Abstract
Background
Concerns have been raised regarding a potential surge of COVID-19 in pregnancy, secondary to the rising numbers of COVID-19 in the community, easing of societal restrictions, and vaccine hesitancy. Although COVID-19 vaccination is now offered to all pregnant women in the United Kingdom; limited data exist on its uptake and safety.
Objective
This study aimed to investigate the uptake and safety of COVID-19 vaccination among pregnant women.
Study Design
This was a cohort study of pregnant women who gave birth at St George’s University Hospitals National Health Service Foundation Trust, London, United Kingdom, between March 1, 2020, and July 4, 2021. The primary outcome was uptake of COVID-19 vaccination and its determinants. The secondary outcomes were perinatal safety outcomes. Data were collected on COVID-19 vaccination uptake, vaccination type, gestational age at vaccination, and maternal characteristics, including age, parity, ethnicity, index of multiple deprivation score, and comorbidities. Further data were collected on perinatal outcomes, including stillbirth (fetal death at ≥24 weeks’ gestation), preterm birth, fetal and congenital abnormalities, and intrapartum complications. Pregnancy and neonatal outcomes of women who received the vaccine were compared with that of a matched cohort of women with balanced propensity scores. Effect magnitudes of vaccination on perinatal outcomes were reported as mean differences or odds ratios with 95% confidence intervals. Factors associated with antenatal vaccination were assessed with logistic regression analysis.
Results
Data were available for 1328 pregnant women of whom 140 received at least 1 dose of the COVID-19 vaccine before giving birth and 1188 women who did not; 85.7% of those vaccinated received their vaccine in the third trimester of pregnancy and 14.3% in the second trimester of pregnancy. Of those vaccinated, 127 (90.7%) received a messenger RNA vaccine and 13 (9.3%) a viral vector vaccine. There was evidence of reduced vaccine uptake in younger women (P=.001), women with high levels of deprivation (ie, fifth quintile of the index of multiple deprivation; P=.008), and women of Afro-Caribbean or Asian ethnicity compared with women of White ethnicity (P<.001). Women with prepregnancy diabetes mellitus had increased vaccine uptake (P=.008). In the multivariable model the fifth deprivation quintile (most deprived) (adjusted odds ratio, 0.10; 95% confidence interval, 0.02–0.10; P=.003) and Afro-Caribbean ethnicity (adjusted odds ratio, 0.27; 95% confidence interval, 0.06–0.85; P=.044) were significantly associated with lower antenatal vaccine uptake, whereas prepregnancy diabetes mellitus was significantly associated with higher antenatal vaccine uptake (adjusted odds ratio, 10.5; 95% confidence interval, 1.74–83.2; P=.014). In a propensity score–matched cohort, the rates of adverse pregnancy outcomes of 133 women who received at least 1 dose of the COVID-19 vaccine in pregnancy were similar to that of unvaccinated pregnant women (P>.05 for all): stillbirth (0.0% vs 0.2%), fetal abnormalities (2.2% vs 2.5%), postpartum hemorrhage (9.8% vs 9.0%), cesarean delivery (30.8% vs 34.1%), small for gestational age (12.0% vs 12.8%), maternal high-dependency unit or intensive care admission (6.0% vs 4.0%), or neonatal intensive care unit admission (5.3% vs 5.0%). Intrapartum pyrexia (3.7% vs 1.0%; P=.046) was significantly increased but the borderline statistical significance was lost after excluding women with antenatal COVID-19 infection (P=.079). Mixed-effects Cox regression showed that vaccination was not significantly associated with birth at <40 weeks’ gestation (hazard ratio, 0.93; 95% confidence interval, 0.71–1.23; P=.624).
Conclusion
Of pregnant women eligible for COVID-19 vaccination, less than one-third accepted COVID-19 vaccination during pregnancy, and they experienced similar pregnancy outcomes with unvaccinated pregnant women. There was lower uptake among younger women, non-White ethnicity, and lower socioeconomic background. This study has contributed to the body of evidence that having COVID-19 vaccination in pregnancy does not alter perinatal outcomes. Clear communication to improve awareness among pregnant women and healthcare professionals on vaccine safety is needed, alongside strategies to address vaccine hesitancy. These strategies include postvaccination surveillance to gather further data on pregnancy outcomes, particularly after first-trimester vaccination, and long-term infant follow-up.
Key words: coverage, COVID-19, immunization, mRNA, pregnancy, safety vaccine uptake, SARS-CoV-2, vaccination, viral vector
Introduction
The COVID-19 pandemic has caused loss of life and poorer health outcomes, outside and in pregnancy, despite worldwide aggressive public health measures to control the spread.1 Mass vaccination is a key method by which countries are aiming to control the pandemic.2
AJOG at a Glance.
Why was this study conducted?
Concerns have been raised regarding a potential surge of COVID-19 in pregnancy, secondary to the rising numbers of COVID-19 in the community, easing of societal restrictions, and vaccine hesitancy. Although COVID-19 vaccination is now offered to all pregnant women in the United Kingdom, limited data exist on its uptake and safety.
Key findings
We found that only 28.5% of pregnant women eligible for a COVID-19 vaccine had accepted it during pregnancy. Pregnancy and neonatal outcomes of women who received the vaccine were similar to that of a propensity score–matched cohort of pregnant women who did not receive the vaccine.
What does this add to what is known?
This study showed that clear communication and targeted strategies are needed to address vaccine hesitancy, including postvaccination surveillance to gather further data on pregnancy outcomes, particularly after first-trimester vaccination, and long-term infant follow-up.
Theoretically, COVID-19 vaccines are safe for use in pregnancy, as they do not contain a live attenuated virus.3 For COVID-19 vaccination in pregnancy, there has been no major safety signal from animal reproductive toxicology studies, the very small number of inadvertent pregnancies in vaccine trials, the Centers for Disease Control and Prevention (CDC) V-safe postvaccination health checker (with limited data on >30,000 pregnant women, but only 827 women have given birth), or a formal pregnancy registry (>1800 enrolled to date).4 A recent report of American health workers who were pregnant (n=84) or lactating (n=31) when vaccinated found that compared with nonpregnant controls (n=16), vaccine-induced humoral immunity was similar, antibody titers were higher following an actual SARS-CoV-2 infection, and antibodies were present in umbilical cord blood and breast milk, suggesting that vaccination can confer maternal and perinatal immunity.5
Based on vast previous experience with other vaccines in pregnancy and no hypothesized mechanism for harm, similar efficacy and side effects are anticipated with COVID-19 vaccination in (vs outside) pregnancy. However, pregnant women were excluded from the initial randomized controlled trials (RCTs) testing the safety and efficacy of COVID-19 vaccines. Although randomized trials of COVID-19 vaccination in pregnancy have now begun,6 , 7 the results will not be available until 2022 at the earliest.
