. 2021;164:271–276. doi: 10.1177/0194599820947686. [DOI] [PubMed] [Google Scholar]
- 41.Riaz A., Shreedhar B., Kamboj M., Natarajan S. Methylene blue as an early diagnostic marker for oral precancer and cancer. Springerplus. 2013;2:95. doi: 10.1186/2193-1801-2-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yaroslavsky A.N., Feng X., Muzikansky A., Hamblin M.R. Fluorescence polarization of methylene blue as a quantitative marker of breast cancer at the cellular level. Sci. Rep. 2019;9:940. doi: 10.1038/s41598-018-38265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scigliano G., Scigliano G.A. Methylene blue in covid-19. Med. Hypotheses. 2021;146 doi: 10.1016/j.mehy.2020.110455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salaris S.C., Babbs C.F., Voorhees W.D. Methylene blue as an inhibitor of superoxide generation by xanthine oxidase. Biochem. Pharmacol. 1991;42:499–506. doi: 10.1016/0006-2952(91)90311-r. [DOI] [PubMed] [Google Scholar]
- 45.Mayer B., Brunner F., Schmidt K. Inhibition of nitric oxide synthesis by methylene blue. Biochem. Pharmacol. 1993;45 doi: 10.1016/0006-2952(93)90072-5. 367-74. [DOI] [PubMed] [Google Scholar]
- 46.Wang J., Zhao C., Kong P., Bian G., Sun Z., Sun Y., Guo L., Li B. Methylene blue alleviates experimental autoimmune encephalomyelitis by modulating AMPK/SIRT1 signaling pathway and Th17/Treg immune response. J. Neuroimmunol. 2016;299:45–52. doi: 10.1016/j.jneuroim.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 47.The photodynamic action of methylene blue on bacteriophage, Proc. R. Soc. London. Ser. B, Contain. Pap. a Biol. Character. 112, 1933, 277–287. 10.1098/RSPB.1933.0010. [DOI]
- 48.Floyd R.A., Schneider J.E., Dittmer D.P. Methylene blue photoinactivation of RNA viruses. Antivir. Res. 2004;61:141–151. doi: 10.1016/j.antiviral.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 49.te Velthuis A.J.W., van den Worml S.H.E., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang W., Jiang J., Su L., Shu T., Liu H., Lai S., Ghiladi R.A., Wang J. The role of NO in COVID-19 and potential therapeutic strategies. Free Radic. Biol. Med. 2021;163:153–162. doi: 10.1016/j.freeradbiomed.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaz A.R., Silva S.L., Barateiro A., Fernandes A., Falcão A.S., Brito M.A., Brites D. Pro-inflammatory cytokines intensify the activation of NO/NOS, JNK1/2 and caspase cascades in immature neurons exposed to elevated levels of unconjugated bilirubin. Exp. Neurol. 2011;229:381–390. doi: 10.1016/j.expneurol.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Shiva S., Wang X., Ringwood L.A., Xu X., Yuditskaya S., Annavajjhala V., Miyajima H., Hogg N., Harris Z.L., Gladwin M.T. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat. Chem. Biol. 2006;2:486–493. doi: 10.1038/nchembio813. [DOI] [PubMed] [Google Scholar]
- 53.Ginimuge P.R., Jyothi S.D. Methylene blue: revisited. J. Anaesthesiol. Clin. Pharmacol. 2010;26:517–520. /pmc/articles/PMC3087269/ (accessed May 31, 2021) [PMC free article] [PubMed] [Google Scholar]
- 54.Ghalayini I.F. Nitric oxide–cyclic GMP pathway with some emphasis on cavernosal contractility. Int. J. Impot. Res. 2004;16:459–469. doi: 10.1038/sj.ijir.3901256. [DOI] [PubMed] [Google Scholar]
- 55.Evora P.R.B. Methylene blue is a guanylate cyclase inhibitor that does not interfere with nitric oxide synthesis. Tex. Heart Inst. J. 2016;43:103. doi: 10.14503/THIJ-15-5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 57.Bojadzic D., Alcazar O., Buchwald P. Methylene blue inhibits the SARS-CoV-2 spike–ACE2 protein-protein interaction–a mechanism that can contribute to its antiviral activity against COVID-19. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.600372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wainwright M., Amaral L. The phenothiazinium chromophore and the evolution of antimalarial drugs. Trop. Med. Int. Heal. 2005;10:501–511. doi: 10.1111/j.1365-3156.2005.01417.x. [DOI] [PubMed] [Google Scholar]
