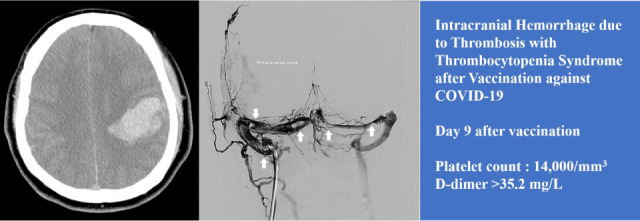
Abstract
Vaccination with an adenoviral vector vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can result in the rare development of thrombosis with thrombocytopenia mediated by platelet-activating antibodies against platelet factor 4 (PF4). This is a life-threating condition that may be accompanied by bleeding due to thrombocytopenia with thrombosis of the cerebral venous sinus or splanchnic vein. Herein, we describe the first fatal case of thrombosis with thrombocytopenia syndrome in Korea, presenting with intracranial hemorrhage caused by cerebral venous sinus thrombosis. A 33-year-old Korean man received the first dose of the ChAdOx1 nCoV-19 vaccination. He developed severe headache with vomiting 9 days after the vaccination. Twelve days after vaccination, he was admitted to the hospital with neurological symptoms and was diagnosed with cerebral venous sinus thrombosis, which was accompanied by intracranial hemorrhage. Thrombocytopenia and D-dimer elevation were observed, and the result of the PF4 enzyme-linked immunosorbent assay antibody test was reported to be strongly positive. Despite intensive treatment, including intravenous immunoglobulin injection and endovascular mechanical thrombectomy, the patient died 19 days after vaccination. Physicians need to be aware of thrombosis with thrombocytopenia syndrome (TTS) in adenoviral vector-vaccinated patients. Endovascular mechanical thrombectomy might be a useful therapeutic option for the treatment of TTS with cerebral venous sinus thrombosis.
Keywords: COVID-19 Vaccines, Platelet Factor 4, Intracranial Sinus Thrombosis, Endovascular Procedures, Mechanical Thrombolysis
Graphical Abstract
INTRODUCTION
Adenoviral vector vaccines against severe acute respiratory syndrome corona virus 2 (SARS-CoV-2) (ChAdOx1 nCoV-19 [AstraZeneca] and Ad26.COV2.S [Johnson & Johnson/Janssen]) are being widely administered worldwide to overcome the coronavirus disease 2019 (COVID-19) pandemic.1 These vaccines show acceptable efficacy and effectiveness in preventing severe infection caused by SARS-CoV-2. However, thrombotic events in unusual locations (cerebral venous sinus, splanchnic vein) with thrombocytopenia have rarely been reported after this vaccination and are referred to as thrombosis with thrombocytopenia syndrome (TTS; also sometimes referred to as vaccine-induced immune thrombotic thrombocytopenia).2 Here, we describe the first fatal case of TTS in Korea in a patient who presented with intracranial hemorrhage and cerebral venous sinus thrombosis (CVT). The patient underwent endovascular mechanical thrombectomy (EMT) with conventional TTS treatment.