Pregnant women have been reluctant to receive COVID-19 vaccination8 and guidance for healthcare professionals has not been consistent. Some guidelines initially recommended against routine COVID-19 vaccination in pregnancy but pivoted as safety data accumulated (eg, the United Kingdom Joint Committee on Vaccination and Immunisation [JCVI]9), whereas others recommended routine vaccination from the start (eg, the International Federation of Gynecology and Obstetrics).10 Therefore, this study aimed to investigate the uptake and safety of COVID-19 vaccination among pregnant women. We studied the determinants of COVID-19 vaccine uptake among eligible pregnant women and compared pregnancy outcomes in women who received COVID-19 vaccination during pregnancy with that of unvaccinated and contemporaneous pregnant controls of similar risk profiles.
Materials and Methods
Study design and population
In this retrospective cohort study, the records of all women who delivered between March 1, 2021, and July 4, 2021, in St George’s University Hospitals NHS Foundation Trust, London, United Kingdom, were screened for eligibility. The first of March was chosen as pregnant women with comorbidities were offered vaccination after this date.11 The inclusion criteria were pregnant women with known vaccination status and complete maternal and fetal outcome data. The exclusion criteria were women who were vaccinated entirely (ie, all doses) before pregnancy or after birth or women who had pregnancies complicated by fetal aneuploidy or genetic syndromes.
Data were obtained from electronic hospital records stored in the following systems: EuroKing E3 maternity information system, Chertsey, United Kingdom; ViewPoint Bildverarbeitung GmbH, Wessling, Germany; and Health Information Exchange—Coordinate My Care, GP Partner 2, One London Hub, One London SWL, MIG Partner, and TPP SystmOne—via Cerner, North Kansas City, Missouri. The quality of data in the later database was checked for accuracy of the COVID-19 vaccination record. For 1 week, women admitted to the postnatal ward were queried about their vaccination status, and their responses were cross-checked with the electronic records. The initial quality control showed no inconsistency between patient responses and electronic records.
Data collected included maternal age, parity, index of multiple deprivation (IMD) score, self-reported ethnicity, body mass index (BMI), alcohol and smoking habits, comorbidities, antenatal complications, and medications. The IMD combines multiple deprivation indices (income, employment, education, health, crime, barriers to housing and services, and living environment) into a single score and is widely used to assess deprivation in the United Kingdom. Other data variables included COVID-19 vaccination uptake, vaccination type, and gestational age (GA) at vaccination. Other data of interest included antenatal complications, including gestational diabetes mellitus, obstetrical cholestasis, and preeclampsia; venous thromboembolism (given the association with the Oxford-AstraZeneca vaccine) or myocarditis or pericarditis (given the association with the Pfizer-BioNTech vaccine); and antenatal medication, including medications for chronic prepregnancy disorders (such as hypothyroidism, epilepsy, diabetes mellitus, or depression) or pregnancy conditions (such as gestational hypertension or preeclampsia), but not nutritional supplements, multivitamins, iron replacement, antibiotics, antiemetics, analgesia or anti-D immunoglobulin.
Vaccine types included messenger RNA (mRNA) vaccines (Moderna, Pfizer-BioNTech) and viral vector vaccine (Oxford-AstraZeneca), which are approved for use in the United Kingdom. As for the general population, pregnant women were eligible for vaccination if they (1) were a health or social care worker, which increased their risk of SARS-Cov-2 infection, or (2) were at high risk of severe COVID-19 because of personal factors (eg, non-White ethnicity) or health conditions (eg, diabetes mellitus or gestational diabetes mellitus specifically). From April 16, 2021, vaccination was offered to those aged 45 years and above, with progressively younger groups offered vaccination from May 28, 2021.12 From April 16, 2021, the JCVI has advised that mRNA vaccines should be used in preference in the United Kingdom for pregnant women.9
Study outcomes
The primary study outcome was COVID-19 vaccine uptake during pregnancy among women eligible for vaccination. Vaccination eligibility was assessed by comparing delivery date with vaccination eligibility date based on the mother’s age and priority category. In the United Kingdom (2021), women became eligible for vaccination based on clinical risk and maternal age, with vaccination offered to pregnant women at the same time as the rest of the population, as follows: >40 years (from April 30, 2021), >30 years (May 26, 2021), >25 years (June 8, 2021), and >18 years (June 18, 2021).13 , 14 Women who delivered after the vaccination eligibility date for their age category were considered eligible for antenatal vaccination. Vaccination uptake rate was calculated as the number of women who received at least 1 dose of any COVID-19 vaccine during pregnancy in a certain group divided by all women eligible for vaccination in that group.
Secondary outcomes included perinatal outcomes to assess the safety of COVID-19 vaccination, which included stillbirth (fetal death at ≥24 weeks’ gestation), neonatal death, fetal abnormalities, preterm birth before 37 weeks’ gestation, GA at birth in weeks, intrapartum complications (pyrexia, suspected chorioamnionitis, placental abruption, and postpartum hemorrhage), birthweight z score, mode of birth (cesarean delivery, instrumental delivery, or unassisted vaginal delivery), maternal high-dependency unit or intensive care unit (ICU) admission (any indication), and neonatal ICU admission (any indication). Postpartum hemorrhage was defined as an estimated blood loss of ≥1 L regardless of the mode of birth.
GA was determined in the first trimester of pregnancy according to the crown-rump length of the fetus (singleton pregnancies) or the larger fetus (twin pregnancies) in cases of spontaneous conception and according to the embryonic age in pregnancies conceived via assisted reproductive technology (ART). After 14 weeks’ gestation, GA was determined using the head circumference of the fetus (singleton pregnancies) or the larger fetus (twin pregnancies) in cases of spontaneous conception and according to the embryonic age in pregnancies conceived via ART. Birthweight z scores were calculated using a reference standard published by Poon et al.15
Statistical analysis
Continuous variables were represented as median and interquartile range (IQR) regardless of the distribution assumptions. Categorical variables were represented as numbers and percentages. The Shapiro-Wilk test and visual inspection of quantile-quantile plots were used for verifying the normality of continuous variables. Mann-Whitney U test, t test, chi-square test, or Fisher-Freeman-Halton test was used for group comparisons as appropriate.
Factors associated with antenatal vaccination were assessed among all women eligible for vaccination, by logistic regression, with factors significant in univariable analyses assessed in a multivariable model to calculate the adjusted odds ratios (ORs). Results of regression analyses were reported as mean difference (MD), OR, or hazard ratio (HR).