- 59.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.V.J. Costela-Ruiz, R. Illescas-Montes, J.M. Puerta-Puerta, C. Ruiz, L. Melguizo-Rodríguez, SARS-CoV-2 infection: The role of cytokines in COVID-19 disease, 2020. 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed]
- 61.Chen I.Y., Moriyama M., Chang M.F., Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front. Microbiol. 2019;10:50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van den Berg D.F., te Velde A.A. Severe COVID-19: NLRP3 inflammasome dysregulated. Front. Immunol. 2020;11:1580. doi: 10.3389/fimmu.2020.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karamyan V.T. Between two storms, vasoactive peptides or bradykinin underlie severity of COVID-19? Physiol. Rep. 2021;9:14796. doi: 10.14814/PHY2.14796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghahestani S.M., Shahab E., Karimi S., Madani M.H. Methylene blue may have a role in the treatment of COVID-19. Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghahestani S.M. Authorea; 2020. Methylene Blue in SARS COV -2: Blue Romance may be a Solution of Black Tragedy. [DOI] [Google Scholar]
- 66.Maslow A.D., Stearns G., Batula P., Schwartz C.S., Gough J., Singh A.K. The hemodynamic effects of methylene blue when administered at the onset of cardiopulmonary bypass. Anesth. Analg. 2006;103:2–8. doi: 10.1213/01.ane.0000221261.25310.fe. [DOI] [PubMed] [Google Scholar]
- 67.Mathew S., Linhartova L., Raghuraman G. Hyperpyrexia and prolonged postoperative disorientation following methylene blue infusion during parathyroidectomy. Anaesthesia. 2006;61 doi: 10.1111/j.1365-2044.2006.04619.x. 580-3. [DOI] [PubMed] [Google Scholar]
- 68.Leyh R.G., Kofidis T., Strüber M., Fischer S., Knobloch K., Wachsmann B., Hagl C., Simon A.R., Haverich T. Methylene blue: the drug of choice for catecholamine-refractory vasoplegia after cardiopulmonary bypass? J. Thorac. Cardiovasc. Surg. 2003;125 doi: 10.1016/S0022-5223(02)73284-4. 1426-31. [DOI] [PubMed] [Google Scholar]
- 69.Levin R.L., Degrange M.A., Bruno G.F., Del Mazo C.D., Taborda D.J., Griotti J.J., Boullon F.J. Methylene blue reduces mortality and morbidity in vasoplegic patients after cardiac surgery. Ann. Thorac. Surg. 2004;77 doi: 10.1016/S0003-4975(03)01510-8. 496-9. [DOI] [PubMed] [Google Scholar]
- 70.Evora P.R.B., Levin R.L., Leyh R.G., Kofidis T., Struber M., Fischer S., Knobloch K., Wachsmann B., Hagl C., Simon A.R., Haverich A. Methylene blue as drug of choice for catecholamine-refractory vasoplegia after cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 2004;127 doi: 10.1016/j.jtcvs.2003.09.046. 895-6. [DOI] [PubMed] [Google Scholar]
- 71.Oliveira Neto A.M., Duarte N.M., Vicente W.V.A., Viaro F., Evora P.R.B. Methylene blue: an effective treatment for contrast medium-induced anaphylaxis. Med. Sci. Monit. 2003;9 102-6. [PubMed] [Google Scholar]
- 72.Cawein M., Behlen C.H., Lappat E.J., Cohn J.E. Hereditary diaphorase deficiency and methemoglobinemia. Arch. Intern. Med. 1964;113 doi: 10.1001/archinte.1964.00280100086014. 578-85. [DOI] [PubMed] [Google Scholar]
- 73.Pelgrims J., De Vos F., Van Den Brande J., Schrijvers D., Prové A., Vermorken J.B. Methylene blue in the treatment and prevention of ifosfamide-induced encephalopathy: report of 12 cases and a review of the literature. Br. J. Cancer. 2000;82 doi: 10.1054/bjoc.1999.0917. 291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Warth A., Goeppert B., Bopp C., Schirmacher P., Flechtenmacher C., Burhenne J. Turquoise to dark green organs at autopsy. Virchows Arch. 2009;454 doi: 10.1007/s00428-009-0734-x. 341-4. [DOI] [PubMed] [Google Scholar]
