Abstract
Background
The present study aims to investigate the effect of Lactobacillus casei on lipid metabolism and intestinal microflora in patients with alcoholic liver injury.
Methods
In a double-blind randomized controlled trial, 158 recruited alcoholic liver injury patients were randomized to three treatments for 60 days: low-dose group (LP, n = 58, 100 ml of Lactobacillus casei strain Shirota (LcS)), high-dose group (HP, n = 54, 200 ml of LcS), and positive control group (PC, n = 46, 100 ml of special drinks without active Lactobacillus casei). Another group of 20 healthy people was served as normal control group (NC).
Results
The serum levels of TG and LDLC in the HP group were significantly decreased by 26.56% and 23.83%, respectively than those in the PC group (P < 0.05). After supplementation of Lactobacillus casei, there was a significant increase in the amount of Lactobacillus and Bifidobacterium when compared with the PC group (P < 0.05).
Conclusions
Supplementation of Lactobacillus casei can improve lipid metabolism and regulate intestinal flora disorders in patients with alcoholic liver injury.
Subject terms: Diseases, Chemistry
Introduction
Alcoholic liver injury caused by long-term and/or excessive drinking has become an international public health problem [1–4]. Alcoholic liver disease (ALD) is a series of liver diseases caused by long-term heavy drinking. Lipid metabolism disorder often occurs in people with alcoholic liver injury [5, 6]. The treatments for chronic alcoholic liver injury mainly are a combinations of alcohol abstinence, improving nutrition, treating the liver injury, and preventing or reversing the progress of liver fibrosis or promoting liver regeneration [7].
Probiotics play an important role in regulating intestinal flora. It has been found that intestinal microecological disorders and endotoxemia caused by excessive alcohol intake also play an important role in the occurrence and development of alcohol-induced liver injury [8–10]. Studies have shown that appropriate supplementation of probiotics can play a good therapeutic role in repairing intestinal mucosal injury, protecting the liver, and preventing cancer and depression [11–14]. Of note, most of these studies are rodent models or non-alcoholic fatty liver population, and most of them use lactobacillus as an intervention [15–18]. There are few studies on the intervention effect of Lactobacillus casei on alcoholic liver injury patients.
Therefore, in order to fill the gap and to confirm the results of the observational studies, a large randomized controlled trial of Lactobacillus casei supplements in ALD patients is highly necessary.
Materials and methods
Trial design
This study was designed as a randomized, double-blind, placebo-controlled trial. Random assignment was performed by one of the investigators who was not involved in the trial by using computer-generated random numbers. During the intervention, neither the participants nor the nurses/physicians in the study center knew the probiotics’ types. Allocation sequence was generated by doctors/nurses in research center and participants were assigned to the intervention groups. All participants, staff members and outcome assessors were blinded to the study assignments until the final analyses were completed. The trial was registered in the Medical Ethics Expert Committee of Qingdao CDC (No. DIC2014J3). All the participants gave written informed consent.
Inclusion and exclusion criteria of the study participants
There is a great disparity in alcohol consumption and rates of dependence between the sexes in China: the rates of alcohol use disorder are 9.3% among men and 0.2% among women, with the male-to-female ratio of 47:1 being substantially higher than in most other countries in the world [19, 20]. Chinese males typically have more social opportunities to drink rather than females. In contrast, the traditional responsibility of Chinese adult females is to manage the daily life of their families, which keeps women in these roles busy caring for children and elderly relatives. They do not have as many opportunities to participate in social engagements as males do. Thus, the delegation of family responsibility restricts the females from frequently consuming alcohol. Our preliminary research also found that there were no female outpatients in the clinic.
The inclusion criteria were: (1) 30–65 years old patients who met the criteria of Alcoholic Liver Disease Diagnosis and Treatment Guidelines (2018 Revision) [21]; (2) those who had not taken probiotic beverage recently, could adhere to probiotic supplementation for 2 months and complete the main observation indicators; (3) those who volunteered to participate and sign the informed consent; (4) patients diagnosed with alcoholic fatty liver by B-ultrasound. The exclusion criteria were: (1) women; (2) people under 30 years old or over 65 years old; (3) relapsed drinkers during treatment; (4) patients with severe complications such as cardiovascular, cerebrovascular, liver and hematopoietic system, or with alcoholic liver fibrosis, alcoholic liver cirrhosis and hepatocellular carcinoma; (5) patients with mental illness who cannot cooperate; (6) those who do not meet the inclusion criteria and cannot judge the efficacy or safety because of incomplete data.
The selection of dosages and experiment beverage preparation
Referring to the dosages of domestic and foreign vivo studies [22–24], which contained Lactobacillus casei strain Shirota (LcS) bacterial cells at 200 × 108/200 ml day, 400 × 108/80 ml day, 400 × 108/80 ml day respectively, patients in the pre-experiment were given 2 bottles/day of beverage containing LcS bacterial cells at 200 × 108/200 ml, and there were no gastrointestinal adverse reactions such as vomiting, diarrhea and constipation, so the maximum dose was set as 2 bottles/day. The low-dose group was given 1 bottle/day of beverage containing LcS bacterial cells at 100 × 108/100 ml to observe the intervention effect. This dosage would ensure the safety of this study.
The fermented milk beverage and placebo (both 100 ml/bottle) were used as the test beverages. The ingredients of the fermented milk beverage were water, sugar, defatted milk, glucose, flavoring agents and Lactobacillus casei. The nutritional contents per bottle (100 ml) were 68 kcal energy, 1.2 g protein, 0.0 g lipid, 15.7 g carbohydrate, and 15 mg sodium. The fermented milk beverage contained 10 billion or more LcS bacterial cells. The placebo was prepared with the same ingredients so it had the same nutritional content and taste as the fermented milk beverage, except that it did not contain Lactobacillus casei.
Sample size calculation and study participants
We employed the liver function index as the primary outcome, as shown in a previous study [25, 26], 43 subjects were required for each group to achieve 80% power of detecting a treatment effect at a two-sided significance level of 5%. Assuming that there were 11 dropouts in each group, a final sample size of at least 54 persons in each group was determined to enable adequate power to evaluate the primary outcome.