The effect of COVID-19 vaccination on perinatal outcomes was assessed among women who had antenatal vaccination, compared with those who did not have the vaccine. Propensity score matching was used to match cases and controls for factors identified from between-group comparisons as potentially differing (P<.10). Groups were matched 1:3 to simulate the observed vaccination uptake. The success of propensity score matching was assessed with propensity score histograms. After matching, the effect of vaccination was estimated using generalized estimation equations using matched group identifiers as cluster labels. Effect magnitudes of vaccination on perinatal outcomes were reported as MD or OR with 95% confidence intervals (CIs). GA at delivery was assessed in a separate cohort matched for the expected date of delivery, and confounders were identified for other perinatal outcomes. Mixed-effects Cox regression was used to estimate the effect of vaccination on GA at birth <40 weeks’ gestation. In a sensitivity analysis for pregnancy outcomes, women with antenatal COVID-19 were excluded. All analyses were performed using R for statistical computing software (version 4.0.2; R Foundation for Statistical Computing, Vienna Austria).
Results
Between March 1, 2021, and July 4, 2021, a total of 1328 eligible women with complete vaccination records were identified (Figure 1 ). This included 140 women who received at least 1 dose of the COVID-19 vaccine in pregnancy before birth and 1188 women who did not. Table 1 shows that women who received antenatal vaccination (vs those who did not) were slightly older, had less deprivation (ie, higher IMD scores), and were more likely to be of White ethnicity than of Afro-Caribbean ethnicity. There was no difference in maternal BMI, alcohol consumption, or smoking habits. Women with COVID-19 vaccination had significantly higher rates of pregestational diabetes mellitus, antenatal medication use, and hypertension than unvaccinated women. There was no difference in antenatal complications, including antenatal SARS-CoV-2 infection (<2% in each group), gestational diabetes mellitus (P=.499), obstetrical cholestasis (P=.646), or cardiac complications (ie, arrhythmia; P=.874).
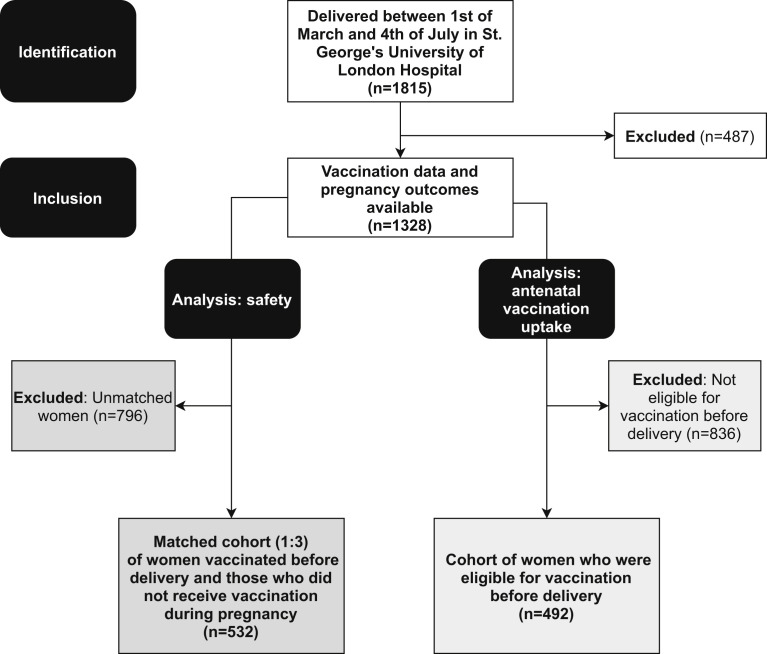
Figure 1.
Study flowchart
Blakeway et al. COVID-19 vaccination during pregnancy. Am J Obstet Gynecol 2022.
Table 1.
Comparison of the baseline and pregnancy characteristics between women who received at least 1 dose of the COVID-19 vaccine during pregnancy and those who did not
| Variables | At least 1 dose during pregnancy (n=140) | Did not receive a vaccine during pregnancy (n=1188) | P valuea |
|---|---|---|---|
| Vaccine typeb | |||
| Pfizer-BioNTech (mRNA) | 109 (77.8) | — | NA |
| Moderna (mRNA) | 18 (12.9) | — | NA |
| Oxford-AstraZeneca (viral vector) | 13 (9.3) | — | NA |
| Vaccination-birth interval (d) | 32.3 (20.2–53.4) | — | NA |
| Trimester at vaccination | |||
| First trimester | 0 (0.0) | — | NA |
| Second trimester | 20 (14.3) | — | NA |
| Third trimester | 120 (85.7) | — | NA |
| Maternal age (y) | 35.0 (31.7–37.0) | 33.0 (30.0–36.0) | .005 |
| Parous | 62 (44.3) | 593 (49.9) | .207 |
| IMD score | 18.9 (14.0–23.7) | 20.2 (14.3–27.6) | .009 |
| IMD quintile | |||
| First quintile | 17 (12.1) | 101 (8.5) | .152 |
| Second quintile | 17 (12.1) | 166 (14.0) | .552 |
| Third quintile | 47 (33.6) | 368 (31.0) | .531 |
| Fourth quintile | 50 (35.7) | 422 (35.5) | .964 |
| Fifth quintile | 5 (3.6) | 102 (8.6) | .039 |
| Not available | 4 (2.9) | 29 (2.4) | .764 |
| Self-reported ethnicity | |||
| White | 80 (57.1) | 551 (46.4) | .015 |
| Asian | 17 (12.1) | 205 (17.3) | .125 |
| Afro-Carribean | 5 (3.6) | 101 (8.5) | .041 |
| Mixed | 13 (9.3) | 156 (13.1) | .196 |
| Not reported | 25 (17.9) | 175 (14.7) | .327 |
| BMI (kg/m2) | 23.8 (21.5–27.5) | 24.2 (21.8–28.3) | .344 |
| Obesity (BMI ≥30 kg/m2) | 15 (10.7) | 173 (14.6) | .216 |
| Smoker | 1 (0.7) | 27 (2.3) | .224 |
| Alcohol use | 1 (0.7) | 5 (0.4) | .624 |
| Pregestational diabetes mellitus | 6 (4.3) | 7 (0.6) | <.001 |
| Antenatal medication | 46 (32.9) | 273 (23.0) | .009 |
| Hypertension on medication | 13 (9.3) | 46 (3.9) | .003 |
| Twin pregnancy | 4 (2.9) | 24 (2.0) | .514 |
| Antenatal complications | |||
| SARS-CoV-2 infection | 2 (1.4) | 16 (1.3) | .936 |
| Gestational diabetes mellitus | 20 (14.3) | 146 (12.3) | .499 |
| Obstetrical cholestasis | 2 (1.4) | 12 (1.0) | .646 |
| Cardiac problems | 1 (0.7) | 10 (0.8) | .874 |
| Any | 25 (17.9) | 175 (14.7) | .327 |
Data are presented as number (percentage) or median (interquartile range), unless otherwise indicated.