- 75.Hamidi-Alamdari D., Hafizi-Lotfabadi S., Bagheri-Moghaddam A., Safari H., Mozdourian M., Javidarabshahi Z., Peivandi-Yazdi A., Ali-Zeraati A., Sedaghat A., Poursadegh F., Barazandeh-Ahmadabadi F., Agheli-Rad M., Tavousi S.M., Vojouhi S., Amini S., Amini M., Majid-Hosseini S., Tavanaee-Sani A., Ghiabi A., Nabavi-Mahalli S., Morovatdar N., Rajabi O., Koliakos G. Methylene blue for treatment of hospitalized COVID-19 patients: a randomized, controlled, open-label clinical trial, phase 2. Rev. Invest Clin. 2021;73:190–198. doi: 10.24875/ric.21000028. [DOI] [PubMed] [Google Scholar]
- 76.Alamdari D.H., Moghaddam A.B., Amini S., Keramati M.R., Zarmehri A.M., Alamdari A.H., Damsaz M., Banpour H., Yarahmadi A., Koliakos G. Application of methylene blue -vitamin C –N-acetyl cysteine for treatment of critically ill COVID-19 patients, report of a phase-I clinical trial. Eur. J. Pharmacol. 2020;885 doi: 10.1016/j.ejphar.2020.173494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arakeri G., Rao US V. Methylene blue as an anti-COVID-19 mouthwash in dental practice. Br. J. Oral. Maxillofac. Surg. 2021;59:135–136. doi: 10.1016/j.bjoms.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. 2020 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tritschler T., Mathieu M.E., Skeith L., Rodger M., Middeldorp S., Brighton T., Sandset P.M., Kahn S.R., Angus D.C., Blondon M., Bonten M.J., Cattaneo M., Cushman M., Derde L.P.G., DeSancho M.T., Diehl J.L., Goligher E., Jilma B., Jüni P., Lawler P.R., Marietta M., Marshall J.C., McArthur C., Miranda C.H., Mirault T., Morici N., Perepu U., Schörgenhofer C., Sholzberg M., Spyropoulos A.C., Webb S.A., Zarychanski R., Zuily S., Le Gal G. Anticoagulant interventions in hospitalized patients with COVID-19: a scoping review of randomized controlled trials and call for international collaboration. J. Thromb. Haemost. 2020;18:2958–2967. doi: 10.1111/JTH.15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lozano M., Cid J., Müller T.H. Plasma treated with methylene blue and light: clinical efficacy and safety profile. Transfus. Med. Rev. 2013;27:235–240. doi: 10.1016/J.TMRV.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 81.Wang J., Jeevarathinam A.S., Humphries K., Jhunjhunwala A., Chen F., Hariri A., Bill I., Miller R., Jokerst J.V. A mechanistic investigation of methylene blue and heparin interactions and their photoacoustic enhancement. Bioconjug. Chem. 2018;29:3768–3775. doi: 10.1021/ACS.BIOCONJCHEM.8B00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gillman P.K. Methylene blue implicated in potentially fatal serotonin toxicity. Anaesthesia. 2006;61 doi: 10.1111/j.1365-2044.2006.04808.x. 1013-4. [DOI] [PubMed] [Google Scholar]
- 83.Bewachter P., Mouton-Faivre C., Tréchot P., Lieu J.C., Mertes P.M. Severe anaphylactic shock with methylene blue instillation. Anesth. Analg. 2005;101 doi: 10.1213/01.ANE.0000153497.60047.80. [DOI] [PubMed] [Google Scholar]
- 84.Clifton J., Leikin J.B. Methylene blue. Am. J. Ther. 2003;10:289–291. doi: 10.1097/00045391-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 85.Sahu K.K., Mishra A.K., Mishra K. Methemoglobinemia in COVID-19. Am. J. Med. Sci. 2021 doi: 10.1016/j.amjms.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dhibar D.P., Sahu K.K., Jain S., Kumari S., Varma S.C. Methemoglobinemia in a case of paint thinner intoxication, treated successfully with vitamin C. J. Emerg. Med. 2018;54:221–224. doi: 10.1016/j.jemermed.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 87.Sahu K.K., Mishra A.K., Lal A., George S.V., Siddiqui A.D. Closing the saturation gap: a ten-year retrospective experience with methemoglobinemia. Intern. Emerg. Med. 2020;15:1109–1112. doi: 10.1007/s11739-020-02332-0. [DOI] [PubMed] [Google Scholar]
- 88.Kelleni M.T. Early use of non-steroidal anti-inflammatory drugs in COVID-19 might reverse pathogenesis, prevent complications and improve clinical outcomes. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.110982. [DOI] [PMC free article] [PubMed] [Google Scholar]