Abstract
Salmonella enterica serovar 4,[5],12:i:- (Salmonella 4,[5],12:i:-) is a monophasic variant of Salmonella Typhimurium that has emerged as a global cause of multidrug resistant salmonellosis. We used Bayesian phylodynamics, genomic epidemiology, and phenotypic characterization to describe the emergence and evolution of Salmonella 4,[5],12:i:- in Australia. We show that the interruption of the genetic region surrounding the phase II flagellin, FljB, causing a monophasic phenotype, represents a stepwise evolutionary event through the accumulation of mobile resistance elements with minimal impairment to bacterial fitness. We identify three lineages with different population dynamics and discrete antimicrobial resistance profiles emerged, likely reflecting differential antimicrobial selection pressures. Two lineages are associated with travel to South-East Asia and the third lineage is endemic to Australia. Moreover antimicrobial-resistant Salmonella 4,[5],12:i- lineages efficiently infected and survived in host phagocytes and epithelial cells without eliciting significant cellular cytotoxicity, suggesting a suppression of host immune response that may facilitate the persistence of Salmonella 4,[5],12:i:-.
Subject terms: Phylogenetics, Bacteriology, Bacterial genetics
Salmonella enterica serovar 4,[5],12:i:- (Salmonella 4,[5],12:i:-) is a major pathogen of humans and animals with a reported incidence in Australia three times higher than the UK and USA. Here, the authors report the circulation, antimicrobial resistance signatures, and effects on host cells, of three Salmonella4,[5],12:i:- lineages within Australia.
Introduction
Non-typhoidal Salmonella enterica (NTS) is a major pathogen of humans and animals, and is responsible for approximately 153 million cases of gastroenteritis and 57,000 deaths globally each year1. Unlike human-restricted typhoidal Salmonella (serovars Typhi and Paratyphi A, B, and C), one of the key features of non-typhoidal Salmonella (NTS) is a wide host range2. In particular, S. enterica serovar Typhimurium (S. Typhimurium) is considered the prototype host generalist, with a broad zoonotic and environmental reservoir3–5. S. Typhimurium is a leading cause of human salmonellosis, with a key epidemiological feature over the past few decades being sequential clonal expansions of multidrug-resistant (MDR) S. Typhimurium lineages, including phage type DT204, DT29, and DT1042,4,6,7. S. Typhimurium lineages are characterized by several multi-locus sequence types (STs), including ST19, ST34, ST36, and ST313, and monophasic variants of significant public health concern have been detected in ST34 and ST3138–10.
Most recently, Salmonella enterica serovar 4,[5],12:i:- (Salmonella 4,[5],12:i:-), a monophasic variant of S. Typhimurium belonging to ST34, has emerged as a major global cause of NTS disease in animals and humans11–17. Key features of this pandemic lineage initially referred to as the European Clone due to its suggested origins18, are (i) deletion(s) in the phase II flagellin locus preventing the expression of the second flagella antigen FljB8, (ii) genes associated with heavy metal resistance, and (iii) increasingly extensive MDR6,13,14,19. The antimicrobial resistance (AMR) profile of resistance to ampicillin, streptomycin, sulfonamides, and tetracycline (ASSuT) initially acted as an epidemiological marker for this lineage16,20,21. Of recent concern, however, are reports of resistance to third-generation cephalosporins and colistin in Salmonella 4,[5],12:i:- from Europe, North America, and parts of Asia11,22,23, raising the prospect of increasingly limited antimicrobial therapeutic options in cases of severe human salmonellosis24.
In addition to bacterial traits, the host range of Salmonella 4,[5],12:i:- also plays a role in the success of this global pathogen, with outbreaks linked to food products derived from livestock11,12,25,26, particularly swine. For example, colistin resistance has been reported in Salmonella 4,[5],12:i:- isolates from pigs and humans in China, where there has been use of polymyxins as growth supplements in swine14,18,27. Further, mcr genes mediating colistin resistance have also been identified on plasmids with resistance genes to multiple antimicrobials, including third-generation cephalosporins (3GCs)14,18,23. IncHI2 plasmids encoding both mcr-3 and blaCTX-M-55 have been linked to clinical disease caused by Salmonella 4,[5],12:i:- in south-east Asia14,18,23.
In Australia, the reported incidence of human salmonellosis is more than three times higher than in the United Kingdom and the United States. Over the past 5 years, Salmonella 4,[5],12:i:- has emerged as one of the major serovars responsible for salmonellosis in Australia, and the most common MDR NTS6,28. To date, however, the specific factors contributing to the emergence of Salmonella 4,[5],12:i:- in Australia have not been assessed. Accordingly, to investigate the origins and establishment of Salmonella 4,[5],12:i:-, we undertook a combined genomic and phenotypic analysis, contextualizing Australian data with Salmonella 4,[5],12:i:- isolates from Europe, Asia, and the Americas. We show the circulation of three major global lineages of Salmonella 4,[5],12:i:- within Australia, each associated with different AMR profiles to key antimicrobials and signature deletions in the phase II flagellin locus. Further, we demonstrate that some Salmonella 4,[5],12:i:- exhibit an increased ability to replicate in host cells without eliciting increased cellular cytotoxicity, suggesting an enhanced capacity for immune evasion and potentially providing a competitive advantage over other NTS.
Results
Global phylogeography of Salmonella 4,[5],12:i:- and multiple introductions into Australia
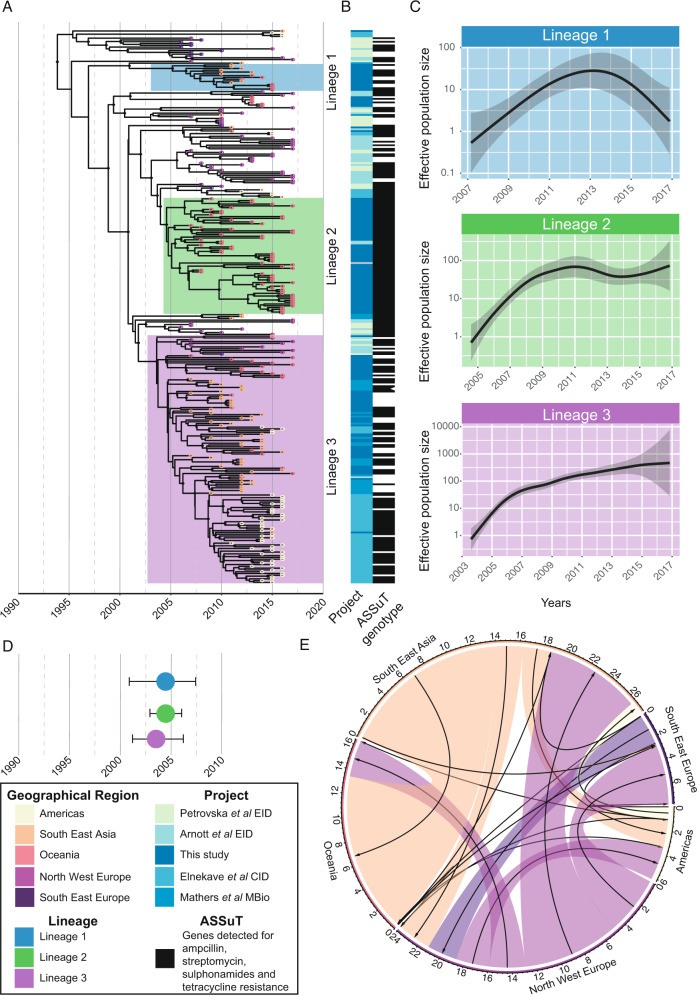
To investigate the introduction of Salmonella 4,[5],12:i:- into Australia and define the geographic distribution of lineages, we undertook phylogenomic analyses of 136 Australian isolates, contextualized with 173 publicly available isolates from multiple geographical regions (Fig. 1A, B and Supplementary Fig. 1A, B, Supplementary Data 1)8,11,12,19. Bayesian phylodynamic analyses from the 2693 core SNP alignment, using the highest supported model of a relaxed clock and coalescent exponential tree prior, revealed three major circulating lineages. These lineages were defined by the most recent common ancestor (MRCA) of the clusters of >10 Australian isolates (Fig. 1A) and had high posterior probability support of ≥0.95 (shown in the maximum-clade-credibility [MCC] tree in Fig. 1A).
Fig. 1. Inferred population dynamics of ST34 Salmonella 4,[5],12:i:-.
A Bayesian evolutionary analysis showing the maximum-clade credibility (MCC) tree of ST34 isolates (n = 309) inferred from 2693 SNPs, demonstrating the timing of divergence and geographical spread of Salmonella 4,[5],12:i:- globally. The tips of the phylogeny are colored by the major geographical regions for the reported country of collection (for publicly available data) or by destination of reported travel (if any) for Australian isolates. The three lineages are highlighted on the phylogeny. The posterior support of ≥0.95 for internal nodes is shown with black circles on the node. The time in years is given on the x axis. Phylogeny available in Newick form from microreact (https://microreact.org/project/mfxxBchBsUpsJu7nvfkFw4). B Heatmap that shows the study from which the isolates were sourced and the presence of the ASSuT genotype (defined by any genes mediating antimicrobial resistance to ampicillin, streptomycin, sulfonamides, and tetracycline). C The estimated effective population size through time for each of the three lineages. The shaded area indicates the 95% confidence interval. D Visualization of the most recent common ancestor (MRCA) of the three lineages with highest posterior density (HPD) intervals shown as the bars. E Circular migration diagram of the migration events between the five geographical regions. The size of the colored block denotes the posterior mean number of inferred migration events from the Bayesian phylogeographical analysis and arrows denote directionality. The inset box is the legend for the different attributes.
One of these lineages, Lineage 2, contained isolates mainly from cases in Australia with no reported overseas travel. Further, Lineage 2 also contained six ST34 Salmonella 4,[5],12:i:- isolated from cattle in Australia and a previously published swine isolate from Australia29. In contrast, Australian isolates in Lineages 1 and 3 were associated with cases that reported recent international travel to South-East Asia. Similar phylogeographic associations were observed for isolates from the United States, which clustered together and formed part of an endemic lineage prevalent in pigs in the United States, as previously reported11. In our phylogeographic analysis, these United States isolates were most closely related to isolates collected from pigs in Vietnam (Fig. 1A)12.
