Abstract
Vigorous exercise countermeasures in microgravity can largely attenuate muscular degeneration, albeit the extent of applied loading is key for the extent of muscle wasting. Running on the International Space Station is usually performed with maximum loads of 70% body weight (0.7 g). However, it has not been investigated how the reduced musculoskeletal loading affects muscle and series elastic element dynamics, and thereby force and power generation. Therefore, this study examined the effects of running on the vertical treadmill facility, a ground-based analog, at simulated 0.7 g on gastrocnemius medialis contractile behavior. The results reveal that fascicle−series elastic element behavior differs between simulated hypogravity and 1 g running. Whilst shorter peak series elastic element lengths at simulated 0.7 g appear to be the result of lower muscular and gravitational forces acting on it, increased fascicle lengths and decreased velocities could not be anticipated, but may inform the development of optimized running training in hypogravity. However, whether the alterations in contractile behavior precipitate musculoskeletal degeneration warrants further study.
Subject terms: Ultrasonography, Outcomes research, Physiology
Introduction
Astronauts exposed to a micro-g-force environment, often referred to as microgravity (μg), experience many physiological adaptations, including musculoskeletal deconditioning, with the plantar flexor muscles appearing particularly susceptible to atrophy1,2. To prevent these detrimental effects, the crewmembers of the International Space Station (ISS) perform daily exercise countermeasures, including treadmill running, cycling, and resistance training3. Due to the implementation of new exercise hardware and improvements of the in-flight exercise hardware and exercise prescriptions, μg-induced physiological deconditioning has been reduced, although variable inter-individual physiological responses to the exercise induced stimuli persist4,5.
For instance, a recent study investigating the plantar flexor muscles of two ISS crewmembers suggests that vigorous treadmill and resistive training reduces the decrements in muscle volume and lower limb strength and the deteriorations in muscle architecture6. Moreover, the muscle wasting seems to affect the organism’s systemic inflammatory/anti-inflammatory balance7, which highlights the requirement to safeguard musculoskeletal health in space. The extent of muscle wasting is likely related to the training volume (sets, repetition, and duration) and training intensity in particular with regard to the maximum external loading that can comfortably be applied during countermeasure exercises6.
ISS crewmembers that are part of U.S. Orbital Segment currently perform locomotion countermeasures on the T2 treadmill. On this treadmill, subject loading is currently provided via a harness system connected to a bungee assembly that is clipped in series with several carabiner clips. The applied harness load is usually an individual crew choice, mainly limited by increasing discomfort of the harness system at higher loads (A. Gerst, Personal Communication 2021, see Supplementary Reference). Running sessions are thus usually performed with ~70% of the equivalent body weight (BW) at 1g (g = 9.81 m s−2)3, resulting in lower peak ground reaction forces (~1.3 BW when running at 2.2 m s−1) compared to terrestrial running8. A ground-based analog to simulate hypogravity running on the ISS is the vertical treadmill facility (VTF), where subjects are suspended horizontally with graded “pull-down” forces toward a vertically mounted treadmill provided via a harness-based subject loading system9–11. Despite marginal differences in joint kinematics and ground reaction forces between running in actual μg (parabolic flight) vs. running in simulated μg (VTF), the latter is still regarded as a valid analog, even though it does not provide a 1:1 representation of running in actual µg since on the VTF the (vertically suspended) body and in particular the suspended limbs are still exposed to gravity12.
Running on the ISS or on ground-based hypogravity simulation systems is not only associated with reduced ground reaction forces but also with lower plantar load13–15. In addition, metabolic cost was found to be reduced during running in simulated hypogravity15–17. Furthermore, estimated ankle joint forces18 and peak ankle dorsiflexion and knee flexion as well as range of motion19 were reported to be reduced when running at different velocities (2.2‒3.5 m s−1) on a lower body positive pressure treadmill to simulate hypogravity.
In contrast, running with additional mass (120% of BW, equivalent to 1.2 g), was found to require more mechanical work at the ankle and knee joints20. Despite these changes in kinetic gait parameters, the overall gastrocnemius medialis (GM) muscle fascicle behavior and peak series elastic element (SEE) length were found to be largely preserved. Interestingly, essentially preserved fascicle−SEE behavior was also observed when walking with only 70% BW achieved by lower body positive pressure21. In 1 g, whilst changes in walking speed between 0.75 and 2.00 m s−1 have been shown to affect fascicle velocity (at the time of peak force), no effects were observed when changing running speed between 2.00 and 3.25 m s−1 22. Taken together, these findings make it difficult to predict if and how the neuromuscular system modulates fascicle‒SEE dynamics when running in simulated hypogravity.
When reducing the loading level, gait transitions occur at a slower preferred walk-to-run transition speed (PTS) but at a similar Froude number, a dimensionless number embedding gait speed, leg lengths, and gravitational acceleration23–25. Thus, to run at “dynamically similar” speeds (i.e., at a similar running speed relative to the PTS) in hypogravity, one must run at the same Froude number, which means a reduction in absolute running speed. However, the influence of hypogravity running at a dynamically similar speed on the interaction between the contractile and series elastic elements within GM’s muscle‒tendon unit (MTU) has not been investigated.
Therefore, the aim of this study was to investigate, via ultrasonography, GM fascicle−SEE behavior in addition to joint kinematics during running with 125% of the PTS at a simulated hypogravity level of 0.7 g (on the VTF) versus 1 g. We hypothesized that fascicle−SEE behavior will be preserved when running in simulated hypogravity at 125% of the PTS.
