Abstract
TroponinT levels are frequently elevated after subarachnoid hemorrhage (SAH). However, their clinical impact on long term outcomes still remains unclear. This study evaluates the association of TroponinT and functional outcomes 3 months after SAH. Data were obtained in the frame of a randomized controlled trial exploring the association of Goal-directed hemodynamic therapy and outcomes after SAH (NCT01832389). TroponinT was measured daily for the first 14 days after admission or until discharge from the ICU. Outcome was assessed using Glasgow Outcome Scale (GOS) 3 months after discharge. Logistic regression was used to explore the association between initial TroponinT values stratified by tertiles and admission as well as outcome parameters. TroponinT measurements were analyzed in 105 patients. TroponinT values at admission were associated with outcome assessed by GOS in a univariate analysis. TroponinT was not predictive of vasospasm or delayed cerebral ischemia, but an association with pulmonary and cardiac complications was observed. After adjustment for age, history of arterial hypertension and World Federation of Neurosurgical Societies (WFNS) grade, TroponinT levels at admission were not independently associated with worse outcome (GOS 1–3) or death at 3 months. In summary, TroponinT levels at admission are associated with 3 months-GOS but have limited ability to independently predict outcome after SAH.
Subject terms: Neuroscience, Neurology
Introduction
Cardiac irregularities after subarachnoid hemorrhage (SAH) are common and include a wide range of manifestations like hyper- and hypotension, arrhythmias, myocardial injury or cardiac failure1,2. A majority of SAH patients develops electrocardiogram (ECG)—changes and more than 50% have increased Troponin levels3. Besides, some patients develop a severe form of stress-induced cardiomyopathy, neurogenic stunned myocardium (NSM) characterized by depressed ventricular function and regional wall motion abnormality (RWMA)4.
Numerous studies have explored the association of these findings with complications and outcome3,5,6. RWMAs, elevated NT-proBNP levels as well as some ECG changes, for example ST-segment depression, were significantly associated with an increased risk of death7. Although the elevation of cardiac troponins has been the topic of several investigations, its impact on outcome remains unclear. While there are studies linking cardiac troponin elevation with long-term outcome8, the association was not present after discharge in other trials3. Therefore, the aim of this study was to evaluate whether hsTroponinT values after SAH are associated with functional outcome at 3 months.
Methods
Study design
Troponin T measurements were obtained prospectively in the frame of a randomized, controlled clinical trial exploring the association of goal-directed hemodynamic therapy and outcomes after SAH at a University Hospital in Germany (Klinikum rechts der Isar, Technical University Munich)9. The study was approved by the local ethics committee (Ethics Commission of the Medical faculty, Technical University of Munich, ID: 5425-12) and was first registered at 16/04/2013 at clinicaltrials.gov; Identifier: NCT01832389. All research was performed in accordance with the Declaration of Helsinki.
Patients older than 18 with an aneurysmal SAH diagnosed by CT and confirmed by angiography were eligible for enrollment. Exclusion criteria were: traumatic SAH, congestive heart failure, severe diseases of aorta or aortic valve, pregnancy, calcium antagonist intolerance. Written consent was obtained from each patient or their legal representative. Clinical management was performed according to current guidelines and was described previously in detail9,10.
Data collection
Outcome was assessed 3 months after discharge using Glasgow Outcome Scale (GOS): GOS 1 = death, GOS 2 = persistent vegetative state, GOS 3 = severe disability, GOS 4 = moderate disability and GOS 5 = good recovery11. The participants were usually seen in the neurosurgical outpatient department by a neurosurgical consultant unaware of measurement results, otherwise they were contacted by telephone.
To describe medical history at admission the following factors were considered: arterial hypertension, history of coronary artery disease, arrhythmia, history of cerebrovascular disease, tobacco use, history of asthma/chronic obstructive pulmonary disease (COPD), diabetes mellitus and chronic kidney disease. Further, the severity of SAH was assessed using Hunt–Hess grade, World Federation of Neurologic Surgeons (WFNS) grade and modified Fisher scale, taking into consideration the amount of blood in the initial CT scan12–14.
Complications were assessed as follows: cardiac complications (myocardial infarction, hemodynamic relevant arrhythmia, heart failure), pulmonary complications (pulmonary edema, pneumonia, pulmonary embolism), cerebrovascular complications (vasospasm, delayed cerebral ischemia (DCI), need for intra-arterial vasodilator therapy). Cardiac and pulmonary complications were assessed based on the current clinical practice involving symptoms, laboratory and radiological parameters, as well as current guidelines15. Vasospasm was diagnosed by daily transcranial Doppler measurements and defined as peak-value increase by > 50 cm/sec/24 h compared to the previous result or a mean value > 120 cm/sec in one of the main supply branches16. DCI was defined as a new focal neurological deficit or a cerebral infarction in the presence of vasospasm revealed by radiologic imaging, or both. Other causes of neurological aggravation had to be excluded17,18.