A total of 193 potential adults with known ALD were screened in the study centers between March 2016 and December 2017. A total of 181 ALD patients were recruited based on the inclusion and exclusion criteria. During the intervention, 14 participants in the PC group, 3 participants in the LP group and 6 participants in the HP group dropped out of the trials due to drinking alcohol or eating foods containing probiotics, leaving 46, 58, and 54 participants in the PC, LP, and HP group, respectively (supplementary material Fig. S1). The positive control group (PC, n = 46) received a routine treatment and 100 ml of placebo beverage were given once a day; the low-dose Lactobacillus casei intervention group (LP, n = 58) which received the routine treatment and 100 ml of LcS (10 billion) was given once a day; the high-dose Lactobacillus casei intervention group (HP, n = 54) which received a routine treatment and 100 ml of LcS (10 billion) was given twice a day; and 20 healthy male people were selected as normal control group (NC).
The patients in each trial group were required to take the daily supplement after meals during a treatment period of 60 days. All patients were given a weekly dose of beverage at baseline, and then given the next week’s dose when they returned to the Study Center for a health check-up 1 week after the intervention. Compliance to this supplement was evaluated indirectly by counting the empty bottles brought back by the patients every week and checking diet diaries (to check if the subjects ate foods containing probiotics). At the beginning of the research, all subjects were asked to provide three dietary records (one weekend day and two weekdays). Based on these 3-day food diaries, the average nutrient intakes of each participant were calculated by Nutrition System of Traditional Chinese Medicine Combining with Western Medicine (version 11.0). Participants were asked not to change their dietary habits, routine physical activities, or use of prescribed medications and to avoid using any probiotics supplements other than those provided to them by the researchers during the intervention.
During the experiment, the subjects were reminded by telephone to take the probiotic preparations and fill in the dietary record form. Missing doses and their reasons were recorded at any time. The tracking methods used were mainly done via phone calls: gave psychological counseling and improved the confidence of patients, reminded patients to keep alcohol abstinence and keep up to date their diet diaries (avoid using others probiotics); regularly informed patients to come back for a health check-up and took the supplements for the following week.
Assessment of anthropometric measures
Anthropometric parameters were measured by well-trained physicians at baseline. The weight and height of the patients were recorded when they were wearing light clothes without shoes, and body mass index (BMI) was calculated as the participant’s weight (kg) divided by the square of standing height (m).
Main reagents and instruments
The quantitative limulus lysate kit with chromogenic matrix was obtained from Xiamen Limulus Reagent Laboratory Co., Ltd., the bacterial genome DNA extraction kit was obtained from Shanghai QIAgen, the real-time fluorescence quantitative PCR detection kit was obtained from Beijing TIANGEN, and the primer synthesis was entrusted to Shanghai biosynthesis. Beckman AU5400 automatic biochemical analyzer (Beckman, USA), Realplex 4 fluorescent quantitative PCR (Eppendorf, Germany).
Sample collection and measure
Fasting blood samples were collected at the baseline and after 60-day intervention. Assays of serum liver function index (alanine aminotransferase [ALT], aspartate aminotransferase [AST], gamma-glutamyltransferase [GGT], total bilirubin [TBIL], direct bilirubin [DBIL] and indirect bilirubin [IBIL]) and lipid profile (total cholesterol [TC], triglyceride [TG], high-density lipoprotein cholesterol [HDL-C], and low-density lipoprotein cholesterol [LDL-C]) were measured using commercially available kits on Beckman AU5400 automatic biochemical analyzer.
The endotoxin level in plasma was detected by quantitative limulus lysate kit with chromogenic matrix. All the containers were treated with pyrogen at 180 °C for 24 h and operated strictly according to the instructions of the kit.
ELISA (enzyme linked immunosorbent assay) assay kits (Cloud-Clone Corp, Katy, USA) were used to determine interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), interleukin-10 (IL-10) in serum, D-lactic acid (D-LA), intestinal fatty acid binding protein (I-FABP) levels, and diamine oxidase (DAO) activity in plasma, in accordance with the instructions of manufacturer.
Regulated upon Activation Normal T cell Expressed and Secreted (RANTES) and Monocyte chemotactic protein-1 (MCP-1) were measured by enzyme immunoassays using commercially available paired antibodies or kits.
Gut microbiota analysis
Genome DNA was extracted from 100 mg samples of feces and stored −20 °C. The contents of bacterial genome DNA were quantified by using real-time fluorescence quantification polymerase chain reaction (PCR) method, involving Escherichia coil, Enterococcus, Bifidobacterium, Lactobacillus, Bacteroides fragilis, and Clostridium tender. The reaction system and reaction condition are similar to standard curve. According to the manufacturers’ instructions, the whole sequence was amplified for fluorescent real-time PCR with the kit (QIAgen, Shanghai, China). Primer sequences and annealing temperature of PCR amplification of specific bacterial are shown in Supplementary material Table S1.
Statistical analysis
We conducted the statistical analyses in SPSS (version 18; Chicago, IL, USA). One-way ANOVA or chi-square test was performed to test the group difference. P (two-tailed) < 0.05 was considered significant. Student’s paired t-test was used to test the difference in markers before and after intervention. Pearson and spearman anlysis were used to analyze the correlation. We conducted the generalized linear models (GLM) in Stata (version 13; StataCorp, College Station, TX, USA). To assess the supplement effects on all the outcomes between groups, the GLM was used to test the difference between groups, with adjustment for age, BMI, liver function, lipid metabolism and inflammatory factor.
Results
At baseline, there was no significant difference in blood lipids, liver function, inflammatory factors or the quantitative of the six bacteria between participants of the three intervention groups (Table 1). Three-day diet assessments at baseline are reported in Supplementary material Table S2. No significant difference in total energy and the other dietary nutrients was found between groups.
Table 1.