Results
Kinetic and spatio-temporal parameters
Running speeds in this study were selected to correspond to 125% of the participants’ PTS, resulting in average running speeds of 2.62 ± 0.08 m s−1 at 1 g and 2.23 ± 0.07 m s−1 at simulated 0.7 g. Participants running on the VTF at simulated hypogravity of 0.7 g were subjected to lower (t(7) = 11.465, P < 0.001, dz = −4.1) mean loading levels than at 1 g, corresponding to 63.4 ± 4.8% (mean ± standard deviation) of the loading levels determined during running on a conventional treadmill. Peak plantar forces (t(7) = 9.070, P < 0.001, dz = −3.2) were reduced by 633.3 ± 197.5 N (95% confidence interval (CI), −798.4 to −468.2) at simulated 0.7 g compared to 1 g (Fig. 1a). In contrast, ground-contact times (t(7) = 5.597, P < 0.001, dz = 2.0) were increased by 0.05 ± 0.02 s (95% CI, 0.03 to 0.07) when running at simulated 0.7 g (Table 1). Accordingly, cadence (t(7) = 5.442, P = 0.001, dz = −1.9) was decreased by 10.1 ± 5.2 steps min−1 (95% CI, −14.4 to −5.7) at simulated 0.7 g compared to 1g.
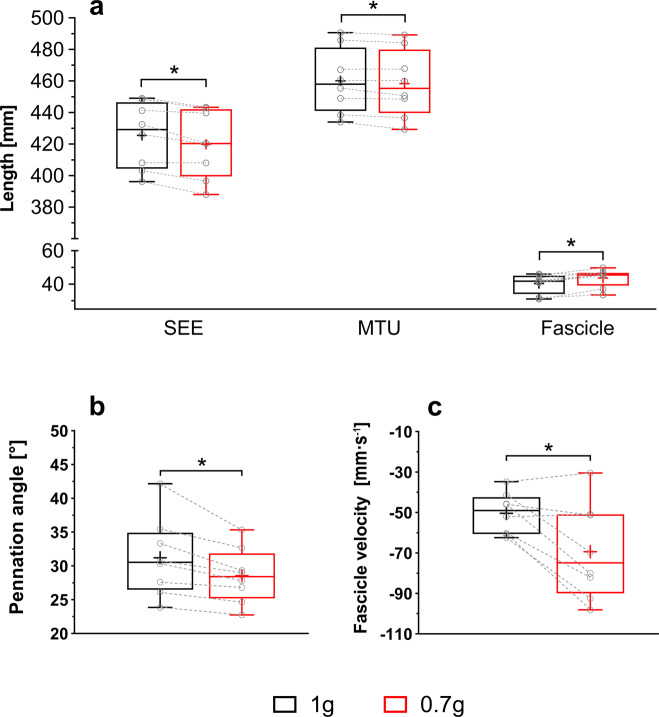
Fig. 1. Kinetic, kinematic, and GM fascicle‒SEE parameters during the stance phase of running at 1 g and simulated 0.7 g.
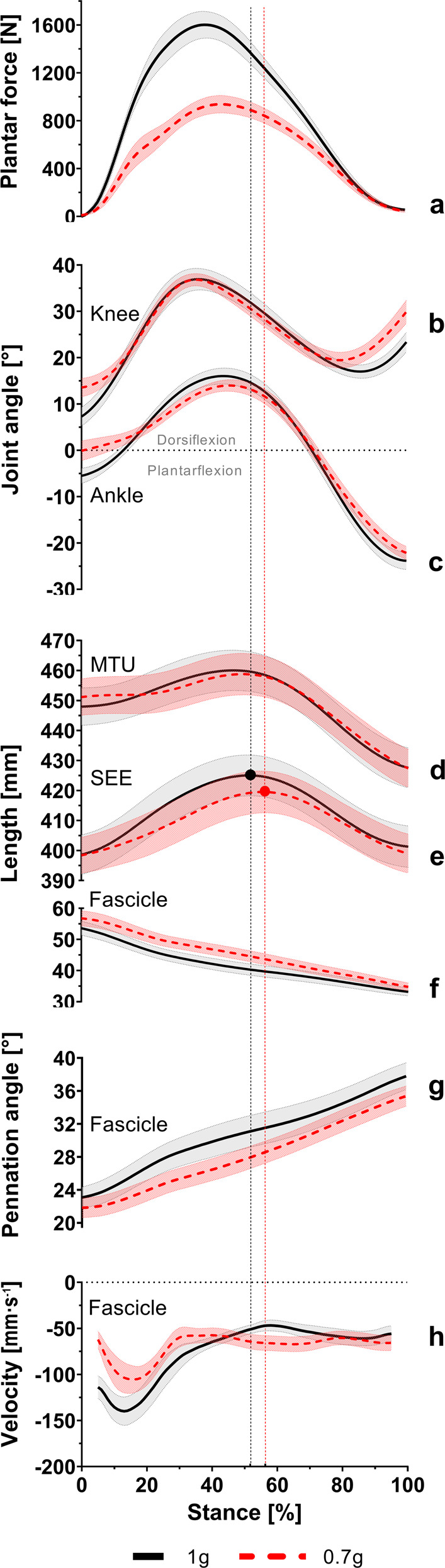
Participants’ average (mean ± standard error of the mean) patterns of plantar forces (a), knee (b) and ankle (c) joint angles, and MTU (d) and SEE (e) lengths as well as muscle fascicle length (f), pennation angle (g), and velocity (h) change during the stance phase of running at 1 g (black line) and simulated 0.7 g (red dashed line). The vertical lines mark the peak SEE length achieved at 1 g (black) and simulated 0.7 g (red). n = 8 participants.
Table 1.
Spatio-temporal and kinematic parameters while participants ran at 125% of their preferred walk-to-run transition speed at 1 g and simulated 0.7 g.
| Parameters | 1 g | 0.7 g | P value |
|---|---|---|---|
| Ground-contact time [s] | 0.30 ± 0.04 | 0.35 ± 0.04a | <0.001 |
| Cadence [steps min−1] | 83.25 ± 5.90 | 73.19 ± 4.21a | 0.001 |
| Ankle joint range of motion [°] | 40.18 ± 7.72 | 36.44 ± 5.99 | 0.155 |
| Knee joint range of motion [°] | 30.03 ± 5.15 | 25.69 ± 3.65a | 0.018 |
| Ankle dorsiflexion [°] | 21.91 ± 3.92 | 15.11 ± 5.02a | <0.001 |
| Knee flexion [°] | 29.98 ± 5.20 | 24.06 ± 4.99a | 0.003 |
| Ankle joint angle at peak SEE length [°] | 15.19 ± 5.09 | 12.81 ± 4.06a | 0.016 |
| Knee joint angle at peak SEE length [°] | 31.92 ± 6.25 | 28.63 ± 4.75a | 0.030 |
Data are presented as mean ± standard deviation.
asignificantly different (two-tailed paired t-test) from 1 g (P ≤ 0.05). Peak SEE length at simulated 0.7 g and 1 g occurred at 57 ± 4% and 52 ± 7% of stance, respectively. n = 8 participants
Joint kinematics
The participant’s knee and ankle joint movement patterns during running at simulated 0.7 g vs. 1 g are displayed in Fig. 1b,c, respectively.