CASE DESCRIPTION
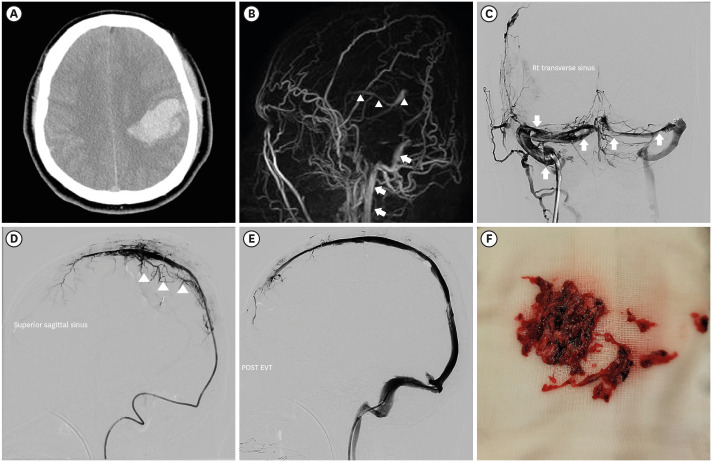
A 33-year-old man with no significant previous medical history received the first dose of the ChAdOx1 nCOV-19 vaccine (day 0). He reported mild symptoms in the evening of the day of vaccination (fever and headache). However, the symptoms improved after 2–3 days. On day 9 after vaccination, he developed a headache again and also experienced vomiting. He received treatment at a private clinic, but his symptoms did not improve and gradually worsened despite of symptomatic care. Twelve days after vaccination, he was admitted to the emergency room by ambulance due to the sudden onset of a tingling sensation in the right arm and mental change. He was alert when he left home, but became drowsy after arriving at the emergency department and developed neurological symptoms with dysarthria and right hemiparesis. He was afebrile, slightly hypertensive (155/90 mmHg), and the SARS-CoV-2 Xpert real-time reverse-transcriptase polymerase chain reaction assay of a nasopharyngeal and oropharyngeal swab was negative. The laboratory results are presented in Table 1. The platelet count was 14,000/mm3, C-Reactive Protein was 3.42 mg/L (reference value, < 5), fibrinogen was 77 mg/dL, and D-dimer was > 35.2 mg/L (reference value, < 0.5). There were no specific findings other than thrombocytopenia in the peripheral blood smear. Non-enhanced brain computed tomography identified a 5 × 4 × 3-cm3-sized subcortical hematoma in the left parietal lobe and an adjacent subarachnoid hemorrhage, which suggested hemorrhagic venous infarction (Fig. 1A). Two hours after arriving at the hospital, he was transferred to the neurosurgical department and admitted to the intensive care unit for further management. Three pint of Fresh Frozen Plasma and 8 pint of Platelet Concentrate were transfused immediately. Seven hours after arriving at the hospital, he became stuporous, and right hemiparesis became more severe from motor grade 2 to 1. On the magnetic resonance (MR) venogram, only the left sigmoid sinus and internal jugular vein were visualized, and the remaining dural venous sinus was not visualized, suggesting extensive CVT (Fig. 1B). According to the Korean TTS guidelines, the patient was considered a probable case of TTS. We requested an enzyme-linked immunosorbent assay (ELISA) for antibodies against platelet factor 4 (PF4 ELISA Ab test) to confirm the diagnosis. Sixteen hours after arriving at the hospital, he received high-dose intravenous immunoglobulin (IVIG) 1,000 mg/kg daily and steroid (methylprednisolone 1.5 mg/kg/day). Heparin was not used upon arrival at the hospital and contraindicated. The symptoms did not improve even during IVIG and steroid maintenance therapy; thirty-six hours after arrival at the hospital, the level of consciousness deteriorated to semi-coma. Endovascular mechanical thrombectomy was performed for recanalization of the occluded dural venous sinus. Venography of the transverse sinus (Fig. 1C) and superior sagittal sinus (Fig. 1D) performed prior to EMT revealed extensive CVT. Endovascular mechanical thrombectomy was performed using a large-caliber suction catheter and stent retriever, which is used for arterial thrombectomy in acute large artery occlusion. After EMT, venous outflow of the superior sagittal sinus and both transverse sinuses was completely restored (Fig. 1E). Although endovascular treatment was successful, the patient became comatose due to extensive brain injury because of severely increased intracranial pressure. On hospital day 8 (day 19 after the vaccination), the result of the PF4 ELISA Ab test was reported as strongly positive (0.72, optical-density units, Lifecodes PF4 IgG assay (Immucor Inc., Norcross, GA, USA) [normal range, < 0.4]). The platelet count did not increase and plasma exchange was initiated; the patient died 20 days after vaccination. He was recognized as the first fatality following and as a result of COVID-19 vaccination in Korea.