BMI, body mass index; IMD, index of multiple deprivation; mRNA, messenger RNA; NA, not applicable.
Blakeway et al. COVID-19 vaccination during pregnancy. Am J Obstet Gynecol 2022.
Wilcoxon signed-rank test, t test, or chi-square test was used, as appropriate
According to the United Kingdom Joint Committee on Vaccination and Immunisation guidance on April 16, 2021, it was preferable for pregnant women to be offered the Pfizer-BioNTech or Moderna vaccines when available (https://www.gov.uk/government/news/jcvi-issues-new-advice-on-covid-19-vaccination-for-pregnant-women).
COVID-19 vaccination uptake
Among the total cohort of 1328 women, vaccination was accepted by 140 of 491 women (28.5%) eligible, based on their age and priority category (Figure 1). Of those vaccinated, 127 (90.7%) received an mRNA vaccine, and 13 (9.3%) received a viral vector vaccine. Regarding the GA at vaccination, 120 women (85.7%) received the first dose during the third trimester of pregnancy and 20 women (14.3%) in the second trimester of pregnancy. None received the vaccine in the first trimester of pregnancy or received a dose before pregnancy. The median interval from vaccination to birth was 32.3 days (IQR, 20.2–53.4 days). There were 26 women (18.6%) who had 2 doses of vaccine during the antenatal period.
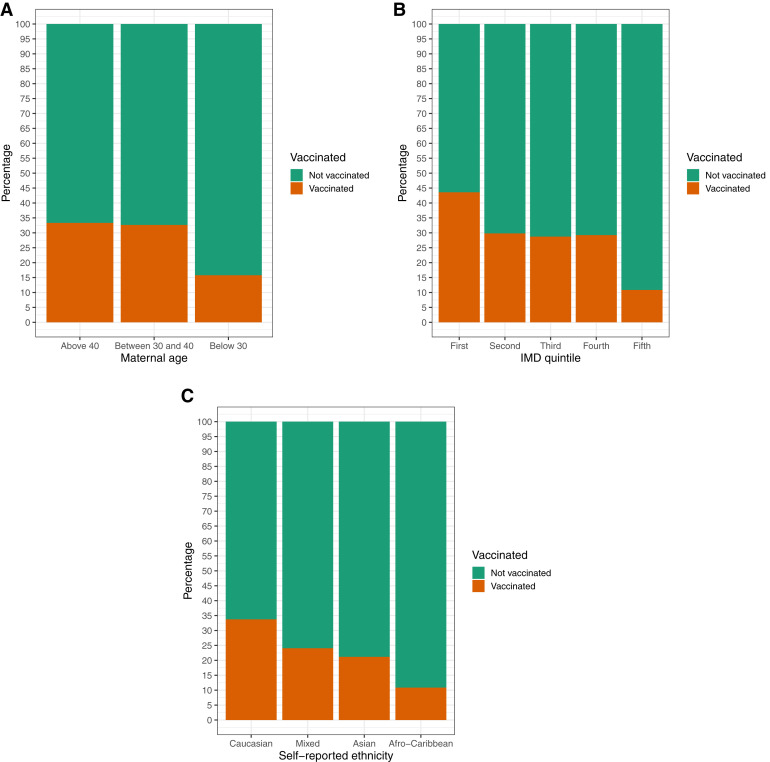
There were significant trends (Cochran-Armitage test) for reduced antenatal vaccine uptake in younger women (P=.001), high levels of deprivation (P=.008), and Afro-Caribbean or Asian ethnicity compared with White or mixed ethnic background (P<.001) (Figure 2 ). Antenatal vaccine uptake was significantly higher among women with prepregnancy diabetes mellitus (OR, 5.19; 95% CI, 1.35–24.9; P=.021) and lower among women with maternal age of <30 years (OR, 0.37; 95% CI, 0.15–0.90; P=.025), fifth deprivation quintile (OR, 0.16; 95% CI, 0.05–0.46; P=.001), Afro-Caribbean ethnicity (OR, 0.24; 95% CI, 0.27–0.89; P=.021), and obesity (defined as maternal BMI at booking of ≥30 kg/m2; OR, 0.55; 95% CI, 0.29–0.99; P=.057) (Table 2 ). Only prepregnancy diabetes mellitus (adjusted OR, 10.5; 95% CI, 1.74–83.2; P=.014), fifth deprivation quintile (adjusted OR, 0.10; 95% CI, 0.02–0.40; P=.003), and Afro-Caribbean ethnicity (adjusted OR, 0.27; 95% CI, 0.06–0.85; P=.044) were significant in multivariable analyses. Maternal age of <30 years (adjusted OR, 0.49; 95% CI, 0.14–1.45; P=.189), and obesity (OR, 0.63; 95% CI, 0.31–1.22; P=.184) were no longer statistically significant (Table 2).
Figure 2.
Bar plots
Vaccine uptake according to maternal age category, index of multiple deprivation quintiles, and self-reported ethnicity. There were significant trends for reduced antenatal vaccine uptake in younger women (P=.001), high levels of deprivation (P=.008), and Afro-Caribbean or Asian ethnicity compared with White or mixed ethnic background (P<.001).
Blakeway et al. COVID-19 vaccination during pregnancy. Am J Obstet Gynecol 2022.
Table 2.
Factors associated with vaccination during the antenatal period in eligible women at the time of birth
| Variables | OR (95% CI) | P valuea | Adjusted OR (95% CI) | P valueb |
|---|---|---|---|---|
| Maternal age (y) | 1.45 (1.18–1.80) | <.001 | — | — |
| >40 | Reference | Reference | ||
| 30–40 | 0.96 (0.46–2.12) | .918 | 1.15 (0.48–3.01) | .751 |
| <30 | 0.37 (0.15–0.90) | .025 | 0.49 (0.17–1.45) | .189 |
| IMD score | 0.72 (0.58–0.89) | .002 | — | — |
| IMD quintile | ||||
| First quintile | Reference | Reference | ||
| Second quintile | 0.55 (0.23–1.28) | .168 | 0.65 (0.25–1.63) | .361 |
| Third quintile | 0.51 (0.25–1.06) | .066 | 0.66 (0.30–1.47) | .310 |
| Fourth quintile | 0.53 (0.26–1.10) | .085 | 0.71 (0.33–1.57) | .403 |
| Fifth quintile | 0.16 (0.05–0.46) | .001 | 0.10 (0.02–0.40) | .003 |
| Self-reported ethnicity | ||||
| White | Reference | Reference | ||
| Afro-Caribbean | 0.24 (0.27–0.89) | .021 | 0.27 (0.06–0.85) | .044 |
| Asian | 0.49 (0.08–0.58) | .003 | 0.61 (0.31–1.16) | .147 |
| Mixed | 0.62 (0.30–1.20) | .171 | 1.01 (0.46–2.10) | .976 |
| Not reported | 1.09 (0.62–1.89) | .761 | 0.87 (0.45–1.65) | .688 |
| Maternal BMI (kg/m2) | 0.84 (0.67–1.04) | .127 | — | — |
| Obesity (BMI ≥30 kg/m2) | 0.55 (0.29–0.99) | .057 | 0.63 (0.31–1.22) | .184 |
| Smoking at the time of birth | 0.35 (0.02–2.01) | .333 | — | — |
| Alcohol use at the time of birth | 2.52 (0.10–63.9) | .515 | — | — |
| Pregestational diabetes mellitus | 5.19 (1.35–24.9) | .021 | 10.5 (1.74–83.2) | .014 |
| Antenatal medication | 1.47 (0.95–2.25) | .077 | 1.43 (0.84–2.41) | .176 |
| Antenatal COVID-19 | 2.53 (0.30–21.2) | .356 | 1.62 (0.06–20.6) | .712 |
| Gestational diabetes mellitus | 1.16 (0.65–2.03) | .603 | — | — |
The overall vaccination uptake rate among those eligible was 28.5% (140/491).
