Abstract
This study is aimed at identifying the chemical composition of the essential oil extracted from the Syzygium aromaticum seeds, as well as investigating its biological activities, insecticide effect, and allelopathic properties. The extraction yield was about 14.3 and 7.14% for grounded and ungrounded seeds, respectively. The GC-MS analysis allowed the identification of 17 heterogeneous compounds, including eugenol (68.7-87.4%), as major compound, cyperene (20.5-7.2%), phenethyl isovalerate (6.4-3.6%), and cis-thujopsene (1.9-0.8%), respectively, for grounded and ungrounded seeds. Concerning the antibacterial activity, the diameter of the inhibition zone reached 35 mm when the essential oil extracted from grounded seeds was applied against Escherichia coli. Regarding the antioxidant activity via the DPPH radical scavenging test, the IC50 varied from 1.2 ± 0.1 to 2.8 ± 0.5 μg/mL. With respect to reducing power, the efficient concentration EC50 ranged from 32 to 50 μg/mL. The essential oil exhibited also an allelopathic effect against seeds of Hyoscyamus niger, as well as an insecticide effect against Sitophilus oryzae with a DL50 value of 252.4 μL/L air. These findings enhance the use of this spice as a natural food preservative and encourage its use in several fields, including pharmaceutical, cosmetics, agriculture, and therapy, that could be a strategic way to guarantee the consumer's health.
1. Introduction
Over the last decades, the use of aromatic plants for traditional medicinal purposes has gained a great interest. Thus, the Arab and Egyptian civilizations have attributed their use to religious recommendations [1]. Thereafter, the healing properties of extracts and essential oils were discovered and became common remedies to traditional medicines. For example, during the great epidemics in ancient Greece, the odorous principles of certain aromatic plants were spread by fumigation in the city streets to prevent the spread of infectious diseases. Fumigation of sick people is indeed one of the oldest therapeutic techniques [2].
Nowadays, modern medicine uses the therapeutic properties of essential oils and their constituents. Actually, many volatile compounds are common ingredients of pharmaceutical preparations. Thymol is used in dental care for its antiseptic properties, and eugenol is recommended due to its analgesic effect [3]. In an attempt to find new treatments to current diseases (cancer, asthma, Alzheimer, etc.), the scientific community has recently focused on the constituents of essential oils, thanks to the higher significant number of volatile compounds, such as sesquiterpenes, which showed remarkable pharmacological activities against diseases, in particular cancer [4]. Moreover, essential oils are used as natural antimicrobial, antioxidant, and bioactive agents against many pathogens [5].
Syzygium aromaticum, also called clove, is a dried flower bud, which belongs to the Myrtaceae family. This species is indigenous to the Maluku islands in Indonesia, and it is the most important and second valuable spice in world trade [6]. Clove bud essential oil is known for its antioxidant, antifungicidal, anticarcinogenic, and anesthetic activities and for its antiprotozoal effects, thanks to its richness in many phytochemicals, such as sesquiterpenes, monoterpenes, hydrocarbons, and phenolic compounds [7, 8].
Several researchers have reported the antibacterial activity of the essential oil from clove buds against different food-borne pathogens [9, 10]. However, to the best of our knowledge, current studies did not take into account the antigerminative effect of this essential oil, and neither did they take into account its insecticide power nor the effect of the size of the plant material on the essential oil chemical composition. All of these traits were the main objectives of this work.
2. Materials and Methods
2.1. Taxonomy and Preparation of Clove Buds
According to the US National Plant Germplasm System, Syzygium aromaticum (S. aromaticum) (synonym: Eugenia cariophylata), commonly known as clove, belongs to the Myrtaceae family, subfamily of Myrtoideae and Syzygiae tribe. It is a median-sized tree (8-12 m) native from the Maluku islands in east Indonesia [11]. It is an evergreen plant, with large leaves and terminal grouped flowers. These latter are initially pale in tint, and then progressively become green, before acquiring a brilliant red hue when harvested at 1.5-2 centimeters long. They are composed of a long calyx, which ends in four extended sepals, and four nonopened petals forming a small central ball. Clove buds were purchased from a local market in Kef (North-west of Tunisia). The buds were cleaned, sorted, and homogenized. The initial particle size for ungrounded buds was about 2 cm. Grounded buds had a size of about 0.6 mm. Two batches were prepared (ungrounded and grounded). Each one is composed of 50 g raw material. The samples were ground using an electric grinder (Moulinex AR 1105, France).
2.2. Hydrodistillation
Both grounded and ungrounded buds were subjected, separately, to hydrodistillation during 6 h using a Clevenger-type apparatus. The raw material was directly submerged in 500 mL distilled water in a round bottom flask (1 L capacity) containing a few pumice stones to regulate boiling. The entire extraction method was operated at atmospheric pressure. This technique consists of boiling the mixture, then condensing the vapors that are released using a water condenser. The condensation of this vapor mixture causes its separation into an organic phase, called essential oil, containing the majority of the odorous compounds, and an aqueous phase, called aromatic water, which contains very few odorous compounds. As the density of clove bud essential oil (1.062) at 20°C was superior to the water density (1.00), the separation of the organic and aqueous phases was a little bit difficult. In fact, the organic phase was below the aqueous one. For this purpose, the distillate was placed in a decanter. Salt-saturated water was then added. After shaking and degassing, the mixture was decanted. This step, called release, allows the separation of the essential oil from the aqueous phase, since the essential oil is less soluble in salt water than in water. The obtained essential oil was then recovered with the help of the decanter. After that, it was dried with Na2SO4 and stored in amber-colored bottles at 4°C for subsequent analysis. The Na2SO4 was removed by filtration of the EO. The extraction yield was calculated as follows:
| (1) |
where DM is dry matter (mg), EOV is essential oil volume (mL).
2.3. Gas Chromatography and Gas Chromatography Coupled with Mass Spectrometry (GC-MS)
The identification of volatile compounds from S. aromaticum bud essential oil used the procedures of Ben Hassine et al. [5]. Analytical gas chromatography was carried out on a Varian Star 3400 (Les Ulis, France) Cx chromatograph fitted with a fused silica capillary DB-5MS column (5% phenyl-methyl-polysiloxane, 30 m × 0.25 mm; film thickness: 0.25 μm). The analysis was performed using the following temperature program: temperature rise from 60°C to 260°C with a gradient of 5°C/min and 15 min isotherm at 260°C. A second gradient was applied to 340°C at 40°C/min. The injection volume was 1 μL in 1 : 10 split mode. The carrier gas was helium with a flow rate of 1 mL/min. Injector temperature was held at 200°C. The mass spectrometer (Varian Saturn GC/MS/MS 4D) was adjusted for an emission current of 10 μA and electron multiplier voltage between 1.400 and 1.500 V. Trap and transfer line temperatures were 220°C and 250°C, respectively. Mass scanning was from 40 to 650 amu. The identification of the oil constituents was based on a comparison of their retention indices (RI) relative to C5-C24 n-alkanes with those of the literature or with those of authentic compounds available in our laboratory. Further identification was made by matching their recorded mass spectra with those stored in NIST 08 and other published mass spectra. The percentage composition of the essential oil was calculated by the normalization method from the GC peak areas, assuming identical mass response factor for all compounds.