Table 1. Laboratory results of the patient.
| Laboratory analysis | Result | Unit | Reference value |
|---|---|---|---|
| Hemoglobin | 17.8 | g/dL | 13.0–18.0 |
| White blood cell count | 7.40 | per mm3 | 4.0–10.0 |
| Platelet count | 14 | per mm3 | 140–450 |
| Erythrocyte sedimentation rate | 2 | mm/h | 0–15 |
| C-reactive protein | 3.42 | mg/L | 0–5 |
| Blood urea nitrogen | 12.5 | mg/dL | 6.0–20.0 |
| Creatinine | 0.97 | mg/dL | 0.70–1.20 |
| Total protein | 7.3 | g/dL | 6.6–8.7 |
| Albumin | 4.3 | g/dL | 3.4–4.8 |
| Total bilirubin | 0.91 | mg/dL | 0.0–1.4 |
| Aspartate aminotransferase | 14 | U/L | 0–40 |
| Alanine aminotransferase | 15 | U/L | 0–40 |
| Alkaline phosphatase | 85 | U/L | 40–129 |
| Lactate dehydrogenase | 203 | U/L | 135–225 |
| Fibrinogen | 77 | mg/dL | 180–415 |
| Antithrombin III | 110.7 | % | 60–120 |
| Fibrin degradation products | 64.6 | µg/mL | < 5.1 |
| Prothrombin time | 14.0 | sec | 10.1–14.0 |
| Activated partial thromboplastin time | 28.0 | sec | 23.4–30.3 |
| D-dimer | > 35.2 | mg/L | 0–0.55 |
| Troponin-T | 13.9 | pg/mL | 0–14 |
| Creatine kinase-MB | 0.721 | pg/mL | 0.0–4.87 |
Fig. 1. Brain CT, MR venogram, and DSA. (A) Axial image of initial non-enhanced brain CT identifies a 5 × 4 × 3-cm3-sized subcortical hematoma in left parietal lobe and an adjacent subarachnoid hemorrhage, which suggests hemorrhagic venous infarction. (B) The lateral view of the MR venogram only demonstrates the left sigmoid sinus and internal jugular vein (arrows), and the remaining dural venous sinus are not visualized. Deep venous drainage systems, such as the internal cerebral vein and great cerebral vein of Galen (arrow heads), can be identified. (C) AP view of DSA performed before mechanical thrombectomy shows multiple filling defects in the right and left transverse sinus (arrows). (D) The lateral view of superior sagittal sinus venography shows a hardly visualized superior sagittal sinus and blockage of the venous outflow to transverse sinus because of extensive thrombosis in the dural venous sinus. Cortical venous reflux due to blockage of venous outflow in superior sagittal sinus was also noted (arrowheads). (E) Lateral view of the superior sagittal sinus venography after endovascular mechanical thrombectomy shows that venous outflow of superior sagittal sinus and transverse sinus was completely restored. (F) A large number of clots which were removed through endovascular mechanical thrombectomy.
CT = computed tomography, MR = magnetic resonance, DSA = digital subtraction angiography, AP = antero-posterior.
DISCUSSION
This is the first fatal case of TTS by vaccination against COVID-19 in Korea. In this case, the initial diagnosis was delayed at the primary clinic. At the time of admission to the emergency room, treatment was difficult because of extensive thrombosis with cerebral hemorrhage. In addition, platelet transfusion was performed before early recognition of TTS, resulting in poor prognosis. Adenoviral vector SARS-CoV-2 vaccines from AstraZeneca (ChAdOx1 nCoV-19, Vaxzevria) and Janssen (Ad26.COV2.S, JNJ-78436735) have been found to very rarely cause TTS. Antibodies related to PF4 induced by adenovector vaccination activate platelets, decrease platelet count, and cause thrombosis.3 The incidence of TTS appears to be between 1 in 125,000 and 1 in 1,000,000 vaccinated individuals in Europe.4 The incidence of TTS in Korea is very low compared to that in other countries. As of July 13, 2021, a total of two confirmed cases of TTS were reported by the Korea Disease Control and Prevention Agency following approximately 12,800,000 adenoviral vector SARS-CoV-2 vaccine doses administered in Korea (incidence 0.156 per 1,000,000). This difference in incidence is thought to be due to racial differences and is indirectly supported by a population-based study.5 Although the incidence of TTS is low, it has very high mortality and morbidity, and early diagnosis and treatment are necessary for a good prognosis, clinicians need to raise awareness of TTS. According to a recently published animal model study, intravenous injection of ChAdOx1 nCov-19 triggers platelet-targeted autoimmunity in spleen, that may result TTS. Hence, safe intramuscular injection, with aspiration prior to injection, could be a potential preventive measure when administering adenovirus-based vaccines.6