Differences in the effective population size (Ne) trajectories, a measure of genetic diversity, were observed between Australian isolates belonging to the three lineages (Fig. 1C), suggesting Lineage 2 and Lineage 3 both underwent population expansion since their emergence in the early 2000s, which has stabilized in recent years. In contrast, the Ne for Lineage 1 suggested a population that initially increased, with a subsequent decrease over the past five years (Fig. 1C).
We assessed for temporal signal within the dataset (Supplementary Fig 1C). To test the robustness of the molecular clock signal, we employed a Bayesian evaluation of temporal signal (BETS)30. A strong temporal signal within ST34 Salmonella 4,[5],12:i:- was shown for the model with correct sampling times with a log Bayes factor of 246 relative to the same model with no sampling times. The substitution rate (the number of expected substitutions per site per year) was estimated at 3.88E−7 (95% highest posterior density [HPD] 3.28E−7 to 4.46E−7) corresponding to previous estimates for non-typhoidal S. enterica serovars31. The year of emergence of the MRCA for all included ST34 Salmonella 4,[5],12:i:- was estimated at 1992 (HPD 1987–1996), with likely concurrent circulation of all three lineages in Australia since the turn of the twenty-first century (Fig. 1D).
We then used a mugration model (which uses stochastic mapping to infer ancestral states) to infer ancestral geographic locations32. We included travel history data, where available for Australian isolates33. The posterior support for the geographical location provided strong evidence supporting the emergence of ST34 Salmonella 4,[5],12:i:- in the northern hemisphere, with subsequent spread to South-East Asia and Australia at the turn of the twenty-first century (Fig. 1A and Supplementary Fig. 2A). We also explored the migration dynamics of ST34 Salmonella 4,[5],12:i:- using a phylogeographic approach that estimated the putative number of migration events between our five included geographical regions33. Most migration events for Oceania were importation events with a total of 13.2 importations [HPD 9-17] from South East Asia and then 3.1 events [HPD 0-5] from North West Europe (Fig. 1E). We found both importation and exportation events between the two European regions, with North West Europe having a mean of 8.3 [HPD 6-11] exportation events to South East Asia. To test the effect of including travel data for the Australian isolates, the same analysis was undertaken but instead, the sampling location was used, which suggested that the main importation event to Oceania was from North West Europe, rather than South East Asia (Supplementary Fig. 2B), an implausible migration route when travel history data were included. Collectively, these data provide strong evidence for multiple importation events of ST34 Salmonella 4,[5],12:i:- into Australia.
Differential interruptions of the fljAB region are found in lineages of monophasic Salmonella
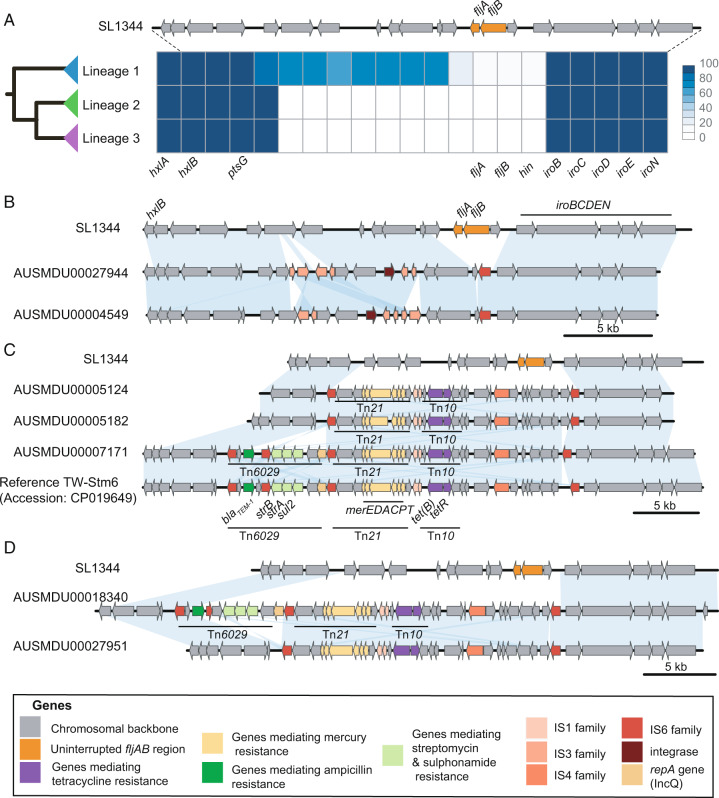
We explored the different patterns of gene interruption of the fljAB region in all isolates, observing that deletion and insertion events of mobile elements were more profound in Lineages 2 and 3 (Fig. 2 and Supplementary Fig. 3). The extent of fljAB interruptions, resulting in the monophasic profile, was characterized using three separate approaches (Supplementary Fig. 3).
Fig. 2. The interruption of the phase II flagella differs between lineages.
A The frequencies that each gene in the phase II flagella region appears in each lineage is shown as a heatmap, with dark blue indicating the gene was detected in all isolates and white indicating that the gene was not detected in any. For comparison, genes in the biphasic ST19 S. Typhimurium SL1344 type are shown above. B–D The different interruption patterns of the phase II flagella in Australian Salmonella 4,[5],12:i:- genomes resolved through long-read sequencing are shown in comparison to the biphasic ST19 S. Typhimurium SL1344 type strain. The blue blocks indicate >95% sequence identity. B Two isolates from humans in Lineage 1. C Two isolates from humans and one bovine isolate from Lineage 2, in addition to the publicly available TW-STm6 isolate collected from a pig in 2014 and D Two isolates from humans in Lineage 3.
In all three lineages, the interrupted flagellar region had a common insertion sequence (IS) element, IS26 (part of the IS6 family)34, located upstream of iroB with identical left direct repeat (DRL) sequences. The similarity of this insertion across all three lineages suggests this element represented an early integration event in the ancestral ST34 Salmonella 4,[5],12:i:- chromosome that has been maintained. Lineage 1 was characterized by deletion of fljAB genes and the two immediate flanking genes (putative transposase and DNA invertase hin), and the interruption of genes putatively classed as inner membrane proteins in the upstream region by IS from two families, IS3 and IS6 (Fig. 2A, B). Twelve Lineage 1 isolates were typed as Salmonella 4,[5],12:i:- from SISTR (an in silico serotyping approach)35, with ten of these phenotypically characterized as Salmonella 4,[5],12:i:-. The remaining three Lineage 1 isolates were typed in silico as S. Typhimurium, and were also characterized phenotypically as S. Typhimurium. This finding was supported by both alignment of short-read data to the fljAB regions and surrounding genes, and pangenome analysis for the presence/absence of these genes (Supplementary Fig. 3).
In contrast to Lineage 1, genomes from Lineage 2 and Lineage 3 had a more extensive chromosomal interruption with the deletion of surrounding genes encoding putative inner membrane, DNA/RNA helicase, and putative solute-binding proteins (Fig. 2A and Supplementary Fig. 3). The resolved chromosomal regions in members of these lineages were characterized by the region bounded by IS6 family elements containing Tn21 and a derivative of Tn10 (Fig. 2C, D). The Tn10-like mobile element was associated with resistance determinants to tetracycline while the merEDACPT operon on Tn21 was associated with resistance determinants to mercury (Fig. 2C, D). These observations suggest that the mobile elements replacing fljAB in Lineages 2 and 3 were acquired once and subsequently maintained within the population, with this event estimated to have occurred around ~2000 (HPD 1998–2002) (Supplementary Fig. 3A).
Further, the previously characterized Tn6029 transposon36,37 was detected in some members of Lineages 2 and Lineages 3 (Fig. 2C, D). This element encodes resistance to ampicillin (blaTEM-1), sulfonamides (sul2), and streptomycin (strAB, also known as aph(3”)-Ib and aph(6)-Id), which together with the tet(B) gene carried on Tn10, confers the ASSuT profile associated with Salmonella 4,[5],12:i:-. The variable presence of Tn6029 in the complete genomes from Lineages 2 and 3 demonstrates that this mobile element does not appear to be fixed in the chromosome. For example, Tn6029 was found in two isolates from Lineage 2, from a human case and the publicly available isolate from a pig but was absent in the second human and cattle isolate. Lineages 2 and 3 were characterized by tet(B) mediating resistance to tetracycline carried on the chromosome with the remaining ASSuT genes integrated into the chromosome of some members of these lineages. This contrasted with Lineage 1 where the ASSuT profile was plasmid-mediated and all Lineage 1 isolates carried a tet(A) gene (Supplementary Fig. 4). Collectively, these data demonstrate stepwise interruptions of fljAB, with a successive accumulation of AMR genes that are distinct across the three major lineages.