BMI, body mass index; CI, confidence interval; IMD, index of multiple deprivation; OR, odds ratio.
Blakeway et al. COVID-19 vaccination during pregnancy. Am J Obstet Gynecol 2022.
Univariable logistic regression
Multivariable logistic regression.
Pregnancy outcomes according to COVID-19 vaccination
Women who had antenatal COVID-19 vaccination (vs those who did not) were matched 1:3 using propensity scores calculated from maternal age, IMD quintile, self-reported ethnicity, prepregnancy diabetes mellitus, antenatal medication (any), and antenatal antihypertensive therapy, with exact matching on antenatal medication use and deprivation quintile.
In a propensity score–matched cohort, 133 women who received at least 1 dose of the COVID-19 vaccine before birth were matched with 399 women who did not (Figure 1); a match could not be found for 8 women in the vaccinated group. The propensity score histograms of both groups before and after matching are shown in the Supplemental Figure. There was no significant difference in intrapartum complications with the exception of intrapartum fever (OR, 3.85; 95% CI, 1.01–14.6; P=.046), or perinatal outcomes between women who received COVID-19 vaccination and unvaccinated women during pregnancy (Table 3 ).
Supplemental Figure.
Propensity score histograms
Antenatal vaccination (treated) and unvaccinated women (control) before (raw) and after matching (matched). The propensity scores of both groups were successfully balanced after the matching.
Blakeway et al. COVID-19 vaccination during pregnancy. Am J Obstet Gynecol 2022.
Table 3.
Pregnancy outcomes among propensity score–matched women who received at least 1 dose of the COVID-19 vaccine
| Variables | At least 1 dose during pregnancy (n=133) | Did not receive a vaccine during pregnancy (n=399) | Effect magnitude (95% CI)a | P valuea |
|---|---|---|---|---|
| Intrapartum complications | ||||
| Pyrexia | 5 (3.7) | 4 (1.0) | 3.85 (1.01–14.6) | .046 |
| Suspected chorioamnionitis | 0 (0.0) | 2 (0.5) | NE | NE |
| Placental abruption | 0 (0.0) | 0 (0.0) | NE | NE |
| Postpartum hemorrhage | 13 (9.8) | 36 (9.0) | 1.09 (0.56–2.12) | .795 |
| Birthweight z score | −0.09 (−0.65 to 0.65) | −0.13 (−0.83 to 0.51) | 0.04 (-0.16–0.24) | .427 |
| Small for gestational age at birth | 16 (12.0) | 48 (12.0) | 1.00 (0.55–1.82) | >.999 |
| Fetal abnormalities | 3 (2.2) | 10 (2.5) | 0.89 (0.24–3.31) | .871 |
| Mode of delivery | ||||
| Unassisted vaginal delivery | 71 (53.4) | 221 (55.4) | 0.92 (0.62–1.36) | .687 |
| Instrumental delivery | 21 (15.8) | 42 (10.5) | 1.59 (0.90–2.80) | .106 |
| Cesarean delivery | 41 (30.8) | 136 (34.1) | 0.86 (0.56–1.31) | .490 |
| Stillbirth | 0 (0.0) | 1 (0.2) | NE | NE |
| High-dependency unit admission | 8 (6.0) | 16 (4.0) | 1.53 (0.64–3.66) | .337 |
| Neonatal intensive care unit admission | 7 (5.3) | 20 (5.0) | 1.05 (0.43–2.54) | .909 |
Data are presented as number (percentage) or mean (interquartile range), unless otherwise indicated. Cases and controls were matched 1:3 using propensity scores calculated from the index of multiple deprivation quintile, self-reported ethnicity, antenatal medication, pregestational diabetes mellitus, maternal age, and antihypertensive medication.
CI, confidence interval; MD, mean difference; NE, not estimable; OR, odds ratio.
Blakeway et al. COVID-19 vaccination during pregnancy. Am J Obstet Gynecol 2022.
Calculated using generalized estimation equations using matched group identifications as clusters.
Here, 3 fetal abnormalities were reported in the women who received COVID-19 vaccination: spina bifida, ventriculomegaly, and hydronephrosis. The spina bifida case was diagnosed before the pregnant woman received the first dose of the vaccine. The ventriculomegaly case was diagnosed at 37 weeks’ gestation and was isolated, with no associated brain abnormalities, as confirmed by fetal brain magnetic resonance imaging. The hydronephrosis was mild, with no associated abnormality at birth.
There were no significant differences in intrapartum complications or perinatal outcomes after excluding women who had COVID-19 during pregnancy (Supplemental Table).
Mixed-effects Cox regression showed that GA at delivery <40 weeks’ gestation did not differ between women vaccinated during pregnancy and those who were not (HR, 0.93; 95% CI, 0.71–1.23; P=.624) (Figure 3 ). The size of the vaccinated cohort (n=133) allowed for the estimation of moderate and high effect sizes of >0.25, with 80% power.
Figure 3.
Gestational age at birth in COVID-19 vaccinated and unvaccinated women
Groups were matched on expected date of delivery, index of multiple deprivation quintile, self-reported ethnicity, antenatal medication, pregestational diabetes mellitus, maternal age, and antihypertensive medication. Mixed-effects Cox regression showed that vaccination was not significantly associated with gestational age at delivery before 40 weeks’ gestation (hazard ratio, 0.93; 95% confidence interval, 0.71–1.23; P=.624).
Blakeway et al. COVID-19 vaccination during pregnancy. Am J Obstet Gynecol 2022.
