Abstract
The coronavirus disease 2019 (COVID-19) has swept the world, posing a serious threat to people's lives and health. Several cases of COVID-19 infection in renal transplant recipients (RTRs) have been reported, but the treatment and prognosis have not been fully elucidated. We followed-up with RTRs infected with SARS-CoV2 in our center and classified them as five clinical types—asymptomatic, mild, moderate, severe, and critical. The immunosuppressive agents were not adjusted in asymptomatic carriers and mild patients, the former was mainly treated by isolation, and the latter was treated by low-dose intravenous immunoglobulin (IVIG) to enhance immunity. For moderate or severe patients, the immunosuppressive agents were largely reduced or even interrupted, low-dose IVIG was adopted, and low-dose methylprednisolone (MP) was used to inhibit inflammation and rejection. Immunosuppressants were discontinued early in critical patients; IVIG, high-dose MP, and antibiotics were used. Meanwhile, all patients received at least one antiviral drugs. After aggressive treatment, three patients developed acute kidney injury, and two showed reversal, while the remaining one lost the allograft kidney; one patient died, while other patients were discharged. For different clinical types of RTRs infected with COVID-19, personalized therapies were essential, Meanwhile, patients with COVID-19 infection may have different outcomes due to their different clinical manifestations.
Keywords: Management, COVID-19, Renal transplant, SARS-CoV-2, Treatment
Abbreviations: ADPKD, autosomal dominant polycystic kidney disease; AKI, acute kidney injury; ARDS, acute respiratory distress syndrome; BP, blood pressure; BNP, brain natriuretic peptide; CNI, calcineurin inhibitor; COVID-19, Coronavirus Disease 2019; CT, computed tomography; CRP, C-reactive protein; Cr, creatine; CRRT, continuous renal replacement therapy; CMV, cytomegalovirus; eGFR, estimated glomerular filtration rate; DCD, donation after cardiac death; ESRD, end-stage renal disease; FiO2, fraction of inspiration O2; HR, heart rate; IVIG, intravenous immunoglobulin; ICU, intensive care unit; MP, methylprednisolone; MMF, mycophenolate mofetil; NAT, nucleic acid testing; PCT, procalcitonin; PaO2, partial pressure of oxygen; PJP, Pneumocystis jiroveci pneumonia; RTRs, renal transplant recipients; SPO2, pulse oxygen saturation; SARS-CoV2, severe acute respiratory syndrome-Coronavirus 2; WHO, world health organization; WBC, white blood cell
1. Introduction
In December 2019, an outbreak of Coronavirus Disease 2019 (COVID-19) was identified in the world. This epidemic rapidly spread globally, and the World Health Organization (WHO) declared COVID-19 to be a pandemic in early March 2020 [1]. As of June 15, 2020, more than 6 million cases and 400,000 deaths have been confirmed worldwide. According to the WHO guidelines, while the overall population is generally susceptible, elderly patients and those with chronic underlying conditions have a poorer prognosis than young, healthy adults [2]. Owing to long-term use of immunosuppressive agents, recipients of renal transplantation comprise an immunocompromised population that are prone to all kinds of opportunistic infection [3]. According to the theory, renal transplant recipients (RTRs) should be susceptible to infection with severe acute respiratory syndrome-coronavirus 2 (SARS-CoV2), which can easily progress to critical type COVID-19 and cause poor prognosis [4].However, based on multiple reports, the COVID-19 infection rate of transplant recipients is similar to that of the general population, most of whom had a good prognosis in Wuhan [5,6]. Moreover, the clinical manifestations of COVID-19 vary widely among patients, from asymptomatic carrier to the most critical type. To cure COVID-19 and protect graft function, different types of patients should choose different treatment options. In this paper, we summarize the clinical data of different clinical types of RTRs in our center to provide reference for the treatment of RTRs infected with COVID-19.
