Abstract
Whether ICIs combined with chemotherapy can improve outcomes in EGFR-mutant non-small cell lung cancer (NSCLC) remains uncertain. Patients with EGFR-mutant NSCLC and who progressed on first-line EGFR-TKIs treatment were retrospectively collected. We reviewed the outcome of these patients treated with ICIs or ICIs combined chemotherapy (ICI + C). Total 30 patients were included. The ORR were 9.1% and 25.0% for the ICI and ICI + C groups. The ICI + C group showed the trend of longer progression-free survival and overall survival periods. Patients without the T790M mutation had a significantly longer PFS than did those without this mutation (4.23 [95% CI: 2.75–5.72] vs. 1.70 [95% CI: 0.00–3.51] months, HR:4.45, p = 0.019). ICIs combined with chemotherapy tended to be more effective than ICIs alone in pretreated EGFR-mutant NSCLC. The T790M mutation may be a potential biomarker.
Subject terms: Cancer therapy, Lung cancer, Oncogenes, Tumour immunology, Oncology
Introduction
The treatment of non–small cell lung cancer (NSCLC) has been transformed considerably in recent decades. Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) have been used as an effective treatment for EGFR-mutant NSCLC1. Compared with traditional chemotherapy, EGFR-TKI treatment is associated with higher response rates and prolonged survival. Nonetheless, most patients typically experience disease progression after 9–14 months of EGFR-TKI treatment2–6, primarily due to acquired resistance, which remains the main clinical challenge. Consequently, third-generation EGFR-TKIs have been developed to overcome TKI resistance, particularly in the driver oncogene T790M5,6. For patients without the T790M mutation or other resistance mechanisms, chemotherapy remains the standard subsequent treatment7,8. Nevertheless, therapeutic options are limited for patients once treatment with TKIs has been exhausted9.
Immune checkpoint inhibitors (ICIs) are emerging as novel therapeutic modalities for various malignancies. ICIs such as anti–programmed death 1 (PD-1) and anti–programmed death-ligand1 (PD-L1) have been approved for first- and second-line treatment of NSCLC. ICIs treatment as monotherapy or in combination with chemotherapy has been associated with greater survival compared with chemotherapy alone10–12. However, the administration of ICIs in EGFR-mutant cohorts has been reported to have suboptimal outcomes13–16. Serial clinical trials and meta-analyses have suggested that treatment with chemotherapy alone engendered higher survival in patients with EGFR mutations than did treatment with ICIs alone8,17. To overcome this obstacle, the administration of ICIs combined with chemotherapy has been associated with favorable outcomes in patients harboring EGFR mutations18–20. The IMpower150 study and PROLUNG study showed the clinical benefits of combination therapy in the EGFR-mutant population20,21. However, a direct comparison of late-line immune monotherapy and combination therapy in patients with oncogene variations is lacking. Furthermore, although patients selected in previous trials may have clinical benefits, information is lacking with regard to optimal predictive biomarkers22–25.
Accordingly, we conducted a retrospective study to compare the efficacy and clinical outcomes of treatment with ICIs alone and with ICIs in combination with chemotherapy in patients with metastatic EGFR-mutant NSCLC. Our objective was to demonstrate real-world applications of immunotherapy combined with chemotherapy in EGFR-mutant NSCLC.
Materials and methods
Patient cohort
This retrospective observational study was conducted in a tertiary medical center. We enrolled patients with stage IV EGFR-mutant NSCLC who received immunotherapy alone or immunotherapy in combination with chemotherapy as their subsequent treatment after disease progression. All patients had been treated with first- or second-generation EGFR-TKIs. We excluded patients with incomplete medical records. In addition, we excluded patients who received a combination of ICIs and anti- vascular endothelial growth factor (VEGF) inhibitors (e.g., bevacizumab) without any chemotoxicity agents. Data were collected between January 2014 and December 2019. We used the staging system described in the 7th edition of the American Joint Committee on Cancer.