Baseline characteristics of study participants involved in the randomized controlled trial.
| PC (n = 46) | LP (n = 58) | HP (n = 54) | |
|---|---|---|---|
| Age, years | 52.60 ± 5.67 | 51.10 ± 3.90 | 49.6 ± 4.17 |
| Height, cm | 166.50 ± 8.02 | 162.05 ± 4.47 | 164.10 ± 10.17 |
| Weight, kg | 69.6 ± 8.30 | 69.87 ± 9.08 | 72.15 ± 10.81 |
| BMI, kg/m2 | 25.06 ± 1.93 | 26.63 ± 3.35 | 26.76 ± 2.71 |
| ALT, U/l | 84.41 ± 23.26 | 81.11 ± 34.66 | 82.37 ± 32.36 |
| AST, U/l | 73.77 ± 44.77 | 82.88 ± 42.84 | 78.12 ± 39.56 |
| GGT, U/l | 181.46 ± 77.40 | 164.69 ± 87.40 | 158.12 ± 82.21 |
| TBIL, μmol/L | 26.52 ± 6.73 | 25.05 ± 9.38 | 24.65 ± 0.38 |
| Endotoxin, EU/ml | 0.35 ± 0.04 | 0.24 ± 0.03 | 0.22 ± 0.02 |
| TG, mmol/l | 2.27 ± 0.82 | 1.97 ± 0.66 | 2.01 ± 0.59 |
| TC, mmol/l | 5.98 ± 0.87 | 5.96 ± 1.05 | 6.12 ± 1.06 |
| LDLC, mmol/l | 5.05 ± 0.85 | 4.07 ± 0.83 | 3.95 ± 0.81 |
| HDLC, mmol/l | 1.33 ± 0.34 | 1.28 ± 0.26 | 1.30 ± 0.31 |
| IL-1β, pg/ml | 49.77 ± 3.56 | 48.84 ± 3.65 | 52.64 ± 4.55 |
| TNF-α, pg/ml | 118.96 ± 13.16 | 107.03 ± 11.52 | 116.54 ± 14.87 |
| IL-6, pg/ml | 209.05 ± 31.64 | 210.23 ± 27.31 | 212.89 ± 24.26 |
| IL-10, pg/ml | 76.55 ± 13.89 | 69.64 ± 14.77 | 69.14 ± 14.84 |
| Escherichia coli, copies/g | 7.88 ± 0.57 | 8.22 ± 0.89 | 7.51 ± 1.05 |
| Enterococcus facalis, copies/g | 5.09 ± 1.11 | 4.96 ± 1.25 | 5.28 ± 1.34 |
| Bacteroides fragilis, copies/g | 7.43 ± 1.22 | 8.27 ± 1.82 | 8.45 ± 1.27 |
| Bifidobacterium longum, copies/g | 5.39 ± 0.38 | 5.23 ± 0.18 | 5.33 ± 0.37 |
| Lactobacillus acidophilus, copies/g | 5.05 ± 0.93 | 4.27 ± 1.02 | 5.12 ± 0.74 |
| Clostridium leptum, copies/g | 8.28 ± 1.40 | 7.88 ± 1.81 | 7.40 ± 1.51 |
Values are presented as mean ± SD. One-way ANOVA (for continuous variables) or chi-square test (for categorical variables) was performed to test the group difference at baseline.
ALT alanine aminotransferase, AST aspartate aminotransferase, GGT gamma-glutamyltransferase, TBIL total bilirubin, TG triglyceride, TC total cholesterol, HDLC: high density lipoprotein cholesterol, LDLC low density lipoprotein cholesterol, IL-1β interleukin-1β, IL-6 interleukin-6, TNF-α tumor necrosis factor-α, IL-10 interleukin-10, PC Positive control group, LP Low dose intervention group, HP High dose intervention group.
Nutritional Risk Screening 2002 (NRS2002)
All patients were screened for nutritional risk with NRS2002 [27]. There were 47 patients (29.75%) whose BMI < 18.5, 77 patients (48.73%) whose BMI were between 18.5 and 23.9, 34 patients (21.52%) whose BMI ≥ 24. There were 57 patients (36.08%) whose NRS ≥ 3.
Effect of probiotics on liver function and lipid metabolism
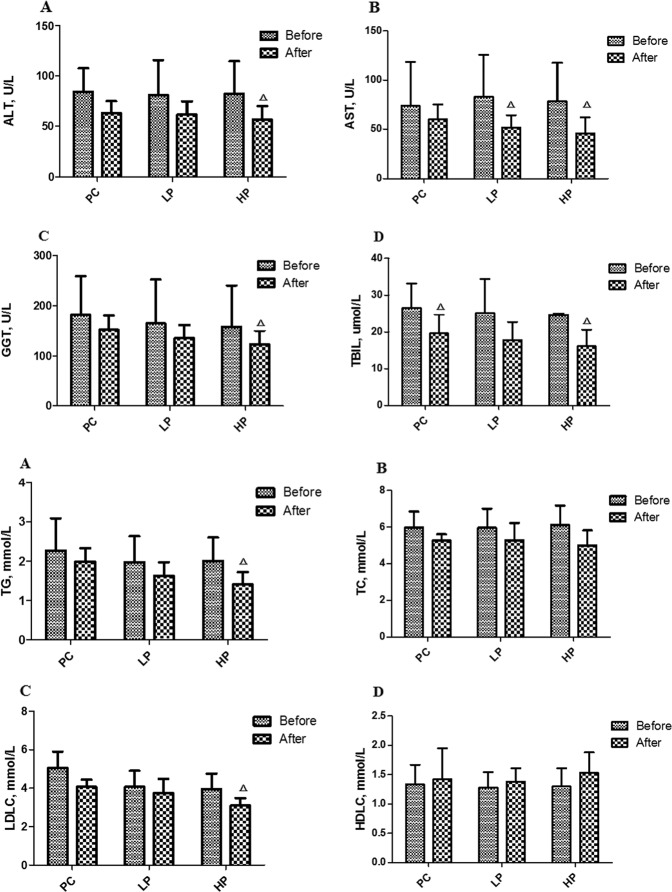
As shown in Table 2, the serum levels of ALT, AST, TBIL, GGT, TG, TC, and LDLC in the PC group were significantly increased than those in the NC group (P < 0.05) and the serum levels of TG, TC and LDLC in the PC group increased by 88.2%, 21.1%, and 27.6%, respectively (P < 0.05). The serum levels of TG and LDLC in the HP group were significantly decreased by 26.56% and 23.83% respectively than those in the PC group (P < 0.05) after treatment. Paired t-test on liver function between baseline and endpoint showed that TBIL in PC group, AST in LP group, ALT, AST, GGT, and TBIL in HP group were significantly decreased by 25.98%, 37.78%, 31.14%, 41.53%, 22.22%, and 34.77%, respectively (P < 0.05); Paired t-test on lipid metabolism between baseline and endpoint showed that TG and LDLC in HP group were significantly decreased by 29.85% and 21.52% (P < 0.05) (Fig. 1).
Table 2.