Acquired resistance determinants to third-generation cephalosporins and colistin further define ST34 Salmonella 4,[5],12:i:- lineages
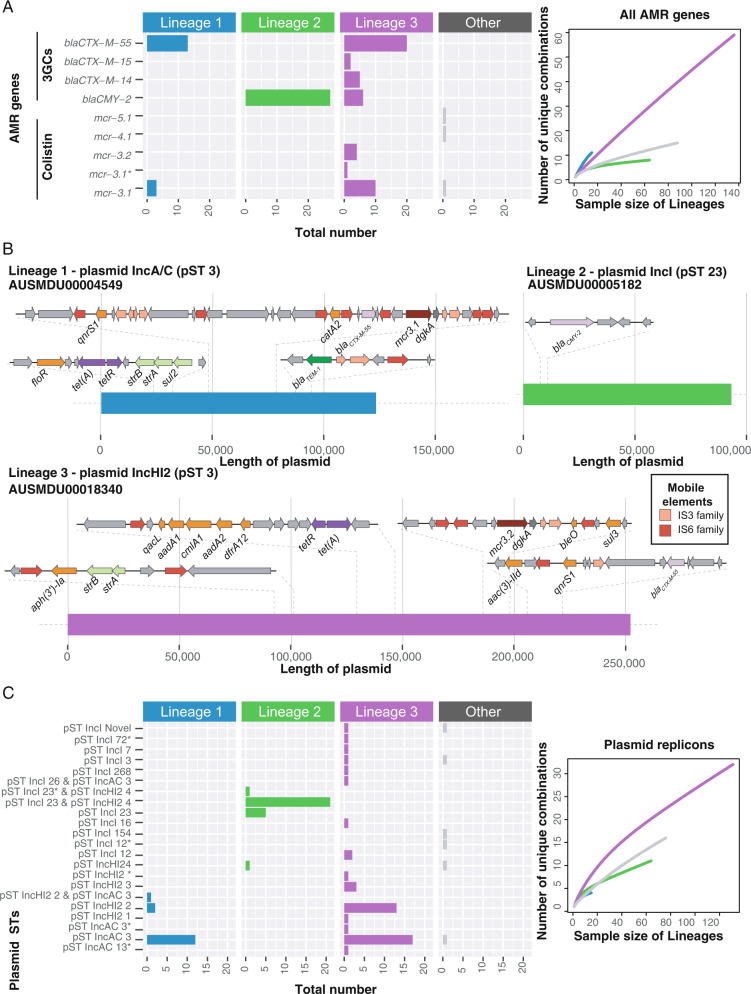
While the study inclusion criterion of exhibiting the epidemiological ASSuT profile for ST34 Salmonella 4,[5],12:i:- isolates resulted in nearly all isolates being considered MDR (defined as resistance determinants to three or more classes)38, extensive diversity was observed in the AMR profiles of all isolates (Supplementary Figs. 4 and 5). Genes mediating the ASSuT profile, blaTEM-1, sul genes, strAB, and tet genes, were found across the entire ST34 Salmonella 4,[5],12:i:- dataset (Supplementary Fig. 4), as were heavy metal resistance genes including those associated with mercury and tellurium resistance, and the Salmonella Genomic Island IV (SGI4), containing genes associated with resistance to silver, arsenic, and copper (Supplementary Figs. 4 and 6). However, there was also a diversity of mechanisms detected to clinically relevant antimicrobials including third-generation cephalosporins (3GC), colistin, and fluoroquinolones (Fig. 3 and Supplementary Fig. 4, Supplementary Data 1).
Fig. 3. Third-generation cephalosporin and colistin resistance profiles differ by lineages and are associated with different plasmids.
A AMR genes mediating resistance to third-generation cephalosporins (3GCs) and colistin was detected in the 309 isolates stratified by lineage membership. The * indicates a close but not exact sequence match. Rarefaction curves of the diversity in the unique AMR gene combinations for each lineage are shown to the right. B Regions encoding AMR in plasmids resolved from long-read data for common AMR profiles that mediate resistance to third-generation cephalosporins and /or colistin in the three lineages. C The plasmid STs in the genomes are shown by lineage membership for the top three plasmid replicons detected from the initial plasmid screen. The * indicates a close but not exact sequence match. Rarefaction curves of the diversity in the unique plasmid replicon combinations for each lineage are shown to the right. AMR antimicrobial resistance.
The blaCMY-2 gene was detected in 27/64 (42.2%) of the endemic Australian Lineage 2 isolates and was harbored on an IncI pST23 plasmid (Fig. 3, Supplementary Figs. 4 and 7). This IncI pST23 plasmid was highly similar (≥ 90% sequence identity) to a previously collected IncI plasmid from a Salmonella Newport strain (CVM 22462) isolated from a dog in 2003 in Arizona, USA39 (Supplementary Fig 7). Lineage 2 had relatively low AMR diversity compared to Lineages 1 and 3, reflected in both the number of drug classes to which the isolates were resistant and the number of unique AMR genes (Fig. 3A and Supplementary Fig. 4).
In both Lineages 1 and 3, which were associated with reported travel to South East Asia, the most frequently detected genes conferring resistance to 3GC and colistin were blaCTX-M-55 and mcr-3.1 (Fig. 3). The blaCTX-M-55 gene was found in 13/15 (86.7%) of Lineage 1 isolates and 20/139 (14.3%) of Lineage 3 isolates, while mcr-3.1 was found in 3/15 (20.0%) Lineage 1 isolates and 11/139 (7.9%) of Lineage 3 isolates (Fig. 3A). Lineage 3 had the greatest diversity of AMR profiles (Fig. 3A and Supplementary Fig. 4, Supplementary Data 1), and the greatest diversity of plasmid replicons (Fig. 3C). While the blaCTX-M-55 gene was only located on an IncAC pST3 plasmid in Lineage 1, in Lineage 3 it was also located on an IncH12 pST3 plasmid (Fig. 3B). The mcr3.2 gene was detected in four Lineage 3 isolates, which co-occurred with IncHI2 pST3 in 3/4 isolates (Fig. 3E and Supplementary Fig. 2). In both IncAC and IncH12 plasmids (Fig. 3B), colistin resistance genes were part of a small transposable unit with dgkA (diacylglycerol kinase), flanked by IS elements previously shown to circularize and integrate into Enterobacterales genomes40. All colistin resistance genes in Lineage 3 co-occurred with blaCTX-M-55, likely on IncAC and IncH12 plasmids.
Of note, the plasmid-mediated qnrs1 gene, which confers reduced susceptibility to ciprofloxacin, was detected in 10/15 (66.7%) of Lineage 1 and 32/139 (23.0%) of Lineage 3 isolates (Supplementary Fig. 4 and Supplementary Data 1). Point mutations in quinolone resistance determining regions (QRDRs) were only detected in three isolates (two from South East Europe and one from South East Asia) that were not members of any lineage, suggesting that QRDR point mutations are rare in ST34 Salmonella 4,[5],12:i:-.
Lineage 2 and 3 ST34 Salmonella 4,[5],12:i:- isolates exhibit increased replication in phagocytic and non-phagocytic human cell lines compared with Lineage 1 isolates
To assess bacterial replication fitness, representative ST34 Salmonella 4,[5],12:i:- isolates Lineages 1–3, along with ST34 Lineage 1-matched biphasic S. Typhimurium isolates (Supplementary Table 1) were assessed for growth in nutrient broth and in a range of cultured human cells in vitro. Biphasic ST34 S. Typhimurium isolates express both flagellar antigens FliC and FljB, whereas the monophasic isolates only express FliC. Lineage 1 biphasic, as well as Lineage 2 and 3 monophasic isolates were comparable in their ability to replicate in nutrient broth over 24 h, with no statistical difference in growth between isolates within a lineage at all time points tested (Supplementary Fig. 8A, C, D). However, significant variation in growth rate was observed across Lineage 1 monophasic isolates (Supplementary Fig. 8B). Lineage 1 monophasic isolate, AUSMDU00004549 was significantly attenuated for growth in nutrient broth which may be attributed to the fact that it carries the most AMR genes of all six Lineage 1 isolates tested (Supplementary Fig. 8B and Supplementary Data 1). All Lineage 1 isolates contained the IncAC pST3 plasmid; however, the AMR profile varied across isolates (Supplementary Data 1).
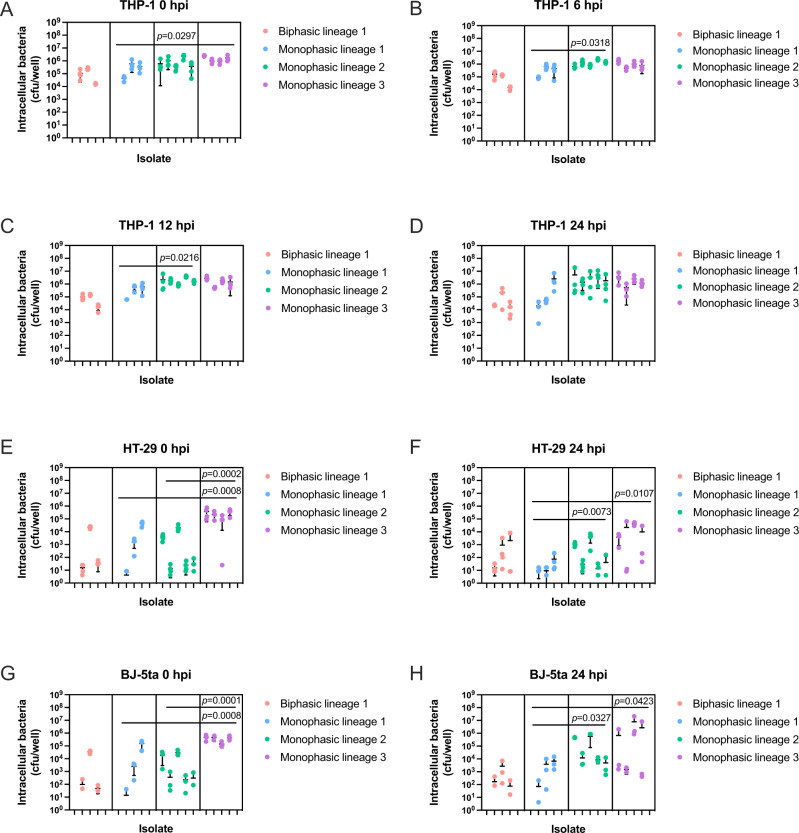
To investigate the in vitro intracellular replication dynamics of ST34 Salmonella 4,[5],12:i:- isolates, human macrophages (THP-1), human colonic epithelial cells (HT-29), and human fibroblasts (BJ-5ta) were infected with the same isolates tested for growth in nutrient broth (Supplementary Table 1). Both Lineage 1 monophasic and biphasic isolates were equally capable of infecting THP-1, HT-29, and BJ-5ta cells (Fig. 4A, E, G), and replication levels of these isolates were comparable across all cell types 24 h post-infection (Fig. 4B–D, F, H).
Fig. 4. Lineage 2 and 3 ST34 Salmonella 4,[5],12:i:- demonstrate increased infectivity and replication in human cell lines.