Study design
We collected information on the patients’ clinical characteristics such as sex, smoking history, age, performance status (Eastern Cooperative Oncology Group [ECOG]), EGFR-mutation status, cancer staging, and treatment lines through a chart review. We divided the enrolled patients into two treatment groups: the ICI group, comprising those who received ICIs alone as their subsequent treatment, and ICI + C group, comprising those who received ICIs along with chemotherapy. The EGFR-mutation profile of each patient was obtained from a chart review. EGFR mutations were detected through the cobas EGFR Mutation Test v2. (Roche Molecular Systems Inc., Pleasanton, CA) using tissue samples or liquid biopsy. We analyzed tumor PD-L1 expression by using a PD-L1 IHC 22C3 assay (Dako, Carpinteria,
CA). The ICIs were anti-PD-1/PD-L1 antibodies, including nivolumab, pembrolizumab, atezolizumab, and durvalumab. We calculated progression-free survival (PFS) as the interval from the date of the treatment to the date of disease progression or death. We calculated overall survival (OS) as the interval from the date of the treatment to the date of death or last follow-up. The treatment response was assessed by a clinical physician according to Response Evaluation Criteria in Solid Tumors analysis (RECIST ver 1.1). All experiments were performed in accordance with the relevant guidelines and regulations. This study was approved and the informed consent was waived by the Institutional Review Board of Taipei Veterans General Hospital (2020-07-046CC).
Statistical analysis
We compared patients’ characteristics by using Pearson’s chi-square test or Fisher’s exact test. We performed a survival analysis using Kaplan–Meier survival curves for PFS and OS. The Cox proportional hazard regression model and logistic regression were applied to analyze clinical features and outcomes. A p value of < 0.05 was considered to be statistically significant, and all p values were two-sided. We used SPSS software (version 24.0, IBM Corp., Armonk, NY, USA) for all statistical analyses.
Ethical disclosure
This study was approved by the Institutional Review Board of Taipei Veterans General Hospital (2020-07-046CC).
Results
Patient characteristics
This study included a total of 30 patients. The median age was 66.5 years (45–85 years). Approximately 86.7% of the patients were never smokers, and 43.3% of the patients were men. Most of the patients (83.3%) had a favorable performance status (ECOG: 0–1). All patients had stage IV NSCLC with adenocarcinoma confirmed by histology. Moreover, all patients possessed EGFR mutations, with 56.7% having exon 19 deletion, 30.0% having an L858R mutation, and 13.3% having another uncommon mutation. The PD-L1 assay had been conducted for only 20% of the patients. The median number of treatment lines before ICIs therapy was 43–11. All patients had been treated with first- or second-generation EGFR-TKIs and subsequent chemotherapy. The objective response rate (ORR) was 76.7%. The median PFS for EGFR-TKIs was 12.20 months (95% confidence interval [CI]: 8.99–15.41). Before subsequent ICIs treatment, 53.3% of the patients had brain metastasis, and 10% of the patients had liver metastasis. Considering immunotherapy, 22 patients received immunotherapy alone (ICI group) and 8 patients received immunotherapy in combination with chemotherapy (ICI + C group). We observed no significant difference between the two treatment groups at baseline. Table 1 presents a summary of the characteristics. Different ICIs were used, including nivolumab (50.0%), pembrolizumab (6.7%), atezolizumab (10.0%), and durvalumab (6.7%). Moreover, 23.3% of the patients received nivolumab combined with chemotherapy, and 3.3% received pembrolizumab with chemotherapy (Supplementary Table 1).
Table 1.
Patients with EGFR mutations (n = 30).
| Patient characteristics (%) | All patients (n = 30) | ICI (n = 22) | ICI + C (n = 8) | p value |
|---|---|---|---|---|
| Sex | ||||
| Male | 13 (43.3) | 10 (45.5) | 3 (37.5) | 1.000 |
| Female | 17 (56.7) | 12 (54.5) | 5 (62.5) | |
| Median age | 66.5 (45–85) | 65.5 (45–78) | 67.5 (55–85) | 0.393 |
| Smoking | ||||
| Never smoker | 26 (86.7) | 18 (81.8) | 8 (100) | 0.550 |
| Ever smoker | 4 (13.3) | 4 (18.2) | 0 (0) | |
| ECOG | ||||
| 0–1 | 25 (83.3) | 18 (81.8) | 7 (87.5) | 1.000 |
| ≥ 2 | 5 (16.7) | 4 (18.2) | 1 (12.5) | |
| Median of previous treatment lines | 4 (3–11) | 5.5 (3–11) | 4 (3–6) | 0.185 |
| EGFR mutation | ||||
| Exon 19 deletion | 17 (56.7) | 13 (59.1) | 4 (50.0) | 0.848 |
| L858R | 9 (30.0) | 6 (27.3) | 3 (37.5) | |
| Uncommon mutation* | 4 (13.3) | 3 (13.6) | 1 (12.5) | |
| Liver metastasis | ||||
| Liver mets( −) | 27 (90.0) | 22 (100) | 5 (62.5) | 0.014 |
| Liver mets( +) | 3 (10.0) | 0 (0) | 3 (37.5) | |
| Brain metastasis | ||||
| Brain mets( −) | 14 (46.7) | 11 (50.0) | 3 (37.5) | 0.689 |
| Brain mets( +) | 16 (53.3) | 11 (50.0) | 5 (62.5) | |
*One exon 20 insertion, two G719X, one L861Q.