Effect of probiotics on liver function and lipid metabolism.
| NC | PC | LP | HP | Between-group comparisons | ||
|---|---|---|---|---|---|---|
| P1 | P2 | |||||
| ALT, U/l | 20.52 ± 6.45 | 63.02 ± 11.88* | 61.41 ± 13.37* | 56.72 ± 13.29* | 0.000 | 0.049 |
| AST, U/l | 19.95 ± 4.44 | 59.78 ± 15.10* | 51.57 ± 12.59* | 45.68 ± 16.24* | 0.000 | 0.004 |
| GGT, U/l | 26.90 ± 9.44 | 151.85 ± 28.55* | 135.07 ± 26.20* | 122.98 ± 27.47* | 0.000 | 0.046 |
| TBIL, μmol/l | 11.13 ± 1.84 | 19.63 ± 5.16* | 17.82 ± 4.88* | 16.08 ± 4.52 | 0.000 | 0.021 |
| DBIL, μmol/l | 4.25 ± 0.68 | 6.23 ± 0.90 | 6.17 ± 1.60 | 5.55 ± 1.39 | 0.000 | 0.328 |
| IBIL, μmol/l | 6.88 ± 2.08 | 13.41 ± 4.78* | 11.67 ± 4.90 | 10.54 ± 3.94 | 0.000 | 0.508 |
| TG, mmol/l | 1.01 ± 0.33 | 1.99 ± 0.34* | 1.62 ± 0.35* | 1.41 ± 0.31 | 0.000 | 0.000 |
| TC, mmol/l | 4.35 ± 0.65 | 5.27 ± 0.34* | 5.28 ± 0.95* | 5.01 ± 0.81* | 0.000 | 0.052 |
| LDLC, mmol/l | 2.53 ± 0.47 | 4.07 ± 0.37* | 3.76 ± 0.72 | 3.10 ± 0.39 | 0.000 | 0.000 |
| HDLC, mmol/l | 1.52 ± 0.30 | 1.42 ± 0.53* | 1.38 ± 0.23* | 1.53 ± 0.35 | 0.129 | 0.062 |
Values are presented as mean ± SD. *P < 0.05 indicated significantly different between the trial arms.
ALT alanine aminotransferase, AST aspartate aminotransferase, GGT gamma-glutamyltransferase, TG triglyceride,TC total cholesterol, HDLC high density lipoprotein cholesterol, LDLC low density lipoprotein cholesterol, TBIL total bilirubin, DBIL direct bilirubin, IBIL indirect bilirubin. NC normal control group, PC positive control group, LP low dose intervention group, HP high dose intervention group. *P < 0.05 versus NC group. P1 value was calculated by a crude general linear model to test the difference in treatment effects between the groups. P2 value was calculated by a full general linear model with adjustments for age, BMI, the liver function, lipid metabolism and inflammatory factor to test the difference in treatment effects between the groups.
Fig. 1. Changes of liver function and lipid metabolism before and after intervention.
Changes of liver function before and after intervention. A Changes of ALT before and after intervention. B Changes of AST before and after intervention. C Changes of GGT before and after intervention. D Changes of TBIL before and after intervention. Changes of lipid metabolism before and after intervention. A Changes of TG before and after intervention. B Changes of TC before and after intervention. C Changes of LDLC before and after intervention. D Changes of HDLC before and after intervention. Before: before intervention, After: after intervention, ALT alanine aminotransferase, AST aspartate aminotransferase, GGT gamma-glutamyltransferase, TBIL totalbilirubin, TG triglyceride, TC total cholesterol, LDL-C low-density lipoproteincholesterol, HDL-C high-density lipoprotein cholesterol. ΔP < 0.05 versus Baseline.
Effect of probiotics on serum inflammatory factors, chemokines, and intestinal barrier function
As shown in Table 3, compared with the NC group, the serum levels of TNF-α and IL-6 in PC, LP, and HP group were significantly increased (P < 0.05); while the serum levels of IL-10 in HP group were significantly increased (P < 0.05); The levels of serum RANTES in PC, LP, and HP group were significantly increased and the levels of serum MCP-1 in PC group were significantly increased (P < 0.05). There were significant differences between the groups after intervention (P < 0.05).
Table 3.
Effect of probiotics on serum inflammatory factors, chemokines, and intestinal barrier function.
| NC | PC | LP | HP | Between-group comparisons | ||
|---|---|---|---|---|---|---|
| P1 | P2 | |||||
| IL-1β, pg/ml | 27.56 ± 6.08 | 40.17 ± 6.66* | 37.27 ± 5.61 | 36.09 ± 8.41 | 0.000 | 0.008 |
| TNF-α, pg/ml | 52.91 ± 12.39 | 103.09 ± 15.39* | 101.40 ± 10.15* | 98.44 ± 11.43* | 0.000 | 0.041 |
| IL-6, pg/ml | 38.67 ± 9.39 | 208.6 ± 78.31* | 203.99 ± 30.77* | 141.6 ± 28.60* | 0.000 | 0.000 |
| IL-10, pg/ml | 102.04 ± 27.60 | 82.29 ± 21.46 | 97.04 ± 32.80 | 155.63 ± 36.92* | 0.000 | 0.000 |
| RANTES, ng/ml | 19.47 ± 6.47 | 52.56 ± 7.37* | 49.35 ± 5.38* | 45.82 ± 10.81* | 0.000 | 0.043 |
| MCP-1, pg/ml | 77.19 ± 9.15 | 115.99 ± 20.93* | 100.30 ± 10.20 | 93.13 ± 14.10 | 0.000 | 0.001 |
| D-lactates, μg/ml | 0.77 ± 0.17 | 1.48 ± 0.15* | 1.37 ± 0.23* | 1.24 ± 0.13* | 0.000 | 0.000 |
| Endotoxin, EU/ml | 0.03 ± 0.02 | 0.24 ± 0.01* | 0.17 ± 0.01 | 0.11 ± 0.02 | 0.000 | 0.000 |
| I-FABP, ng/ml | 109.79 ± 25.19 | 233.79 ± 49.43* | 206.19 ± 39.02* | 154.49 ± 29.08 | 0.000 | 0.000 |
| DAO, U/ml | 21.10 ± 4.49 | 38.91 ± 5.65* | 34.88 ± 4.35* | 23.53 ± 3.88 | 0.000 | 0.000 |
Values are presented as mean ± SD. P < 0.05 indicated significantly different between the trial arms
NC normal control group, PC positive control group, LP low dose intervention group, HP high dose intervention group.