In the majority of cases of TTS, the site of thrombosis was the cerebral veins, and splanchnic vein thrombosis and pulmonary embolism were other common manifestations.4 Clinical presentations of CVT can be diverse and highly variable, ranging from the non-specific symptoms, such as headache or blurred vision, to coma.7 Ilyas et al.8 summarized a large number of case series of CVT; according to their report, headache was the most common presentation of CVT, which was followed by focal neurologic deficit, seizure, and altered mental status. Since these symptoms are highly likely to be confused with conventional adverse events that can occur after SARS-CoV-2 vaccination, diagnosis of TTS with CVT requires a high index of suspicion.9 For early diagnosis of TTS, it is helpful to check for thrombocytopenia by performing CBC when a young person known to be at high risk has a headache or abdominal pain. Symptoms such as fever and headache that occur immediately after vaccination usually disappear after 2–3 days later; however, TTS occurs a median of 10 days (range 5–24 days) after vaccination.4 Crescendo-type progression of symptoms over a few days with a recent history of adenoviral vector SARS-CoV-2 vaccination raises a strong possibility of CVT, which should be followed by appropriate laboratory and imaging studies for the diagnosis of TTS.10
The clinical presentations and prognosis of TTS with CVT depend on the location and extent of the thrombosis.7 The mainstay of treatment of TTS with CVT is high-dose IVIG and anticoagulation using direct oral anticoagulants (DOAC).4,11,12 IVIG is effective in interrupting platelet activation by PF4 antibodies, manifesting as a rapid restoration of the platelet count. Anticoagulation is another important treatment for TTS with CVT; unfractionated heparin or low-molecular-weight heparin should be avoided as they can exacerbate TTS through a mechanism similar to autoimmune heparin-induced thrombocytopenia. Therefore, anticoagulation for the treatment of TTS with CVT must be performed using DOACs.
Meanwhile, anticoagulation in patients with very low platelet counts has the potential to cause fatal bleeding. Moreover, special therapeutic modalities are needed for patients with rapid progression of symptoms due to extensive CVT. To solve these problems, a special strategy is required, and EMT may be the answer. Endovascular mechanical thrombectomy can restore normal venous outflow, alleviate venous congestion, and reduce increased intracranial pressure through rapid and definite recanalization of occluded venous sinuses.7,8 Ilyas et al.8 analyzed a total of 17 studies comprising 235 CVT patients treated by EMT, 40.2% of patients included in the study were severe CVT which presented with encephalopathy or coma, but 67% of patients had good clinical outcome after EMT. In addition, society of NeuroInterventional Surgery recommended EMT for severe CVT patients with clinical deterioration despite anticoagulation, or with severe neurological deficits or coma.7 In case of TTS, Wolf et al.13 reported 3 cases which was successfully treated by EMT. Endovascular mechanical thrombectomy for TTS with CVT could be a good therapeutic adjuvant to anticoagulation which shortens the duration of DOAC use, thus reducing the risk of fatal bleeding and stopping progression of symptoms with rapid and definite recanalization of occluded venous sinuses. Although the outcome of endovascular thrombectomy in this case was not good, complete recanalization of the whole cerebral venous sinus was achieved; therefore, a better outcome might be expected if the endovascular treatment was performed earlier before the beginning of cortical venous occlusion, which can cause severely increased intracranial pressure and subsequent brain injury. Therefore, if CVT caused by TTS is clinically suspected and the patient's symptoms worsen quickly, early EMT is necessary without waiting for the confirmation test result or the effect of IVIG.
In conclusion, the diagnosis of CVT due to TTS has to be made with high suspicion because of its rapid and diverse clinical manifestations. Endovascular mechanical thrombectomy might be a useful therapeutic option for the treatment of TTS with CVT.
Ethics statement
This report was approved by our Institutional Review Board, and the requirement for informed consent was waived (subject number: HC21ZASI0073).
Footnotes
Funding: This work was supported by a Korea National Enterprise for Clinical Trials (KONECT) grant funded by the Korean Government (No. HE21C0006030021).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Yoo JH, Kim SR.
- Data curation: Choi JK, Kim S, Kim SR, Choi SW, Kim H.
- Formal analysis: Choi JK.
- Funding acquisition: Choi JK.
- Investigation: Choi JK, Kim S, Kim SR.
- Methodology: Choi JK, Kim S, Yoo JH, Kim SR.
- Software: Choi JK.
- Validation: Jin JY, Yoo JH, Park IS, Kim SR.
- Visualization: Kim S.
- Writing - original draft: Choi JK, Kim S.
- Writing - review & editing: Choi JK, Kim S, Kim SR, Jin JY, Choi SW, Kim H, Yoo JH, Park IS, Kim SR.
References
- 1.Yoo JH. What we do know and do not yet know about COVID-19 vaccines as of the beginning of the year 2021. J Korean Med Sci. 2021;36(6):e54. doi: 10.3346/jkms.2021.36.e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(23):2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franchini M, Liumbruno GM, Pezzo M. COVID-19 vaccine-associated immune thrombosis and thrombocytopenia (VITT): Diagnostic and therapeutic recommendations for a new syndrome. Eur J Haematol. 2021;107(2):173–180. doi: 10.1111/ejh.13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huh K, Na Y, Kim YE, Radnaabaatar M, Peck KR, Jung J. Predicted and observed incidence of thromboembolic events among Koreans vaccinated with ChAdOx1 nCoV-19 vaccine. J Korean Med Sci. 2021;36(27):e197. doi: 10.3346/jkms.2021.36.e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicolai L, Leunig A, Pekayvaz K, Anjum A, Riedlinger E, Eivers L, et al. Thrombocytopenia and splenic platelet directed immune responses after intravenous ChAdOx1 nCov-19 administration. bioRxiv. 2021 doi: 10.1101/2021.06.29.450356. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SK, Mokin M, Hetts SW, Fifi JT, Bousser MG, Fraser JF Society of NeuroInterventional Surgery. Current endovascular strategies for cerebral venous thrombosis: report of the SNIS Standards and Guidelines Committee. J Neurointerv Surg. 2018;10(8):803–810. doi: 10.1136/neurintsurg-2018-013973. [DOI] [PubMed] [Google Scholar]
- 8.Ilyas A, Chen CJ, Raper DM, Ding D, Buell T, Mastorakos P, et al. Endovascular mechanical thrombectomy for cerebral venous sinus thrombosis: a systematic review. J Neurointerv Surg. 2017;9(11):1086–1092. doi: 10.1136/neurintsurg-2016-012938. [DOI] [PubMed] [Google Scholar]
- 9.Mowla A, Shakibajahromi B, Shahjouei S, Borhani-Haghighi A, Rahimian N, Baharvahdat H, et al. Cerebral venous sinus thrombosis associated with SARS-CoV-2; a multinational case series. J Neurol Sci. 2020;419:117183. doi: 10.1016/j.jns.2020.117183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SK, Kim BS, Terbrugge KG. Clinical presentation, imaging and treatment of cerebral venous thrombosis (CVT) Interv Neuroradiol. 2002;8(1):5–14. doi: 10.1177/159101990200800102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cattaneo M. Thrombosis with thrombocytopenia syndrome associated with viral vector COVID-19 vaccines. Eur J Intern Med. 2021;89:22–24. doi: 10.1016/j.ejim.2021.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warkentin TE. High-dose intravenous immunoglobulin for the treatment and prevention of heparin-induced thrombocytopenia: a review. Expert Rev Hematol. 2019;12(8):685–698. doi: 10.1080/17474086.2019.1636645. [DOI] [PubMed] [Google Scholar]
- 13.Wolf ME, Luz B, Niehaus L, Bhogal P, Bäzner H, Henkes H. Thrombocytopenia and intracranial venous sinus thrombosis after “COVID-19 vaccine AstraZeneca” exposure. J Clin Med. 2021;10(8):1599. doi: 10.3390/jcm10081599. [DOI] [PMC free article] [PubMed] [Google Scholar]