Treatment outcomes of immunotherapy and combination chemotherapy
The ORRs in the ICI and ICI + C groups were 9.1% and 25.0%, respectively. Furthermore, the disease control rates in the ICI and ICI + C groups were 54.6% and 87.5%, respectively (Fig. 1; Supplementary Table 2). Among the 30 patients, only 8 had documented immunotherapy-related toxicity, which ranged from grade 1 to 2 (skin rash, liver enzyme elevation, fatigue). The median follow-up period of this cohort was 16.76 months (95% CI: 8.49–25.04). The median PFS was 3.57 months (95% CI: 2.18–4.95) and OS was 22.77 months (95% CI: 7.18–38.36) of this study cohort. To divide the patients into two groups by their treatment: the ICI + C group had a slightly longer PFS period than did the ICI group (4.23 [95% CI: 3.03–5.43] vs. 2.93 [95% CI: 1.67–4.20] months; p = 0.599; Fig. 2A). In addition, the ICI + C group tended to have a longer OS period than did the ICI group (not reached vs. 19.67 [95% CI: 8.11–31.22] months; p = 0.270; Fig. 2B).
Figure 1.

Treatment response to ICI and ICI + C.
Figure 2.
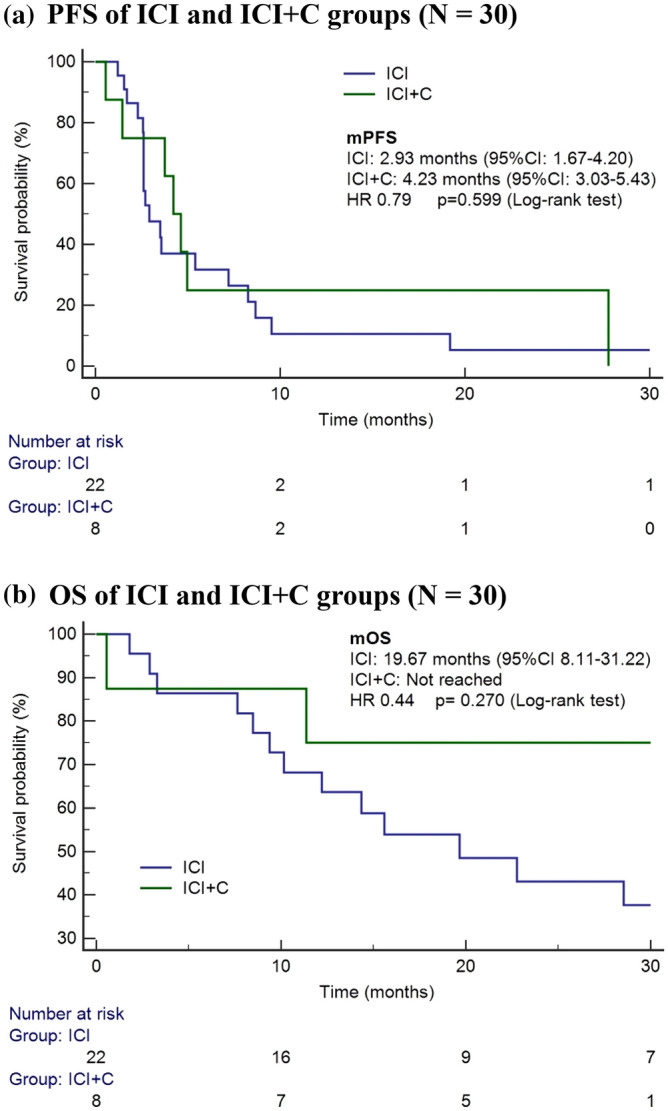
(a) PFS of ICI and ICI + C groups (n = 30). (b) OS of ICI and ICI + C groups ( n= 30).