*P < 0.05 versus NC group; P1 value was calculated by a crude general linear model to test the difference in treatment effects between the groups. P2 value was calculated by a full general linear model with adjustments for age, BMI, the liver function, lipid metabolism, and inflammatory factor to test the difference in treatment effects between the groups.
Compared with the NC group, the levels of D-lactates, Endotoxin, I-FABP, and DAO in plasma were significantly increased in the PC group (P < 0.05). The levels of Endotoxin, I-FABP, and DAO in HP group were significantly decreased by 54.17%, 33.93%, and 38.78%, respectively than those in PC group (P < 0.05). The level of DAO in HP group were significantly decreased by 26% than that in LP group (P < 0.05).
Quantitative analysis of 16S rDNA of dominant strains of fecal intestinal flora
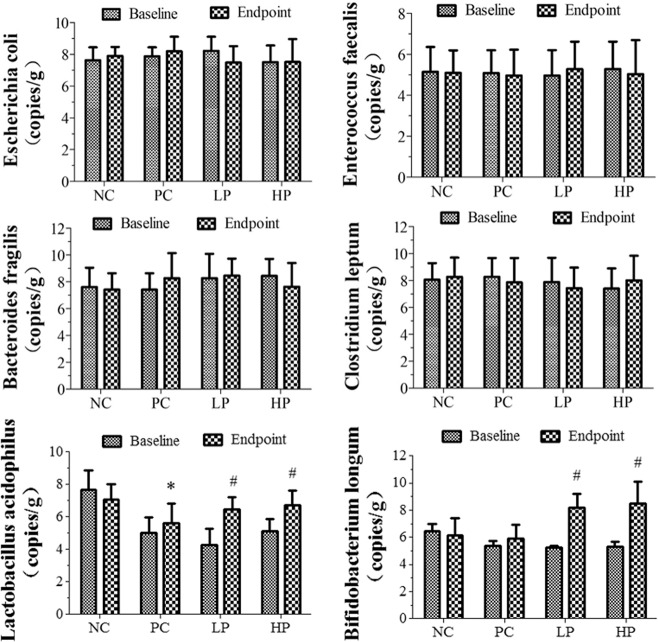
As shown in Fig. 2, the amount of Lactobacillus in the PC group was significantly decreased by 20.7% than that in the NC group (P < 0.05), while the amount of Escherichia coli, Enterococcus, Bifidobacterium, Bacteroides, and Clostridium leptum had no significant difference compared with the NC group (P > 0.05). After supplementation of Lactobacillus casei, the amounts of Lactobacillus and Bifidobacterium in the LP group were significantly increased by 15.3 and 19.9% than those in the PC group (P < 0.05); The amounts of Lactobacillus and Bifidobacterium in the HP group were significantly increased by 39.5 and 43.6% than those in the PC group (P < 0.05); There was no significant difference in the amount of Escherichia coli, Enterococcus, Bacteroides, and Clostridium leptum compared with the PC group (P > 0.05).
Fig. 2. The quantitative test results of the six bacteria in the intestinal flora.
NC Normal control group; PC Positive control group; LP Low dose intervention group; HP High dose intervention group. *P < 0.05 versus NC group; #P < 0.05 versus PC group.
Correlation analysis of liver function, lipid metabolism, and probiotics
As shown in Table 4, The ALT was positively correlated with TG and LDLC (r = 0.455 and 0.373, p < 0.05); the AST was positively correlated with TG, TC, and LDLC(r = 0.483, 0.339, and 0.507, P < 0.05); the TG was positively correlated with TBIL, DBIL, and IBIL(r = 0.416, 0.411, and 0.321, P < 0.05). No significant correlation was observed between liver function, lipid profile, and intestinal microflora.
Table 4.
Correlation analysis of liver function, lipid metabolism, and probiotics.
| TG | TC | LDLC | HDLC | TBIL | DBIL | IBIL | Escherichia coli | Bacteroides fragilis | Lactobacillus acidophilus | Enterococcus facalis | Bifidobacterium longum | Clostridium leptum | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALT | 0.4550 | 0.2080 | 0.3730 | 0.0558 | 0.5002 | 0.4236 | 0.4553 | −0.0486 | 0.0075 | −0.0775 | 0.1264 | 0.1924 | −0.0890 |
| 0.0032 | 0.1971 | 0.0176 | 0.7324 | 0.0010 | 0.0065 | 0.0032 | 0.7658 | 0.9636 | 0.6347 | 0.4372 | 0.2342 | 0.5849 | |
| AST | 0.4834 | 0.3390 | 0.5075 | 0.1153 | 0.4177 | 0.4574 | 0.3544 | 0.0400 | 0.2360 | −0.2371 | 0.0144 | 0.1001 | −0.1078 |
| 0.0016 | 0.0324 | 0.0008 | 0.4785 | 0.0073 | 0.0030 | 0.0248 | 0.8067 | 0.1427 | 0.1408 | 0.9299 | 0.5389 | 0.5079 | |
| GGT | 0.3013 | 0.0500 | 0.3716 | −0.1951 | 0.4628 | 0.5347 | 0.3744 | 0.0379 | 0.1281 | −0.1246 | 0.1373 | 0.1965 | −0.0964 |
| 0.0589 | 0.7594 | 0.0182 | 0.2276 | 0.0026 | 0.0004 | 0.0173 | 0.8163 | 0.4310 | 0.4438 | 0.3981 | 0.2242 | 0.5542 | |
| TG | 0.4805 | −0.0560 | 0.4164 | 0.4105 | 0.3214 | 0.1521 | 0.0293 | −0.1578 | 0.1611 | −0.1300 | −0.2339 | ||
| 0.0017 | 0.7317 | 0.0075 | 0.0085 | 0.0432 | 0.3487 | 0.8577 | 0.3309 | 0.3207 | 0.4241 | 0.1463 | |||
| TC | 0.3085 | −0.0159 | 0.1458 | 0.0950 | 0.1443 | −0.1483 | 0.0842 | −0.1486 | 0.1095 | 0.0999 | −0.0415 | ||
| 0.0528 | 0.9222 | 0.3695 | 0.5599 | 0.3744 | 0.3612 | 0.6053 | 0.3603 | 0.5010 | 0.5395 | 0.7993 |
Values are presented as correlation coefficient. P < 0.05 indicated significantly different between the trial arms.