2. Methods
2.1. Diagnosis standard
Epidemiological history (≤14 days): travel to or residence in Wuhan and its surrounding areas, or other communities where COVID-19-positive cases had been found; contact with COVID-19 patients or contact with patients with fever or respiratory symptoms and from Wuhan and its surrounding areas, or from communities where COVID-19 has been found; clustered cases.
Clinical symptoms: fever and/or respiratory symptoms; normal or decreased white blood cell (WBC) count, normal or decreased lymphocyte count; imaging characteristics of COVID-19.
Any one criteria of epidemiological history plus any two clinical symptoms, or all three clinical symptoms were considered as suspected cases. A confirmed case of COVID-19 was made based on the suspected cases with etiological or serological evidence: (1) RT-PCR positivity in respiratory specimens for SARS-CoV-2 or (2) serological evidence with specific IgG or IgM.
2.2. Clinical classification methods
According to the diagnosis and treatment guidelines of the national health commission of China (7th edition), all COVID-19 confirmed cases were classified into different types [2]. The classification criteria are described below:
Asymptomatic infected people: no related clinical symptoms such as fever, cough, or sore throat and no evidence of pneumonia on computed tomographic (CT) images, but positive nucleic acid test or specific antibody.
Mild type: mild clinical manifestation, without pneumonia.
Moderate type: fever and/or respiratory symptoms with mild pneumonia.
Severe type: dyspnea or respiratory Rate ≥ 30/min or SpO2 < 93% or PaO2/FiO2 < 300 mmHg, or lung infiltrates >50% within 24–48 h.
Critical type; Needs mechanical ventilation or shock or complicated with other organ failure and requiring ICU admission.
3. Results
3.1. Clinical cases series
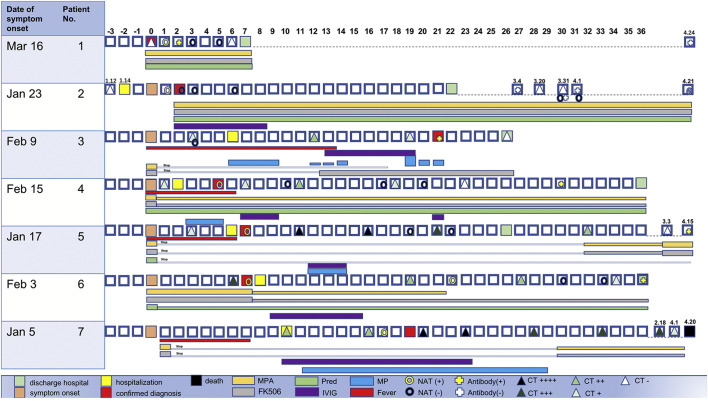
The mean age of the seven RTRs who developed COVID-19 pneumonia was 52 years (range: 37–64 years, four male and three female). The transplant length covered from 10 days to 80 months after renal transplantation. Seven patients were associated with one or more of the following diseases: hypertension (n = 6), myocardial infarction (n = 1), and polycystic kidney disease (n = 1). The maintenance immunosuppression regimen was MMF + Tacrolimus (FK) + prednisone. Serum creatinine and eGFR was maintained at normal levels during follow-up. In terms of epidemiology, all seven patients lived in Wuhan, but only one family member was infected with SARS-CoV-2. Fever developed in five of the seven patients, with the highest recorded body temperature being 38.9 °C; fatigue and myalgia were seen in three patients; productive cough in two, and dyspnea and dysphonia in one. None of the patients exhibited significant gastrointestinal symptoms. The duration of 7 cases of COVID-19 -19 infection after renal transplantation is shown in Fig. 1 .Demographic and clinical details of the seven patients infected with COVID-19 were shown in Table 1 .
Fig. 1.
This is an overview of the course of 7 patients with COVID-19 infection after renal transplantaion.
Table 1.