Knee joint range of motion (t(7) = 3.057, P = 0.018, dz = −1.1) was lower by 4.3 ± 4.0° (95% CI, −7.7 to −1.0) at simulated 0.7 g compared to 1 g, whereas ankle joint range of motion (t(7) = 1.595, P = 0.155, dz = −0.6) was not affected by unloading, with a mean difference of 3.7 ± 6.6° (95% CI, −9.3 to 1.8) (Table 1). Furthermore, ankle dorsiflexion (t(7) = 6.629, P < 0.001, dz = −2.3) and knee flexion (t(7) = 4.503, P = 0.003, dz = −1.6) during the first half of the stance phase were both lower at simulated 0.7 g, by 6.8 ± 2.9° (95% CI, −9.2 to −4.4) and 5.9 ± 3.7° (95% CI, −9.0 to −2.8), respectively (Table 1). At the time of peak SEE length, both ankle (t(7) = 3.144, P = 0.016, dz = −1.1) and knee (t(7) = 2.706, P = 0.030, dz = −1.0) joint angles were lower during running at simulated 0.7 g, by 3.0 ± 2.7° (95% CI, −5.3 to −0.7) and 3.3 ± 3.4° (95% CI, −6.2 to −0.4), respectively (Table 1).
GM muscle and SEE parameters
Temporal differences in muscle−SEE parameters within the single stance phase between running at simulated 0.7 g and 1 g are depicted in Fig. 1d−h.
Loading level had no effect upon overall fascicle shortening (t(7) = 1.646, P = 0.144, dz = 0.6), with a mean difference of 1.6 ± 2.7 mm (95% CI, −0.7 to 3.9). However, at the time of peak SEE length, muscle fascicles operated at a longer length (∆ = 3.3 ± 1.9 mm, 95% CI 1.7 to 4.9, t(7) = 4.922, P = 0.002, dz = 1.7, Fig. 2a), smaller pennation angle (∆ = −2.7 ± 2.0°, 95% CI −4.3 to −1.0, t(7) = 3.789, P = 0.007, dz = −1.3, Fig. 2b), and faster shortening velocity (∆ = 19.0 ± 16.6 mm s−1, 95% CI −32.9 to −5.1, t(7) = 3.230, P = 0.014, dz = −1.1, Fig. 2c) at simulated 0.7 g compared to 1 g.
Fig. 2. GM fascicle‒SEE behavior at the time of peak SEE length when running at 1 g and simulated 0.7 g.
SEE length (a, left), MTU length (a, middle), fascicle length (a, right), pennation angle (b), and fascicle velocity (c) at the time of the peak SEE length differ between running at 1 g (black box) and simulated 0.7 g (red box). The lower and upper parts of the box represent the first and third quartile, respectively. The length of the whisker represents the minimum and maximum values. The horizontal line in the box represents the statistical median of the sample; + the mean of the sample; ○ individual data points; * significantly different (two-tailed paired t-test) from 1 g (P ≤ 0.05). n = 8 participants.
The peak SEE length (t(7) = 4.315, P = 0.004, dz = −1.5) and the MTU length at the time of peak SEE length (t(7) = 2.547, P = 0.038, dz = −0.9) were shorter during running at simulated 0.7 g compared to 1 g, by 5.6 ± 3.7 mm (95% CI, −8.7 to −2.5) and 1.8 ± 2.0 mm (95% CI, −3.5 to −0.1), respectively (Fig. 2a).
The time at which peak SEE length was achieved (t(7) = 1.860, P = 0.105, dz = 0.7) did not differ between loading levels although peak SEE length was attained slightly later (56.9 ± 4.1% vs. 51.5 ± 7.5% of stance phase, 95% CI, −1.5 to 12.2) at simulated 0.7 g compared to 1 g.
Discussion
Running on the VTF with 125% of the PTS at simulated 0.7 g vs. 1 g induced peak plantar forces corresponding to ~1.3 BW, which are similar to those observed when running with bungee loading on board the ISS8. The main findings of the present study were that simulated 0.7 g running increased ground-contact time, reduced cadence, and lowered ankle dorsiflexion and knee flexion at the time of peak SEE length. Concurrently, GM fascicles operated at longer lengths, smaller pennation angles, and faster shortening velocities, whilst MTU and SEE lengths were shorter.
Hypogravity (0.7 g) running induced a significant reduction in peak plantar forces (−39.3%), whilst ground-contact times were slightly increased, resulting in a greater time available for the neuromuscular system to adopt GM’s contractile behavior. In addition, ankle and knee joint angles at the time of the peak SEE length were significantly reduced, consistent with a previous lower body positive pressure study reporting that participants adapted their running pattern when the loading level was <0.8 g19. Smaller ankle joint flexions were also observed in studies investigating running on a treadmill equipped with a vertical body weight support system26 or a subject loading system as used during parabolic flights10. Changes in GM’s MTU behavior may thus result from an altered movement pattern induced by prolonged stance phase durations and altered joint kinematics. For instance, lower ankle dorsiflexion at peak SEE length may compensate for the less-flexed knee joint, significantly shortening MTUs.
Furthermore, the present study indicates that at the time of peak SEE length, where the force acting on the SEE is at its greatest, GM fascicles are less contracted compared to 1 g running. Simultaneously, the pennation angle was found to be significantly smaller, potentially facilitating fascicles to operate at longer lengths. However, this contrasts with preliminary findings indicating that overall fascicle behavior is relatively stable against a reduction or increase in loading by 30% BW21 or 20% BW20, respectively.