2.4. Free Radical Scavenging Activity: DPPH• Assay
Spectrophotometric measurements were assessed according to the method of Ben Hassine et al. [5]. A volume of 1.5 mL essential oil dilution was added to 1.5 mL of DPPH• solution (0.2 mM). After 30 min of incubation at room temperature, the absorbance was measured at 520 nm. The DPPH• inhibition percentage (IP) was expressed as follows: IP(%) = ((Ablank − Asample)/Ablank)∗100. Here, Ablank is the absorbance of the control containing only the solvent and DPPH• solution. Radical scavenging activity was expressed in terms of IC50 (μg/mL), and BHT was used as standard. IC50 represents the concentration at which clove EO exerts half of its maximal DPPH• inhibitory effect. It indicates how much EO is needed to inhibit the radical by half, thus providing a measure of potency. A lower value of IC50 indicates more potent inhibitors. All measurements were replicated three times.
2.5. Ferric-Reducing Antioxidant Power: FRAP Assay
Experiments were done according to Kammoun El Euch et al. [12]. One mL of S. aromaticum essential oil was mixed with 2.5 mL of sodium phosphate buffer (0.2 M; pH = 6.6) and 2.5 mL of 1% K3Fe (CN)6. The whole mixture was incubated in a water bath at 50°C for 20 min. Then, 2.5 mL of a 10% TCA solution was added to the mixture, which was centrifuged at 6500 rpm for 10 min. The supernatant (2.5 mL) was then mixed with 2.5 mL of distilled water and 0.5 mL of a 0.1% FeCl3 solution. The intensity of the blue-green color was measured at 700 nm, and BHT was used as a positive control. The IC50% value (μg/mL) is the effective concentration at which the absorbance was 0.5 for reducing antioxidant power and was obtained by interpolation from linear regression analysis. All measurements were replicated three times.
2.6. Antimicrobial Activity
The antimicrobial activity of clove buds essential oil was achieved according to the disc diffusion method [5]. Three types of Gram-positive bacteria (Staphylococcus aureus ATCC 6538, Listeria monocytogenes FMCC B-128, and Bacillus cereus ATCC 168) and two Gram-negative types (Escherichia coli ATCC 35150 and Salmonella enteritidis ATCC 13076) were used. A suspension of the tested microorganism (0.1 mL of 108 cells per mL) was spread on nutrient agar. The discs have a 6 mm diameter, and against each microbial strain, one dose each of 1, 5, and 10 μL of pure EO was applied to each disc, respectively, and separately, by injecting and placing on the inoculated plates. Penicillin (P), chloramphenicol (C), gentamicin (GM), and erythromycin 500 (E) were used as standard antibiotics to determine the sensitivity of the bacterial species tested. These plates, after staying at 4°C for 2 h to allow the diffusion of volatile compounds inside the Petri dishes, were incubated for 48 h at 37°C. Antimicrobial activity was evaluated by measuring the inhibition zone diameter (IZD) (mm) against the tested microorganism. The strain's sensitivity was evaluated according to IZD (Ф) as follows: (-) 6 mm < Ф < 10 mm: the strain is insensitive to the EO action; (+) 10 mm ≤ Ф < 15 mm: the strain is sensitive; (+ +) 15 mm ≤ Ф < 20 mm: the strain is very sensitive; (+ + +) Ф > 20 mm: the strain is extremely sensitive to the EO action.
2.7. Allelopathic and Insecticidal Effects
In light of previous results, tests for the allelopathic and insecticidal effects were carried out with the grounded clove bud essential oil, since it was responsible for the highest biological activity results.
2.7.1. Allelopathic Effect
The allelopathic effects of clove EO on H. niger germination was tested following the method adopted by Zahed et al. with slight modifications [13]. Five concentrations (1, 5, 10, 20, and 30 μL/mL) were used. Essential oil was diluted in 1‰ MeOH. Distilled water served as the control. Whatman No. 2 filter paper was placed in 9 cm diameter Petri dishes moistened with 5 mL of EO solutions. Five replications of 25 seeds were used for each treatment. To prevent evaporation, Petri dishes were sealed with parafilm and placed in an incubator at 25°C in the dark. After 10 days, the number of germinated seeds was recorded and the percentage of germination was calculated using the following formula:
| (2) |
where n is the number of germinated seeds in the test solution, and N is the total number of seeds. Seeds showing radicle emergence (2 mm) were recorded as germinated.
2.7.2. Insecticide Effect
(1) Insect Rearing. Sitophilus oryzae adults were obtained from cultures initiated in the Laboratory of Materials, Molecules, and Applications at IPEST, La Marsa, Tunisia. Corn (Zea mays) seeds were used as a host. Insects were reared in darkness at 25°C in a relative humidity (R.H.) of 65 ± 5% and 16/8 (L/D) photoperiod. The old adult insects (7-10 days) were used for fumigant assay.
(2) Fumigant Assay. Fumigant toxicity assay of S. aromaticum essential oil was evaluated using the method described by Bachrouch et al. [14]. Two-centimeter-diameter filter papers (Whatman No. 1) were impregnated with the tested EO doses. The impregnated filter papers were then attached to the screw caps of 44 mL plexiglas bottles. Caps were screwed tightly on the vials; each one contained S. oryzae adults (1–7 days old). When no leg or antennal movements were observed, insects were considered dead. To evaluate the insects' mortality, amounts of 2.5, 5, 7.5, and 10 μL EO from clove grounded buds, corresponding to concentrations of 41.7, 83.3, 125, and 166.7 μL/L air, respectively, were prepared. Control insects were kept under the same conditions without any EO, and each dose was replicated three times. The number of dead and alive insects in each bottle was counted 24 h after initial exposure. The mortality was evaluated by direct hourly observation of the insects. Probit analysis [15] was used to estimate LC50 and LC95 values after 24 h of treatment (lethal concentration was designed to assess 50% and 95% of insect mortality).
2.8. Statistical Analysis
All data were expressed as means ± standard deviations of triplicate measurements. The confidence limits were set at P < 0.05. LC50 and LC95 were calculated by Probit analysis using IBM SPSS statistics version 19 (2010). Data were calculated for significance by ANOVA (one-way analysis of variance) using SPSS 20.1 (version IBM, 20.0.2004). Statistical differences between the essential oils were estimated by Tukey's test. Student's t-test analysis was used to compare between essential oil extraction yields. Multiple range tests were conducted at 5% significance level.
