Abstract
We report the case of a 10-year-old boy with acute–onset diplopia and ptosis in the right eye. CR was positive for SARS-CoV-2. The patient was managed successfully with corticosteroids. We highlight the need for heightened suspicion of occult COVID-19 infection among children presenting with unusual III nerve palsy.
Abbreviations: COVID‐19, coronavirus disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; PCR, polymerase chain reaction; RT-PCR, real-time reverse transcription-PCR; MRI, magnetic resonance imaging; HSV, herpes simplex virus; EBV, Epstein–Barr virus; CMV, cytomegalovirus; PMIS, Pediatric Multisystem Inflammatory Syndrome
Keywords: Third cranial nerve palsy, Child, COVID-19, Corticosteroids
Introduction
The coronavirus disease (COVID-19) has appeared in China in December 2019 [1] and is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). When compared to adults, children were less frequently infected with SARS-CoV-2 during 2020 COVID-19 pandemic [2]. We describe a rare case of asymptomatic SARS-CoV-2 infection associated with isolated acute unilateral palsy of the third oculomotor nerve.
Case description
A previously healthy 10-year-old boy, with no medical history (such as diabetes, high blood pressure, dyslipidemia, vasculitis, obesity, familial neurological disease, or other risk factors for ischemic ophthalmoplegia), consulted the Emergency Department for acute ptosis and diplopia of the right eye, evolving for 3 days. The patient had previous contacts with her father, who tested positive for COVID-19 five days before.
He was afebrile. He had no dry cough, nor anosmia. Blood pressure level and hemodynamic state were within normal ranges (Bp = 105/60 mmHg, SpO2 = 100%, heart rate = 80 ppm, respiratory rate = 23 bpm). On physical examination, abduction of the right eye was painful, and diplopia increased with ocular adduction. A superior eyelid ptosis and anisocoria with a discrete mydriasis were noted in the right eye. The visual acuity by Teller visual acuity cards at 84 cm was 7 cycles/cm (Snellen equivalent, 30/63) in the right eye. Cycloplegic refraction was +1.50 D sphere in each eye. Cover testing at near and distance revealed an exotropia in the right eye. The Krimsky test, without correction, revealed an exotropia at near of 45Δ, fixating with the left eye. Ocular versions showed adduction limitation (right medial rectus muscle, −1), uplift limitation (right superior rectus muscle, −2), and depression limitation (right inferior rectus muscle, ≤1). Intraocular pressure, biomicroscopy, and fundoscopy were normal in both eyes. No neurological impairment was found, and the rest of the physical examination was unremarkable. A complete palsy of the third cranial nerve was diagnosed.
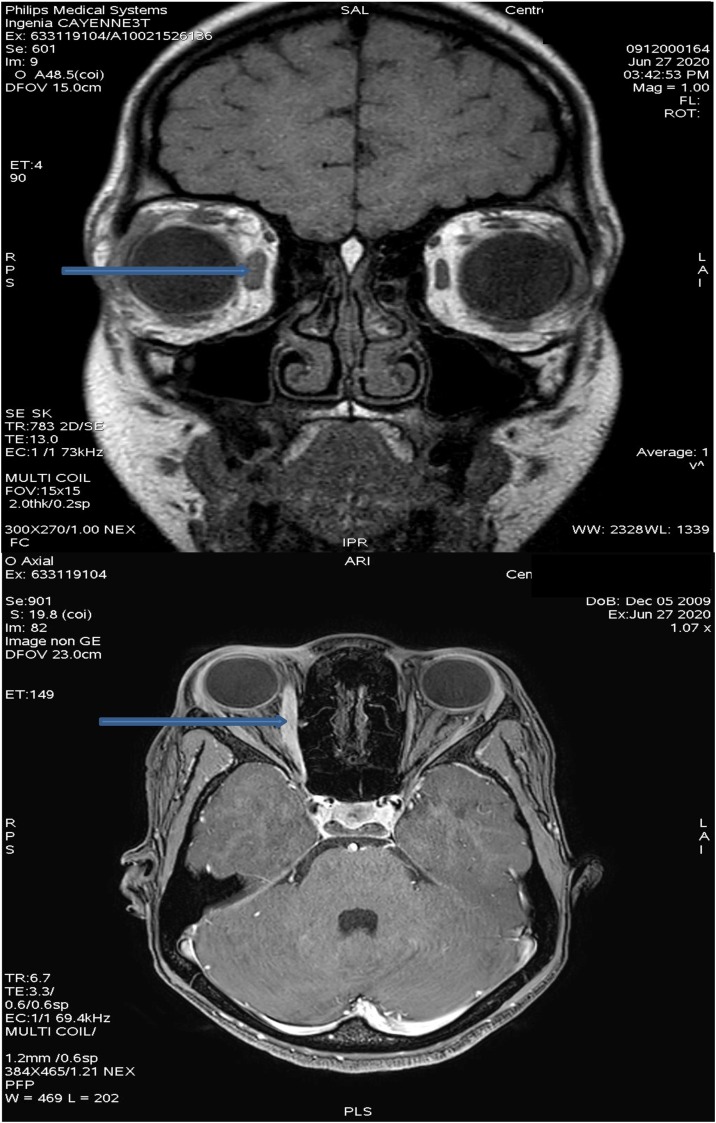