During 1 g running, fascicles barely reach the plateau region of the force−length relationship27,28, thereby limiting their ability to generate force. Thus, the increase in fascicle length observed during simulated (0.7 g) hypogravity running may enable the GM to operate closer to its optimum length, thereby enhancing force-generation ability. By using a normalized active force−length relationship represented by a Gaussian function29 and an optimum GM fascicle length of 51.0 ± 9.8 mm (99% confidence interval, 45.0–58.0 mm), as determined by a cadaveric study30, we estimate an increase in GM’s force-generation ability by as much as 6% when running at simulated 0.7 g. Moreover, a shift in the fascicles’ operating range toward longer lengths may result in an increased strain on the z-disks, potentially preserving or increasing the number of sarcomeres in-series, which in turn may be beneficial for muscle mass preservation31.
On the other hand, fascicle-shortening velocity at peak SEE length was found to be significantly increased during running at simulated 0.7 g. This may result in less-favorable contractile conditions as fascicles are less able to generate force with increasing speed of contraction32. In fact, it has been reported that fascicle-shortening velocity is a major determinant of the preferred walk-to-run transition by improving fascicles’ contractile conditions after switching gait to counteract impaired muscle force production22,33. Indeed, a change in fascicle velocity is noted to have a greater impact on muscular performance than a change in fascicle length, especially at high running speeds34.
Interestingly, at 1 g, fascicles operated at a sub-optimal length but at a slower and more optimal shortening velocity for generating force. Assuming that fascicle neuro-motor control is optimally adapted to 1 g, an increase in fascicle velocity and thus induction of less-favorable contractile conditions in simulated 0.7 g may outweigh the benefits from an increase in fascicle length. In fact, the significantly shorter peak SEE length observed during simulated 0.7 g running may be the direct result of lower muscular forces acting on the SEE. In addition, according to the MTU’s stretch-shortening cycle, the smaller SEE strain should result in less stored and thus released elastic energy. However, as an exact replication of running in actual microgravity is not possible using the VTF, the present results strongly suggest but do not prove that running on the ISS induces a significant change in muscle‒tendon dynamics in response to lower musculoskeletal loading.
Although, given a largely preserved fascicle behavior when running with increased loading20, one might speculate that when the musculoskeletal loading is increased, it is more important to preserve the well-adapted contractile conditions to favor economical force production.
It has been proposed that the provision of (non-standardized) external force loading while exercising in μg may underlie the observation of variable muscular degeneration during long-term spaceflight6. The present (in vivo) study supports this notion as significant alterations in GM fascicle−SEE outcome parameters were observed between running at simulated 0.7 g on a ground-based analog and 1 g. Such alterations point to functional adaptations in response to a reduced locomotor demand during hypogravity running, involving not only lower gravitational but also muscular forces that may precipitate musculoskeletal degeneration35. Thus, it appears that the consequences of hypogravity running are not limited to a mere reduction in mechanical loading but also to an altered contractile behavior, which could affect the muscle’s work capacity upon the return to daily activities in a 1 g environment. The longer fascicles may be beneficial to preserve muscle mass but may also result in long-term adaptations in optimal fascicle length that are no longer functional for the requirements on Earth and may require specific attention during the rehabilitation phase upon return to Earth’s 1 g environment31,36.
To increase the mechanical loading on the MTU during hypogravity running, and hence to induce muscle fascicle−SEE behavior that is similar to that in 1 g conditions (to ensure the stimuli exerted on the muscle remain the same), it would be required that the harness applies a higher external force to the body than what is typically chosen by crewmembers. This force is mainly limited by considerable discomfort of the harness at higher loads, especially during gait cycle phases with maximum stretch of the bungee assembly3,37. Moreover, whether the provision of full BW loading is actually optimal, is subject to further research. An alternative approach may be to increase running speed8,15,38, which has been shown to augment maximum plantar force13. Interestingly, many ISS crewmembers appear to intuitively increase their running speed to achieve a perceived workout intensity that is similar to what they are used to on Earth despite the reduced external loading (A. Gerst, Personal Communication 2021, see Supplementary Reference). This sensation may relate to the fact that increasing running speed reduces the ground-contact time but requires a higher force production, evident by greater plantar flexor muscle activity20. However, further research is needed to investigate the interaction between loading level and running speeds on fascicle−SEE behavior in vivo. Another possibility to mitigate μg-induced muscle wasting would be to increase the volume of in-flight treadmill running. However, one goal of optimizing exercise countermeasures in space is the reduction of crew time spent on exercise whilst maintaining or improving the effectiveness of currently prescribed exercise countermeasures39.
To conclude, simulated 0.7 g running significantly alters fascicle-SEE interaction. For instance, a shorter peak SEE length seems to be the result of lower muscular forces acting on it. However, to answer the question as to whether there is a loading and running speed combination above which muscular deconditioning is prevented, additional measurements of torque and neuromuscular activation are required to estimate the effects of (various-level) hypogravity running on GM strain and resultant contractility/excitability. Such knowledge is crucial to inform the development of optimized running training in hypogravity but may also inform the mechanisms of contractile behavior regulation on Earth.
Data processing
For each participant and each outcome measure at each loading level, the first eight consecutive left foot stance phases (from the 30 s of data recording) were analyzed using a custom-made script (MATLAB R2018a, MathWorks, Inc., Natick, United States). Fascicle length and pennation angle data were smoothed with a five-point moving average, whereas electrogoniometer signals were smoothed with a fifth-order Butterworth low-pass filter at a 10-Hz cut-off frequency. Muscle fascicle velocities were calculated as the time derivative of the respective length using the central difference method40. Data were time-normalized by being resampled to 101 data points per stance phase.