Demographic and clinical details of the seven patients infected with COVID-19.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | mean ± SD |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 37 | 59 | 52 | 64 | 56 | 54 | 48 | 52.86 ± 8.65 |
| Sex | Male | Male | Female | Female | Female | Male | Male | – |
| Height(cm)/weight (Kg) | 174/74 | 173/59 | 162/52 | 158/58 | 160/50 | 170/67 | 168/60 | 166.43 ± 6.43/60.00 ± 8.31 |
| Comorbidities | HTN | ADPKD | HTN | HTN | HTN | HTN,MI | HTN | – |
| Length after transplant | 2 mo | 10 d | 48 mo | 80 mo | 64 mo | 73 mo | 4 mo | 38.76 ± 35.67mo |
| IS protocol | M + FK + P | M + FK + P | M + FK + P | M + FK + P | M + FK + P | M + FK + P | M + FK + P | – |
| SCr (μmol/L) | 141 | 58 | 127 | 77 | 44 | 114 | 133 | 99.14 ± 38.98 |
| eGFR (mL/min) | 42 | 106 | 42 | 70 | 116 | 62 | 54 | 70.29 ± 29.72 |
| Onset to admission (d) | 0 | 0 | 6 | 2 | 6 | 7 | 10 | 4.43 ± 3.82 |
| Onset to diagnosis (d) | 0 | 1 | 11 | 5 | 9 | 6 | 17 | 7 ± 5.92 |
| Symptom | – | |||||||
| Fever (°C) | 36.2 | 37.3 | 38.2 | 38 | 38.9 | 36.5 | 38.6 | 37.67 ± 1.04 |
| Respiratory symptoms | – | fatigue | dyspnea, myalgia | poor appetite | fatigue, myalgia | cough, sputum production, hemoptysis | fatigue, cough, sputum production, myalgia | – |
| Gastrointestinal symptoms | – | – | – | – | – | – | – | – |
Note: COVID-19 = coronavirus disease 2019; HTN = hypertension; ADPKD = autosomal dominant poly cystic kidney disease; MI = myocardial infarction; IS = immunosuppressant; M = mycophenolic acid; FK = tacrolimus; P = prednisone; SCr = serum creatinine; eGFR = estimated glomerular filtration rate; mo = month(s); d = day(s).
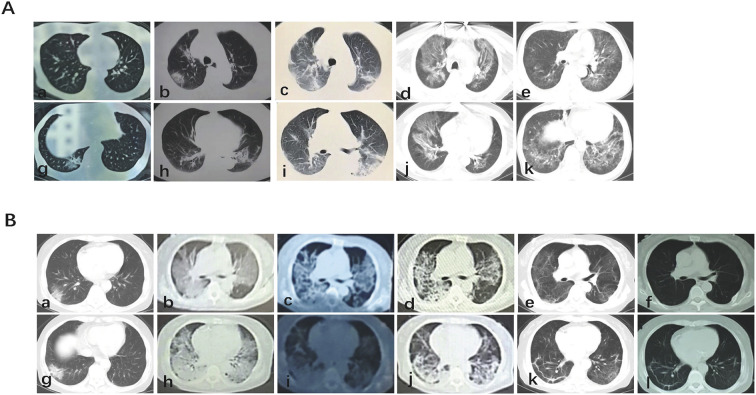
According to the diagnosis criteria and clinical classification method, patient 1 and patient 2 were confirmed as asymptomatic carrier and mild type respectively, patient 3 was moderate type, patients 4 and 5 were severe type, and patients 6 and 7 were critical type. Laboratory results showed that seven patients had normal WBC and reduced lymphocyte counts, and four patients had significantly elevated levels of C-reactive protein (CRP). All patients had normal procalcitonin levels. Chest CT was performed in each patient, and abnormalities were observed in both lungs in all patients except for one asymptomatic patient and one mild patient. Six patients were confirmed with positive nucleic acid testing (NAT), and one patient was confirmed by specific SARS-CoV2 IgM and IgG antibody titers. Five patients produced specific SARS-CoV2 antibody. Data on patients' laboratory tests and computed tomographic imaging were shown in Table 2 . The chest CT image of all patients were characterized by typical COVID-19 image features including multiple patchy ground glass shadows in the pulmonary peripheral zone (Fig. 2A). However, If the patients were accompanied by other basic diseases or in different periods of the course of the COVID-19, the chest CT images were not typical (Fig. 2B).