Differentiated THP-1 (human monocyte) cells (A–D), HT-29 (human intestinal epithelial) cells (E, F), or BJ-5ta (human fibroblast) cells (G, H) were infected at an MOI:10 with selected ST34 Salmonella 4,[5],12:i:- and ST34 matched-Lineage 1 biphasic S. Typhimurium isolates (Supplementary Table 1). THP-1 cells were lysed and intracellular bacteria enumerated as CFU/well at times 0, 6, 12, and 24 hpi. HT-29 and BJ-5ta cells were lysed and intracellular bacteria enumerated as CFU/well at times 0 and 24 hpi. Isolates depicted on the x axis correspond to isolates listed in Supplementary Table 1. Each dot represents CFU/well of a biological replicate (performed in technical duplicate), with error bars indicating ±1 standard deviation of n = 3 or 4 biological replicates. Statistical significance was determined by nested one-way ANOVA with Fisher’s uncorrected multiple comparisons test. MOI multiplicity of infection, hpi hours post-infection, CFU colony-forming units.
In contrast, Lineage 2 isolates replicated to significantly higher levels than Lineage 1 isolates in THP-1 cells over 12 h (Fig. 4B, C) and to significantly higher levels 24 h post-infection in HT-29 and BJ-5ta cells (Fig. 4F, H). Lineage 3 isolates infected all cell types more efficiently than Lineage 1 monophasic isolates (Fig. 4A, E, G) and infected both HT-29 and BJ-5ta cells more efficiently than Lineage 2 isolates (Fig. 4E, G). Lineage 3 isolates showed no significant replication advantage over lineages 1 and 2 in THP-1 cells (Fig. 4B–D) but exhibited significantly higher bacterial load in HT-29 and BJ-5ta cells compared to Lineage 1 monophasic isolates at 24 h post-infection (Fig. 4F, H). The growth of all individual bacterial isolates in each cell type is depicted in Supplementary Fig. 9A–L.
Of note, the higher bacterial load of Lineage 2 and 3 Salmonella 4,[5],12:i:- isolates did not negatively impact the viability of the cell types tested, with cellular cytotoxicity remaining comparable to biphasic Lineage 1 isolates over 24 h of infection (Supplementary Fig. 10A–E). Cytotoxicity was higher overall in THP-1 cells for all lineages, as expected in immune cells (Supplementary Fig. 10A–C), and significantly lower in HT-29 and BJ-5ta cells (Supplementary Fig. 10D, E). Collectively, these results demonstrate that Lineages 2 and 3 ST34 Salmonella 4,[5],12:i:- efficiently infect and survive in both phagocytic and non-phagocytic cells without compromising cellular viability.
Discussion
Overall, our data demonstrate the co-circulation of distinct lineages of ST34 Salmonella 4,[5],12:i:- both in Australia and globally. Two lineages (Lineages 1 and 3) were associated with travel-acquired infections (predominantly from South-East Asia, a common travel destination from Australia), while one lineage (Lineage 2) was endemic to Australia. Our phylodynamic analyses are consistent with earlier reports of the global dissemination of the Salmonella 4,[5],12:i:- initially from Europe, then to Asia and the USA11,12,41. Importantly, our analyses explicitly included travel history data in our phylodynamic model, in the absence of which a different migration route would have been misleadingly supported. Our detailed genomic analysis of phase II flagellar interruption shows this key evolutionary event differentiates between lineages, with the integration and maintenance of Tn21 and Tn10 in Lineages 2 and 3 associated with a population expansion of these lineages over the past decade. IS elements were critical in mobilizing these transposons, and future research should investigate the influence of IS on the transcription of fljAB in monophasic and biphasic ST34 Salmonella. Our data also reveal distinct population dynamics relating to cephalosporin and colistin resistance within the three lineages. We hypothesize that differing AMR and plasmid profiles in ST34 Salmonella 4,[5],12:i:- reflect adaptation to distinct ecological niches, with potential differences in regional drug usage acting as a selective pressure for acquisition and retention of accessory genome content, as observed in other members of the Enterobacterales42–44.
Although the Australian endemic Lineage 2 isolates had the least AMR and plasmid diversity, ~40% of isolates in this lineage harbored an IncI pST23 plasmid carrying blaCMY-2, including all isolates from cattle. IncI plasmids have previously been associated with Salmonella enterica isolated from livestock species including cattle in the USA and poultry in Europe11,45,46. For example, cattle were identified as a key reservoir for Salmonella Newport strains harboring an IncI plasmid carrying blaCMY-2 in the USA in the early 2000s45,46, and two US isolates included in this study (one from cattle and one from food product)11 had blaCMY-2 co-occurring with IncI pST12. In this present study, isolates from Australian cattle and swine were closely associated with human isolates in Lineage 2, suggesting a possible livestock reservoir in Australia, with spill-over infections in humans. This hypothesis is supported by a previous molecular epidemiological study demonstrating endemic circulation of ST34 Salmonella 4,[5],12:i:- in Australian pig herds47. However, it is also plausible that the initial spill-over event was from humans to animals, with subsequent dissemination throughout both animal and human reservoirs. Of note, third-generation cephalosporin use in Australia is highly restricted, requiring a veterinary prescription48. Our finding of endemic circulation of a third-generation cephalosporin-resistant lineage amongst livestock and humans in Australia warrants further investigation, and further highlights the need for integrated, cross-sectoral AMR surveillance.
In contrast to Lineage 2, isolates in Lineages 1 and 3 had a diverse range of AMR profiles and plasmids and were associated with introductions from returned travelers. It is possible this diversity may reflect a range of host reservoirs in South-East Asia, where there are strong antimicrobial selection pressures for the emergence and spread of resistant Enterobacterales42,43. Large MDR IncAC pST3 and IncHI2 pST2 plasmids from Salmonella serovars (including S. Typhimurium and Salmonella 4,[5],12:i:-) have been previously reported from several parts of Asia13,15,23. In particular, resistance genes to 3GC (blaCTX-M-55) and colistin (mcr3-1) have been reported from human salmonellosis and livestock isolates from Asia14,15,23,49, and were identified in Lineages 1 and 3 in our study. Notably, US isolates included in this study formed a distinct subclade in Lineage 3, and were closely related to isolates collected from pigs in Vietnam11,12, further illustrating the propensity for global dissemination of AMR NTS.
In addition to the association of AMR profiles with host and geographic reservoirs, our data suggest that the successful emergence of Salmonella 4,[5],12:i:-, particularly Lineages 2 and 3, may also be driven by genetic factors that promote intracellular survival in host cells. Isolates from Lineages 2 and 3 were infected and replicated in both phagocytic and non-phagocytic human cells more efficiently than Lineage 1 monophasic and biphasic isolates, without inducing a significant increase in cellular cytotoxicity. Although AMR is clearly a major factor in the global spread of monophasic Salmonella enterica, in particular S. Typhimurium ST313, the leading cause of endemic NTS in Africa50–52, recent studies have also implicated loss-of-function mutations (genome degradation) in host-adaptation of S. Typhimurium ST31310. Further, a recent study showed that Lineage 2 isolates of S. Typhimurium ST313 escape early immune recognition by MAIT cells (mucosal immune cells), via overexpression of the bacterial enzyme RibB53. Similar to ST313, our initial observations suggest that Salmonella 4,[5],12:i:- Lineages 2 and 3 may have also evolved specific immune evasion mechanisms to maintain the viability of host cells and confer a competitive advantage over Lineage 1 isolates and possibly other biphasic NTS. These mechanisms could facilitate rapid and efficient bacterial dissemination of Salmonella 4,[5],12:i:- in human and animal hosts and may have potentially contributed to the population expansion of Lineages 2 and 3 and the subsequent decrease of Lineage 1 as reported here. A comprehensive future study of potential genome degradation events promoting bacterial virulence across ST34 Salmonella 4,[5],12:i:- lineages could provide valuable insights into not only functional relevance, but also the evolutionary trajectory and epidemiological relevance of loss- and gain-of-function mutations.
Although our data do not provide a direct causal link between the loss of phase II flagellin in Salmonella 4,[5],12:i:- and increased virulence potential, flagellin is a major pathogen-associated molecular pattern (PAMP) that potently activates host innate immune responses54,55. Recent studies on S. Typhimurium ST313 demonstrated a correlation between lower FliC expression, reduced inflammatory responses, and enhanced survival in macrophages; these phenotypes were linked to the high invasiveness and bacteremia associated with clinical S. Typhimurium ST313 infections56–58. One caveat of these studies, however, was that conclusions were drawn from the comparison of in vitro cellular responses between ST313 and ST19 isolates, two distinct lineages of NTS. Further, an association between flagellin deletion and increased invasiveness has been described in other host-adapted serovars, including S. enterica serovar Dublin in cattle and S. enterica serovar Gallinarium in poultry59,60. Overall, loss of flagellin expression appears to be a common theme in Salmonella serovars that cause invasive disease, a trait shared with ST34 Salmonella 4,[5],12:i:-. Although FljB may play a role in the ability of Salmonella 4,[5],12:i:- to evade host immune responses and survive inside host cells, further studies utilizing isogenic strains of biphasic ST34 S. Typhimurium deleted for fljAB are required to make causal associations of FljB loss with immune evasion and invasive capacity. Moreover, the extent of infectivity and replication differed both between and within ST34 Salmonella 4,[5],12:i:- lineages, suggesting that loss of FljB expression alone is not solely responsible for replicative fitness.
Our phylodynamic analyses represent one of the most diverse collections of ST34 Salmonella 4,[5],12:i:- to date, providing a snapshot of the global spread of this extensively drug-resistant pathogen. An important caveat of all phylogeographic analyses is that they are contingent on the sampling strategy across the actual geographic range of the organism in question. The availability of samples from currently unsampled locations will be important for our future understanding of the emergence of ST34 Salmonella 4,[5],12:i:-. Our data draws on Bayesian phylodynamics, microbial genomic epidemiology, and phenotypic analyses to investigate the origin, spread, adaptation, and replicative fitness of ST34 Salmonella 4,[5],12:i:- in Australia and provides a framework for ongoing surveillance of this important public health pathogen.
Methods
Setting and data sources
In Australia, the National Enteric Pathogens Surveillance Scheme (NEPSS) is a surveillance system for human, animal, and environmental enteric pathogens (including Salmonella) that has been operated by the Microbiological Diagnostic Unit Public Health Laboratory (MDU-PHL) at the University of Melbourne since 1978. Data in NEPSS include (i) epidemiological typing data, (ii) antimicrobial susceptibility data, (iii) phenotypic serovar and (iv) basic demographic data for human salmonellosis cases (e.g., age, gender, state of residence). When provided, travel history for patients with salmonellosis is also recorded in NEPSS.