Association between clinical significance and treatment outcomes
Among the 30 patients with EGFR mutations who were subsequently treated with immunotherapy or combination chemotherapy, those with liver metastasis had a higher risk of disease progression than did those without liver metastasis (hazard ratio [HR]: 11.07, 95% CI: 1.11–110.48; p = 0.041). Other clinical factors, including age, sex, smoking history, performance status, and brain metastasis, were not associated with PFS. A higher number of previous treatment lines was not associated with a higher risk of progression. We used the median PFS for prior EGFR-TKI treatment as the cutoff value and observed that the PFS for prior EGFR-TKI treatment was not associated with the PFS for ICIs treatment. After adjustment for clinical characteristics, the ICI + C group was associated with a longer PFS period compared with the ICI group (HR: 0.24, 95% CI: 0.06–0.97; p = 0.045; Table 2). However, factors such as liver metastasis (HR: 0.968, 95% CI: 0.04–32.28; p = 0.968) and combination chemotherapy (HR: 0.586, 95% CI: 0.05–5.32; p = 0.586) were not statistically associated with OS. Only the performance status of patients was significantly associated with OS; patients with poor status had shorter OS (HR: 24.09, 95% CI: 3.70–157.06; p = 0.001; Table 3).
Table 2.
Cox regression of factors related to PFS (n= 30).
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p value | Hazard ratio | 95% CI | p value | |
| Age | 1.00 | 0.96–1.05 | 0.992 | 1.03 | 0.96–1.09 | 0.431 |
| Female | 1.13 | 0.51–2.50 | 0.760 | 2.07 | 0.54–8.02 | 0.291 |
| Smoking history | 1.42 | 0.42–4.77 | 0.573 | 1.50 | 0.29–7.72 | 0.626 |
| ECOG ≥ 2 | 2.60 | 0.70–9.75 | 0.156 | 3.41 | 0.77–15.02 | 0.105 |
| Previous treatment lines > 4 | 0.89 | 0.41–1.95 | 0.773 | 0.59 | 0.21–1.64 | 0.312 |
| Median PFS of prior EGFR-TKI over 12 months | 0.84 | 0.39–1.82 | 0.660 | 0.61 | 0.21–1.77 | 0.366 |
| Uncommon mutation | 0.52 | 0.15–1.88 | 0.319 | 0.42 | 0.07–2.51 | 0.345 |
| Brain metastasis | 0.94 | 0.44–2.04 | 0.877 | 1.20 | 0.41–3.55 | 0.743 |
| Liver metastasis | 1.97 | 0.56–6.91 | 0.288 | 11.07 | 1.11–110.48 | 0.041 |
| Combination chemotherapy | 0.79 | 0.33–1.91 | 0.604 | 0.24 | 0.06–0.97 | 0.045 |
Table 3.
Cox regression of factors related to OS (n = 30).
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p value | Hazard ratio | 95% CI | p value | |
| Age | 0.98 | 0.93–1.03 | 0.429 | 0.98 | 0.92–1.05 | 0.602 |
| Female | 0.98 | 0.36–2.66 | 0.966 | 0.91 | 0.19–4.38 | 0.910 |
| Smoking history | 1.54 | 0.44–5.46 | 0.501 | 1.77 | 0.21–14.83 | 0.598 |
| ECOG ≥ 2 | 14.61 | 4.03–52.99 | <0.001 | 24.09 | 3.70–157.06 | 0.001 |
| Previous treatment lines > 4 | 1.95 | 0.72–5.31 | 0.190 | 1.08 | 0.24–4.79 | 0.921 |
| Median PFS of prior EGFR-TKI over 12 months | 0.93 | 0.34–2.51 | 0.881 | 0.58 | 0.16–2.11 | 0.406 |
| Uncommon mutation | 0.29 | 0.04–2.29 | 0.242 | 0.15 | 0.01–1.72 | 0.128 |
| Brain metastasis | 1.54 | 0.57–4.14 | 0.395 | 3.32 | 0.86–12.85 | 0.082 |
| Liver metastasis | 0.82 | 0.11–6.35 | 0.852 | 1.07 | 0.04–32.28 | 0.968 |
| Combination chemotherapy | 0.44 | 0.10–1.97 | 0.283 | 0.53 | 0.05–5.32 | 0.586 |
T790M mutation and treatment response
We then analyzed the patients for their EGFR mutation status and their treatment response to immunotherapy. We re-evaluated a total of 21 patients for their EGFR mutation profile (re-biopsy tissue or liquid biopsy) before ICIs treatment. These patients were divided into a T790M-positive group, namely T790M(+), and a T790M-negative group, namely T790M(−). Eighteen patients had no T790M mutation, and only 3 patients were found to be T790M positive. The baseline characteristics are presented in Supplementary Table 3, revealing no obvious difference between the two mutation groups. The PFS period was significantly longer in the