Comment
Principal findings
The overall rate of antenatal COVID-19 vaccine uptake in this cohort of pregnant women who were eligible for vaccination and gave birth in an inner London maternity hospital was 28.5%. When offered during pregnancy, the most striking determinants of COVID-19 vaccination uptake were prepregnancy diabetes mellitus (a facilitator) and deprivation (a barrier). Women from the most deprived socioeconomic background were less likely to receive a vaccine, whereas women with prepregnancy diabetes mellitus were more likely to receive a vaccine. Possible additional factors were maternal age of <30 years and Afro-Caribbean ethnicity, both associated with lower vaccine uptake. Following propensity score matching for differences between women vaccinated and those not vaccinated, there was no difference seen in pregnancy outcomes associated with COVID-19 vaccination in pregnancy except intrapartum pyrexia, although 95% CIs were wide. However, it is extremely unlikely that cases of intrapartum pyrexia were related to vaccination because the shortest vaccination-delivery interval in women with intrapartum fever was 2 weeks. According to the CDC, side effects related to the COVID-19 vaccine are short-lived, resolving within a few days.16 Moreover, the statistical significance was lost after excluding women with antenatal COVID-19 from the analysis, implying a spurious association, that is, a type I error.
Interpretation of study findings and comparison with the published literature
Our study has important implications for improving COVID-19 vaccine uptake among pregnant women by identifying facilitators and barriers to vaccine uptake.
In the United Kingdom, the JCVI initially stated that COVID-19 vaccination should be offered only to those pregnant women with underlying health conditions that put them at increased risk of severe COVID-19 or where exposure to COVID-19 could not be avoided.11 In addition, pregnant women were excluded from initial COVID-19 vaccine trials, meaning that there are currently limited data on their safety and efficacy in pregnancy.17 A key factor in determining uptake of these vaccines is public trust. A survey of 16 countries, including the United Kingdom, found that skepticism around the disease, concern regarding vaccine safety, and lack of trust in government advice and guidelines were significant indicators in predicting vaccine uptake.18 Consequently, changes in the United Kingdom guidance along with the lack of safety and efficacy data likely contributed to vaccine hesitancy among pregnant women. In contrast, this may explain why we found that women with prepregnancy diabetes mellitus were more likely to have the vaccine; their underlying health condition was an indication for vaccination in and outside pregnancy from the start. Clear government communication and advice are needed to help build trust in the system and improve vaccine uptake.
Our results showed that pregnant women of Afro-Caribbean ethnicity were less likely to receive the vaccine; this is consistent with recent questionnaire data both in pregnancy and outside of pregnancy.8 However, people from ethnic minority groups are more likely to suffer from severe COVID-19, with increased risk of hospitalization, higher ICU admissions, and higher death rates in those of South Asian ethnicity in the United Kingdom.19 Of note, ethnicity did not seem like an indication for COVID-19 vaccination in pregnancy until March 24, 2021,12 emphasizing the importance of tailoring counseling to individual pregnant women to encourage uptake.20
To receive COVID-19 vaccination in the United Kingdom, pregnant women must attend a vaccine center rather than their local general practitioner. A United Kingdom study that interviewed 31 pregnant women in April 2020 about the possibility of a COVID-19 vaccine in pregnancy identified concerns about attending hospital or clinical settings for a vaccine because of the perceived risk of exposure to COVID-19.21 Additional challenges have included reduced public transport and difficulty in accessing child care.22 Importantly, maternity care providers and healthcare professionals in vaccine centers, who are less familiar with maternity care, are not always comfortable discussing the benefits and risks of vaccination in pregnancy, even for the current antenatal immunization program.23 This is likely to be the case with the COVID-19 vaccine. As the COVID-19 vaccine is so new, the advice for vaccination changes so frequently, and as mentioned before, there is currently no United Kingdom data on its safety and efficacy in pregnant women. When the vaccine rollout started in the United Kingdom, many pregnant women turned to their midwives and obstetricians for advice, but with a lack of clear guidance at that point, it was difficult for healthcare professionals to advise women.17
In addition, safety concerns are cited as a reason why pregnant women are hesitant to have the COVID-19 vaccine. A multimethods study into women’s views on the COVID-19 vaccine found that 81% of nonpregnant women were willing to accept the vaccine immediately, compared with only 62% of pregnant women for whom vaccine hesitancy was most commonly related to safety concerns. Concerns were particularly related to long-term effects and about the speed at which vaccines were developed and tested.8 There is additional evidence that pregnant women in the United Kingdom have concerns about vaccination in general. Although the United Kingdom has an extensive antenatal immunization program, with a routine offer of vaccination against pertussis, diphtheria, tetanus, polio, and seasonal influenza,24 a multicenter questionnaire study into vaccine hesitancy for influenza and pertussis vaccination in pregnancy found that, most commonly, vaccination was declined for fear of adverse fetal effects.23
To date, only observational data have been published on vaccination in pregnancy, including a cohort of 3958 pregnant women, of whom 827 completed their pregnancy.25 The main focus of that study was safety, but the conclusions drawn are potentially limited as the outcomes of pregnant women were compared with historic background rates instead of a contemporaneous control group. Moreover, this is the case for another study that assessed short-term outcomes of COVID-19 vaccination in pregnancy.26 To date, there is little published evidence comparing outcomes of pregnant women who had the COVID-19 vaccine with other pregnant women who did not have the vaccine but had the same exposure to the pandemic.25 , 26 This is important, as it has been shown that pregnancy outcomes may be altered by the indirect effects of the pandemic, such as changes in the provision of healthcare services and the behavior of pregnant women.22 , 27
Clinical and research implications
Our study findings have both clinical and research implications. Our study had contemporaneous pregnant controls, matched for factors associated with vaccination. This is important, as both vaccinated and unvaccinated pregnant women were equally exposed to the indirect effects of the pandemic on maternity care and outcomes. This means that our findings of no significant difference in perinatal outcomes were more robust. Clinically, healthcare professionals should use this knowledge to encourage pregnant women to accept COVID-19 vaccination and reassure them with the growing evidence that the vaccines are safe during pregnancy.