Troponin T measurement
Troponin T was measured daily starting from admission for consecutive 14 days or, if shorter, until discharge from ICU. For troponin T measurements the Roche high-sensitive troponin T Elecsys-assay was used in our laboratory.
Statistical analysis
Variables are presented as median and interquartile ranges or as mean and standard deviation, as appropriate. Continuous, normally distributed variables were compared using a two-sided Student’s t test, and a Mann Whitney-U-test was used for variables with skewed distributions. Spearman rank test was used to test correlations in non-normally distributed variables. Troponin T values at admission were divided into tertiles. Logistic regression models were applied to assess the relation between initial troponin T values and admission as well as outcome parameters. In order to test whether troponin T is an independent risk factor for worse outcome, a multivariate regression was performed considering age, history of arterial hypertension and WFNS score according to SAHIT core criteria19. All statistical tests were done using SPSS statistics 27 (IBM SPSS, Inc.). A p value < 0.05 was considered statistically significant.
Results
Patients were enrolled between March 2013 and December 20159. Both, outcome and troponin t data, were available in 106 patients. From the initially included 108 patients two were excluded: one due to missing outcome data, the other due to missing troponin T data.
The median age was 55 years and 81% of the patients were female. Almost half of the patients had a history of arterial hypertension and 8% had a history of coronary artery disease. An overview of baseline characteristics is presented in Table 1. 54% of the aneurysms were secured by coiling and 46% were clipped. The median interval from bleeding to surgery was 10 h [6–20 h] (median [interquartile range]). At admission, mean arterial pressure (MAP) was 95 mmHg [83–110 mmHg] and systolic blood pressure was 140 mmHg [120–160 mmHg].
Table 1.
Patient characteristics at admission.
| Characteristics | n (%) |
|---|---|
| Age—year [median (interquartile range)] | 55 (47–69) |
| Female sex | 81 (76) |
| Hunt–Hess grade | |
| 1 | 14 (13) |
| 2 | 34 (32) |
| 3 | 27 (26) |
| 4 | 23 (22) |
| 5 | 8 (8) |
| WFNS grade | |
| 1 | 45 (43) |
| 2 | 13 (12) |
| 3 | 8 (8) |
| 4 | 7 (7) |
| 5 | 32 (30) |
| Modified Fisher scale | |
| 1 | 23 (22) |
| 2 | 10 (9) |
| 3 | 29 (27) |
| 4 | 44 (42) |
| Aneurysm location | |
| AcoA/ACA | 38 (36) |
| MCA | 23 (22) |
| ACoP | 14 (13) |
| ICA | 5 (5) |
| Other | 26 (24) |
| Medical history at admission | |
| Arterial hypertension | 50 (47) |
| Coronary artery disease | 8 (8) |
| Cardiac arrhythmia | 8 (8) |
| Cerebrovascular disease | 1 (1) |
| Asthma/COPD | 11 (10) |
| Tobacco use | 32 (30) |
| Diabetes mellitus | 5 (5) |
| Chronic kidney disease | 4 (4) |
WFNS World Federation of Neurosurgeons, ACoA Arteria communicans anterior, ACA Arteria cerebri anterior, MCA Arteria cerebri media, ACoP Arteria communicans posterior, ICA Arteria carotis interna, COPD Chronic obstructive pulmonary disease.
Troponin T profiles
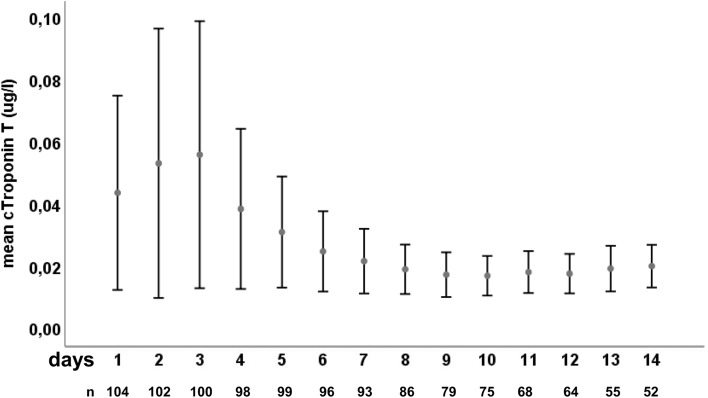
Troponin T measurements were available in 104 patients (98%) at admission (ICU day 1). The median interval between admission and troponin T peak was 2 days [1–5 days]. In 36% of the patients, the first measurement with a value of 0.011 µg/L [0.005–0.031 µg/L] was the highest. There was a close correlation between the initial troponin T measurement and the peak troponin T value (r = 0.79, p < 0.01). Mean troponin T values were higher (p < 0.001) on days 1–3 (0.054 ± 0.14 µg/L; mean ± SD) compared to days 4–7 (0.027 ± 0.053 µg/L) and days 8–14 (0.016 ± 0.021 µg/L). An overview of troponin T distribution over time is shown in Fig. 1. Troponin T levels at admission were significantly higher in patients with Hunt–Hess grades 3–5 (p < 0.001) and WFNS grades 4–5 (p < 0.001), whereas there was no difference in modified Fisher scale (p = 0.054).