T790M(−) group than in the T790M(+) group (4.23 [95% CI: 2.75–5.72] vs. 1.70 [95% CI: 0.00–3.51] months; p = 0.019; Fig. 3A). Furthermore, the T790M( −) group had a longer OS period than did the T790M( +) group (28.53 [95% CI: 16.81–40.26] vs. 10.17 [95% CI: 0.00–25.53] months; p = 0.014; Fig. 3B). Multivariate analysis also revealed that patients with T790M had greater risk of disease progression (HR: 35.46, 95% CI: 3.18–395.41; p = 0.004). The superior treatment response observed for front-line EGFR-TKI treatment (PFS longer than 12 months) was associated with the longer PFS for ICI treatment, regardless of whether combination chemotherapy was administered (HR: 0.16, 95% CI: 0.03–0.80; p = 0.025; Supplementary Table 4). These associations among clinical factors and prognosis were not noted in the OS analysis (Supplementary Table 5).
Figure 3.

(a) PFS of T790M mutation status (n = 21). (b) OS of T790M mutation status (n = 21).
Discussion
We present the results of patients with EGFR-mutant NSCLC who were treated with ICIs or ICIs combined with chemotherapy in a real-world setting. We found that ICIs in combination with chemotherapy showed a trend of superior efficacy along with acceptable treatment response compared with monotherapy involving ICIs. These results are consistent with those reported by previous studies18–20. Several possible mechanisms underlie the unsatisfactory efficacy of ICI therapy in patients with EGFR mutations. Patients with EGFR mutations have lower tumor mutation burden (TMB) levels and reduced tumor-infiltrating lymphocytes compared with other patients; they thus have lower immunogenicity and antitumor immunity22,23. The application of TKIs may affect PD-L1 expression and the tumor microenvironment25–29, both of which may have an adverse effect on the efficacy of immunotherapy. For example, a study proposed that chemotherapy may contribute to an increase in neoantigens and boost the reaction to immunotherapy30. Kuo et al. demonstrated that compared with ICI therapy alone, combining ICI therapy with chemotherapy improved survival in patients with NSCLC18. We confirmed this beneficial effect of combination chemotherapy with ICIs in the EGFR-mutant population. Cox regression analysis revealed improved PFS for the ICI + C group compared with the ICI group (HR: 0.24, 95% CI: 0.06–0.97; p = 0.045). This finding demonstrates a synergistic interaction between chemotherapy and ICIs. For clinicians, this study demonstrated that ICI alone in EGFR-mutant NSCLC has poor outcome. Concomitant chemotherapy may improve the benefits of immunotherapy, however, the optimal strategy for such combinations requires further investigation.
We evaluated the possible clinical characteristics affecting outcomes. After adjusting for age, sex, smoking history, performance status, and uncommon mutations, we observed that patients with liver metastasis may have poorer PFS than did those without such metastasis. A previous study also indicated that liver metastasis is an issue affecting immunotherapy because ICIs alone provide minimal therapeutic benefits31. This might be associated with the specific microenvironments and immunoregulation of the liver20,32. We observed that patients with EGFR-mutant NSCLC and liver metastasis showed inferior PFS. By analyzing our data, we identified that in late-line settings, liver metastasis was predictive of poor response. However, not all clinical factors were associated with OS; only poor ECOG showed any predictive value for patient survival. This might be because the patients were all heavily pretreated and the small number of study groups caused no significant OS results. Nevertheless, one can reasonably assume that the performance status may be useful for predicting OS in these patients, given the complicated condition of patients in this late-line setting.