Our study did not include any pregnant women who had the vaccine in the first trimester of pregnancy, and there are conflicting reports about the effect of first-trimester SARS-CoV-2 infection on miscarriage rates.28 , 29
Currently, the Royal College of Obstetricians and Gynaecologists (RCOG) does not specify any gestation to avoid COVID vaccination but mentions that in low-risk situations, pregnant women may choose to delay vaccination until 12 weeks’ gestation: “COVID-19 vaccines can be given at any time in pregnancy. In low-risk situations, some women may choose to delay vaccination until 12 weeks of gestation, aiming for vaccination as soon as possible thereafter.”12 More data are needed on safety of the vaccine when administered in the first trimester of pregnancy so that clearer, informed guidance can be developed. Moreover, data on the long-term outcomes of infants born following COVID-19 vaccination in pregnancy are needed (as they are for infants born to women who had COVID-19 in pregnancy). Furthermore, it is important to investigate the safety and efficacy of COVID-19 vaccination in pregnancy in an RCT setting. This is currently proposed in a United Kingdom multicenter study (PregCOV), in an international RCT by Pfizer, and in another trial proposed by Janssen. In the United States, an observational study of vaccinated pregnant women, sponsored by the National Institutes of Health, is now underway.6 , 7 , 30
Strengths and limitations
We conducted this analysis to rapidly evaluate vaccine uptake, factors associated with vaccine acceptance, and outcomes in women who received a COVID-19 vaccine compared with a contemporaneous group of pregnant women who were not vaccinated. Vaccine uptake was low at the time, but that may change over time as more data emerge, and women are more reassured with COVID-19 vaccination during pregnancy. Furthermore, we used a matched cohort of pregnant women with balanced propensity scores. The propensity score was calculated using variables that were significantly different between vaccinated and unvaccinated women and were likely to affect adverse pregnancy outcomes. Although propensity score matching is no substitute for randomization, it remains one of the best methods for causal inference from observational data. Moreover, we provided effect magnitudes with P values, so that the clinical significance of any differences observed could be interpreted, whether statistically significant or not. Finally, a mixed-effects Cox regression was performed in a cohort of expected delivery date matched women to investigate any differences in anticipated delivery date of vaccinated and unvaccinated women.
Some limitations apply to our findings. First, the median time to birth after vaccination was only a month. Consequently, insufficient time may have passed between exposure and birth for some outcomes to be affected (eg, small for GA and preterm birth). However, when analyzed on the continuous scale (ie, for GA at birth and birthweight z score), there was no systematic difference. Second, none of the women were vaccinated in the first trimester of pregnancy, and only 15% of women were vaccinated in the second trimester of pregnancy, so our findings primarily applied to women vaccinated in the third trimester of pregnancy. Third, women without vaccination records were not included in this study, which may have led to a selection bias and reduced any potential differences between vaccinated and unvaccinated women. However, the observed vaccination uptake was similar to vaccination hesitancy rates in the United Kingdom according to a recent RCOG survey,31 so we believe that our data are a good approximation of the actual picture. Finally, our sample was powered to detect small to moderate effect sizes down to Cohen’s d of 0.25, so small and very small differences (Cohen’s d <0.2) may have been undetected, and our 95% CIs were wide. However, we reported effect magnitudes for safety data, and the clinical significance of any type II error we may have encountered was uncertain.
Conclusions
Our findings have contributed to the mounting evidence that supports the safety of COVID-19 vaccination in pregnancy. In our study cohort, less than one-third of eligible women received the COVID-19 vaccination during pregnancy. This rate was much lower than that for nonpregnant women, despite the known increased risk of severe COVID-19 in pregnant women. The facilitators and barriers of vaccine uptake in pregnancy should be used to design targeted educational campaigns to reduce COVID-19 vaccine hesitancy in pregnancy. Moreover, postvaccine surveillance data are needed on pregnancy outcomes following first-trimester vaccination, and long-term outcomes following vaccination at any time in pregnancy, to support pregnant women in deciding whether to accept the offer of COVID-19 vaccination.
Footnotes
The authors report no conflict of interest.
The authors report no funding sources.
Cite this article as: Blakeway H, Prasad S, Kalafat E, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol 2022;226:236.e1-14.
Supplementary Materials
Supplemental Table.
Pregnancy outcomes in women who received at least 1 dose of the COVID-19 vaccine, excluding women who had antenatal COVID-19
| Variables | At least 1 dose during pregnancy (n=131) | Did not receive a vaccine during pregnancy (n=393) | Effect magnitude: odds ratio or mean difference (95% CI)a | P valuea |
|---|---|---|---|---|
| Intrapartum complications | ||||
| Pyrexia | 5 (3.8) | 5 (1.3) | 3.07 (0.87–10.8) | .079 |
| Suspected chorioamnionitis | 0 (0.0) | 2 (0.5) | NE | NE |
| Placental abruption | 0 (0.0) | 0 (0.0) | NE | NE |
| Postpartum hemorrhage | 13 (9.9) | 33 (8.4) | 1.20 (0.61–2.35) | .593 |
| Birthweight z score | −0.08 (−0.66 to 0.66) | −0.07 (−0.67 to 0.56) | −0.02 (−0.23 to 0.18) | .849 |
| Small for gestational age at birth | 16 (12.2) | 45 (11.4) | 1.07 (0.58–1.97) | .813 |
| Fetal abnormalities | 3 (2.2) | 11 (2.8) | 0.98 (0.79–1.20) | .754 |
| Mode of delivery | ||||
| Unassisted vaginal delivery | 71 (54.2) | 213 (54.2) | 1.00 (0.67–1.49) | .541 |
| Instrumental delivery | 20 (15.3) | 46 (11.7) | 1.35 (0.77–2.39) | .288 |
| Cesarean delivery | 40 (30.5) | 134 (34.1) | 0.85 (0.55–1.30) | .453 |
| Stillbirth | 0 (0.0) | 1 (0.2) | NE | NE |
| High-dependency unit admission | 8 (6.1) | 11 (2.8) | 2.25 (0.88–5.74) | .087 |
| Neonatal intensive care unit admission | 7 (5.3) | 24 (6.1) | 0.86 (0.36–2.06) | .748 |
Data are presented as number (percentage) or mean (interquartile range), unless otherwise indicated. Case and controls were matched 1:3 using propensity scores calculated from the index of multiple deprivation quintile, self-reported ethnicity, antenatal medication, pregestational diabetes mellitus, maternal age, and antihypertensive medication.
CI, confidence interval; NE, not estimable.
Blakeway et al. COVID-19 vaccination during pregnancy. Am J Obstet Gynecol 2022.
Calculated using generalized estimation equations using matched group identifications as clusters.