ALT alanine aminotransferase, AST aspartate aminotransferase, GGT gamma-glutamyltransferase, TG triglyceride, TC total cholesterol, HDLC high density lipoprotein cholesterol, LDLC low density lipoprotein cholesterol, TBIL total bilirubin, DBIL direct bilirubin, IBIL indirect bilirubin.
Discussion
In the present study, paired t-test on lipid metabolism between baseline and endpoint showed that TG and LDLC in HP group were significantly different before and after intervention (P < 0.05) and the serum TG and LDLC in the patients treated with Lactobacillus casei supplements significantly decreased than those in the positive control group (P < 0.05). After supplementation of Lactobacillus casei, there was a significant increase in the amount of Lactobacillus and Bifidobacterium. We found that Lactobacillus casei supplements might potentially improve lipid metabolism and regulate intestinal flora disorders in patients with alcoholic liver injury over the past 2 months.
In several other trials, the authors suggested intestinal flora disorders may be the initiating factor of alcoholic liver injury and play a key role in its occurrence and development [28, 29]. Alcohol destroys the balance of intestinal microorganisms and makes the colonization and reproduction of pathogenic bacteria advantageous. Inokuchi et al. [30]. found that alcohol promoted the growth of Gram-negative bacteria such as Proteus in the intestinal tract, thereby reducing the amounts of anaerobic bacteria such as bifidobacteria. Our current trial supported that after Lactobacillus casei supplementation, bifidobacteria significantly increased in patients with alcoholic liver disease. Improvement of intestinal microecology can inhibit oxidative stress, alleviate liver inflammation and accelerate lipid metabolism [31]. Our findings showed that lipid metabolism was improved after intervention and the decrease in TG and LDL-C may be attributed to the aforementioned evidence. Abstinence from alcohol is the most effective approach to mitigating or ceasing alcohol-related liver injury [32]. All recruited patients were asked to abstain from drinking, we speculated that this might be why all liver function indexes decreased in the PC group. The result showed that TBIL in PC group, AST in LP group, ALT, AST, GGT, and TBIL in HP group were significantly different before and after intervention (P < 0.05) and there were significant differences among the groups after intervention (P < 0.05), indicating that supplementation of Lactobacillus casei could improve liver function. Consistent with our findings, another two studies showed that Lactobacillus could improve alcoholic liver injury [33, 34].
TNF-α, IL-1β, and IL-6 are considered to be important indicators reflecting the severity of inflammatory reactions and play a vital role in the occurrence and development of liver injury. On the one hand, TNF-α has direct cytotoxic effect, causing hepatocyte necrosis, on the other hand, it can cause microcirculation disorder leading to hepatocyte necrosis. At the same time, it also interacts with other inflammatory factors such as IL-6 to further aggravate liver injury [35, 36]. Our results showed that after alcoholic liver injury, the levels of TNF-α, IL-1β, and IL-6 significantly increased and it showed that there was inflammation in patients with alcoholic liver injury. Interleukin 10 (IL-10) is an effective anti-inflammatory factor and one of its key functions is to limit inflammation. Previous study showed that IL-10 plays a key role in regulating the production of TNF-α and so as to reduce inflammation [37]. Our study also found that IL-10 in HP group was significantly increased than that in NC group. There were significant differences for inflammatory markers between the groups after intervention, suggesting that the protective effect of Lactobacillus casei on liver injury may be associated to the reduction of the levels of inflammatory factors.
The “Gut–liver Axis” makes intestinal microecological disorders and intestinal barrier function damage become one of the important pathogenesis of alcoholic liver injury [38]. Our trial suggested that Lactobacillus casei supplementation could inhibit the increase of the endotoxin, D-lactates, I-FABP, and DAO which caused by alcohol. In addition, Some studies suggest probiotics supplementation to ALD patients can maintain the normal function of the intestinal barrier, rebuild the balance of intestinal microorganisms, and prevent intestinal bacterial translocation and inflammation [17, 26, 33, 39]. In line with these studies, the present results suggested that Lactobacillus casei supplementation could improve the intestinal barrier function.
Metagenomic sequencing technology can determine the species and percentage of microorganisms in a sample, while the real-time fluorescence quantitative PCR is more economical and can determine the specific amount of a certain bacteria in the sample [40]. The defect of the real-time fluorescence quantitative PCR is that it can only detect a limited number of bacteria. In the future, we will use metagenomic probiotic study for further research. Real-time fluorescent quantitative PCR was used to quantitatively analyze six species of intestinal flora, including Escherichia coli, Enterococcus, Bifidobacterium, Lactobacillus, Bacteroides and Clostridium tenella [41]. In several other studies, the authors suggested intestinal flora was usually in a symbiotic equilibrium state, but long-term excessive alcohol intake would lead to intestinal flora imbalance [42–44], which was consistent with our findings. In addition, our trial also found that after supplementation of probiotics, there was a significant increase in the amount of Lactobacillus and Bifidobacterium. These results indicated that the Lactobacillus casei supplementation can regulate intestinal flora disorders in patients with alcoholic liver injury, which may be one of the mechanisms of its improving lipid metabolism.
In our current trial, 57 of 158 patients with alcoholic liver injury had nutritional risk by NRS2002. The reasons for the nutritional risk may be: (1) patients with liver function damage had digestive and absorption dysfunction, which led to imbalance of nutrients and energy metabolism; (2) the gastrointestinal symptoms of patients with alcoholic liver injury are more obvious, which affects appetite. Malnutrition was common in patients with alcoholic liver disease [45]. Long term excessive drinking can cause intestinal flora imbalance and intestinal mucosal damage, leading to the absorption function of nutrients be weakened, which would cause or aggravate the liver damage [46]. Studies have shown that probiotics could significantly relieve gastrointestinal symptoms and improve appetite in patients with colorectal cancer during chemotherapy and could also significantly improve the nutritional status of patients with partial resection of gastric cancer [47, 48]; on the other hand, probiotics could improve intestinal flora disorder, repair the intestinal mucosal barrier damage and liver tissue damage [49]. Probiotics were used as intervention in this trail. In addition, we gave the general advice of nutrition and lifestyle for patients: abstinence from alcohol and keep a healthy lifestyle, such as good nutrition and exercise habits, and maintaining appropriate weight; those are very important for the recovery of ALD patients [50].