To identify patients that may have benefited from therapy, several biomarkers have been studied for ICIs in EGFR-positive cohorts. Smoking status have been reported as a clinical predictor of response to ICIs in EGFR-mutant NSCLC33. A previous cohort study reported that T790M-negative patients may benefit from ICI treatment after TKI failure25. Yamada et al. also demonstrated that uncommon mutations and the absence of T790M mutations are predictive of positive ICIs outcomes24. A study on the IMMUNOTARGET cohort also supported the finding that different subtypes of EGFR mutations have different PFS periods after ICI treatment34. Lau et al. also reported the significant difference of treatment response to ICIs between common and uncommon mutations in retrospective study35. Patients with uncommon mutations may have a higher TMB16. In addition, patients showing acquired resistance not engendered by T790M mutations are likely to exhibit high PD-L1 levels24,25. High PD-L1 expression may be result from the activation of other alternative oncogenic pathway and these pateints may benefit from ICIs administration36. Our study confirmed that T790M remains a poor prognostic marker not only for ICIs alone but also for ICIs combined with chemotherapy. In addition, a longer duration of treatment with first-line EGFR-TKIs was associated with a longer PFS, but this finding is not consistent with previous reports. Ichihara et al. reported that the PFS for prior treatment with EGFR-TKIs was negatively associated with that for prior treatment with ICIs37. Liu et al. also reported a better response to subsequent immunotherapy in EGFR-mutant NSCLC with shorter PFS during EGFR-TKIs treatment38. They also conducted single cell RNA-sequencing to prove the different tumor microenvironment between longer and shorter first-line TKI treatment groups. Comparing to their study, our cohort focusing on those with late-line treatment group. Our patients may receive more lines of treatment after EGFR-TKIs failure. In this cohort, tumor mutation loads were more strongly affected by T790M mutation than by previous EGFR-TKI treatment response. Therefore, for PFS, prior treatment with EGFR-TKIs was less informative than T790M as a biomarker under clinical circumstances.
Our study has several limitations. First, the retrospective observational cohort study design has inherent restrictions on the data available for analysis. For example, some molecular profiles, such as PD-L1 expression, was evaluated only in a subset of patients. This was probably due to PD-L1 expression was not extensively applied in the late-line settings. However, PD-L1 expression has been reported to be a significant predictor of ICIs efficacy for EGFR-mutated NSCLC patients in the subgroup analysis of the ATLANTIC study39. Further investigation focusing on the role of PD-L1 expression in ICIs combined chemotherapy may offer more information. Similarly, there was no data of TMB reported. Second, the total sample size was small. Thirdly, the chemotherapy regimens in our study were heterogeneous, including single agent navelbine, docetaxel, and doublet combining navelbine plus gemcitabine (Supplement Table 1). One patient received paclitaxel, carboplatin and bevacizumab. The heterogenicity was due to the lack of standard-of-care in late-line treatment. Meanwhile, it also reflected the unmet need in clinical practice. Finally, the previous treatment pathways and chemotherapy combinations may have differed among patients. Nevertheless, this is the first study examining the efficacy and outcomes of ICIs administered alone and in combination with chemotherapy in patients with EGFR-mutant NSCLC in an area with a high prevalence of EGFR mutations.
Conclusion
ICIs combined with chemotherapy may be more effective and beneficial than ICIs alone in pretreated patients with EGFR-mutant NSCLC in the real world. Furthermore, the T790M mutation can be used as a predictive biomarker for poor response to treatments comprising both ICIs alone and ICIs combined chemotherapy.
Supplementary Information
Acknowledgements
The authors thank Wallace Academic Editing for English editing.
Author contributions
C.I.S and Y.M.C contributed to the conception establishment and drafting the manuscript. C.I.S, C.L.C, H.C.H and Y.H.L interpreted the clinical data and statistics. H.S.C, T.H.S, C.H.C and Y.M.C helped acquisition of the data and revise the work. All authors approve of the version to be published.
Funding
This work was supported by Taipei Veterans General Hospital, Taiwan [V109A–003] and Ministry of Science and Technology, Taiwan (MOST 109–2314-B-075–083-MY3).
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to patients’ privacy but are available from the corresponding author on reasonable request.