Figure 1.
Troponin T levels over time. Mean high sensitive Troponin T levels on the first 14 days after subarachnoid hemorrhage (SAH). Bars represent 95% confidence interval (CI). Day 1 refers to admission.
Outcome
GOS was assessed 3 months after discharge: GOS5: 58 patients (55%), GOS4: 10 (9%), GOS3: 12 (11%), GOS2: 12 (11%), GOS1: 14 (13%). There was a significant association between GOS and troponin T values at all 14 days (p < 0.05). Patients with good recovery (GOS4–5) 3 months after admission had lower initial troponin T levels than patients with GOS 1–3 (0.009 µg/L [0.005–0.019] vs. 0.015 µg/L [0.009–0.065], p = 0.013) (Fig. 2). Further, patients with GOS 1 (death) at 3 months had higher troponin T levels at admission than patients who survived after 3 months (GOS 2–5) (0.023 µg/L [0.011–0.045] vs. 0.010 µg/L [0.005–0.022], p = 0.024).
Figure 2.
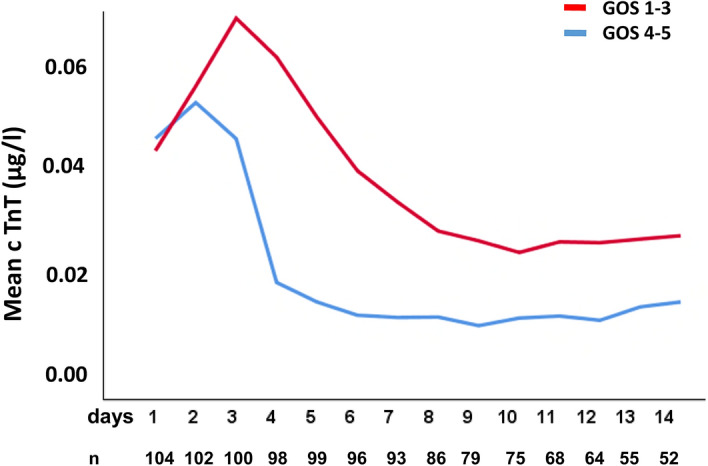
Comparison of Troponin T levels in patients with good and poor recovery. Troponin T levels were significantly higher from admission for further 14 days in patients with moderate to poor recovery (GOS1–3) than in patients with good recovery (GOS4–5), p < 0.05.
Cardiac complications occurred in 9% of the cases. Ten patients (9%) had a therapy-relevant arrhythmia and one case of congestive heart failure was registered (1%). None of the patients developed a myocardial infarction. Troponin T values at admission were associated with cardiac complications: patients with cardiac complications had higher troponin T levels than those without (0.027 µg/L [0.009–0.071] vs. 0.011 µg/L [0.005–0.025], p = 0.040). 23% of the patients developed pulmonary complications and showed higher troponin T levels at admission compared to those who did not (0.026 µg/L [0.008–0.077] vs. 0.010 µg/L [0.005–0.020], p = 0.007). Vasospasm was observed in 55% of the patients and occurred on day 4 [3–6]. There was no significant difference in initial troponin T levels between those who developed vasospasm and those who did not (0.010 µg/L [0.005–0.029] vs. 0.014 µg/L [0.006–0.032], p = 0.325). Intra-arterial vasodilator therapy was performed in 10% of the cases with no significant differences in initial troponin T levels between patients requiring the therapy and those who did not (0.005 µg/L [0.004–0.065] vs. 0.012 µg/L [0.005–0.03], p = 0.695). Further, 23% of the patients developed DCI on day 5 [4–8]. Similarly, no significant difference was observed in patients developing DCI (23%) and those who did not (initial troponin T: 0.010 µg/L [0.005–0.029] vs. 0.015 µg/L [0.005–0.025], p = 0.830).