This study has several strengths. First, the sample size of this study provided relatively more power to examine the effect of Lactobacillus casei on lipid metabolism and intestinal microflora in patients with alcoholic liver injury. Second, research duration was longer than most of previous trials in the alcoholic liver injury patients. The limitation of this trial was that 60-day of intervention was still too short for us to examine the effect of Lactobacillus casei on lipid metabolism and intestinal microflora in patients with alcoholic liver injury complications, and we didn’t observe significant correlation between liver function, lipid profile and intestinal microflora. In addition, we recommended that the patients maintained their daily diet and physical activity, but did not monitor their lifestyles during the intervention period, although we got oral agreements from the patients that they would not alter their diet or lifestyles during the intervention period. Third, the sample size has a limited statistic power to find significant difference in other indicators, so large-scale researches are required to confirm our findings. Fourth, this randomized controlled trial was a single-center study because of budget limitations, so it may not be generalizable to the overall alcoholic liver injury patients.
Conclusions
In summary, there were disorder of lipid metabolism and intestinal flora imbalance in patients with alcoholic liver injury. Lactobacillus casei supplementation can improve lipid metabolism and regulate intestinal flora disorders in patients with alcoholic liver injury.
Supplementary information
Funding
This work was funded and supported by National Natural Science Foundation of China (Grant Nos. 81573137 and 81872605).
Author contributions
HL, YL, and XL conceived and designed the experiments; XL, XG, HZ, YM, and YL performed experiments and analyzed the data. HL and YL were responsible for the critical review of the study design, protocol, and methodology, providing scientific advice to this study. XL and YL contributed to interpreting the data as well as writing and editing the manuscript. All authors approved the final version of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41430-020-00852-8.
References
- 1.Dunn W, Shah VH. Pathogenesis of Alcoholic Liver Disease. Clin Liver Dis. 2016;20:445–56. doi: 10.1016/j.cld.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res Curr Rev. 2017;38:147. [PMC free article] [PubMed] [Google Scholar]
- 3.Mathurin P, Bataller R. Trends in the management and burden of alcoholic liver disease. J Hepatol. 2015;62:S38–S46. doi: 10.1016/j.jhep.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felix S, Christian D, Jochen H, Ramon B. Pathophysiology and management of alcoholic liver disease: update 2016. Gut Liver. 2017;11:173–88. doi: 10.5009/gnl16477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099–108. doi: 10.1002/hep.27406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ge N, Liang H, Liu Y, Ma AG, Han L. Protective effect of Aplysin on hepatic injury in ethanol-treated rats. Food Chem Toxicol. 2013;62:361–72.. doi: 10.1016/j.fct.2013.08.071. [DOI] [PubMed] [Google Scholar]
- 7.MD Z. Abstinence: the best way to control alcoholic liver diseases. Chin J Hepatol. 2003. [PubMed]
- 8.Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30–6. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mcc C, Nl L, Cm F, Jl G, D A, C G, et al. Comparing the effects of acute alcohol consumption in germ-free and conventional mice: the role of the gut microbiota. BMC Microbiol. 2014;14:240. doi: 10.1186/s12866-014-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Zhang J, Gao SL, Li TJ, Liang H. Quantitative analysis of gut flora in patients with alcoholic liver disease. Acta Nutrimenta Sin. 2018;40:36–41. [Google Scholar]
- 11.LI TJ, Zhang YJ, Wang ZL, Liu Y, Liang H. Protective Effect of Lactobacillus casei on Acrylamide-Induced Intestinal Injury in Rats. Food Science. 2018;39(9):128–33. [Google Scholar]
- 12.Zhang YH, Cong WH, Liu JX. Effect of crocin on mitochondrial dynamics in SH-SY5Y cells against injury induced by oxygen-glucose deprivation. Chinese Pharmacol. Bull. 2016;32:986–91. [Google Scholar]
- 13.Liang ZY, Liu Y, Xue ML, Liu J, Liang H. Inhibitory effect and immunological mechanism of Lactobacillus casei on induction of breast cancer by 7,12-dimethylbenz(a)anthracene in rats. Carcinog, Teratog Mutagen. 2017;029:7–12. [Google Scholar]
- 14.Wu YY, Dou M, Ma AG, Liu Y, Liang H. Effect of Lactobacillus casei on behavior and intestinal flora in rats with depression. Acta Acad Med Qingdao Univ. 2017;53:163–7. [Google Scholar]
- 15.Arora S, Kaur IP, Chopra K, Rishi P. Efficiency of double layered microencapsulated probiotic to modulate proinflammatory molecular markers for the management of alcoholic liver disease. Mediators Inflamm. 2014;2014:715130. doi: 10.1155/2014/715130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritze Y, Bárdos G, Claus A, Ehrmann V, Bergheim I, Schwiertz A, et al. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PloS ONE. 2014;9:e80169. doi: 10.1371/journal.pone.0080169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang B, Sang L, Wang Y, Tong J, Zhang D, Wang B. The protective effect of VSL#3 on intestinal permeability in a rat model of alcoholic intestinal injury. BMC Gastroenterol. 2013;13:151. doi: 10.1186/1471-230X-13-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim WG, Kim HI, Kwon EK, Han MJ, Kim DH. Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 mitigate alcoholic steatosis in mice by inhibiting LPS-mediated NF-κB activation through restoration of the disturbed gut microbiota. Food Funct. 2018;9:4255–65.. doi: 10.1039/C8FO00252E. [DOI] [PubMed] [Google Scholar]
- 19.Organization WH. Global Status Report on Alcohol And Health: World Health Organization, Geneva (Switzerland); 2014.