3. Results and Discussion
3.1. Extraction Yield
Obtained results showed that grounded samples (particle size = 0.6 mm) present the highest extraction yield. While the ungrounded clove buds (particle size between 1.5 and 2 cm) exhibited an EO extraction yield of 7.1 ± 0.8%, the grounded ones showed an extraction yield two times higher than the first EO and reached the value of 14.3 ± 0.6% (Figure 1). These results were in agreement with those found by Selles et al. [16] when working on the grounded buds of S. aromaticum essential oil from Algeria. They obtained an extraction yield of 11.6%. Similarly, Kapadiya and Desai [17] have found an extraction yield equal to 11.35% when the clove bud's particle size was about 565 microns. The current data showed the influence of the grinding method on the EO extraction yield, which is a technical practice, which enlarges the contact area between solid medium and the boiled water [18]. The decrease of particle size was related to the increase in essential oil extraction yield [17]. In addition, grinding improves the extraction speed and the hydrodistillation yield. However, extreme grinding may disturb the steam circulation, and thus slow down the extraction speed [19].
Figure 1.
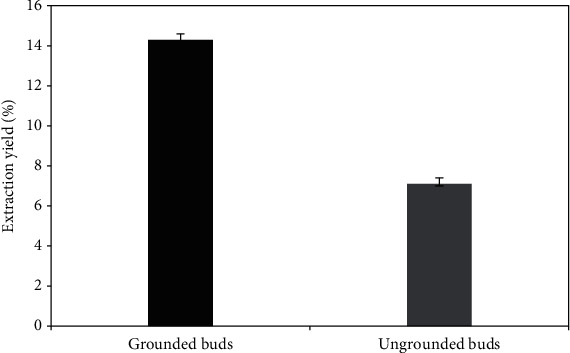
Extraction yield from grounded and ungrounded clove buds essential oil (%).
3.2. GC-MS Analysis
The volatile compounds of the different essential oils (grounded and ungrounded) of clove buds were analyzed by GC-MS (Figure 2). A comparison between the EO chemical composition obtained from grounded and ungrounded buds was elaborated. Results shown in Table 1 and Figures 2(a) and 2(b) indicate that eugenol was the major component for both ungrounded (87.4%) and grounded buds (68.7%). The current result was higher than that determined by Aguilar-González et al. [20], who found a peak area value of 75.4% for ungrounded clove buds. Cyperene ranks second with an average peak area of about 7.2 and 20.5%, respectively, for ungrounded and grounded clove buds. However, it was reported that β-caryophyllene (11.5%) was one of the major components [21]. This latter compound was not detected in this study. Furthermore, it was reported that eugenol, eugenol acetate, β-cariofilen, α-humulen, β-pinene, limonene, farnesol, benzaldehyde 2-heptanone, and ethylanoate were the most common compounds [21]. Accordingly, it was demonstrated that eugenol (75.4%) and caryophyllene oxide (11.2%) were the major compounds [20]. This variability in the extraction yield value and EO composition can be explained by the maturity stage and environmental factors (temperature, photoperiod, and hygrometry), the analytical methods, and the major effects of geographical and ecological variations among habitats [14]. The particle size has an effect on the extraction efficiency, which depends on the extraction conditions. Indeed, on the one hand, a decrease in the size of the particles will lead to an improvement in the specific surface area; the solvent, which is distilled water, penetrates more easily into the particles, thus increasing the quantity of EO and decreasing the internal transfer time of solutes. On the other hand, the process of grinding can lead indirectly to a decomposition of the plant material and thus to a reduction of the amount of volatile chemical compounds. These results are in accordance with those stipulated by Herzi [22]. Kapadiya and Desai [17] also indicated that size parameter is one of the most important factors influencing the essential oil extraction of clove buds. Their findings highlighted that the decrease of particle size involved an increase in the extraction yield and eugenol (10.30%), which was different from our results. In the current study, the amount of eugenol decreased with the grounding process, from 87.39% to 68.73%, respectively, for ungrounded and grounded clove bud essential oils; however, it is still much more higher than the values reported by Kapadiya and Desai [17] which is equal to eugenol at 10.36%. These differences could be related to the technical procedure, hydrodistillation time, heating, etc. In light of these results, one recommendation could be established. If the objective of the valorization is to have an essential oil concentrated in eugenol, the grinding procedure is not necessary. On the other hand, if the objective is to exploit the other active biomolecules, such as cyperene, cis-thujopsene, and phenyl isovalerate, it would be preferable to ground the clove buds to improve their contents in the obtained essential oil.
Figure 2.
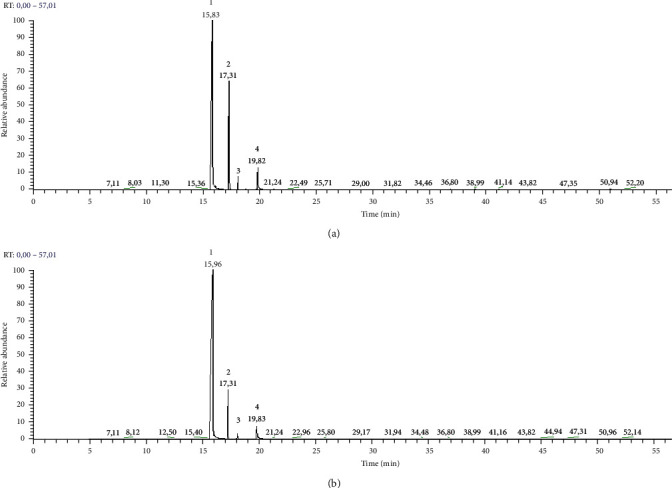
GC-MS chromatograms of (a) grounded and (b) ungrounded clove buds essential oils. (1) Eugenol; (2) cyperene; (3) cis-thujopsene; (4) phenethyl isovalerate.
Table 1.
Chemical composition of S. aromaticum essential oil extracted from ungrounded and grounded clove buds.
| Compounds | RTC | RIC | RIL | % peak area of the chemical component | |
|---|---|---|---|---|---|
| Ungrounded buds | Grounded buds | ||||
| Eugenol | 15.83-15.96 | 1344 | 1351 [56] | 87.39 | 68.73 |
| Cyperene | 17.31 | 1396 | 1398 [57] | 7.22 | 20.52 |
| cis-Thujopsene | 18.02-18.10 | 1427 | 1426 [58] | 0.75 | 1.94 |
| Phenethyl isovalerate | 19.82-19.83 | 1498 | 1495 [59] | 3.63 | 6.41 |
| Total identified compounds | 98.99 | 97.77 | |||
| Phenolic compounds | 87.39 | 68.73 | |||
| Sesquiterpenes | 7.97 | 22.63 | |||
| Others | 3.63 | 6.41 | |||
RIC: calculated retention index of the chemical compound; RTC: calculated retention time; RIL: retention index from literature.
3.3. Antioxidant Activities: DPPH• and FRAP Assays
Several researchers have reported the efficacy of employing DPPH radical and FRAP methods to evaluate the antioxidant potential by estimating the radical scavenging activity [23, 24]. Comparative evaluation of antioxidant potential of the different essential oils (grounded and ungrounded) of clove buds was established. Overall, clove essential oil is constituted of many powerful antioxidant components like eugenol, β-caryophyllene, and eugenyl acetate, which are known to inhibit reactive oxidative species by scavenging free radicals [6]. Figure 3 illustrates that the antioxidant activity of grounded bud EO (IC50 = 1.2 ± 0.1 μg/mL) was about two times greater than that of ungrounded ones (IC50 = 2.8 ± 0.5 μg/mL). These results were very significant when compared to BHT (IC50 = 35.5 ± 0.57 μg/mL). These findings were in perfect agreement with Gülçin et al. [25] who reported that DPPH• scavenging activity of clove bud EO was more important than various synthetic antioxidants, namely, BHT, α-tocopherol, and Trolox. This important activity could be related to the high content of eugenol according to many scientific reports proving that eugenol is a potent chelator with values of IC50, ranging from 4.4 to 130.5 μg/mL [26, 27]. Results depicted in Figure 4 show that the efficiency concentration was dependent on clove bud particle size, which is a crucial parameter affecting the chemical composition as described in the previous section, and thus affecting the biological activities. In fact, when comparing this result to the one of chemical composition, we can deduce that the essential oil with the lower eugenol amount (68.73% for grounded clove buds) possesses a lower EC50 value (32 ± 1.3 μg/mL) than theEC50value of intact buds (50 ± 4.5 μg/mL) with eugenol amount of 87.39%.
Figure 3.
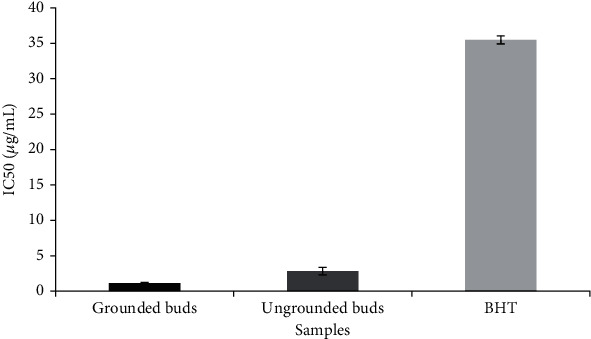
The antioxidant activity of clove buds essential oil expressed as IC50 (μg/mL) using the free radical scavenging capacity by DPPH assay compared to a standard BHT.
Figure 4.
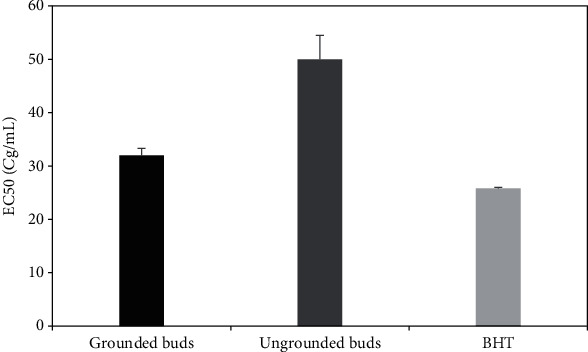
The equivalent reducing power from different samples of clove buds essential oil compared to the standard BHT expressed as EC50 (μg/mL).
The BHT EC50 was equal to 25.8 ± 0.2 μg/mL in the same experimental conditions. This finding could be related to the loss and the decomposition of clove buds during the grinding process, and thus to a reduction of the amount of volatile chemical compounds. It is important to note that there are no previous citations concerning the FRAP test on clove buds EO. These results were obtained after three measurements for both clove batches (ungrounded and grounded).
3.4. Antibacterial Activity: Disc Diffusion Assay
Summarized results in Table 2 demonstrate that clove buds EO was very active against Gram+ and Gram− bacteria with IZD ranging between 17.2 ± 4.5 and 35.5 ± 2.6 mm. Statistical analysis showed a significant difference between EO from grounded and ungrounded clove buds. These findings were in perfect agreement with previous reports which elucidated important MIC values for L. monocytogenes (0.05 mg/mL) and S. enteritidis (0.1 mg/mL) [28]. This activity could be attributed to the major compound “eugenol (>85%).” Indeed, it was demonstrated in an in vitro study that the MIC values of clove oil and eugenol to Bacillus cereus were 25 and 15 μg/mL, and their MBC values were 40 and 25 μg/mL, respectively, whereas the half maximal inhibitory concentration (IC50) of clove oil against this important food source gram-positive pathogen was 5.02 μg/mL, and that of eugenol was 4.05 μg/mL [29]. Radünz et al. [30] demonstrated that S. aromaticum EO containing 56.06% eugenol showed a strong inhibitory effect up to a concentration of 0.30 mg/mL against pathogenic bacteria (E. coli O157:H7, L. monocytogenes, and S. aureus) [30]. In addition, according to Thielmann et al. [31], Syzygium aromaticum revealed promising growth inhibitory activity with MICs of 100 and 400 μg/mL, respectively, against S. aureus and E. coli. Abdullah et al. recorded an IZD against S. aureus of 13 ± 2.2 and 22 ± 1.6 mm at 1.3 and 10% concentration, respectively, [32]. However, antimicrobial activity cannot be attributed to the action of the major chemical component. Eugenol followed by aldehydes, ketones, and alcohols are the responsible compounds [33]. Besides, from Table 2, it is shown that E. coli is the most sensitive recording the highest IZD of about 28.7, 34, and 35.5 mm, respectively, to 1, 5, and 10 μL applied doses. Besides, these results conventionally indicated that the increase of bioactive inhibitory power was closely correlated to EO type (from grounded or ungrounded buds) and concentration (Table 2). All these findings enhance the use of this spice as a natural food preservative and encourage its use in several fields: pharmaceutical, cosmetics, agriculture, and therapy. Indeed, eugenol besides other components such as β-caryophyllene and eugenol acetate [34, 35] has the main essential effects of clove as an anti-inflammatory, analgesic, antimicrobial, antiparasitic, antioxidant, antimutagenic, anticonvulsant, and anticarcinogenic agent [36, 37]. They are also known for their therapeutic potential to control vomiting, nausea, diarrhoea, flatulence, cough, and stomach distension; to relieve pain; and to cause uterine contraction [38]. All these biological activities contribute to the improvement of consumers' health. Clove EO has been found to show antifungal activity against pathogenic fungi, namely, Tricophyton rubrum, Tricophyton mentagrophytes var. interdigitale, Epidermophyton flocosum, Candida spp., and Aspergillus spp. [39, 40]. The antibacterial activity of clove EO alone and in combination with different antimicrobial compounds against common food-borne pathogens has been evaluated in a wide variety of food products such as cucumber [41], rainbow trout fillets [42], ground beef [43], cooked pork sausages [37], and ground chicken meat [44]. These findings allowed the application of this raw material as a natural bioconservative, as an alternative to synthetic ones, and thus preserving the wellbeing of consumers.
Table 2.
Antimicrobial activity of clove buds essential oil.
| Doses (μL)/strains | EO from (grounded buds) | EO from (ungrounded buds) | Antibiotics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 10 | 1 | 5 | 10 | GM | C | E | P | |
| Diameter of inhibition zone (mm) | ||||||||||
| B. cereus | 27.3 ± 4.9a | 29.0 ± 3.2a | 32.1 ± 4.0a | 22.3 ± 1.6a | 26.3 ± 2.4a | 29.0 ± 5.0a | — | — | 25 ± 0a | — |
| S. aureus | 29.5 ± 6.5a | 30.0 ± 2.7a | 32.0 ± 3.6a | 21.6 ± 1.4a | 25.3 ± 5.3a | 26.5 ± 4.0a | — | — | — | 38 ± 2a |
| L. monocytogenes | 28.0 ± 5.6a | 29.8 ± 1.0a | 31.5 ± 3.7a | 17.2 ± 4.5b | 20.3 ± 4.3b | 21.6 ± 2.1b | 24 ± 0b | — | — | — |
| E. coli | 28.6 ± 1.4a | 34.0 ± 3.5a | 35.4 ± 2.6a | 26.0 ± 3.2b | 26.8 ± 1.6b | 28.0 ± 1.6b | — | 30 ± 1a | — | — |
| S. enteritidis | 25.8 ± 1.2b | 28.5 ± 1.8b | 29.6 ± 3.3b | 21.4 ± 3.1c | 22.2 ± 1.9c | 25.0 ± 3.7c | — | — | 36 ± 2a | — |
The different superscripts in the same line means significant difference (P ≤ 0.05). All antibiotics were applied at a concentration of 0.33 μg/mL. GM: gentamicin; C: chloramphenicol; E: erythromycin; P: penicillin. Pure essential oil was applied on a surface of 6 mm disc.
3.5. Allelopathic Effect
Figure 5 illustrates the H. niger germination rate under different clove buds EO concentrations. Noticeably better results were demonstrated for the essential oil (30 μL) in comparison with the control seeds. Indeed, clove buds EO achieved complete inhibition of H. niger germination during the whole experiment period; however, only 91% was recorded for the control seeds. Controls prepared with a methanol/water (1‰) mixture did not affect H. niger germination in comparison with controls in water. In addition, obtained results showed the dose-dependent manner of the allelopathic effect. These findings were in line with those reported by Amparo-Blazquez [45], which indicated that S. aromaticum EO caused an important inhibition of seedling growth against broccoli, lambsquarters, and redroot pigweed. The mechanism of germination inhibition is not well known, but some studies reported that a lack of electrolyte, cell destruction, and membrane permeability caused by EO could be implicated [46]. An inhibition of water uptake in the plant cell, as well as some physiological and biochemical processes may also lead to germination inhibition [47]. EOs caused many changes in plant seedlings leading to reduction in some organelles, such as mitochondria and, in certain cases, inhibition of DNA synthesis [47]. These factors may explain the blockage of seed germination and embryo death. The application of eugenol on hybrid rice decreased its seed germination speed at 2 g/concentration [48]. Kegley et al. reported that the effectiveness of clove EO is only important as burnt herbicide used on growing plant leaves [49].
Figure 5.
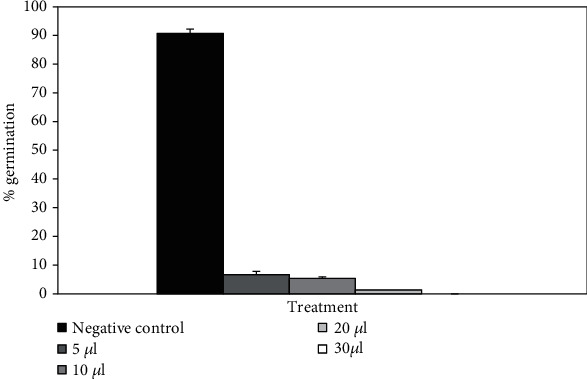
The germination percentage of H. niger decreased in a dose-dependent manner in the presence of grounded clove buds essential oil.
3.6. Insecticide Effect
The insecticidal activity was examined using the paper filter contact method. S. oryzae was significantly susceptible to the EO after 24 h of exposure. Probit analysis showed that 50% of mortality was achieved at 252.4 μL/L air concentration, when the confidence limits were about 95%. LD95 was estimated at 426.2 μL/L air. Cardiet et al. [50] mentioned that clove EO has an insecticidal activity against S. oryzae with LC90 observed at 355 μL/L. According to Devi and Devi [51], S. oryzae was susceptible to the toxic effect of clove oil resulting in 92% mortality by 21 days. Vendan et al. [52] registered a44.90 ± 6.91%adult mortality in S. oryzae recorded at a concentration of 400 μL/L air of clove oil and during 72 h of exposure time. Mishra et al. [53] reported that the essential oils extracted from S. aromaticum killed the adults of S. oryzae by fumigant action. They also mentioned that the fumigant toxicity was proportional to exposure time and concentration. The authors indicated that S. aromaticum EO had stronger toxicity in comparison to A. marmelos EO against S. oryzae. For that reason, Jairoce et al. [54] recommended clove bud EO as a promising alternative to be used under storage conditions for the integrated management of stored grains pest. Besides, Trivedi et al. [55] indicated that the LC50 value obtained for clove oil after 24 h exposure against C. chinensis adults was about 0.3 μg/mL. The efficacy of clove oil is due to its major components, such as phenylpropanoids like carvacrol, thymol, eugenol, and cinnamaldehyde. The authors indicated that clove EO may be developed as possible natural fumigants or repellents to control the pulse beetle.