Competing interests
Yuh-Min Chen has received honoraria from Boehringer Ingelheim, Eli Lilly, Roche/Genentech/Chugai, MSD, Pfizer, Novartis, BMS, Ono Pharmaceutical, AstraZeneca, and Takeda Oncology; and served as advisor for Boehringer Ingelheim, Eli Lilly, Roche/Chugai, MSD, AstraZeneca, and Takeda Oncology. Chao-Hua Chiu has received honoraria from AstraZeneca, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Ono Pharmaceutical, Pfizer, and Roche. Chi-Lu Chiang has received honoraria from AstraZeneca, Boehringer Ingelheim, Roche, Merck Sharp & Dohme, Bristol-Myers Squibb and Ono Pharmaceutical. Other authors have nothing to disclose.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-95628-w.
References
- 1.Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J. Clin. Oncol. 2011;29(21):2866–2874. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 2.Yoshioka H, Shimokawa M, Seto T, Morita S, Yatabe Y, Okamoto I, et al. Final overall survival results of WJTOG3405, a randomized phase III trial comparing gefitinib versus cisplatin with docetaxel as the first-line treatment for patients with stage IIIB/IV or postoperative recurrent EGFR mutation-positive non-small-cell lung cancer. Ann. Oncol. 2019;30(12):1978–1984. doi: 10.1093/annonc/mdz399. [DOI] [PubMed] [Google Scholar]
- 3.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 4.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 5.Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small cell lung cancer. N. Engl. J. Med. 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 6.Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N. Engl. J. Med. 2017;376(7):629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N. Engl. J. Med. 2015;372(18):1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 8.Cavanna L, Citterio C, Orlandi E. Immune checkpoint inhibitors in EGFR-mutation positive TKI-treated patients with advanced non-small-cell lung cancer network meta-analysis. Oncotarget. 2019;10(2):209–215. doi: 10.18632/oncotarget.26541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piper-Vallillo, A. J., Sequist, L. V. & Piotrowska, Z. Emerging treatment paradigms for EGFR-mutant lung cancers progressing on osimertinib: A review. J. Clin. Oncol. JCO1903123 (2020). [DOI] [PubMed]
- 10.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small cell lung cancer. N. Engl. J. Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 11.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 12.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small cell lung cancer. N. Engl. J. Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 13.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small cell lung cancer. N. Engl. J. Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 15.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hastings K, Yu HA, Wei W, Sanchez-Vega F, DeVeaux M, Choi J, et al. EGFR mutation subtypes and response to immune checkpoint blockade treatment in non-small-cell lung cancer. Ann. Oncol. 2019;30(8):1311–1320. doi: 10.1093/annonc/mdz141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gettinger S, Rizvi NA, Chow LQ, Borghaei H, Brahmer J, Ready N, et al. Nivolumab monotherapy for first-line treatment of advanced non-small cell lung cancer. J. Clin. Oncol. 2016;34(25):2980–2987. doi: 10.1200/JCO.2016.66.9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo CS, Wang CC, Huang YC, Pavlidis S, Liu CY, Ko HW, et al. Comparison of a combination of chemotherapy and immune checkpoint inhibitors and immune checkpoint inhibitors alone for the treatment of advanced and metastatic non-small cell lung cancer. Thorac. Cancer. 2019;10(5):1158–1166. doi: 10.1111/1759-7714.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arrieta O, Barron F, Ramirez-Tirado LA, Zatarain-Barron ZL, Cardona AF, Diaz-Garcia D, et al. Efficacy and safety of pembrolizumab plus docetaxel vs docetaxel alone in patients with previously treated advanced non-small cell lung cancer: The PROLUNG phase 2 randomized clinical trial. JAMA Oncol. 2020;6:856–864. doi: 10.1001/jamaoncol.2020.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir. Med. 2019;7(5):387–401. doi: 10.1016/S2213-2600(19)30084-0. [DOI] [PubMed] [Google Scholar]