To estimate the loading achieved on the VTF, average simulated gravity levels over the stance phase were calculated via plantar force and impulse and expressed as percentage of the average gravity levels determined similarly during running on a conventional treadmill. Peak plantar force was defined as the maximum force value observed during stance. Ground-contact times were calculated as the time between left foot touchdown and toe-off. Cadence was defined as steps (duration from touchdown to the next ipsilateral touchdown) per minute. Ankle and knee joint angles as well as SEE-, fascicle-, and MTU lengths in addition to fascicle pennation angle and velocity were determined at the time of the peak SEE length, where the force acting on the SEE is at its greatest. Overall fascicle shortening was calculated by subtracting the minimum from the maximum fascicle length. Ankle and knee joint ranges of motion were defined as the differences between their respective minimum and maximum joint angles. The differences in knee and ankle joint angles between touchdown to the time of first local maximum and maximum dorsiflexion were defined as knee flexion and ankle dorsiflexion, respectively.
Statistical analysis
Data distribution for all outcome measures was assessed using the Shapiro−Wilk normality test. As normal distribution was confirmed for all outcome measures, a two-tailed paired t-test (n = 8 participants) was performed to test for significant differences in joint kinematics and fascicle‒SEE outcomes between loading levels (1 g vs. simulated 0.7 g). All statistical analysis was performed in GraphPad Prism (v 7.04) with α set to 0.05. Data is reported as mean (±standard deviation). Effect sizes (dz) were calculated using the G*Power software version 3.1.9.441. Thresholds of 0.2, 0.5, and 0.8 were defined as small, moderate, and large effects between the two comparison groups42.
Methods
Participants
Eight healthy male volunteers (31.9 ± 4.7 years, 178.4 ± 5.7 cm heights, 94 ± 6 cm leg lengths, and 73.5 ± 7.3 kg body masses) provided informed written consent to participate in this study, which received approval from the “Ärztekammer Nordrhein” Ethical Committee of Düsseldorf, Germany, in accordance with the ethical standards of the 1964 Helsinki declaration. All participants were examined medically. Exclusion criteria included cardiovascular, musculoskeletal, or neurological diseases and/or surgery within 2 years prior to participation.
Study design and experimental protocol
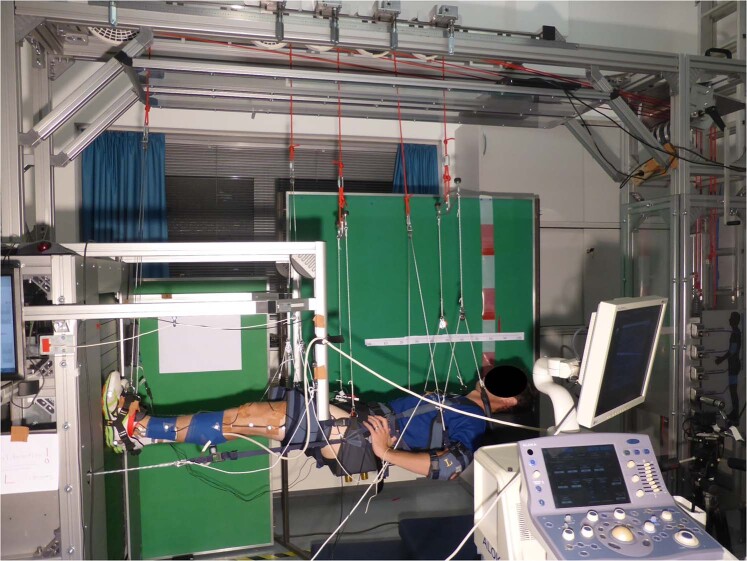
Participants attended the laboratory on a single occasion and familiarized themselves with running on the vertical treadmill facility (VTF; Arsalis, Glabais, Belgium, Fig. 3) at their predefined running speed (125% PTS). After achieving a stable gait, 30 s were recorded while they ran on the VTF at simulated 0.7 g in addition to on a conventional treadmill at 1 g.
Fig. 3. VTF experimental set-up.
Participant being suspended horizontally on the vertical treadmill facility (VTF) with an ultrasound transducer attached to the mid-belly of the GM muscle and electrogoniometers to record knee and ankle joint angles. Photo credit Charlotte Richter; participant provided written informed consent to publish this photo.
The VTF comprises a customized, motorized treadmill (Woodway, Waukesha, WI, USA) mounted vertically onto a chassis with an overhead suspension system, allowing supine suspension of the participant using a customized cradle and harness. Fabric cuffs attached to cords support the participants’ torso and pelvis and each foot, thigh, and arm. An adjustable piston-based loading system generates a constant controllable force pulling the participant toward the treadmill belt via fixation to the harness at the pelvis (Fig. 3). Written informed consent was obtained for publication of this photograph (Fig. 3).
At both loading levels, running speeds were defined as 125% of the PTS to obtain mechanically equivalent running speeds. PTS, expressed as a Froude number (PTSFR), was estimated by fitting an exponential regression equation with a least-squares method (r2 = 0.99) to the data provided by Kram, et al.23 using the resulting acceleration (a) as the independent variable. Hence, for a = 0.7 g, a PTSFR value of 0.49 was obtained. By accounting for the participants’ leg length (l), the individual was determined for each participant; moreover, adding 25% to this PTS resulted in running speeds of 2.62 ± 0.08 m s−1 at 1 g and 2.23 ± 0.07 m s−1 at simulated 0.7 g.
Data collection
Joint kinematics
Knee and ankle joint angles were recorded using a twin-axis (Penny and Giles Biometrics Ltd., Blackwood Gwent, UK) and a custom-made 2D-electrogoniometer, respectively. The end blocks of the knee electrogoniometer were positioned along the line from the greater trochanter to the lateral femur epicondyle and from the lateral femur epicondyle to the lateral malleolus. The end blocks of the ankle electrogoniometer were placed along the line from the lateral femur epicondyle to the lateral malleolus and from the lateral malleolus to the most distal end of the fifth metatarsal bone. Before each running trial, the goniometers were zeroed when in the anatomical neutral position (standing). Electrogoniometry data were sampled at 1500 Hz via the TeleMyo 2400 G2 Telemetry System (Noraxon USA., Inc., Scottsdale, USA) using the MyoResearch XP software (Master Edition 1.08.16). Electrogoniometry has been revealed to produce reliable and reproducible knee and ankle joint kinematics43–45 and has already been used during running with reduced loading19,26,46,47.