A nasopharyngeal swab for SARS-CoV-2 real-time reverse transcription-polymerase chain reaction (RT-PCR) (GeneFinder™ COVID-19 Eurobio Kit) was positive. After admission to the pediatric COVID-19 ward, laboratory workup and radiological investigations were performed. Orbital and Brain Magnetic resonance angiography showed increased enhancement of the internal rectus muscle on the right side; there were no lesions or aneurysmal compression of the third left cranial nerve (Fig. 1 ). Blood tests, including infectious disease investigation, complete blood count, and inflammatory markers, were normal (Table 1 ). The corrected QT interval was 380 ms on electrocardiogram. Treatment was started with corticosteroids. The patient received prednisone (2 mg/kg per day for 10 days) with, calcium (500 mg once a day for 10 days) and vitamin D (440 UI once a day for 10 days). Evolution was marked by improvement of clinical signs by the third day. The ptosis and diplopia of the right eye resolved rapidly, and complete recovery was achieved by the seventh day of treatment. Fortunately, no adverse effects of the treatment were noted, and discharge was possible after five days of hospital stay. To date, our patient is healthy and doing well.
Fig. 1.
Magnetic resonance imaging (MRI) of the brain showing an increased enhancement of the internal rectus muscle on the right side (see blue arrows). The rest of the brain MRI examination was unremarkable, without any signs of nerve compression, masses, or aneurysms.
Table 1.
Results of laboratory workup.
| Elements | Patient’s values | Normal values |
|---|---|---|
| Hemoglobin | 11.9 g/dL | 12–16 g/dL |
| MCV | 751 fL | 77–98 |
| MCH | 25.8 pg | 25–35 |
| MCHC | 34,5/dL | 31–37 |
| Leucocytes | 8.0 G/L | 3.75–13 G/L |
| Neutrophils | 2.66 G/L | 1.5-6.3 G/L |
| Lymphocytes | 2.38 G/L | 1.3–4.5 G/L |
| Monocytes | 0.50 G/L | 0.15–1.3 G/L |
| Eosynophils | 2.37 G/L | 0.04–0.89 G/L |
| Basophils | 0.08 G/L | 0.01–0.23 G/L |
| Platelets | 257 G/L | 166–385 G/L |
| Erythrocytes | 4.61 T/L | 4.2–5.6 T/L |
| TP | 93% | 70–100% |
| TCA | 34.3 s | 25.1–36.5 s |
| INR | 1.05 | |
| Fibrinogene | 2.87 g/L | 2.38–4.98 g/L |
| K+ | 4.6 mmol/L | 3.50–5.10 |
| NA+ | 142 mmol/L | 136.0–145.0 |
| Cl– | 108 mmol/L | 98.0–107.0 |
| Urea | 3.4 mmol/L | 2.8-8.1 mmol/L |
| Creatinine | 54 μmol/L | 39–60 μmol/L |
| Glycaemia | 4.8 mmol/L | 5.85–6.05 mmol/L |
| AST | 33 U/L | <40 UI/L |
| ALT | 37 U/L | <41 UI/L |
| GGT | 21 U/L | <60 UI/L |
| ALP | 211 U/L | <390 UI/L |
| LDH | 203 U/L | 120–300 UI/L) |
| CRP | 0.6 mg/L | <5 mg/L |
| Ferritin | 50 μg/L | 15.0–80.0 μg/L |
| d-dimer | 0.009 dOD | (0.00–0.05) |
| Troponin | 0.008 μg/L | <0.014 μg/L |
| TSH | 2.07 mUI/L | 0.27–4.20 mUI/L |
| T4 | 16.0 pmol/L | 12–22 pmol/L |
| T3 | 5.8 pmol/L | 31–68 pmol/L |
| Cerebrospinal fluid | Normal |
Discussion
To our knowledge, this is the second reported case indicating a possible association between COVID-19 and acquired oculomotor nerve palsy in a previously asymptomatic child. To date, 8 cases of cranial nerve palsy associated with COVID-19 infection have been reported, one involving a child [3]. Although isolated post-COVID cranial neuropathies in children are rare and may be favored by underlying comorbidities, our patient had no comorbidities.