4. Conclusion
The main objectives of this study were the chemical and biological evaluations of S. aromaticum essential oil. The assessment of the grinding effect on clove buds EO was performed initially. It was demonstrated that the grounded buds gave higher essential oil yield than intact ones, with respective values of 14.3 and 7.1%. Chemical composition analysis identified particularly four major compounds, respectively, as mentioned below for unground and ground clove buds EOs: eugenol (87.4-68.7%), cyperene (7.2-20.5%), phenethyl isovalerate (3.6-6.4%), and cis-thujopsene (0.8-1.9%). α-Patchoulene was present at 0.2% in the chemical composition of grounded clove bud EO. For the antibacterial activity, the best inhibition zone diameter was observed against E. coli(35.5 ± 2.6 mm), when the grounded clove buds EO was applied. This important antimicrobial activity enhances the potential use of essential oil as a food preservative. Regarding the antioxidant power, both tested EOs were more potent radical DPPH scavengers (IC50 values of 2.8 ± 0.5 and 1.2 ± 0.1 μg/mL, respectively, for ungrounded and grounded buds) than α-tocopherol, as they exhibited noticeable ferric-reducing power (EC50 = 32 and 50 μg/mL, respectively, for intact and grounded clove buds). The significant antioxidant power encourages the application of clove buds essential oil in the composition of several drugs as a natural remedy. Furthermore, our results highlighted the potent herbicidal effect of S. aromaticum oil on seed germination of H. niger, as well as its allelopathic effect. These findings promote the use of clove buds essential oil as a synthetic pesticide alternative to preserve maize, wheat, or other grass seeds during their storage period.
Acknowledgments
The authors are grateful to Miss Amina Gammoudi, English teacher at the Higher Institute of Applied Biology of Medenine (Tunisia) for her support and English revision.
Abbreviations
- ATCC:
American Type Culture Collection
- BHT:
Butylated hydroxytoluene
- C:
Chloramphenicol
- DPPH:
2,2-Diphenyl-1-picrylhydrazyl
- E:
Erythromycin 500
- EO:
Essential oil
- FeCl3:
Iron chloride (III)
- FRAP:
Ferric-reducing antioxidant power
- GC-MS:
Gas chromatography-mass spectrometry
- GM:
Gentamicin
- IP:
Inhibition percentage
- IZD (Ф):
Inhibition zone diameter
- K3Fe (CN)6:
Potassium ferricyanide solution
- MBC:
Minimum bactericidal concentration
- MIC:
Minimum inhibitory concentration
- Na2SO4:
Anhydrous sodium sulfate
- NIST:
National Institute of Standards and Technology
- P:
Penicillin
- RI:
Retention index
- TCA:
Trichloroacetic acid.
Contributor Information
Dorsaf Ben Hassine, Email: dorsaf_benhassine@yahoo.fr.
Jalloul Bouajila, Email: jalloul.bouajila@univ-tlse3.fr.
Data Availability
The datasets generated during the current study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Giannenas I., Sidiropoulou E., Bonos E., Christaki E., Florou-Paneri P. Feed Additives: Aromatic Plants and Herbs in Animal Nutrition and Health. 1st. Academic Press; 2020. The history of herbs, medicinal and aromatic plants, and their extracts: past, current situation and future perspectives; pp. 1–18. [Google Scholar]
- 2.Stein S. W., Thiel C. G. The history of therapeutic aerosols: a chronological review. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2017;30(1):20–41. doi: 10.1089/jamp.2016.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kouidhi B., al Qurashi Y. M. A., Chaieb K., Chaieb K. Drug resistance of bacterial dental biofilm and the potential use of natural compounds as alternative for prevention and treatment. Microbial Pathogenesis. 2015;80:39–49. doi: 10.1016/j.micpath.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Izneid T., Rauf A., Ali Shariati M., et al. Sesquiterpenes and their derivatives—natural anticancer compounds: an update. Pharmacological Research. 2020;161:105–165. doi: 10.1016/j.phrs.2020.105165. [DOI] [PubMed] [Google Scholar]
- 5.Ben Hassine D., Abderrabba M., Yvon Y., et al. Chemical composition and in vitro evaluation of the antioxidant and antimicrobial activities of Eucalyptus gillii essential oil and extracts. Molecules. 2012;17(8):9540–9558. doi: 10.3390/molecules17089540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batiha G. E. S., Alkazmi L. M., Wasef L. G., Beshbishy A. M., Nadwa E. H., Rashwan E. K. Syzygium aromaticum L. (Myrtaceae): traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules. 2020;10:1–16. doi: 10.3390/biom10020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Immacolata A., Sabia C., de Niederhäusern S., et al. In vitro evaluation of the amoebicidal activity of rosemary (Rosmarinus officinalis L.) and cloves (Syzygium aromaticum L. Merr. & Perry) essential oils against Acanthamoeba polyphaga trophozoites. Natural Product Research. 2017;33(4):606–611. doi: 10.1080/14786419.2017.1399390. [DOI] [PubMed] [Google Scholar]
- 8.Sharifi-Rad J., Sureda A., Tenore G. C., et al. Biological activities of essential oils: from plant chemoecology to traditional healing systems. Biomolecules. 2017;22:1–55. doi: 10.3390/molecules22010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budiati T., Suryaningsih W., Umaroh S., Poerwanto B., Bakri A., Kurniawati E. Antimicrobial activity of essential oil from Indonesian medicinal plants against food-borne pathogens. Earth and Environmental Science. 2018;207:1–6. [Google Scholar]
- 10.Chouhan S., Sharma K., Guleria S. Antimicrobial activity of some essential oils—present status and future perspectives. Medicine. 2017;4(3):p. 58. doi: 10.3390/medicines4030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortès-Rojas D. F., Fernandes de Souza C. R., Oliveira W. P. Clove (Syzygium aromaticum): a precious spice. Asian Pacific Journal of Tropical Biomedicine. 2014;4(2):90–96. doi: 10.1016/S2221-1691(14)60215-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.el Euch S. K., Bouajila J., Bouzouita N. Chemical composition, biological and cytotoxic activities of Cistus salviifolius flower buds and leaves extracts. Industrial Crops and Products. 2015;76:1100–1105. doi: 10.1016/j.indcrop.2015.08.033. [DOI] [Google Scholar]
- 13.Zahed N., Hosni K., Ben Brahim N., Kallel M., Sebei H. Allelopathic effect of Schinus molle essential oils on wheat germination. Acta Physiologiae Plantarum. 2010;32(6):1221–1227. doi: 10.1007/s11738-010-0492-z. [DOI] [Google Scholar]
- 14.Bachrouch O., Ferjani N., Haouel S., Jemâa J. M. B., Ben J. M. Major compounds and insecticidal activities of two Tunisian Artemisia essential oils toward two major coleopteran pests. Industrial Crops and Products. 2015;65:127–133. doi: 10.1016/j.indcrop.2014.12.007. [DOI] [Google Scholar]
- 15.Thomas A., Mazigo H. D., Manjurano A., Morona D., Kweka E. J. Evaluation of active ingredients and larvicidal activity of clove and cinnamon essential oils against Anopheles gambiae (sensu lato) Parasites & Vectors. 2017;10(1):1–7. doi: 10.1186/s13071-017-2355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selles S. M. A., Kouidri M., Belhamiti B. T., Ait Amrane A. Chemical composition, in-vitro antibacterial and antioxidant activities of Syzygium aromaticum essential oil. Journal of Food Measurement and Characterization. 2020;14(4):2352–2358. doi: 10.1007/s11694-020-00482-5. [DOI] [Google Scholar]
- 17.Kapadiya S., Desai M. A. Isolation of essential oil from buds of Syzygium aromaticum using hydrodistillation: multi-response optimization and predictive modelling. International Journal of Advanced Science and Technology. 2017;6(1):405–418. [Google Scholar]
- 18.Johner J. C. F., Hatami T., Carvalho P. I. N., Meireles M. A. A. Impact of grinding procedure on the yield and quality of the extract from clove buds using supercritical fluid extraction. Open Food Science Journal. 2018;10(1):1–7. doi: 10.2174/1874256401810010001. [DOI] [Google Scholar]
- 19.Ben Hassine D., Ben Ismail H., Jribi C., Khouja M. L., Abderrabba M. Chemical composition of some Tunisian Eucalyptus essential oils as obtained by hydrodistillation using Clevenger type apparatus. Biosciences, Biotechnology Research Asia. 2010;7(2):647–656. [Google Scholar]
- 20.Aguilar-González A. E., Palou E., López-Malo A. Antifungal activity of essential oils of clove (Syzygium aromaticum) and/or mustard (Brassica nigra) in vapor phase against gray mold (Botrytis cinerea) in strawberries. Innovative Food Science & Emerging Technologies. 2015;32:181–185. [Google Scholar]
- 21.Xu J. G., Liu T., Hu Q. P., Cao X. M. Chemical composition, antibacterial properties and mechanism of action of essential oil from clove buds against Staphylococcus aureus. Molecules. 2016;21(1194):1–13. doi: 10.3390/molecules21091194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herzi N. These de doctorat. Extraction et purification de substances naturelles: comparaison de l’extraction au CO2-supercritique et des techniques conventionnelles, [Ph.D. thesis] 2013. [Google Scholar]
- 23.Andrade J. K. S., Denadai M., De Oliveira C. S., Nunes M. L., Narain N. Evaluation of bioactive compounds potential and antioxidant activity of brown, green and red propolis from Brazilian northeast region. Food Research International. 2017;101:129–138. doi: 10.1016/j.foodres.2017.08.066. [DOI] [PubMed] [Google Scholar]
- 24.Sultana K., Jayathilakan K., Pandey M. C. Evaluation of antioxidant activity, radical scavenging, and reducing power of clove oil and clove oleoresin in comparison with natural and synthetic antioxidants in chevon (Capra aegagrus hircus) and chicken meat. Defence Life Science Journal. 2017;3(1):51–88. doi: 10.14429/dlsj.3.12016. [DOI] [Google Scholar]
- 25.Gülçin I., Elmastaş M., Aboul-Enein H. Y. Antioxidant activity of clove oil: a powerful antioxidant source. Arabian Journal of Chemistry. 2012;5(4):489–499. doi: 10.1016/j.arabjc.2010.09.016. [DOI] [Google Scholar]
- 26.Mahboub R., Memmou F. Antioxidant activity and kinetics studies of eugenol and 6-bromoeugenol. Natural Product Research. 2015;29(10):966–971. doi: 10.1080/14786419.2014.958738. [DOI] [PubMed] [Google Scholar]
- 27.da Silva F. F. M., Monte F. J. Q., de Lemos T. L. G., do Nascimento P. G. G., de Medeiros Costa A. K., de Paiva L. M. M. Eugenol derivatives: synthesis, characterization, and evaluation of antibacterial and antioxidant activities. Chemistry Central Journal. 2018;12(1):p. 34. doi: 10.1186/s13065-018-0407-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Somrani M., Debbabi H., Palop A. Antibacterial and antibiofilm activity of essential oil of clove against Listeria monocytogenes and Salmonella enteritidis. Food Science and Technology International. 2021;0(0):1–9. doi: 10.1177/10820132211013273. [DOI] [PubMed] [Google Scholar]
- 29.Hu Q., Zhou M., Wei S. Progress on the antimicrobial activity research of clove oil and eugenol in the food antisepsis field. Journal of Food Science. 2018;83(6):1476–1483. doi: 10.1111/1750-3841.14180. [DOI] [PubMed] [Google Scholar]
- 30.Radünz M., Martins da Trindade M. L., Camargo T. M., et al. Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food Chemistry. 2019;276:180–186. doi: 10.1016/j.foodchem.2018.09.173. [DOI] [PubMed] [Google Scholar]
- 31.Thielmann J., Muranyi P., Kazman P. Screening essential oils for their antimicrobial activities against the foodborne pathogenic bacteria Escherichia coli and Staphylococcus aureus. Heliyon. 2019;5(6):1–6. doi: 10.1016/j.heliyon.2019.e01860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdullah H. B., Hatem S. F., Jumaa W. A comparative study of the antibacterial activity of clove and rosemary essential oils on multidrug resistant bacteria. Pharmaceutical and Biosciences. 2016;3(1):18–22. [Google Scholar]
- 33.Javed S., Mushtaq S., Khokhar I., Ahmad R., Haider M. S. Comparative antimicrobial activity of clove and fennel essential oils against food borne pathogenic fungi and food spoilage bacteria. African Journal of Biotechnology. 2012;11(94):16065–16070. [Google Scholar]
- 34.Toledano-Medina M. A., Pérez-Aparicio J., Moreno-Rojas R., Merinas-Amo T. Evolution of some physicochemical and antioxidant properties of black garlic whole bulbs and peeled cloves. Food Chemistry. 2016;199:135–139. doi: 10.1016/j.foodchem.2015.11.128. [DOI] [PubMed] [Google Scholar]
- 35.Teixeira B., Marques A., Pires C., et al. Characterization of fish protein films incorporated with essential oils of clove, garlic and origanum: physical, antioxidant and antibacterial properties. LWT - Food Science and Technology. 2014;59(1):533–539. doi: 10.1016/j.lwt.2014.04.024. [DOI] [Google Scholar]
- 36.Sanuja S., Agalya A., Umapathy M. J. Studies on magnesium oxide reinforced chitosan bionanocomposite incorporated with clove oil for active food packaging application. International Journal of Polymeric Materials. 2014;63(14):733–740. doi: 10.1080/00914037.2013.879445. [DOI] [Google Scholar]
- 37.Lekjing S. A chitosan-based coating with or without clove oil extends the shelf life of cooked pork sausages in refrigerated storage. Meat Science. 2016;111:192–197. doi: 10.1016/j.meatsci.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Karuppiah P., Rajaram S. Antibacterial effect of Allium sativum cloves and Zingiber officinale rhizomes against multiple-drug resistant clinical pathogens. Asian Pacific Journal of Tropical Biomedicine. 2012;2(8):597–601. doi: 10.1016/S2221-1691(12)60104-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gayoso C. W., Lima E. O., Oliveira V. T., et al. Sensitivity of fungi isolated from onychomycosis to Eugenia cariophyllata essential oil and eugenol. Fitoterapia. 2005;76(2):247–249. doi: 10.1016/j.fitote.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Viuda-Martos M., Ruiz-Navajas Y., Fernández-López J., Pérez-Álvarez J. A. Antifungal activities of thyme, clove and oregano essential oils. Journal of Food Safety. 2007;27(1) doi: 10.1111/j.1745-4565.2007.00063.x. [DOI] [Google Scholar]
- 41.Cui H., Bai M., Rashed M. M. A., Lin L. The antibacterial activity of clove oil/chitosan nanoparticles embedded gelatin nanofibers against Escherichia coli O157:H7 biofilms on cucumber. International Journal of Food Microbiology. 2018;266:69–78. doi: 10.1016/j.ijfoodmicro.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 42.Dehghani P., Hosseini S. M. H., Golmakani M.-T., Majdinasab M., Esteghlal S. Shelf-life extension of refrigerated rainbow trout fillets using total Farsi gum-based coatings containing clove and thyme essential oils emulsions. Food Hydrocolloids. 2018;77:677–688. doi: 10.1016/j.foodhyd.2017.11.009. [DOI] [Google Scholar]
- 43.Khaleque M. A., Keya C. A., Hasan K. N., Hoque M. M., Inatsu Y., Bari M. L. Use of cloves and cinnamon essential oil to inactivate Listeria monocytogenes in ground beef at freezing and refrigeration temperatures. LWT. 2016;74:219–223. doi: 10.1016/j.lwt.2016.07.042. [DOI] [Google Scholar]
- 44.Muppalla S. R., Kanatt S. R., Chawla S. P., Sharma A. Carboxymethyl cellulose-polyvinyl alcohol films with clove oil for active packaging of ground chicken meat. Food Packaging and Shelf Life. 2014;2(2):51–58. doi: 10.1016/j.fpsl.2014.07.002. [DOI] [Google Scholar]
- 45.Blázquez M. Role of natural essential oils in sustainable agriculture and food preservation. Journal of Scientific Research and Reports. 2014;3(14):1843–1860. doi: 10.9734/JSRR/2014/11376. [DOI] [Google Scholar]
- 46.Rassaeifar M., Hosseini N., Haji-Hasani A. N., Zandi P., Moradi-Aghdam A. Allelopathic effect of Eucalyptus globulus essential oil on seed germination and seedling establishment of Amaranthus blitoides and Cydon dactylon. Trakia Journal of Sciences. 2013;11(1):73–81. [Google Scholar]
- 47.Amri I., Hamrouni L., Hanana M., Jamoussi B. Chemical composition of Juniperus oxycedrus L. subsp. macrocarpa essential oil and study of their herbicidal effects on germination and seedling growth of weeds. Asian Journal of Applied Sciences. 2011;4(8):771–779. [Google Scholar]
- 48.Hu Q., Lin C., Guan Y., Sheteiwy M. S., Hu W., Hu J. Inhibitory effect of eugenol on seed germination and pre-harvest sprouting of hybrid rice (Oryza sativa L.) Scientific Reports. 2017;7(1):p. 5295. doi: 10.1038/s41598-017-04104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kegley S., Conlisk E., Moses M. Marin municipal water district herbicide risk assessment. Overview and Executive Summary. 2010;1:1–20. [Google Scholar]
- 50.Cardiet G., Fuzeau B., Barreau C., Fleurat-Lessard F. Contact and fumigant toxicity of some essential oil constituents against a grain insect pest Sitophilus oryzae and two fungi, Aspergillus westerdijkiae and Fusarium graminearum. Journal of Pest Science. 2012;85(3):351–358. doi: 10.1007/s10340-011-0400-3. [DOI] [Google Scholar]
- 51.Devi K. C., Devi S. S. Insecticidal and oviposition deterrent properties of some spices against coleopteran beetle, Sitophilus oryzae. Journal of Food Science and Technology. 2013;50(3):600–604. doi: 10.1007/s13197-011-0377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vendan S. E., Manivannan S., Sunny A. M., Murugesan R. Phytochemical residue profiles in rice grains fumigated with essential oils for the control of rice weevil. PLoS One. 2017;12(10, article e0186020) doi: 10.1371/journal.pone.0186020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mishra B. B., Tripathi S. P., Tripathi C. P. M. Bioactivity of two plant-derived essential oils against the rice weevils Sitophilus oryzae (L.) (Coleoptera: Curculionidae) Proceedings of the National Academy of Sciences, India Section B: Biological Sciences. 2012;83(2):3–7. [Google Scholar]
- 54.Jairoce C. F., Teixeira C. M., Nunes C. F. P., Nunes A. M., Pereira C. M. P., Garcia F. R. M. Insecticide activity of clove essential oil on bean weevil and maize weevil. Revista Brasileira de Engenharia Agrícola e Ambiental. 2016;20(1):72–77. doi: 10.1590/1807-1929/agriambi.v20n1p72-77. [DOI] [Google Scholar]
- 55.Trivedi A., Nayak N., Kumar J. Fumigant toxicity study of different essential oils against stored grain pest Callosobruchus chinensis. Journal of Pharmacognosy and Phytochemistry. 2017;6(4):1708–1711. [Google Scholar]
- 56.Choi H.-S. Character impact odorants of citrus hallabong [(C. unshiu Marcov x C. sinensis Osbeck) x C. reticulata Blanco] cold-pressed peel oil. Journal of Agricultural and Food Chemistry. 2003;51(9):2687–2692. doi: 10.1021/jf021069o. [DOI] [PubMed] [Google Scholar]
- 57.Adams R. P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Carol Stream, IL, USA: Allured Publishing Corporation; 1995. [Google Scholar]
- 58.Senatore F., Arnold N. A., Bruno M. Volatile components ofCentaurea eryngioidesLam. andCentaurea ibericaTrev. var.hermonisBoiss. Lam., two Asteraceae growing wild in Lebanon. Nat. Prod. Res. 2005;19(8):749–754. doi: 10.1080/14786410412331302136. [DOI] [PubMed] [Google Scholar]
- 59.Leffingwell J. C., Alford E. D. Volatile constituents of perique tobacco. Electronic Journal of Environmental, Agricultural and Food Chemistry. 2005;4:899–915. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are available from the corresponding authors upon reasonable request.