Spatio-temporal parameters
Shoe insoles (novel GmbH, loadsol® version 1.4.60, Munich, Germany) were used to measure plantar forces during running and hence to determine the stance phase. Touchdown and toe-off were automatically detected from the signal acquired with a sampling rate of 83 Hz via a custom-made script (MATLAB R2018a, MathWorks, Inc., Natick, United States) using a 20 N force threshold for 0.1 s. Insole and electrogoniometer signals were time-synchronized via recording of a rectangular TTL pulse generated by pressing on a custom-made pedal before each running trial.
GM muscle fascicle length and pennation angle
Real-time B-mode ultrasonography (Prosound α7, ALOKA, Tokyo, Japan) was used to image the GM fascicles at a frame rate of 73 Hz. The T-shaped 6-cm linear array transducer (13 MHz), placed inside a custom-made cast to prevent shifting, was positioned at the intersection of the mediolateral and proximodistal midline over the GM mid-belly and secured with elastic Velcro. The ultrasound recordings and electrogoniometer signals were time-synchronized via a rectangular TTL pulse generated by a hand switch recorded on the electrocardiography channel of the ultrasound device and the MyoResearch XP software. Ultrasonography has been frequently used in dynamic conditions48 and is regarded as a reliable method to quantify fascicle architecture. Fascicle length and pennation angle show good reproducibility not only within sessions but also between sessions49,50.
A semi-automatic tracking algorithm (UltraTrack Software, version 4.2)51 was used to quantify GM fascicle lengths and pennation angles. Manual correction of the digitized fascicle and the deep aponeurosis, defined as a second fascicle, was performed where appropriate. Fascicle length was defined as the distance between the insertions to the superficial and deep aponeurosis parallel to the lines of collagenous tissue (Fig. 4). If the transducer’s field of view was too small to display the entire fascicle, the missing portion was extrapolated, assuming that the fascicle and the aponeuroses extended linearly. The pennation angle (φ) was defined as the angle between the fascicle and the deep aponeurosis (Fig. 4).
Fig. 4. Ultrasound image of the gastrocnemius medialis with schematic representation of the extracted fascicle parameters.
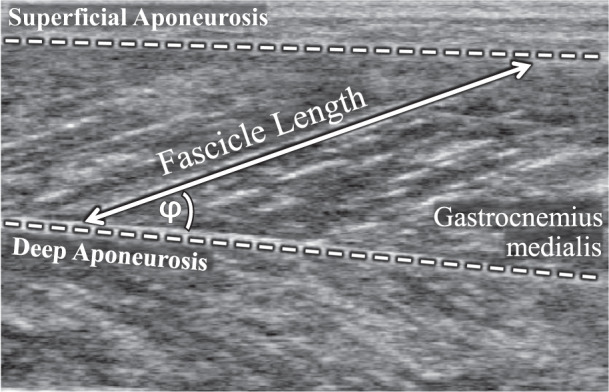
The pennation angle (φ) of the muscle fascicles (double-headed arrow) is defined with respect to the deep aponeurosis (lower dashed line). Fascicle length is measured as the length following the pennation between the deep and the superficial (upper dashed line) aponeuroses.
SEE and MTU length
To calculate SEE length (Achilles tendon, aponeuroses, and proximal tendon) on the basis of an MTU model52, muscle fascicle lengths multiplied by the cosine of their pennation angles were subtracted from the MTU lengths. MTU length was calculated via a multiple linear regression equation53 using the participant’s shank length as well as their knee and ankle joint angles.
Supplementary information
Acknowledgements
This study was supported with funding from the ESA Space Medicine Team (HRE-OM) of the European Astronaut Centre in Cologne, Germany, and the University of Applied Science Aachen obtained funding from the Federal Ministry for Economic Affairs and Energy (50WB1728). ESA provided the VTF used in this study.
Author contributions
B.B., T.W., and D.A.G. conceptualized research. B.B., J.A., T.W., K.N.M., J.R., D.A.G., and K.A. designed the research. C.R., B.B., B.S., J.A., and A.S. acquired the data; C.R., B.B., B.S., A.S., and K.A. analyzed the data; and C.R., B.B., J.R., D.A.G., and K.A. interpreted the data. C.R. and D.A.G. drafted the manuscript; B.B., B.S., J.A., T.W., K.N.M., J.R., D.A.G., and K.A. revised the manuscript. All authors approved the manuscript and account for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The data that support the findings of this are available from the corresponding author upon reasonable request.
Code availability
A custom-made MATLAB code (MathWorks, Inc., Natick, United States) used for data analysis is available from the corresponding author upon reasonable request.
Competing interests
Authors Dr. David A. Green and Dr. Tobias Weber are employed by KBR on behalf of the European Space Agency. All authors declare that the research was conducted in the absence of any commercial and financial relationships or non-financial interests that could be construed as a potential conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41526-021-00155-7.
References
- 1.Narici MV, de Boer MD. Disuse of the musculo-skeletal system in space and on earth. Eur. J. Appl. Physiol. 2011;111:403–420. doi: 10.1007/s00421-010-1556-x. [DOI] [PubMed] [Google Scholar]
- 2.Gopalakrishnan R, et al. Muscle volume, strength, endurance, and exercise loads during 6-month missions in space. Aviat. Space Environ. Med. 2010;81:91–104. doi: 10.3357/ASEM.2583.2010. [DOI] [PubMed] [Google Scholar]
- 3.Petersen N, et al. Exercise in space: the European Space Agency approach to in-flight exercise countermeasures for long-duration missions on ISS. Extrem. Physiol. Med. 2016;5:1–13. doi: 10.1186/s13728-016-0050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott, J. P., Weber, T. & Green, D. A. Introduction to the frontiers research topic: optimization of exercise countermeasures for human space flight–lessons from terrestrial physiology and operational considerations. Front. Physiol.10.3389/fphys.2019.00173 (2019). [DOI] [PMC free article] [PubMed]
- 5.Scott JPR, Kramer A, Petersen N, Green DA. The role of long-term head-down bed rest in understanding inter-individual variation in response to the spaceflight environment: a perspective review. Front. Physiol. 2021;12:9. doi: 10.3389/fphys.2021.614619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rittweger J, et al. Sarcolab pilot study into skeletal muscle’s adaptation to long-term spaceflight. npj Microgravity. 2018;4:18. doi: 10.1038/s41526-018-0052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capri M, et al. Recovery from 6-month spaceflight at the International Space Station: muscle-related stress into a proinflammatory setting. FASEB J. 2019;33:5168–5180. doi: 10.1096/fj.201801625R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Witt JK, Ploutz-Snyder LL. Ground reaction forces during treadmill running in microgravity. J. Biomech. 2014;47:2339–2347. doi: 10.1016/j.jbiomech.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 9.De Witt, J. K. et al. Kinematic and Electromyographic Evaluation of Locomotion on the Enhanced Zero-gravity Locomotion Simulator: A Comparison of External Loading Mechanisms. TP-2007-214764, 1-27. (US: National Aeronautics and Space Administration, 2008).
- 10.Gosseye TP, Willems PA, Heglund NC. Biomechanical analysis of running in weightlessness on a treadmill equipped with a subject loading system. Eur. J. Appl. Physiol. 2010;110:709–728. doi: 10.1007/s00421-010-1549-9. [DOI] [PubMed] [Google Scholar]
- 11.Genc KO, Mandes VE, Cavanagh PR. Gravity replacement during running in simulated microgravity. Aviat. Space Environ. Med. 2006;77:1117–1124. [PubMed] [Google Scholar]
- 12.De Witt JK, et al. Locomotion in simulated and real microgravity: horizontal suspension vs. parabolic flight. Aviat. Space Environ. Med. 2010;81:1092–1099. doi: 10.3357/ASEM.2413.2010. [DOI] [PubMed] [Google Scholar]
- 13.Thomson A, Einarsson E, Witvrouw E, Whiteley R. Running speed increases plantar load more than per cent body weight on an AlterG® treadmill. J. Sports Sci. 2017;35:277–282. doi: 10.1080/02640414.2016.1163401. [DOI] [PubMed] [Google Scholar]
- 14.Genc K, et al. Foot forces during exercise on the International Space Station. J. Biomech. 2010;43:3020–3027. doi: 10.1016/j.jbiomech.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Grabowski AM, Kram R. Effects of velocity and weight support on ground reaction forces and metabolic power during running. J. Appl. Biomech. 2008;24:288–297. doi: 10.1123/jab.24.3.288. [DOI] [PubMed] [Google Scholar]
- 16.Teunissen LP, Grabowski A, Kram R. Effects of independently altering body weight and body mass on the metabolic cost of running. J. Exp. Biol. 2007;210:4418–4427. doi: 10.1242/jeb.004481. [DOI] [PubMed] [Google Scholar]
- 17.Farley CT, McMahon TA. Energetics of walking and running: insights from simulated reduced-gravity experiments. J. Appl. Physiol. 1992;73:2709–2712. doi: 10.1152/jappl.1992.73.6.2709. [DOI] [PubMed] [Google Scholar]
- 18.Jensen BR, Hovgaard-Hansen L, Cappelen KL. Muscle activation and estimated relative joint force during running with weight support on a lower-body positive-pressure treadmill. J. Appl. Biomech. 2016;32:335–341. doi: 10.1123/jab.2015-0075. [DOI] [PubMed] [Google Scholar]
- 19.Neal M, Fleming N, Eberman L, Games K, Vaughan J. Effect of body-weight-support running on lower-limb biomechanics. J. Orthop. Sports Phys. Ther. 2016;46:784–793. doi: 10.2519/jospt.2016.6503. [DOI] [PubMed] [Google Scholar]
- 20.Werkhausen, A., Cronin, N. J., Albracht, K., Bojsen-Møller, J. & Seynnes, O. R. Distinct muscle-tendon interaction during running at different speeds and in different loading conditions. J. Appl. Physiol.10.1152/japplphysiol.00710.2018 (2019). [DOI] [PubMed]
- 21.Richter C, et al. Gastrocnemius medialis contractile behavior is preserved during 30% body weight supported gait training. Front. Sports Act. Living. 2020;2:204. doi: 10.3389/fspor.2020.614559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farris DJ, Sawicki GS. Human medial gastrocnemius force-velocity behavior shifts with locomotion speed and gait. Proc. Natl Acad. Sci. USA. 2012;109:977–982. doi: 10.1073/pnas.1107972109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kram R, Domingo A, Ferris DP. Effect of reduced gravity on the preferred walk-run transition speed. J. Exp. Biol. 1997;200:821–826. doi: 10.1242/jeb.200.4.821. [DOI] [PubMed] [Google Scholar]
- 24.Labini FS, Ivanenko YP, Cappellini G, Gravano S, Lacquaniti F. Smooth changes in the EMG patterns during gait transitions under body weight unloading. J. Neurophysiol. 2011;106:1525–1536. doi: 10.1152/jn.00160.2011. [DOI] [PubMed] [Google Scholar]
- 25.Minetti AE. Invariant aspects of human locomotion in different gravitational environments. Acta Astronaut. 2001;49:191–198. doi: 10.1016/S0094-5765(01)00098-4. [DOI] [PubMed] [Google Scholar]
- 26.Ferris DP, Aagaard P, Simonsen EB, Farley CT, Dyhre-Poulsen P. Soleus H-reflex gain in humans walking and running under simulated reduced gravity. J. Physiol. 2001;530:167–180. doi: 10.1111/j.1469-7793.2001.0167m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishikawa M, Pakaslahti J, Komi P. Medial gastrocnemius muscle behavior during human running and walking. Gait Posture. 2007;25:380–384. doi: 10.1016/j.gaitpost.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Lichtwark G, Bougoulias K, Wilson A. Muscle fascicle and series elastic element length changes along the length of the human gastrocnemius during walking and running. J. Biomech. 2007;40:157–164. doi: 10.1016/j.jbiomech.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 29.Thelen DG. Adjustment of muscle mechanics model parameters to simulate dynamic contractions in older adults. J. Biomech. Eng. 2003;125:70–77. doi: 10.1115/1.1531112. [DOI] [PubMed] [Google Scholar]
- 30.Ward SR, Eng CM, Smallwood LH, Lieber RL. Are current measurements of lower extremity muscle architecture accurate? Clin. Orthop. Relat. Res. 2009;467:1074–1082. doi: 10.1007/s11999-008-0594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams PE, Goldspink G. The effect of immobilization on the longitudinal growth of striated muscle fibres. J. Anat. 1973;116:45. [PMC free article] [PubMed] [Google Scholar]
- 32.Hill AV. The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. Lond. B. 1938;126:136–195. doi: 10.1098/rspb.1938.0050. [DOI] [Google Scholar]
- 33.Neptune RR, Sasaki K. Ankle plantar flexor force production is an important determinant of the preferred walk-to-run transition speed. J. Exp. Biol. 2005;208:799–808. doi: 10.1242/jeb.01435. [DOI] [PubMed] [Google Scholar]
- 34.Miller RH, Umberger BR, Caldwell GE. Limitations to maximum sprinting speed imposed by muscle mechanical properties. J. Biomech. 2012;45:1092–1097. doi: 10.1016/j.jbiomech.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 35.Kohrt WM, Barry DW, Schwartz RS. Muscle forces or gravity: what predominates mechanical loading on bone? Med. Sci. Sports Exerc. 2009;41:2050. doi: 10.1249/MSS.0b013e3181a8c717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herzog W, Guimaraes A, Anton M, Carter-Erdman K. Moment-length relations of rectus femoris muscles of speed skaters/cyclists and runners. Med. Sci. Sports Exerc. 1991;23:1289–1296. [PubMed] [Google Scholar]
- 37.De Witt JK, Schaffner G, Ploutz-Snyder LL. Bungee force level, stiffness, and variation during treadmill locomotion in simulated microgravity. Aviat. Space Environ. Med. 2014;85:449–455. doi: 10.3357/ASEM.3217.2014. [DOI] [PubMed] [Google Scholar]
- 38.Fomina E, Savinkina A, Yarmanova E. Ground reaction force values in cosmonauts during locomotor exercises on board the International Space Station. Hum. Physiol. 2017;43:542–548. doi: 10.1134/S0362119717050048. [DOI] [Google Scholar]
- 39.Weber, T., Scott, J. P. R. & Green, D. A. Optimization of Exercise Countermeasures for Human Space Flight – Lessons from Terrestrial Physiology and Operational Implementation (Frontiers Media SA, 2020). [DOI] [PMC free article] [PubMed]
- 40.Robertson, G. E., Caldwell, G. E., Hamill, J., Kamen, G. & Whittlesey, S. Research Methods In Biomechanics (Human kinetics, 2013).
- 41.Faul F, Erdfelder E, Lang A-G, Buchner A. G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 42.Cohen, J. Statistical Power Analysis For The Behavioral Sciences (Academic, 1988).
- 43.Bronner S, Agraharasamakulam S, Ojofeitimi S. Reliability and validity of electrogoniometry measurement of lower extremity movement. J. Med. Eng. Technol. 2010;34:232–242. doi: 10.3109/03091900903580512. [DOI] [PubMed] [Google Scholar]
- 44.Piriyaprasarth P, Morris ME, Winter A, Bialocerkowski AE. The reliability of knee joint position testing using electrogoniometry. BMC Musculoskelet. Disord. 2008;9:6. doi: 10.1186/1471-2474-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urwin SG, Kader DF, Caplan N, Gibson ASC, Stewart S. Validation of an electrogoniometry system as a measure of knee kinematics during activities of daily living. J. Musculoskelet. Res. 2013;16:1350005. doi: 10.1142/S021895771350005X. [DOI] [Google Scholar]
- 46.Liebenberg J, et al. Determination of muscle activity during running at reduced body weight. J. Sports Sci. 2011;29:207–214. doi: 10.1080/02640414.2010.534806. [DOI] [PubMed] [Google Scholar]
- 47.Mercer JA, Applequist BC, Masumoto K. Muscle activity while running at 20%-50% of normal body weight. Res. Sports Med. 2013;21:217–228. doi: 10.1080/15438627.2013.792084. [DOI] [PubMed] [Google Scholar]
- 48.Cronin NJ, Lichtwark G. The use of ultrasound to study muscle-tendon function in human posture and locomotion. Gait Posture. 2013;37:305–312. doi: 10.1016/j.gaitpost.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 49.Van Hooren B, Teratsias P, Hodson-Tole EF. Ultrasound imaging to assess skeletal muscle architecture during movements: a systematic review of methods, reliability, and challenges. J. Appl. Physiol. 2020;128:978–999. doi: 10.1152/japplphysiol.00835.2019. [DOI] [PubMed] [Google Scholar]
- 50.Giannakou E, Aggeloussis N, Arampatzis A. Reproducibility of gastrocnemius medialis muscle architecture during treadmill running. J. Electromyogr. Kinesiol. 2011;21:1081–1086. doi: 10.1016/j.jelekin.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Farris DJ, Lichtwark GA. UltraTrack: software for semi-automated tracking of muscle fascicles in sequences of B-mode ultrasound images. Comput. Methods Prog. Biomed. 2016;128:111–118. doi: 10.1016/j.cmpb.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 52.Fukunaga T. In vivo behaviour of human muscle tendon during walking. Proc. R. Soc. Lond. B. 2001;268:229–233. doi: 10.1098/rspb.2000.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hawkins D, Hull M. A method for determining lower extremity muscle-tendon lengths during flexion/extension movements. J. Biomech. 1990;23:487–494. doi: 10.1016/0021-9290(90)90304-L. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this are available from the corresponding author upon reasonable request.
A custom-made MATLAB code (MathWorks, Inc., Natick, United States) used for data analysis is available from the corresponding author upon reasonable request.