Oculomotor nerve palsies caused by presumed inflammation usually present with brain magnetic resonance imaging (MRI) findings confined to the oculomotor nerve, without further brain or orbital imaging findings, which is the case in our patient. Our patient had a normal infectious and inflammatory workup apart from the presence of SARS-COV2. It is known that penetration of SARS-COV-2 virus through the riddled lamina of ethmoid bone can damage the olfactory bulb, causing anosmia, and may be the route of entry of the virus into the nervous system [4]. The neuroinvasive mechanism of SARS-CoV-2 is not yet fully understood, although previous studies have suggested that the virus infects the host cell via the interaction of angiotensin converting enzyme 2 receptors bound to the membrane, which is also expressed in various organ systems, including the neurological system [5]. Previous studies hypothesized that the neurological spectrum of COVID-19 could be due to direct viral neurological injury or indirect neuroinflammatory and autoimmune mechanisms [6]. The aberrant immune response mediated by SARS-CoV-2 might be responsible for neuroinflammation. This inflammation will, in turn, cause swelling of the third oculomotor nerve swelling [7]. Our patient did not develop any other signs or symptoms suggesting immune involvement. Therefore, we suggest that the oculomotor nerve palsy may have been caused by the direct action of the virus, causing inflammation of the third cranial nerve. However, the indirect action of the virus via an immune response cannot be ruled out. Although improvement in the present case followed corticosteroid-treatment, spontaneous improvement has been described in a previous case [3]. Indeed, the clinical features of cranial nerve palsies in childhood are affected by children’s ability to repair and regenerate after injury [8]. Other neurotropic viruses such as herpes (HSV, herpes zoster virus, EBV, CMV) and, chikungunya also can cause cranial nerve palsy [9]. Although the vast majority of children with COVID-19 have a favorable clinical course, pediatricians should be aware of the possibility of particular clinical manifestations such as Pediatric Multisystem Inflammatory Syndrome (PMIS) temporally associated with COVID-19, and now isolated cranial nerve palsy, which until then had been the prerogative of adults. This requires further vigilance for early and careful diagnosis and therapy.
Authors’ contributions
NE conceived the study, analyzed data and drafted the manuscript; EM, MG, LO, and NR critically revised the manuscript for intellectual content.
Consent for publication
Consent for publication has been received from the parents.
Funding
No funding sources.
Competing interests
None declared.
Ethical approval
Consent to participate has been received from the parents.
Acknowledgments
The authors wish to thank Prof. Mathieu NACHER from the INSERM U1424 of Cayenne Hospital, Rue des Flamboyants, BP 6006, 97306 Cayenne Cedex, French Guiana for his input and corrections.
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmermann P., Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39(5):355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Oliveira M.R., Lucena A.R.V.P., Higino T.M.M., Ventura C.V. Oculomotor nerve palsy in an asymptomatic child with COVID-19. J AAPOS. 2021;25(3):169–170. doi: 10.1016/j.jaapos.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belghmaidi S., Nassih H., Boutgayout S., et al. Third cranial nerve palsy presenting with unilateral diplopia and strabismus in a 24-year-old woman with COVID-19. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.925897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrantes F.J. Central nervous system targets and routes for SARS-CoV-2: current views and new hypotheses. ACS Chem Neurosci. 2020;11(18):2793–2803. doi: 10.1021/acschemneuro.0c00434. [DOI] [PubMed] [Google Scholar]
- 6.Rehmani R., Segan S., Maddika S.R., Lei Y.W., Broka A. Spectrum of neurologic & neuroimaging manifestation in COVID-19. Brain Behav Immun Health. 2021;13 doi: 10.1016/j.bbih.2021.100238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costello F., Dalakas M.C. Cranial neuropathies and COVID-19: neurotropism and autoimmunity. Neurology. 2020;95(5):195–196. doi: 10.1212/WNL.0000000000009921. [DOI] [PubMed] [Google Scholar]
- 8.Lyons C.J., Godoy F., ALQahtani E. Cranial nerve palsies in childhood. Eye (Lond) 2015;29(2):246–251. doi: 10.1038/eye.2014.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa A.L.F.A., Martins T.G.D.S., Martins D.G.D.S. Third cranial nerve palsy after a chikungunya virus infection. Strabismus. 2017;25(December (4)):172–175. doi: 10.1080/09273972.2017.1391851. [DOI] [PubMed] [Google Scholar]