Table 2.
Data on patients' laboratory tests and computed tomographic imaging.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Mean ± SD |
|---|---|---|---|---|---|---|---|---|
| WBC(×109/L) | 7.8 | 8.5 | 5.2 | 4.42 | 5.2 | 8.7 | 11.5 | 7.33 ± 2.53 |
| Lymphocyte(×109/L) | 1.44 | 0.17 | 0.7 | 0.91 | 0.85 | 0.34 | 0.34 | 0.68 ± 0.44 |
| CRP (mg/L) | None | None | 0.06 | 45.3 | 46.5 | 80.5 | 90 | – |
| PCT (ng/mL) | None | <0.1 | 0.15 | 0.02 | <0.1 | 0.14 | 0.92 | – |
| CT image | Normal | Normal | Typical | Typical | Typical | Atypical | Atypical | – |
| Link to Wuhan | Wuhan | Wuhan | Wuhan | Wuhan | Wuhan | Wuhan | Wuhan | – |
| No. of family members infected | 0 | 0 | 0 | 0 | 1 | 0 | 0 | – |
| NAT | Positive | Positive | Negative | Positive | Positive | Positive | Positive | – |
| SARS-CoV2 Antibody | Positive | Negative | Positive | Positive | Positive | Positive | Negative | – |
| Disease severity | Asymptomatic infection | Mild | Moderate | Severe | Severe | Critical | Critical | – |
Note:WBC = white blood cell; CRP = C-reactive protein;PCT = procalcitonin;CT = computed tomography;NO. = number; NAT = nucleic acid test;SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Fig. 2.
COmputed tomography after covid-19 infection.
2A) It is a typical imaging feature of covid-19, showing multiple patchy ground glass shadows around the lung.
2B) In diffent stages of the course of covid-19, chest CT showed atypical manifestations.
3.2. Treatment
For the asymptomatic patient 1, we did not adjust the immunosuppressive regimen. In the case of mild COVID-19, the patient's dose of immunosuppressant remained unchanged. We choose the IVIG, and umifenovir (Arbidol) for antivirus therapy. For patients with moderate- and severe-type patients, the immunosuppressive agents were first largely reduced and then interrupted if symptom or CT lesion worsen. A low-dose IVIG (2.5–10 g/d) was administered to restore immune function for fighting infection, and low-dose methylprednisolone (20–40 mg/d) was used to inhibit inflammation and prevent rejection. The patients were given common antiviral drugs including Arbidol, lopinavir/ritonavir, and chloroquine phosphate. These patients were only prescribed one of the above drugs at a given time. For patients 6 and 7 with critical type illness, the treatment regimen included interruption of immunosuppressive agents, cefoperazone sodium+sulbactam sodium to fight infection, high-dose IVIG (10–20 g/d) to enhance immune function, and methylprednisolone (40–80 mg/d) to inhibit inflammation and prevent rejection. The antiviral regimen included Arbidol or lopinavir/ritonavir tablets.
3.3. Prognosis
The timeline of clinical characteristic, key treatment, and outcome of RTRs infected with COVID-19 were shown in Fig. 2. The asymptomatic patient was discharged after 6 days of isolation. The patient's renal function returned to normal and a series of NAT and chest CT images were negative for COVID-19. The mild type patient was cured after 2 weeks of therapy. In addition, the series of NAT and chest CT images were negative, the antibody test was also negative upon evaluation for the next two months.
The moderate-type patient's fever disappeared after 2 weeks of treatment, and CT image showed that the lesion was gradually absorbed. AKI which was defined as increase of serum creatinine ≥26.5 μ mol/L within 48 h appeared during the treatment. Based on the clinical symptom and doppler ultrasound results, the clinical rejection was diagnosed, so the renal function was restored to normal after MP shock therapy. The patient was cured after 24 days of treatment.
After the first 10 days of symptom aggravation and evidence of progression on chest CT images, both patients with severe-type illness recovered very smoothly, and the renal function was always maintained at normal levels. After a month of oxygen therapy and antiviral therapy, the lung lesions were gradually absorbed, and the patients were discharged. Both patients developed protective IgG antibodies.
The two critical patients were admitted to the intensive care unit (ICU) for further treatment. Patient 6 developed acute heart failure and fast ventricular rate of atrial fibrillation causing AKI; the patient's serum creatine level was elevated to 305 μmol/L. A series of treatments including furosemide diuresis, sodium bicarbonate, and continuous renal replacement therapy (CRRT) were administered to improve the heart failure. The further synchronized cardioversion was provided to reverse atrial fibrillation to a sinus rhythm. The patient gradually recovered from heart failure and renal insufficiency, and finally, the pneumonia symptoms and pulmonary infection lesions also gradually disappeared. After two consecutive negative NAT results, the patient was discharged after 40 days treatment. During the most recent one-month follow-up, the renal function of both patients remained normal, and there was no indication of heart failure. The SARS-CoV2 antibody was positive.
Patient 7 also was admitted to the ICU as the infection aggravated. Invasive ventilation was used to improve the oxygen saturation. Then, he developed oliguria and acutely increased serum creatinine levels (>700 μmol/L) a month after cessation of immunosuppressive drugs; further, no significant improvement was observed with 3 days of 500 mg/d MP treatment. The patient resumed dialysis treatment. Although nutritional support and antiviral treatment relieved the pulmonary inflammation gradually, the patient suffered from coagulation dysfunction, gastrointestinal bleeding, cerebral infarction, and other organs dysfunction, and died on April 20, 2020.
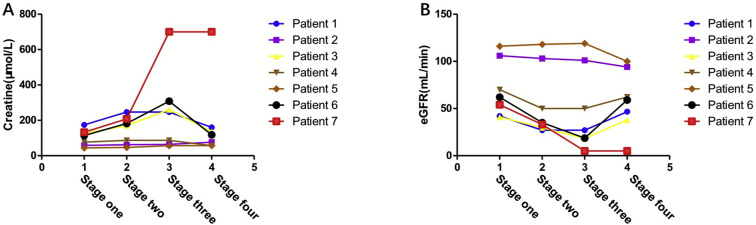
Follow-up serum Cr and eGFR values showed that the renal function of surviving patients was damaged when the disease occurred and deteriorated further during the treatment. After recovery from COVID-19, the renal function of most patients returned to the baseline level (Fig. 3 ). Three patients experienced significant AKI, in which rejection and side effects of drugs were considered the main causes; one patient needed the MP treatment for reversal and one patient eventually returned to dialysis.
Fig. 3.
This figure shows the changes of creatinine and eGFR in 7 patients throughout the course of the disease.
3A). shows the changes of creatinine in 7 patients;
3B) Shows the changes of eGFR in & patients.
Stage one is the test result at the last follow-up, stage two is the test result on the day of diagnosis.
Stage three is the test result when the disease progresses most seriously after infection with covid-19, Stage four is the test result after recovery, discharge of death.
4. Discussion
In our hospital, there was currently nearly 1200 renal transplantation recipients being followed-up, and COVID-19 infection was detected in seven patients. While the overall incidence was similar to the confirmed incidence reported in Wuhan, there was no large-scale outbreak of COVID-19 among transplant recipients.
The clinical manifestations of the transplant recipients were consistent with the general population [[4], [5], [6], [7], [8], [9]]. In our current reported cases, fever was the most common symptom, accompanied by other respiratory symptoms such as cough, expectoration, and myalgia. The low level of blood lymphocytes is most likely because of the viral infection and long-term use of antiproliferative drugs to inhibit lymphocyte proliferation. Acute kidney injury appeared in some patients to an extent, the causes of which include various drugs' side effects, acute rejection, and kidney injury possibly caused by the virus [10]. Although the SARS-CoV2 binding receptor, ACE2, is highly expressed in the kidney, studies have differing views on whether it directly causes renal pathological changes [11,12].
The CT images of recipient patients infected with COVID-19 showed similar typical lesions as the general population, characterized by bilateral, multiple peripheral patchy shadows [13,14]. However, in case of complications such as kidney dysfunction, heart failure, the CT images were not typical. To illustrate, the CT findings of a critical type patient showed pulmonary edema, while that of another patient with critical type showed early Cytomegalovirus (CMV) pneumonia; the superposition of the two images resulted in an atypical presentation.
Nucleic acid detection can identify the virus, but nucleic acid detection often shows false negative results [15]. Hence, the test should be repeated, and multi-site nucleic acid detection should be used. At the same time, serum antibody should be used as a supplementary basis for diagnosis of the virus. However, some patients did not produce antibodies against SARS-CoV2 for a long time despite positive NAT results [16]. Of the seven patients we followed-up, only five produced antibodies. Therefore, patients who have recovered from COVID-19 infection still have the possibility of reinfection in future.
The current treatment options are similar to those used in the past for lung infections in RTRs, including dose reduction or discontinuation of immunosuppressive agents, rebuilding immune function, antiviral therapy, and anti-inflammatory responses(Table 3 ).
Table 3.
Treatment choice and complications.
| Patient |
|||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| The severity of the disease | Asymptomatic | Mild | Moderate | Severe | Severe | Critical | Critical |
| IS adjustment | |||||||
| MMF reduction or interruption | No | No | I | R | I | R | I |
| FK506 reduction or interruption | No | No | I | R | I | R | I |
| Treatment | |||||||
| Antivirus therapy | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Arbidol | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Lopinavir/ritonavir | No | No | Yes | No | No | No | Yes |
| Chloroquine phosphate | No | No | Yes | No | No | No | No |
| IVIG/days | No | Yes/7d | Yes/7d | Yes/5d | Yes/3d | Yes/7d | Yes/14d |
| MP/days | No | No | Yes/10d | Yes/3d | Yes/3d | No | Yes/18d |
| Other complications and support | |||||||
| Septic shock | No | No | No | No | No | No | No |
| ICU admission | No | No | No | No | No | Yes | Yes |
| AKI | No | No | Yes | No | No | Yes | Yes |
| Rejection | No | No | Yes | No | No | No | Yes |
| Acute cardiac injury | No | No | No | No | No | Yes | Yes |
| ARDS | No | No | No | Yes | Yes | Yes | Yes |
| Life support | |||||||
| Initiation of invasive ventilation | No | No | No | No | No | No | Yes |
| Initiation of noninvasive ventilation | No | No | No | No | No | Yes | Yes |
| Initiation of renal replacement therapy | No | No | No | No | No | Yes | Yes |
| Outcome | Discharge | Discharge | Discharge | Discharge | Discharge | Discharge | Death |
Note: IS = immunosuppressant; MPA = mycophenolic acid; FK506 = tacrolimus; IVIG = intravenous immunoglobulin; MP = methylprednisolone; ICU = intensive care unit, AKI = acute kidney injury; ARDS = acute respiratory distress syndrome.
Transplant recipients are an immunocompromised population, and physicians initially considered reducing the dose of or discontinuing immunosuppressant drugs after pneumonia [17]. However, this increases the risk of organ rejection, which will lead to kidney damage and possible irreversible failure. As described in a patient with moderate-type illness, we discontinued immunosuppressants; after a continuous fever for 10 days thereafter, the first signs of organ rejection appeared, and MP shock therapy was administered to reverse this rejection. Fortunately, the patient's renal function recovered, and she recovered from COVID-19. Thus, in the case of mild and moderate type COVID-19 illness, it is not recommended to reduce the immunosuppressant use. For severe and critical types, we suggest to discontinue MMF and reduce CNI. If the immunosuppressant drugs have been stopped for long term, possible rejection reactions should be closely monitored and CNI drugs restarted in a timely manner. In addition, moderate immunosuppressive agents can avoid inflammatory factor storm and alleviate the progression of lung infection; hence, it is necessary to detect inflammatory factors in the treatment of COVID-19 and choose tocilizumab therapy depending on the severity of the inflammatory storm [[18], [19], [20]].
Whether MP therapy is beneficial for the COVID-19 infection, there is a big dispute, while the Chinese and WHO guidelines do not advocate the use of MP, except in the case of progressive deterioration of oxygenation indicators, rapid disease progression verified by imaging, and excessive activation of inflammatory response in the body. Even then, the MP dose should not exceed 1–2 mg/kg [21]. The main concern is that hormone-induced immunosuppression slows virus clearance. According to our case summary, patients with mild and moderate COVID-19 do not need to use MP, while those with the severe and critical type with MMF and CNI reduction can use a low dose of MP for short term.For low-dose hormone, we use 40-80 mg per day. In case of pulmonary edema, excessive exudation and fever, we recommend using low-dose hormone for 5–10 days in the early stage. When the symptoms improve, we can reduce it to 20 mg per day.
Another preferred treatment choice for transplant physicians is reconstruction of immune function. The theoretical basis for this is the long-term immunosuppression in cellular immunity and humoral immunity function in recipients with impaired cellular and humoral immune function [22]. Therefore, IVIG was widely used to treat infection among transplant recipients, especially for the severe or critical type patient. There is no standard for the dosage and course of IVIG treatment, For critically ill patients, IVIG in our center is used 5-10 g per day for 5–10 consecutive days [19]..
Several existing drugs have been recommended for antiviral treatment and written into the national guide, such as Arbidol, chloroquine phosphate, and oral lopinavir/ritonavir [23].Arbidol was mostly chosen for antiviral treatment for transplant recipients of COVID-19, as this drug is cheaper and easier to obtain than others. Several doctors have tried other drugs such as colchicine, which inhibit inflammation in transplant patients and reduce the production of IL-6, leading to recovery [4]. Overall, there is no conclusive evidence to control the development of severe pneumonia. However, it is preferable to not routinely use more than three antiviral drugs at a given time and/or use them for more than ten days. The use of these drugs is not an issue for transplant patients, as it is important to consider the risk of renal function damage as well as the interaction with CNI drugs.
In summary, the clinical manifestations, disease progression, and prognosis of infection in RTRs were different in different clinical type.Classification treatment according to the severity of different diseases can control the progress of pneumonia, while protecting the function of the transplanted kidney and reducing side effects of drugs.
Ethics approval and consent to participate
The study was proved by ethics committee of Renmin Hospital of Wuhan University.
Consent for publication
All the authors approved of this version of the manuscript for publication.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 81870067, 81400753).
Declaration of Competing Interest
The authors have no conflicts of interest.
Acknowledgments
We wish to thank Kang Jing from Renmin Hospital of Wuhan University for offering the CT image analysis. Thanks Yao Di from Renmin Hospital of Wuhan University for offering some clinical information.