Sampling strategy
The final dataset comprised 309 genomes; these included 127 ST34 Salmonella 4,[5],12:i:- and nine S. Typhimurium (n=9) genomes from Australia, and 173 publicly available ST34 Salmonella 4,[5],12:i:- (Supplementary Data 1 and Supplementary Fig. 1A, B)
For Australian isolates, our sampling strategy involved two approaches. First, to ensure coverage of highly drug-resistant ST34 Salmonella 4,[5],12:i:-, we included all Salmonella 4,[5],12:i:- isolates that were phenotypically resistant to third-generation cephalosporins received at MDU-PHL over the study period. Second, to capture the diversity of ST34 Salmonella 4,[5],12:i:- over the study period, we included a random sample of Australian isolates from 2007-2017; these isolates also included ST34 S. Typhimurium that had the phenotypic AMR profile of ASSuT (a known epidemiological marker for ST34 Salmonella 4,[5],12:i:-). Six available ST34 Salmonella 4,[5],12:i:- isolates sourced from cattle were included. This gave a total of 136 Australian genomes included in this study; ST34 Salmonella 4,[5],12:i:- (n = 127) and ST34 S. Typhimurium (n = 9). All Australian isolates were sequenced at MDU-PHL (see below).
For publicly available isolates, our aim was to capture the diversity of ST34 Salmonella 4,[5],12:i:- circulating globally over the past decade by including isolates that formed part of several key studies reporting Salmonella 4,[5],12:i:-. To be included, isolates needed to have accessions for short-read data available, with geographic location (by country), year of collection, and source (human, food, or animal). To capture the diversity of Salmonella 4,[5],12:i:- circulating in the United States (US) from different sources, a random subset of isolates, stratified by human, animal, or food source, was included that was representative of lineages in the study from Elnekave et al.11. The final number of isolates comprised those from Italy (n = 13), the United Kingdom (n = 65), the US (n = 63), Australia (n = 2), and Vietnam (n = 30)8,11,12,19 (Supplementary Data 1).
Whole-genome sequencing
Genomic DNA was extracted from a single colony using a QIAsymphony™ DSP DNA Mini Kit (Qiagen) according to the manufacturer’s instructions. Sequence libraries were prepared using NexteraXT with random library selection and whole-genome sequencing (WGS) was performed using Illumina NextSeq500, generating 150 bp paired-end reads.
Details of the included genomes are available in Supplementary Data 1. Genomes had a phred score 33, a depth of ≥50 relative to the reference genome, and filtering for isolates with the number of contigs ≤200 (filtering contigs <500 bases) (for details see assemblies below). The short reads of isolates sequenced at MDU-PHL are available on the NCBI Sequence Read Archive (BioProject PRJNA319593 or PRJNA556438).
Phylogenetic analysis
The 309 genomes were mapped to the reference Salmonella 4,[5],12:i:- genome TW-Stm6 that was isolated from a pig fecal sample in Victoria, Australia in 201429 (NCBI accession: CP019649) using Snippy (v4.3.7)/BWA MEM 1.2.061 (https://github.com/tseemann/snippy). This reference was selected as it was a publicly available complete genome from Australia obtained during the study period. Single-nucleotide polymorphisms (SNPs) were called using Snippy (v4.3.7)/Freebayes (v1.0.2)62. Phage regions were identified using Phaster and masked from the alignment63. Recombinant regions were identified using Gubbins64 and removed from the 2713 base alignment. The final core alignment of 2693 bases was extracted with SNP-sites65. A maximum likelihood (ML) phylogenetic tree was inferred using IQ-TREE (v1.6.10) modeled using a general time-reversible (GTR) substitution model +Γ, including invariable sites as a constant pattern and 1000 ultra-fast bootstrap replicates66.
Genome assemblies and screening
De novo assemblies of the genomes were constructed using Spades (v3.13)67. In silico multi-locus sequence types (STs) were determined using the program mlst with the senterica database (https://github.com/tseemann/mlst), and the in silico serovars of all genomes were determined using SISTR35. The genome assemblies of all isolates were screened for acquired AMR determinants using abriTAMR (https://github.com/MDU-PHL/abritamr) in conjunction with the NCBI AMRFinder database v3.2.1 (https://github.com/ncbi/amr)68. MDR isolates were those where mechanisms mediating resistance to three or more drug classes were detected. Known point mutations in quinolone resistance determining regions in gyrA and parC were investigated from the snippy output. Specifically, the variant calls for each isolate were investigated for non-synonymous point mutations in codons 83 and 87 in gyrA and codon 80 in parC. Known plasmid replicons were screened using ABRicate (https://github.com/tseemann/abricate) against the PlasmidFinder database69, with a minimum identity and minimum coverage thresholds of 95%. The diversity of unique combinations of AMR genes and plasmid replicons was assessed using the R package vegan (v2.5-6)70. The input was the number of unique combinations of either AMR genes or plasmid replicons detected in each of the three lineages or other for isolates that were not a member of one of the three lineages. These data were visualized with the rarecurve function. The number of AMR genes belonging to different drug classes (Supplementary Data 1) was plotted using ggplot2 (v3.2.1)71 by both lineage membership and geographical membership.
Long-read sequencing and complete genome assembly
Seven Australian genomes underwent long-read sequencing, three with PacBio and four with Oxford Nanopore (see Supplementary Data 2). Isolates were selected for sequencing based on the diversity of AMR profiles and lineage membership. PacBio data was sequenced and assembled as previously described72. Briefly, raw sequence data were assembled using HGAP v3 (SMRT- Portal v2.3.0)73 with default parameters except for seed length set to 1 kb and genome size to 5 Mb. For isolates sequenced with the Oxford Nanopore GridION X5, genomic DNA (gDNA) of isolates was prepared from solid media scrapings of pure culture using the Genelute Bacterial Genomic DNA Kit (Sigma-Aldritch), and the Gram-negative protocol. Libraries were prepared through a 1D native barcoding genomic DNA approach (SQK-LSK109), and sequenced on an R9.4 flowcell before basecalled using Guppy (3.1.5) and samples de-multiplexed using Porechop (v0.2.4) (https://github.com/rrwick/Porechop), using the --require_two_barcodes option. The long reads were first filtered for minimum length 1 kb and target bases 5 Mb using Filtlong (v0.2.0) (https://github.com/rrwick/Filtlong), then assembled using both the hybrid mode of Unicycler74 and Flye75. Post-assembly comparison, the Unicycler outputs were kept as this approach produced complete genomes for all four isolates, with the chromosome structure, and plasmid type and size, consistent for each respective lineage. Genomes were annotated with Prokka (v1.14.0)76. The complete assemblies of the PacBio isolates and Oxford Nanopore long-read data are available at the European Nucleotide Archive (ENA) under PRJEB41036.
Phylogeographical reconstruction of Salmonella 4,[5],12:i:-
To investigate temporal signal in the ST34 Salmonella 4,[5],12:i:- genomes, we first used TempEst (v1.5)77. A regression analysis was performed of the root-to-tip branch distances within the ST34 ML phylogeny as a function of year of collection, using the heuristic residual mean squared method and to select the best-fitting root. The resulting data was visualized in R using ggplot271.
The final alignment was analyzed in BEAST v1.10.478. We calibrated the molecular clock using including isolation dates for each genome (by year of collection) and specified locations based on major geographical regions (defined by the Australian Bureau of Statistics 1269.0 Standard, 2016). A GTR+Γ substitution model was specified with different combinations of molecular clock models (strict or uncorrelated relaxed with an underlying lognormal distribution) and tree priors (constant coalescent or exponential growth coalescent). Each model was fit using a Markov chain Monte Carlo of 200 million iterations, sampling every 20,000 iterations. We assessed sufficient sampling from the stationary distribution by verifying that the effective sample size (ESS) of key parameters was at least 200. We assessed convergence by repeating the analyses and verifying that they reached the same stationary distribution. Because our alignments consisted of SNPs only, we specified the number of constant nucleotides in the model.
We assessed statistical support for models by approximating their log marginal likelihood using generalized stepping-stone (GSS) and calculating the Bayes factors79. The ESS of key parameters was>200. The highest supported model was the relaxed lognormal clock with a coalescent exponential population model. We used the Bayesian evaluation of temporal signal (BETS) method to explicitly assess temporal signal under the Bayesian framework30 in Beast v1.10.478 with the best-fitting model parameters with and without sampling dates. The premise of this approach is to test two competing models, one with and one without sampling times using their log marginal likelihood.
The frequency of importation or exportation events into Australasia was inferred using a discrete phylogeographic approach (i.e., a migration model), also known as Bayesian stochastic mapping, as implemented in Beast 1.10.478. Our locations consisted of the five major geographical regions (defined by the Australian Bureau of Statistics 1269.0 Standard, 2016) from which the isolate was collected. These five geographical regions were the Americas, North West Europe, Oceania, South East Asia, and South East Europe. To exploit available metadata, we included travel history data from returning Australian travelers under the highest supported model selected above. This approach consists of specifying singleton internal nodes with the travel origin as their location for the Australian isolates (where there was reported travel). For comparison, we also conducted these analyses without such travel history data (using Oceania as the sampling location)33. We assessed convergence of the MCMC and sufficient sampling as above. We inferred the posterior number of migration events between the five possible regions and the amount of time spent at each state, known as Markov rewards. These data were visualized in R using circlize v0.4.880.
We obtained the maximum-clade credibility tree (MCC) using TreeAnnotator v1.10.4 using the keep heights option from the run with the highest ESS values where the reported travel history for the Australian isolates was included. The final MCC tree was visualized in R using ggtree v1.16.681. To investigate the lineages in circulation in Australia, three lineages were defined using the getMRCA function in ape v5.382 for each of the three groups with >10 Australian isolates.
Modeling effective population size within Australian lineages
The effective population size (Ne) of the three Salmonella 4,[5],12:i:- lineages was modeled using SkyGrowth83. To address the population dynamics of the three lineages in Australia, phylogenies were extracted from the MCC phylogeny using ape82 for each of the three lineages circulating in Australia. These three trees were used as input for Skygrowth83, with res parameter equal to an Ne changing every month for nine years in Lineage 1 and eleven years in Lineages 2 and 3 and a tau0 smoothing parameter of 0.1.
Interruption of the fljAB region
To explore the interruption of the fljAB region in both public and Australian data, chromosomal regions surrounding the phase II flagellar region were extracted from the publicly available genomes S. Typhimurium SL1344 (accession: NC_016810) and the complete Australian genomes using seqret in EMBOSS (v6.6.0)84. BLAST comparisons were undertaken and visualized in R using GenoPlotR (v0.8.9)85 to show regions with ≥ 95% sequence homology.
The coverage of phase II flagellar region in all isolates was explored in detail using two complementary approaches. First, all isolates were mapped to the publicly available ST19 SL1344 (accession: NC_016810) type strain using Snippy (v4.6.0) with all regions masked except the phase II flagellar region (shown in Fig. 2) and the percentage coverage of reads across the genomic region determined. Second, the presence/absence of genes in the phase II flagellar region in all isolates was explored using Panaroo (v1.2.4)86. The gff files of the annotated assemblies were used as input for Panaroo to cluster the pangenome using strict clean-mode and default parameters. The protein sequences for the annotated genes from the SL1344 phase II flagellar region were used to identify the gene clusters from panaroo in the Australian isolates typed as Typhimurium from SISTR. The presence/absence of these genes in all isolates was extracted from the Panaroo output tables. The individual genes for each isolate were plotted against the MCC tree using ggtree81. The frequencies of the individual genes within each of the three lineages were visualized in R using pheatmap v1.0.12 (https://cran.r-project.org/web/packages/pheatmap/)
Characterization of plasmids
Plasmids were further characterized by plasmid multi-locus sequence typing (pMLST) for three plasmid replicons detected most frequently within the isolates, specifically IncA/C, IncHI2, IncI. The schemes for these plasmid types were obtained from https://pubmlst.org/plasmid/ and formatted for use with ARIBA (v2.14.1)87. The IncI replicon plasmid from AUSMDU00005182 was then compared to publicly available data using BLAST and found to be homologous to Salmonella Newport pCVM22462 (Accession CP009566). The homology was visualized using GenoPlotR85. Further investigation of the IncI plasmid was undertaking by mapping all isolates to the complete AUSMDU00005182 genome from Lineage 2 and the presence of the same IncI plasmid was inferred if reads mapped to ≥ 95% of the reference. The regions encoding AMR genes on the plasmids were resolved by long-read sequencing and plotted in R with GenoPlotR85.
Screening for elements associated with heavy metal resistance
Genome assemblies were screened for the SGI48 (SO4698-09, accession LN999997) with blastn. SGI4 was determined as being present with >90% coverage and >99% identity to the reference. Genes mediating heavy metal resistance were identified from the AMRFinderPlus database v3.2.1 and screened with ABRicate using a minimum identity of 75% and minimum coverage of 90%. The resulting data were transformed in binary presence/absence data in R using tidyverse (v1.2.1)88.
Selection of isolates for use in phenotypic assays
Between 3-5 representative Salmonella 4,[5],12:i:- isolates were selected for phenotypic characterization from each Lineage 1, 2, and 3, along with three Lineage 1-matched biphasic isolates. All isolates selected for phenotypic analysis were utilized for complete genome assembly and are represented in the phylogenetic trees depicted in Fig. 1 and Supplementary Figs. 3, 4. Further, at least two of each monophasic Lineage 1, 2, and 3 isolates were used in additional genomic analyses represented in Figs. 2B, C and 3B. The isolates selected are listed in Supplementary Table 1 and details of plasmid and AMR profiles for each isolate are listed in Supplementary Data 1.
Phenotypic comparison of growth rates in liquid culture medium
Isolates were plated onto Luria Bertani (LB) agar and incubated at 37 °C overnight (+50 μg/ml w/v streptomycin for SL1344). For CFU/ml readings in broth culture, single colonies of each bacterial isolate were inoculated into 10 ml of LB broth and incubated in a shaking 37 °C incubator at 200 rpm for ~16 h. From these cultures, 1/100 starter cultures (5 ml total) were inoculated and incubated for 3–4 h as above. Following the 3–4 h incubation, all cultures were standardized to OD600 0.05 and 200 µl of each culture was added to a 96 well plate in duplicate and grown for 24 h in a BMG FLUOstar Omega heated to 37 °C and shaking at 200 rpm. The plate reader was stopped for no longer than 3 min to remove 10 µl of culture from each well using a multichannel pipette at times 0, 3, 6, 9, 12, and 24 hr. Serial dilution of the culture was performed in PBS and plated onto LB agar in duplicate. This experiment was performed three independent times (biological triplicate) on separate days. Differences between isolates at each time point were determined by two-way ANOVA or mixed-effects analysis with Tukey’s multiple comparisons post-test (GraphPad Software v9.0). Statistical significance was determined to be p < 0.05.
Phenotypic comparison of growth rates in and human cell lines
Human macrophage (THP-1) (ATCC® TIB-202™) and colonic epithelial (HT-29) (ATCC® HTB-38™) cell lines were maintained in Roswell Park Memorial Institute (RPMI) 1640 media + 200 mM GlutaMAX (Life Technologies) supplemented with 10% v/v Fetal Bovine Serum (Bovogen) and grown in a humidified 5% CO2 37 °C incubator. Human h-TERT immortalized foreskin (BJ-5ta) (ATCC® CRL-4001™) fibroblasts were maintained in Dulbecco’s Modified Eagle Medium (DMEM) containing l-glutamine (Life Technologies) and 10% FCS, 50 ng/ml hygromycin. Prior to infections, THP-1 cells were differentiated for 3 days with 25 ng/ml phorbol 12-myristiate-12 acetate (PMA, Sigma-Aldrich). For intracellular replication experiments, single colonies of each bacterial isolate (Supplementary Table 1) were inoculated separately (triplicate) in LB broth and incubated overnight at 37 °C at 200 rpm, then sub-cultured (at 1/100) for 3 h in the same conditions in 5 ml LB. Mammalian cells were infected at a multiplicity of infection (MOI) of 10 in RPMI or DMEM (depending on cell type) in duplicate and centrifuged at 525×g to synchronize bacterial uptake. Following 30 min incubation at 37 °C in 5% CO2, cells were incubated with 100 μg/ml gentamicin in RPMI for 1 h to inhibit extracellular bacterial growth in the media, then replaced with 10 μg/ml gentamicin in 1% v/v FBS/RPMI or FBS/DMEM for the remainder of the infection. For THP-1 cells, samples were collected at times 0-, 6-, 12- and 24-h post-infection. For HT-29 and BJ-5ta cells, samples were collected at 0- and 24-h post-infection. All Lineage 1 and 2 isolates were sensitive to gentamicin. We observed that all lineage 3 isolates were resistant to gentamicin which resulted in overgrowth of these isolates in the tissue culture media. Therefore, for all Lineage 3 isolates, tissue culture media was supplemented with 0.5 µg/ml meropenem (to which Lineage 3 isolates were susceptible) from 6 h post-infection (in addition to the gentamicin treatment applied to Lineages 1 and 2 isolates) to restrict extracellular bacterial growth. Previous work suggests meropenem has limited ability to cross the plasma membrane at very low concentrations (0.5 µg/ml)89. To confirm this, we assessed the intracellular viability of isolates with either gentamicin alone or gentamicin in combination with meropenem and found no difference (Supplementary Fig. 11).
Cell viability was measured by lactate dehydrogenase (LDH) release into the supernatant of infected cells. Here, cell supernatant was collected before cell lysis and LDH release was quantified as per the manufacturer’s instructions (Promega). The percentage of cytotoxicity was calculated by comparison to LDH release from 100% lysed uninfected control cells. For enumeration of intracellular bacteria, media was removed, and cells were washed twice with PBS to remove extracellular bacteria, followed by lysis in 0.1% Triton X-100. Lysates were serially diluted in PBS in duplicate and plated onto LB agar. Bacterial enumeration and cell viability assays were performed over 3–4 biological replicates, each performed independently, with a new passage of host cells, on a separate day. Within each biological replicate, technical duplicates were performed for each isolate. All statistical analyses were performed using Prism software (GraphPad Software v9.0) and determined by two-way ANOVA with Tukey’s post-test for multiple comparisons. Statistical significance was determined to be p < 0.05. To compare groups of isolates between lineages, we performed a nested one-way ANOVA with Tukey’s multiple comparisons test, which allowed for the inclusion of individual isolates in sub-groups of one lineage, and then the comparison between the different lineages.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This work was supported by the National Health and Medical Research Council (NHMRC), Australia Project Grant (APP1129770). D.J.I. is supported by an NHMRC Investigator Grant (APP1195210). S.D. was funded by the ARC (DE190100805). M.D.K. is supported by an NHMRC Career Development Fellowship (APP1145997). B.P.H. is supported by an NHMRC Investigator Grant (APP1196103). J.S.P. is supported by an NHMRC Career Development Fellowship (APP1159230). D.A.W. is supported by an NHMRC Investigator Grant (APP1174555). The Microbiological Diagnostic Unit Public Health Laboratory is funded by the Department of Health and Human Services, Victoria.
Source data
Author contributions
D.J.I. and D.A.W. conceptualized the study and M.D.K., B.P.H., and J.S.P. contributed to the design of the study. D.J.I. performed the genomic analyses with input from S.D. and S.L.B. D.J.I., R.A., S.L.B., S.D., A.G.S., M.D.K., B.P.H., J.S.P., and D.A.W. contributed to the interpretation of the data. D.J.I., R.A., S.L.B., S.D., and J.S.P. prepared the figures. M.V., S.L.B., G.T., D.Y.J.L., A.G.S., and S.A.B. performed whole-genome sequencing. R.A., M.J., and J.S.P. performed the phenotypic analyses. D.J.I. and D.A.W. prepared the first draft of the manuscript, which was revised by all authors.
Data availability
Supplementary Data 1 lists the individual accessions for all isolates, with associated metadata. Short-read data for Australian isolates in this study are available from the NCBI Sequence Read Archive (BioProject PRJNA319593 or PRJNA556438). Long-read data are available at the European Nucleotide Archive (ENA) under PRJEB41036, and individual accessions are provided in Supplementary Data 2. An interactive annotated phylogeny is available in Microreact https://microreact.org/project/mfxxBchBsUpsJu7nvfkFw4. Antimicrobial resistance genes were detected using AbritAMR (https://github.com/MDU-PHL/abritamr) in conjunction with the AMRFinder database v3.2.1 (https://github.com/ncbi/amr). Source data for phenotypic work are provided with this paper. Data supporting the findings of this study are available within the text and in Supplementary files. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review informationNature Communications thanks Jasna Kovac and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Danielle J. Ingle, Email: danielle.ingle@unimelb.edu.au
Deborah A. Williamson, Email: deborah.williamson@unimelb.edu.au
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-25073-w.
References
- 1.Kirk MD, et al. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: A Data Synthesis. PLoS Med. 2015;12:e1001921. doi: 10.1371/journal.pmed.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crump JA, Sjölund-Karlsson M, Gordon MA, Parry CM. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin. Microbiol. Rev. 2015;28:901–937. doi: 10.1128/CMR.00002-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabsch, W. in Salmonella: Methods and Protocols (eds. Schatten, H. & Eisenstark, A.) 177–211 (Humana Press, 2007). [DOI] [PubMed]
- 4.Bawn M, et al. Evolution of Salmonella enterica serotype Typhimurium driven by anthropogenic selection and niche adaptation. PLoS Genet. 2020;16:e1008850–29. doi: 10.1371/journal.pgen.1008850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mastrorilli E, et al. A comparative genomic analysis provides novel insights into the ecological success of the monophasic Salmonella serovar 4,[5],12:i. Front. Microbiol. 2018;9:4–18. doi: 10.3389/fmicb.2018.00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williamson, D. A. et al. Increasing antimicrobial resistance in nontyphoidal Salmonella isolates in Australia from 1979 to 2015. Antimicrob. Agents Chemother.62, e02012–17–9 (2018). [DOI] [PMC free article] [PubMed]
- 7.Leekitcharoenphon P, et al. Global genomic epidemiology of Salmonella enterica Serovar Typhimurium DT104. Appl. Environ. Microbiol. 2016;82:2516–2526. doi: 10.1128/AEM.03821-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrovska L, et al. Microevolution of monophasic Salmonella Typhimurium during epidemic, United Kingdom, 2005–2010. Emerg. Infect. Dis. 2016;22:617–624. doi: 10.3201/eid2204.150531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Puyvelde, S. et al. An African Salmonella Typhimurium ST313 sublineage with extensive drug-resistance and signatures of host adaptation. Nat. Commun.10, 4280 (2019). [DOI] [PMC free article] [PubMed]
- 10.Pulford, C. V. et al. Stepwise evolution of Salmonella Typhimurium ST313 causing bloodstream infection in Africa. Nat. Microbiol.6, 327–338 (2020). [DOI] [PMC free article] [PubMed]
- 11.Elnekave E, et al. Salmonella enterica Serotype 4,[5],12:i:- in swine in the United States Midwest: an emerging multidrug-resistant clade. Clin. Infect. Dis. 2017;66:877–885. doi: 10.1093/cid/cix909. [DOI] [PubMed] [Google Scholar]
- 12.Mather AE, et al. New variant of multidrug-resistant Salmonella enterica serovar Typhimurium associated with invasive disease in immunocompromised patients in Vietnam. mBio. 2018;9:882–11. doi: 10.1128/mBio.01056-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monte DF, et al. Multidrug- and colistin-resistant Salmonella enterica 4,[5],12:i:- sequence type 34 carrying the mcr-3.1 gene on the IncHI2 plasmid recovered from a human. J. Med. Microbiol. 2019;68:986–990. doi: 10.1099/jmm.0.001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun R-Y, et al. Global clonal spread of mcr-3-carrying MDR ST34 Salmonella enterica serotype Typhimurium and monophasic 1,4,[5],12:i:− variants from clinical isolates. J. Antimicrob. Chemother. 2020;18:136–10. doi: 10.1093/jac/dkaa115. [DOI] [PubMed] [Google Scholar]
- 15.Lu X, et al. Epidemiologic and genomic insights on mcr-1-harbouring Salmonella from diarrhoeal outpatients in Shanghai, China, 2006-2016. EBioMedicine. 2019;42:133–144. doi: 10.1016/j.ebiom.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucarelli C, et al. Nucleotide sequence of the chromosomal region conferring multidrug resistance (R-type ASSuT) in Salmonella Typhimurium and monophasic Salmonella Typhimurium strains. J. Antimicrob. Chemother. 2011;67:111–114. doi: 10.1093/jac/dkr391. [DOI] [PubMed] [Google Scholar]
- 17.Lu J, et al. Prevalence and molecular characteristics of mcr-1 gene in Salmonella Typhimurium in a tertiary hospital of Zhejiang Province. IDR. 2019;12:105–110. doi: 10.2147/IDR.S190269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biswas S, Li Y, Elbediwi M, Yue M. Emergence and dissemination of mcr-carrying clinically relevant Salmonella Typhimurium monophasic clone ST34. Microorganisms. 2019;7:298–16. doi: 10.3390/microorganisms7090298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnott A, et al. Multidrug-resistant Salmonella enterica 4,[5],12:i:- Sequence Type 34, New South Wales, Australia, 2016–2017. Emerg. Infect. Dis. 2018;24:751–753. doi: 10.3201/eid2404.171619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García P, et al. Horizontal acquisition of a multidrug-resistance module (R-type ASSuT) is responsible for the monophasic phenotype in a widespread clone of Salmonella Serovar 4,[5],12:i. Front. Microbiol. 2016;7:e48228–7. doi: 10.3389/fmicb.2016.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barco L, et al. Molecular characterization of Salmonella enterica Serovar 4,[5],12:i:- DT193 ASSuT strains from two outbreaks in Italy. Foodborne Pathog. Dis. 2014;11:138–144. doi: 10.1089/fpd.2013.1626. [DOI] [PubMed] [Google Scholar]
- 22.Branchu P, et al. SGI-4 in monophasic Salmonella Typhimurium ST34 is a novel ICE that enhances resistance to copper. Front. Microbiol. 2019;10:e00118. doi: 10.3389/fmicb.2019.01118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadimpalli M, et al. CTX-M-55-type ESBL-producing Salmonella enterica are emerging among retail meats in Phnom Penh, Cambodia. J. Antimicrob. Chemother. 2018;23:29–7. doi: 10.1093/jac/dky451. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019 1–148 (U.S. Centers for Disease Control and Prevention, 2019).
- 25.Arai N, et al. Salmonella Genomic Island 3 is an integrative and conjugative element and contributes to copper and arsenic tolerance of Salmonella enterica. Antimicrob. Agents Chemother. 2019;63:711–711. doi: 10.1128/AAC.00429-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helmuth IG, et al. An outbreak of monophasic Salmonella Typhimurium associated with raw pork sausage and other pork products, Denmark 2018–19. Epidemiol. Infect. 2019;147:1–7. doi: 10.1017/S0950268819002073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carattoli A, Carretto E, Brovarone F, Sarti M, Villa L. Comparative analysis of an mcr-4 Salmonella enterica subsp. enterica monophasic variant of human and animal origin. J. Antimicrob. Chemother. 2018;21:449–4. doi: 10.1093/jac/dky340. [DOI] [PubMed] [Google Scholar]
- 28.Ford L, et al. Increasing Incidence of Salmonella in Australia, 2000-2013. PLoS ONE. 2016;11:e0163989–11. doi: 10.1371/journal.pone.0163989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dyall-Smith, M. L., Liu, Y. & Billman-Jacobe, H. Genome sequence of an Australian monophasic Salmonella enterica subsp. enterica Typhimurium isolate (TW-Stm6) carrying a large plasmid with multiple antimicrobial resistance genes. Genome Announ.5, e00793–17–2 (2017). [DOI] [PMC free article] [PubMed]
- 30.Duchêne S, et al. Bayesian evaluation of temporal signal in measurably evolving populations. Mol. Biol. Evol. 2020;29:59–17. doi: 10.1093/molbev/msaa163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duchêne S, et al. Genome-scale rates of evolutionary change in bacteria. Microb. Genom. 2016;2:1–12. doi: 10.1099/mgen.0.000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemey P, Suchard M, Rambaut A. Reconstructing the initial global spread of a human influenza pandemic: a Bayesian spatial-temporal model for the global spread of H1N1pdm. PLoS Curr. 2009;1:RRN1031–13. doi: 10.1371/currents.RRN1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemey, P. et al. Accommodating individual travel history and unsampled diversity in Bayesian phylogeographic inference of SARS-CoV-2. Nat. Commun.1, 5110 (2020). [DOI] [PMC free article] [PubMed]
- 34.Harmer CJ, Hall RM. An analysis of the IS6/IS26 family of insertion sequences: is it a single family? Microb. Genom. 2019;5:15–19. doi: 10.1099/mgen.0.000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida CE, et al. The Salmonella In Silico Typing Resource (SISTR): an open web-accessible tool for rapidly typing and subtyping draft Salmonella genome assemblies. PLoS ONE. 2016;11:e0147101–e0147117. doi: 10.1371/journal.pone.0147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cain AK, Liu X, Djordjevic SP, Hall RM. Transposons related to Tn1696 in IncHI2 plasmids in multiply antibiotic resistant Salmonella enterica serovar Typhimurium from Australian animals. Micro. Drug Resist. 2010;16:197–202. doi: 10.1089/mdr.2010.0042. [DOI] [PubMed] [Google Scholar]
- 37.Reid CJ, Chowdhury PR, Djordjevic SP. Tn6026 and Tn6029 are found in complex resistance regions mobilised by diverse plasmids and chromosomal islands in multiple antibiotic resistant Enterobacteriaceae. Plasmid. 2015;80:127–137. doi: 10.1016/j.plasmid.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Magiorakos AP, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2014;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 39.Cao G, et al. Complete sequences of six IncA/C plasmids of multidrug-resistant Salmonella enterica subsp. enterica Serotype Newport. Genome Announ. 2015;3:e55687–2. doi: 10.1128/genomeA.00027-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiang R, et al. Colocation of the polymyxin resistance gene mcr-1 and a variant of mcr-3 on a plasmid in an Escherichia coli isolate from a chicken farm. Antimicrob. Agents Chemother. 2018;62:161–165. doi: 10.1128/AAC.00501-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elnekave E, et al. Transmission of multidrug-resistant Salmonella enterica subspecies enterica 4,[5],12:i:- Sequence Type 34 between Europe and the United States. Emerg. Infect. Dis. 2020;26:3034–3038. doi: 10.3201/eid2612.200336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ingle DJ, Levine MM, Kotloff KL, Holt KE, Robins-Browne RM. Dynamics of antimicrobial resistance in intestinal Escherichia coli from children in community settings in South Asia and sub-Saharan. Afr. Nat. Microbiol. 2018;3:1–14. doi: 10.1038/s41564-017-0093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duy, P. T. et al. Commensal Escherichia coli are a reservoir for the transfer of XDR plasmids into epidemic fluoroquinolone-resistant Shigella sonnei. Nat. Microbiol.5, 256-264 (2020). [DOI] [PMC free article] [PubMed]
- 44.Shen, C. et al. Dynamics of mcr-1 prevalenvce and mcr-1-postiive Escherichia coli after cessation of colistin use as a feed additive for animals in China: a prospective cross-section and whole genome sequencing based molecular epidemiological study. Lancet Microbe1, e34–e43 (2020). [DOI] [PubMed]
- 45.Michael GB, Schwarz S. Antimicrobial resistance in zoonotic nontyphoidal Salmonella: an alarming trend? Clin. Microbiol. Infect. 2016;22:968–974. doi: 10.1016/j.cmi.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 46.Varma, J. K. et al. Highly resistant Salmonella Newport-MDR AmpC transmitted through the domestic US Food Supply: a FoodNet case-control study of sporadic Salmonella Newport infections, 2002–2003. J. Infect. Dis.194, 222–230 (2006). [DOI] [PubMed]
- 47.Weaver T, et al. Longitudinal study of Salmonella 1,4,[5],12:i:- shedding in five Australian pig herds. Prev. Vet. Med. 2017;136:19–28. doi: 10.1016/j.prevetmed.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 48.Rajkhowa, A. et al. Animals, agriculture and antimicrobial stewardship. Med. J. Austral. Insighthttps://insightplus.mja.com.au/2020/2/animals-agriculture-and-antimicrobial-stewardship/ (2020).
- 49.Litrup, E. et al. Plasmid-borne colistin resistance gene mcr-3 in Salmonella isolates from human infections, Denmark, 2009–17. Euro Surveill. 22, e00543–17–7 (2017). [DOI] [PMC free article] [PubMed]
- 50.Feasey NA, et al. Three epidemics of invasive multidrug-resistant Salmonella bloodstream infection in Blantyre, Malawi, 1998. Clin. Infect. Dis. 2015;61:S363–S371. doi: 10.1093/cid/civ691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reddy EA, Shaw AV, Crump JA. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect. Dis. 2010;10:417–432. doi: 10.1016/S1473-3099(10)70072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okoro CK, et al. Intracontinental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat. Genet. 2012;44:1215–1221. doi: 10.1038/ng.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Preciado-Llanes L, et al. Evasion of MAIT cell recognition by the African Salmonella Typhimurium ST313 pathovar that causes invasive disease. Proc. Natl Acad. Sci. USA. 2020;117:20717–20728. doi: 10.1073/pnas.2007472117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayashi F, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 55.Zhao, Y. et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature477, 596–600 (2011). [DOI] [PubMed]
- 56.Ramachandran G, Perkins DJ, Schmidlein PJ, Tulapurkar ME, Tennant SM. Invasive Salmonella Typhimurium ST313 with naturally attenuated flagellin elicits reduced inflammation and replicates within macrophages. PLoS Negl. Trop. Dis. 2015;9:e3394–12. doi: 10.1371/journal.pntd.0003394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carden S, Okoro C, Dougan G, Monack D. Non-typhoidal Salmonella Typhimurium ST313 isolates that cause bacteremia in humans stimulate less inflammasome activation than ST19 isolates associated with gastroenteritis. Pathog. Dis. 2014;73:2247–2248. doi: 10.1093/femspd/ftu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lê-Bury G, et al. Increased intracellular survival of Salmonella Typhimurium ST313 in HIV‐1‐infected primary human macrophages is not associated with Salmonella hijacking the HIV compartment. Biol. Cell. 2020;112:92–101. doi: 10.1111/boc.201900055. [DOI] [PubMed] [Google Scholar]
- 59.de Freitas Neto OC, et al. A flagellated motile Salmonella Gallinarum mutant (SG Fla+) elicits a pro-inflammatory response from avian epithelial cells and macrophages and is less virulent to chickens. Vet. Microbiol. 2013;165:425–433. doi: 10.1016/j.vetmic.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 60.Yim L, et al. Repression of flagella is a common trait in field isolates of Salmonella enterica Serovar Dublin and is associated with invasive human infections. Infect. Immun. 2014;82:1465–1476. doi: 10.1128/IAI.01336-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garrison, E. & Marth, G. Haplotype-based variant detection from short-read sequencing. Preprint at https://arxiv.org/abs/1207.3907 (2012).
- 63.Arndt D, et al. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016;44:W16–W21. doi: 10.1093/nar/gkw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Croucher NJ, et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res. 2015;43:e15. doi: 10.1093/nar/gku1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Page AJ, et al. SNP-sites: rapid efficient extraction of SNPs from multi-FASTA alignments. Microb. Genom. 2016;2:e000056. doi: 10.1099/mgen.0.000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nguyen LT, Schmidt HA, Haeseler, von A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating Maximum-Likelihood phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bankevich A, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell Sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feldgarden M, et al. Validating the AMRFinder tool and resistance gene database by using antimicrobial resistance genotype-phenotype correlations in a collection of isolates. Antimicrob. Agents Chemother. 2019;63:1–20. doi: 10.1128/AAC.00483-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carattoli A, et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014;58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oksanen, J. et al. vegan: Community Ecology Package (2015).
- 71.Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer International Publishing, 2016).
- 72.Baines SL, et al. Complete microbial genomes for public health in Australia and the Southwest Pacific. Microb. Genom. 2020;6:e000471–12. doi: 10.1099/mgen.0.000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chin C-S, et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat. Methods. 2013;10:563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- 74.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017;13:e1005595–22. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kolmogorov, M., Yuan, J., Lin, Y. & Pevzner, P. A. Assembly of long, error-prone reads using repeat graphs. Nat. Biotechnol. 37, 540–546 (2019). [DOI] [PubMed]
- 76.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 77.Rambaut A, Lam TT, Max Carvalho L, Pybus OG. Exploring the temporal structure of heterochronous sequences using TempEst (formerly Path-O-Gen) Virus Evol. 2016;2:vew007. doi: 10.1093/ve/vew007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suchard MA, et al. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018;4:170–175. doi: 10.1093/ve/vey016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baele G, Lemey P, Suchard MA. Genealogical working distributions for Bayesian model testing with phylogenetic uncertainty. Syst. Biol. 2016;65:250–264. doi: 10.1093/sysbio/syv083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gu Z, Gu L, Eils R, Schlesner M, Brors B. circlize implements and enhances circular visualization in R. Bioinformatics. 2014;30:2811–2812. doi: 10.1093/bioinformatics/btu393. [DOI] [PubMed] [Google Scholar]
- 81.Yu G, Smith DK, Zhu H, Guan Y, Lam TT-Y. ggtree: an rpackage for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 2017;8:28–36. doi: 10.1111/2041-210X.12628. [DOI] [Google Scholar]
- 82.Paradis E, Claude J, Strimmer K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 83.Volz EM, Didelot X. Modeling the growth and decline of pathogen effective population size provides insight into epidemic dynamics and drivers of antimicrobial resistance. Syst. Biol. 2018;67:719–728. doi: 10.1093/sysbio/syy007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rice P, Longden I, Bleasby A. EMBOSS: The European Molecular Biology Open Software Suite. Trends Genet. 2000;16:276–277. doi: 10.1016/S0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- 85.Guy L, Kultima JR, Andersson SGE. genoPlotR: comparative gene and genome visualization in R. Bioinformatics. 2010;26:2334–2335. doi: 10.1093/bioinformatics/btq413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tonkin-Hill, G. et al. Producing polished prokaryotic pangenomes with the Panaroo pipeline. Genome Biol.21, 180 (2020). [DOI] [PMC free article] [PubMed]
- 87.Hunt M, et al. ARIBA: rapid antimicrobial resistance genotyping directly from sequencing reads. Microb. Genom. 2017;3:e000131. doi: 10.1099/mgen.0.000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wickham H, et al. Welcome to the Tidyverse. JOSS. 2019;4:1686–1686. doi: 10.21105/joss.01686. [DOI] [Google Scholar]
- 89.Sueke H, et al. Pharmacokinetics of meropenem for use in bacterial keratitis. Invest. Ophthalmol. Vis. Sci. 2015;56:5731–5738. doi: 10.1167/iovs.15-17077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Supplementary Data 1 lists the individual accessions for all isolates, with associated metadata. Short-read data for Australian isolates in this study are available from the NCBI Sequence Read Archive (BioProject PRJNA319593 or PRJNA556438). Long-read data are available at the European Nucleotide Archive (ENA) under PRJEB41036, and individual accessions are provided in Supplementary Data 2. An interactive annotated phylogeny is available in Microreact https://microreact.org/project/mfxxBchBsUpsJu7nvfkFw4. Antimicrobial resistance genes were detected using AbritAMR (https://github.com/MDU-PHL/abritamr) in conjunction with the AMRFinder database v3.2.1 (https://github.com/ncbi/amr). Source data for phenotypic work are provided with this paper. Data supporting the findings of this study are available within the text and in Supplementary files. Source data are provided with this paper.