- 20.Tang YL, Xiang XJ, Wang XY, Cubells JF, Babor TF, Hao W. Alcohol and alcohol-related harm in China: policy changes needed. Bull World Health Organ. 2013;91:270–6. doi: 10.2471/BLT.12.107318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association; Fatty Liver Expert Committee, Chinese Medical Doctor Association. [Guidelines of prevention and treatment for alcoholic liver disease: a 2018 update]. Zhonghua Gan Zang Bing Za Zhi. 2018;26:188–94. [DOI] [PubMed]
- 22.Zhang XS, Liu YH, Zhang Y, Xu Q, Liu Z, Yang XY, et al. probiotics supplementation on respiratory infection and body immune function of soldiers in military training. Acad J Chin PLA Med Sch. 2018;39:54–8. [Google Scholar]
- 23.Matsumoto K, Takada T, Shimizu K, Moriyama K, Kawakami K, Hirano K, et al. Effects of a probiotic fermented milk beverage containing Lactobacillus casei strain Shirota on defecation frequency, intestinal microbiota, and the intestinal environment of healthy individuals with soft stools. J Biosci Bioeng. 2010;110:547–52.. doi: 10.1016/j.jbiosc.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto K, Takada T, Shimizu K, Kado Y, Kawakami K, Makino I, et al. The effects of a probiotic milk product containing lactobacillus casei strain shirota on the defecation frequency and the intestinal microflora of sub-optimal health state volunteers: a randomized placebo-controlled cross-over study. Bioence Microbiota Food Health. 2006;25:39–48. [Google Scholar]
- 25.Li XS, Liu JZ, Li SF. Effect of Jiedu Qinggan decoction combined with reduced glutathione in treating alcoholic hepatitis. Chinese J Integr Tradit West Med Dig. 2012;20:52–5. [Google Scholar]
- 26.Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675–82.. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kondrup J, Allison SP, Elia M, Vellas B. § MP. ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22:415–21. doi: 10.1016/S0261-5614(03)00098-0. [DOI] [PubMed] [Google Scholar]
- 28.Federico A, Dallio M, Caprio GG, Ormando VM, Loguercio C. Gut microbiota and the liver. Minerva Gastroenterol Dietol. 2017;63:385–98. doi: 10.23736/S1121-421X.17.02375-3. [DOI] [PubMed] [Google Scholar]
- 29.Hartmann P, Hochrath K, Horvath A, Chen P, Seebauer CT, Llorente C, et al. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology. 2018;67:2150–66.. doi: 10.1002/hep.29676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inokuchi S, Tsukamoto H, Park E, Liu ZX, Brenner DA, Seki E. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin Exp Res. 2011;35:1509–18. doi: 10.1111/j.1530-0277.2011.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazagova M, Wang L, Anfora AT, Wissmueller M, Lesley SA, Miyamoto Y, et al. Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice. FASEB J. 2015;29:1043–55. doi: 10.1096/fj.14-259515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang WJ, Xiao P, Xu HQ, Niu JQ, Gao YH. Hepatology do. Growing burden of alcoholic liver disease in China: a review. World J Gastroenterol. 2019;25:1445–56. doi: 10.3748/wjg.v25.i12.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han SH, Suk KT, Kim DJ, Kim MY, Baik SK, Kim YD, et al. Effects of probiotics (cultured Lactobacillus subtilis/Streptococcus faecium) in the treatment of alcoholic hepatitis: randomized-controlled multicenter study. Eur J Gastroenterol Hepatol. 2015;27:1300–6. doi: 10.1097/MEG.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 34.Barone R, Rappa F, Macaluso F, Caruso Bavisotto C, Sangiorgi C, Di Paola G, et al. Alcoholic liver disease: a mouse model reveals protection by Lactobacillus fermentum. Clin Transl Gastroenterol. 2016;7:e138. doi: 10.1038/ctg.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albano E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol Asp Med. 2008;29:9–16. doi: 10.1016/j.mam.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Kohn G, Wong HR, Bshesh K, Zhao B, Vasi N, Denenberg A, et al. Heat shock inhibits tnf-induced ICAM-1 expression in human endothelial cells via I kappa kinase inhibition. Shock. 2002;17:91–7. doi: 10.1097/00024382-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 37.McClain CJ, Hill DB, Song Z, Chawla R, Watson WH, Chen T, et al. S-Adenosylmethionine, cytokines, and alcoholic liver disease. Alcohol. 2002;27:185–92.. doi: 10.1016/S0741-8329(02)00224-0. [DOI] [PubMed] [Google Scholar]
- 38.Szabo Gyongyi. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16:1321. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS ONE. 2013;8:e53028. doi: 10.1371/journal.pone.0053028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colborn JM, Byrd BD, Koita OA, Krogstad DJ. Estimation of copy number using SYBR Green: confounding by AT-rich DNA and by variation in amplicon length. Am J Trop Med Hyg. 2008;79:887–92.. doi: 10.4269/ajtmh.2008.79.887. [DOI] [PubMed] [Google Scholar]
- 41.Ma Y, Li R, Liu Y, Liu M, Liang H. Protective Effect of Aplysin Supplementation on Intestinal Permeability and Microbiota in Rats Treated with Ethanol and Iron. Nutrients. 2018;10:681.. doi: 10.3390/nu10060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen TD, Pyrsopoulos N, Rustgi VK. Microbiota and the liver. Liver Transplant. 2018;24:539–50. doi: 10.1002/lt.25008. [DOI] [PubMed] [Google Scholar]
- 43.Frosali S, Pagliari D. How the intricate interaction among toll-like receptors, microbiota, and intestinal immunity can influence gastrointestinal. Pathology. 2015;2015:489821. doi: 10.1155/2015/489821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen P, Torralba M, Tan J, Embree M, Zengler K, Stärkel P, et al. Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology. 2015;148:203–14.e16. doi: 10.1053/j.gastro.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McClain CJ, Barve SS, Barve A, Marsano L. Alcoholic liver disease and malnutrition. Alcohol Clin Exp Res. 2011;35:815–20.. doi: 10.1111/j.1530-0277.2010.01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang XY, Li GP, Kou JG, Li L, Xia XM, Yang L, et al. Relationship between intestinal dysbacteriosis and Child-Pugh classification in patients with cirrhosis. J Clin Hepatol. 2015;31:392–5. [Google Scholar]
- 47.Chen QX, Zhu JL, Xu Y. Effect of probiotics on nutritional status, inflammatory reaction and chemotherapy-related complications in patients having undergone chemotherapeutic treatment of colorectal cancer. Health Res. 2020;40:68–71. [Google Scholar]
- 48.Zhang L, Sun YS, He TZ. Effects of viable Tri-Bifidobacteria on inflammatory factors, immunity and nutritional status of patients with gastric cancer after partial resection. Chin J Microecol. 2019;31:680–3. doi: 10.1097/CM9.0000000000000125. [DOI] [Google Scholar]
- 49.Liang H, Lyu R, Fu Y, Zhou ZT, Liu Y, Zhou XB, et al. Effects of probiotics on alcoholic liver injury in rats and its mechanisms. Chinese Pharmacol Bull. 2016;32:991–7. [Google Scholar]
- 50.Marsano LS, Mendez C, Hill D, Barve S, Mcclain CJ. Diagnosis and treatment of alcoholic liver disease and its complications. Alcohol Res. 2003;27:247–56. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.