- 21.Bylicki O, Guisier F, Monnet I, Doubre H, Gervais R, Janicot H, et al. Efficacy and safety of programmed cell-death-protein-1 and its ligand inhibitors in pretreated patients with epidermal growth-factor receptor-mutated or anaplastic lymphoma kinase-translocated lung adenocarcinoma. Medicine (Baltimore) 2020;99(3):e18726. doi: 10.1097/MD.0000000000018726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mhanna L, Guibert N, Milia J, Mazieres J. When to consider immune checkpoint inhibitors in oncogene-driven non-small cell lung cancer? Curr. Treat. Options Oncol. 2019;20(7):60. doi: 10.1007/s11864-019-0652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin R, Zhao J, Xia L, Li Q, Li W, Peng L, et al. Application of immune checkpoint inhibitors in EGFR-mutant non-small-cell lung cancer: From bed to bench. Ther. Adv. Med. Oncol. 2020;12:1758835920930333. doi: 10.1177/1758835920930333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamada T, Hirai S, Katayama Y, Yoshimura A, Shiotsu S, Watanabe S, et al. Retrospective efficacy analysis of immune checkpoint inhibitors in patients with EGFR-mutated non-small cell lung cancer. Cancer Med. 2019;8(4):1521–1529. doi: 10.1002/cam4.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haratani K, Hayashi H, Tanaka T, Kaneda H, Togashi Y, Sakai K, et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann. Oncol. 2017;28(7):1532–1539. doi: 10.1093/annonc/mdx183. [DOI] [PubMed] [Google Scholar]
- 26.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3(12):1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong ZY, Zhang JT, Liu SY, Su J, Zhang C, Xie Z, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology. 2017;6(11):e1356145. doi: 10.1080/2162402X.2017.1356145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann. Oncol. 2014;25(10):1935–1940. doi: 10.1093/annonc/mdu242. [DOI] [PubMed] [Google Scholar]
- 29.Lin A, Wei T, Meng H, Luo P, Zhang J. Role of the dynamic tumor microenvironment in controversies regarding immune checkpoint inhibitors for the treatment of non-small cell lung cancer (NSCLC) with EGFR mutations. Mol. Cancer. 2019;18(1):139. doi: 10.1186/s12943-019-1062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Donnell T, Christie EL, Ahuja A, Buros J, Aksoy BA, Bowtell DDL, et al. Chemotherapy weakly contributes to predicted neoantigen expression in ovarian cancer. BMC Cancer. 2018;18(1):87. doi: 10.1186/s12885-017-3825-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol. Res. 2017;5(5):417–424. doi: 10.1158/2326-6066.CIR-16-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pao W, Ooi CH, Birzele F, Ruefli-Brasse A, Cannarile MA, Reis B, et al. Tissue-specific immunoregulation: A call for better understanding of the “immunostat” in the context of cancer. Cancer Discov. 2018;8(4):395–402. doi: 10.1158/2159-8290.CD-17-1320. [DOI] [PubMed] [Google Scholar]
- 33.Garassino MC, Gelibter AJ, Grossi F, Chiari R, Soto Parra H, Cascinu S, et al. Italian nivolumab expanded access program in nonsquamous non-small cell lung cancer patients: Results in never-smokers and EGFR-mutant patients. J. Thorac. Oncol. 2018;13(8):1146–1155. doi: 10.1016/j.jtho.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 34.Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann. Oncol. 2019;30(8):1321–1328. doi: 10.1093/annonc/mdz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau, S. C. M., Fares, A. F., Le, L. W., Mackay, K. M., Soberano, S., Chan, S. W. et al. Subtypes of EGFR- and HER2-mutant metastatic NSCLC influence response to immune checkpoint inhibitors. Clin. Lung Cancer (2021). [DOI] [PubMed]
- 36.Liu J, Itchins M, Nagrial A, Cooper WA, De Silva M, Barnet M, et al. Relationship between PD-L1 expression and outcome in EGFR-mutant lung cancer patients treated with EGFR tyrosine kinase inhibitors. Lung Cancer. 2021;155:28–33. doi: 10.1016/j.lungcan.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Ichihara, E., Harada, D., Inoue, K., Shibayama, T., Hosokawa, S., Kishino, D. et al. Characteristics of patients with EGFR-mutant non-small-cell lung cancer who benefited from immune checkpoint inhibitors. Cancer Immunol. Immunother. (2020). [DOI] [PMC free article] [PubMed]
- 38.Liu S, Wu F, Li X, Zhao C, Jia Y, Jia K, et al. Patients with short PFS to EGFR-TKIs predicted better response to subsequent anti-PD-1/PD-L1 based immunotherapy in EGFR common mutation NSCLC. Front. Oncol. 2021;11:639947. doi: 10.3389/fonc.2021.639947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garassino MC, Cho BC, Kim JH, Mazieres J, Vansteenkiste J, Lena H, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): An open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19(4):521–536. doi: 10.1016/S1470-2045(18)30144-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available due to patients’ privacy but are available from the corresponding author on reasonable request.