References
- 1.Roberton T., Carter E.D., Chou V.B., et al. Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8:e901–e908. doi: 10.1016/S2214-109X(20)30229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viana J., van Dorp C.H., Nunes A., et al. Controlling the pandemic during the SARS-CoV-2 vaccination rollout. Nat Commun. 2021;12:3674. doi: 10.1038/s41467-021-23938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalafat E., O’Brien P., Heath P.T., et al. Benefits and potential harms of COVID-19 vaccination during pregnancy: evidence summary for patient counseling. Ultrasound Obstet Gynecol. 2021;57:681–686. doi: 10.1002/uog.23631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynaecologists Vaccinating pregnant and lactating patients against COVID-19 practice advisory. 2021. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19 Available at:
- 5.Gray K.J., Bordt E.A., Atyeo C., et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. medRxiv. https://doi.org/10.1101/2021.03.07.21253094 Preprint posted online March 8, 2021. [DOI] [PMC free article] [PubMed]
- 6.Pfizer Pfizer and BioNTech commence global clinical trial to evaluate COVID-19 vaccine in pregnant women. 2021. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-commence-global-clinical-trial-evaluate Available at:
- 7.US National Library of Medicine ClinicalTrials.gov. A study of Ad26.COV2.S in healthy pregnant participants (COVID-19) (HORIZON 1). 2021. https://clinicaltrials.gov/ct2/show/NCT04765384 Available at:
- 8.Skirrow H., Barnett S., Bell S., et al. Women’s views on accepting COVID-19 vaccination during and after pregnancy, and for their babies: a multi-methods study in the UK. medRxiv. https://doi.org/10.1101/2021.04.30.21256240 Preprint posted online May 3, 2021. [DOI] [PMC free article] [PubMed]
- 9.Public Health England JCVI issues new advice on COVID-19 vaccination for pregnant women. 2021. https://www.gov.uk/government/news/jcvi-issues-new-advice-on-covid-19-vaccination-for-pregnant-women Available at:
- 10.International Federation of Gynecology and Obstetrics COVID-19 vaccination for pregnant and breastfeeding women. 2021. https://www.figo.org/covid-19-vaccination-pregnant-and-breastfeeding-women Available at:
- 11.Joint Committee on Vaccination and Immunisation Advice on priority groups for COVID-19 vaccination. 2021. https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-30-december-2020/joint-committee-on-vaccination-and-immunisation-advice-on-priority-groups-for-covid-19-vaccination-30-december-2020 Available at:
- 12.Royal College of Obstetricians and Gynaecologists Coronavirus (COVID-19) vaccination in pregnancy, information for healthcare professionals. 2021. https://www.rcog.org.uk/covid-vaccine Available at:
- 13.Public Health England COVID-19 vaccination: a guide to phase 2 of the programme. 2020. https://www.gov.uk/government/publications/covid-19-vaccination-guide-for-older-adults Available at:
- 14.National Health Service Coronavirus (COVID-19) vaccines. 2021. https://www.nhs.uk/conditions/coronavirus-covid-19/coronavirus-vaccination/coronavirus-vaccine/?gclid=Cj0KCQjw1dGJBhD4ARIsANb6OdlYLbBGAHY51nDmCX0kLdSWHwASh_O3cFPiLSBk7aM1CFXmPE5tBzYaArHXEALw_wcB Available at:
- 15.Poon L.C., Tan M.Y., Yerlikaya G., Syngelaki A., Nicolaides K.H. Birth weight in live births and stillbirths. Ultrasound Obstet Gynecol. 2016;48:602–606. doi: 10.1002/uog.17287. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention Pfizer-BioNTech COVID-19 vaccine overview and safety. 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Pfizer-BioNTech.html#:∼:text=These%20side%20effects%20happen%20within,getting%20a%20COVID%2D19%20vaccine Available at:
- 17.Wang E.W., Parchem J.G., Atmar R.L., Clark E.H. SARS-CoV-2 vaccination during pregnancy: a complex decision. Open Forum Infect Dis. 2021;8:ofab180. doi: 10.1093/ofid/ofab180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skjefte M., Ngirbabul M., Akeju O., et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. 2021;36:197–211. doi: 10.1007/s10654-021-00728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathur R., Rentsch C.T., Morton C.E., et al. Ethnic differences in SARS-CoV-2 infection and COVID-19-related hospitalisation, intensive care unit admission, and death in 17 million adults in England: an observational cohort study using the OpenSAFELY platform. Lancet. 2021;397:1711–1724. doi: 10.1016/S0140-6736(21)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donaldson B., Jain P., Holder B.S., Lindsey B., Regan L., Kampmann B. What determines uptake of pertussis vaccine in pregnancy? A cross sectional survey in an ethnically diverse population of pregnant women in London. Vaccine. 2015;33:5822–5828. doi: 10.1016/j.vaccine.2015.08.093. [DOI] [PubMed] [Google Scholar]
- 21.Anderson E., Brigden A., Davies A., Shepherd E., Ingram J. Maternal vaccines during the COVID-19 pandemic: a qualitative interview study with UK pregnant women. Midwifery. 2021;100:103062. doi: 10.1016/j.midw.2021.103062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chmielewska B., Barratt I., Townsend R., et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Glob Health. 2021;9:e759–e772. doi: 10.1016/S2214-109X(21)00079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilcox C.R., Calvert A., Metz J., et al. Determinants of influenza and pertussis vaccination uptake in pregnancy: a multicenter questionnaire study of pregnant women and healthcare professionals. Pediatr Infect Dis J. 2019;38:625–630. doi: 10.1097/INF.0000000000002242. [DOI] [PubMed] [Google Scholar]
- 24.Gall S.A., Myers J., Pichichero M. Maternal immunization with tetanus-diphtheria-pertussis vaccine: effect on maternal and neonatal serum antibody levels. Am J Obstet Gynecol. 2011;204:334.e1–334.e5. doi: 10.1016/j.ajog.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 25.Shimabukuro T.T., Kim S.Y., Myers T.R., et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bookstein Peretz S., Regev N., Novick L., et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet Gynecol. 2021 doi: 10.1002/uog.23729. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Townsend R., Chmielewska B., Barratt I., et al. Global changes in maternity care provision during the COVID-19 pandemic: a systematic review and meta-analysis. EClinicalMedicine. 2021;37:100947. doi: 10.1016/j.eclinm.2021.100947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sacinti K.G., Kalafat E., Sukur Y.E., Koc A. Increased incidence of first-trimester miscarriage during the COVID-19 pandemic. Ultrasound Obstet Gynecol. 2021;57:1013–1014. doi: 10.1002/uog.23655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.la Cour Freiesleben N., Egerup P., Hviid K.V.R., et al. SARS-CoV-2 in first trimester pregnancy: a cohort study. Hum Reprod. 2021;36:40–47. doi: 10.1093/humrep/deaa311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Institutes of Health . US Department of Health and Human Services; 2021. NIH begins study of COVID-19 vaccination during pregnancy and postpartum.https://www.nih.gov/news-events/news-releases/nih-begins-study-covid-19-vaccination-during-pregnancy-postpartum Available at: [Google Scholar]
- 31.Royal College of Obstetricians and Gynaecologists Maternity colleges express concern over vaccine hesitancy in pregnant women. 2021. https://www.rcog.org.uk/en/news/maternity-colleges-express-concern-over-vaccine-hesitancy-in-pregnant-women/ Available at: