Because patients with cancers were excluded from COVID-19 vaccine clinical trials, there is concern about efficacy and the safety profile of vaccines in this setting. Publications focussing on patients with cancer reported weak immunogenicity after a single dose [1] but efficient immunogenicity after two doses of the BNT162b2 COVID-19 vaccine [2,3], as well as lower titres of neutralising antibodies after 1 dose of the vaccine in patients receiving checkpoint inhibitors [4]. However, limited data are available for patients enrolled in early-phase clinical trials. We thus aimed to assess safety and immunogenicity of the vaccine in a cohort of early-phase trial oncology patients.
We retrospectively collected data from patients who received 2 doses of the BNT162b2 COVID-19 vaccine, between January 2021 and April 2021. COVID-19 immunisation had been monitored, as part of routine care: samples were taken before the first dose (T0), before the second dose (T1) and one month after the second dose of the vaccine (T2) with a 21- to 28-day interval between the doses, as recommended in France. The vaccine was injected in between experimental treatment administrations, except for patients on a continuous regimen. Polymerase chain reaction swab tests were performed at each patient's venue to rule out asymptomatic SARS-Cov-2 infections. Serum samples were tested for quantitative detection of anti-SARS-Cov-2 spike (S1) IgG antibodies (Euroimmun®, Luebeck, Germany). In samples with an enzyme-linked immunosorbent assay ratio ≥0.7, neutralising antibodies against SARS-Cov-2 were detected using a virus neutralisation test (VNT100). The study was approved by the data protection committee of AP-HM.
Of a total population of 86 patients, 17 (37%) refused vaccination; 48 were vaccinated including 35 in our centre from which 22 were studied. Patients' characteristics are detailed in Sup. Material. All had metastatic disease; the most frequent tumour types were lung (43%) and melanoma (38%). Cancer treatment at the time of vaccination consisted of an immunotherapy regimen in 64% (from which 50% were provided treatment in combination with an anti-angiogenic therapy), targeted therapy in 27% and chemotherapy for the remaining 9% (Sup. Material). Fifty-seven percent of patients reported G1 adverse events related to vaccination that did not postpone experimental treatment administration. None of the patients was diagnosed with SARS-Cov-2 infection during or after vaccine administration.
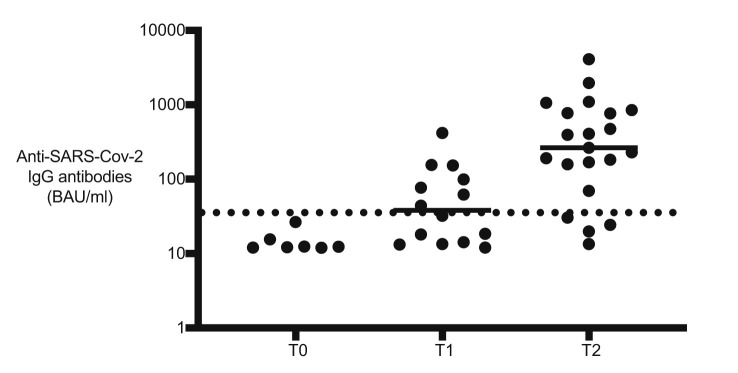
Seroconversion rates were 37% at T1 and 77% at T2 (Fig. 1 ). Among patients who seroconverted, 2 of 8 had positive neutralising antibodies at T1, whereas 16 of 20 had positive neutralising antibodies at T2. We could identify no difference depending on the patient's age, treatment type or lymphocyte count.
Fig. 1.
Anti–SARS-CoV-2 IgG antibodies directed against the S1 domain of the spike protein of the virus using a commercial ELISA kit (Euroimmun, Lübeck, Germany) and quantitative results were expressed in standardised units (binding antibody units [BAU] per mL) as recommended by the manufacturer. The dotted line represents the positive threshold of 35.2 BAU/mL. ELISA, enzyme-linked immunosorbent assay.
The 77% seroconversion rate achieved after 2 doses of the vaccine in our cohort is in line with the data reported in other oncology patients treated with this vaccine scheme, without unexpected toxicity or treatment delay. This is lower than reported in a small series of adolescents and young adults [5]. The lower rate of neutralising antibodies among seropositive patients might be explained by differences in assays.
Conclusion
Two injections of the BNT162b2 COVID-19 vaccine seem efficient and safe in patients with cancer in early-phase trials.
Funding
None declared.
Conflict of interest statement
N.M. declares having received advisory fees from Bristol Myers Squibb and payment for lectures and meeting invitations from Novartis, Bristol Myers Squibb and MSD.
N.A. reports receiving grants and drugs for a trial from Bristol Myers Squibb; receiving drugs for a trial from Pierre Fabre; receiving drugs and grants for a clinical trial from Bristol Myers Squibb outside the area of work commented on here; receiving travel support from Bristol Myers Squibb for an International Society of Paediatric Oncology meeting and participating as a scientific advisory board member (without receiving personal fees) for Bayer, Bristol Myers Squibb and Partner Therapeutics outside the area of work commented on here.
C.G-M has received consulting fees from Bristol Myers Squibb and payment for lectures from Bristol Myers Squibb and Roche. All other authors have declared no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2021.07.040.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Palich R., Veyri M., Marot S., et al. Weak immunogenicity after a single dose of SARS-CoV-2 mRNA vaccine in treated cancer patients. Ann Oncol. 2021. Apr 29 doi: 10.1016/j.annonc.2021.04.020. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monin L., Laing A.G., Muñoz-Ruiz M., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massarweh A., Eliakim-Raz N., Stemmer A., et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol. 2021. May 28 doi: 10.1001/jamaoncol.2021.2155. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terpos E., Zagouri F., Liontos M., et al. Low titers of SARS-CoV-2 neutralizing antibodies after first vaccination dose in cancer patients receiving checkpoint inhibitors. J Hematol Oncol. 2021. May 31 doi: 10.1186/s13045-021-01099-x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Revon-Riviere G., Ninove L., Min V., et al. The BNT162b2 mRNA COVID-19 vaccine in adolescents and young adults with cancer: A monocentric experience. Eur J Cancer. 2021;154:30–34. doi: 10.1016/j.ejca.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.