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Apr 7;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Liu N., He G., Yang X., et al. Dynamic changes of chest CT follow-up in coronavirus Disease-19 (COVID-19) pneumonia: relationship to clinical typing. BMC Med. Imaging. 2020;20:92. doi: 10.1186/s12880-020-00491-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu L., Gong N., Liu B., Lu X., Chen D., Chen S., Shu H., Ma K., Xu X., Guo Z., Lu E., Chen D., Ge Q., Cai J., Jiang J., Wei L., Zhang W., Chen G., Chen Z. Coronavirus Disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur Urol. 2020 Jun;77(6):748–754. doi: 10.1016/j.eururo.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gandolfini I., Delsante M., Fiaccadori E., et al. COVID-19 in kidney transplant recipients. Am. J. Transplant. 2020 Jul;20(7):1941–1943. doi: 10.1111/ajt.15891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H., Chen Y., Yuan Q., et al. Identification of Kidney Transplant Recipients with Coronavirus Disease 2019. Eur. Urol. 2020;77(6):742–747. doi: 10.1016/j.eururo.2020.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu L., Gong N., Liu B., et al. Coronavirus Disease 2019 Pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur. Urol. 2020 Jun;77(6):748–754. doi: 10.1016/j.eururo.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez V., Vazquez T., Alonso-Titos J., et al. Recommendations on management of the SARS-CoV-2 coronavirus pandemic (Covid-19) in kidney transplant patients. Nefrologia. 2020 May-Jun;40(3):265–271. doi: 10.1016/j.nefro.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu L., Xu X., Ma K., et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am. J. Transplant. 2020 Jul;20(7):1859–1863. doi: 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J., Li X., Cao G., Wu X., Wang Z., Yan T. COVID-19 in a kidney transplant patient. Eur. Urol. 2020 Jun;77(6):769–770. doi: 10.1016/j.eururo.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanelli V., Fiorentino M., Cantaluppi V., et al. Acute kidney injury in SARS-CoV-2 infected patients. Crit. Care. 2020;24:155. doi: 10.1186/s13054-020-02872-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L., Li X., Chen H., et al. Coronavirus Disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am. J. Nephrol. 2020:1–6. doi: 10.1159/000507471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su H., Yang M., Wan C., et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020 Jul;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kates O.S., Fisher C.E., Stankiewicz-Karita H.C., et al. Earliest cases of coronavirus disease 2019 (COVID-19) identified in solid organ transplant recipients in the United States. Am. J. Transplant. 2020 Jul;20(7):1885–1890. doi: 10.1111/ajt.15944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan Y.N., Qin J. Pre- and Posttreatment chest CT findings: 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295:21. doi: 10.1148/radiol.2020200323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Team C-I Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Nat. Med. 2020 Jun;26(6):861–868. doi: 10.1038/s41591-020-0877-5. [DOI] [PubMed] [Google Scholar]
- 16.Infantino M., Grossi V., Lari B., et al. Diagnostic accuracy of an automated chemiluminescent immunoassay for anti-SARS-CoV-2 IgM and IgG antibodies: an Italian experience. J. Med. Virol. 2020 Sep;92(9):1671–1675. doi: 10.1002/jmv.25932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martino F., Plebani M., Ronco C. Kidney transplant programmes during the COVID-19 pandemic. Lancet Respir. Med. 2020 May;8(5):e39. doi: 10.1016/S2213-2600(20)30182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 2020 Jul;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao W., Liu X., Bai T., et al. High-Dose Intravenous Immunoglobulin as a Therapeutic Option for Deteriorating Patients With Coronavirus Disease 2019. Open Forum Infect Dis. 2020;7:ofaa102. doi: 10.1093/ofid/ofaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontana F., Alfano G., Mori G., et al. Covid-19 pneumonia in a kidney transplant recipient successfully treated with Tocilizumab and Hydroxychloroquine. Am. J. Transplant. 2020 Jul;20(7):1902–1906. doi: 10.1111/ajt.15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie L.X. Interpretation of the 7th edition of the “diagnosis and treatment guidelines of coronavirus disease 2019 in China”: Progress and challenges. Chronic Dis Transl Med. 2020 Apr;6(2):75–78. doi: 10.1016/j.cdtm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi H., Zhou C., He P., et al. Successful treatment of plasma exchange followed by intravenous immunogloblin in a critically ill patient with 2019 novel coronavirus infection. Int. J. Antimicrob. Agents. 2020;105974 doi: 10.1016/j.ijantimicag.2020.105974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakraborty C., Sharma A.R., Sharma G., Bhattacharya M., Lee S.S. SARS-CoV-2 causing pneumonia-associated respiratory disorder (COVID-19): diagnostic and proposed therapeutic options. Eur. Rev. Med. Pharmacol. Sci. 2020;24:4016–4026. doi: 10.26355/eurrev_202004_20871. [DOI] [PubMed] [Google Scholar]