In order to assess the relationship between troponin T levels and admission as well as outcome parameters, troponin T values at admission were stratified by tertiles (lowest: troponin T < 0,007 µg/L; intermediate: 0.007–0.019 µg/L; highest > 0.019 µg/L). An overview is shown in Table 2. Troponin T tertiles at admission were significantly associated with death (GOS = 1) or worse outcome at 3 months (GOS = 1–3) (Table 3). After adjustment for age and Hunt–Hess grade, this association was no longer present for death (OR [95% CI] 1.808 [0.717–4.563], p = 0.21) or for worse outcomes at 3 months (GOS1–4) (OR [95% CI] 0.977 [0.507–1.884], p = 0.946). Similarly, there was no association in the multivariate analysis when troponin T was considered as a continuous variable for death (mortality: OR [95% CI] 2.769 [0.102–75.29], p = 0.546, or for unfavorable outcomes at 3 months (GOS1–3) (OR [95% CI] 0.093 [0.003–3.163], p = 0.187)).
Table 2.
Complications after SAH stratified by Troponin T tertiles at admission.
| Troponin T tertiles at admission | ||||
|---|---|---|---|---|
| Total n(%) | Lowest 0–0.007[µg/L] n = 34 |
Intermediate 0.007–0.019[µg/L] n = 34 |
Highest > 0.019[µg/L] n = 36 |
|
| GOS 1–3 (unfavourable outcome) | 38 (37) | 7 (21) | 13 (38) | 18 (50) |
| GOS 1 (death) | 14 (13) | 1 (3) | 5 (15) | 8 (22) |
| Vasospasm | 56 (54) | 21 (62) | 16 (47) | 19 (53) |
| Intra-arterial vasodilator therapy | 11 (10) | 6 (18) | 1 (3) | 4 (11) |
| Delayed cerebral ischemia (DCI) | 22 (21) | 9 (26) | 5 (15) | 8 (22) |
| Cardiac complications | 10 (10) | 1 (3) | 2 (6) | 7 (19) |
| Pulmonary complications | 24 (23) | 5 (15) | 5 (15) | 14 (39) |
GOS Glasgow Outcome Scale.
Table 3.
Admission characteristics and outcomes associated with Troponin T.
| n (%) | OR (95% CI) for Troponin T tertiles at admission | P | |
|---|---|---|---|
| Admission characteristics | |||
| Hunt–Hess grade 3–5 | 58 (56) | 2.703 (1.589–4.598) | < 0.001 |
| WFNS grade 4–5 | 39 (38) | 2.545 (1.480–4.375) | 0.001 |
| Modified Fisher Scale 3–4 | 73 (70) | 1.582 (0.934–2.678) | 0.088 |
| Outcome parameters | |||
| GOS 1–3 (unfavourable outcome) | 38 (37) | 1.933 (1.153–3.240) | 0.012 |
| GOS 1 (death) | 14 (13) | 2.514 (1.121–5.640) | 0.025 |
| Vasospasm | 56 (54) | 0.837 (0.522–1.342) | 0.461 |
| Intra-arterial vasodilator therapy | 11 (10) | 0.713 (0.328–1.551) | 0.394 |
| Delayed cerebral ischemia (DCI) | 22 (21) | 0.885 (0.498–1.572) | 0.677 |
| Cardiac complications | 10 (10) | 3.094 (1.115–8.591) | 0.030 |
| Pulmonary complications | 24 (23) | 2.076 (1.13–3.814) | 0.019 |
GOS Glasgow Outcome Scale.
Discussion
In this prospective study, we were able to show that troponin T levels measured for the first 14 days are associated with 3 months outcomes assessed by GOS. However, when adjusted for age and Hunt–Hess scores, troponin T levels at admission were not independently predictive of death (GOS 1) or worse outcomes (GOS 1–3) at 3 months.
Cardiac impairment and its impact on outcomes after SAH has been an object of great interest in recent years20–23. While there is widely agreement about the mechanism behind these findings involving abnormal sympathetic innervation with an excessive norepinephrine release, their impact on short and long term outcome remains controversial24. There are only very few studies who have prospectively evaluated the association between cardiac troponins and outcome after SAH, and only two of them used a high sensitive troponin essay similar to the one in the present study8,25. To the best of our knowledge, the present study is the first one prospectively conducting serial hs troponin T measurements for 14 days after admission.
We were able to show that initial troponin T levels are strongly correlated to peak troponin T levels. Additionally, there was an association in the univariate analysis between troponin T levels at admission and severity scores like Hunt-Hess and WFNS grade. Both findings confirm previously published results and support the hypothesis that troponin T elevation is likely to reflect an initial SAH severity with its influence on myocardial dysfunction26. Neither vasospasm nor DCI nor the need for intra-arterial vasodilator therapy showed an association with initial troponin T levels in our study. Neither did the modified Fisher scale which is closely related to vasospasm14. Due to the disparity of definitions of vasospasm and DCI in previous trials, the results in this field are poorly comparable. While no association between cardiac troponins and vasospasm was found by some investigators27, other reported a relation between cardiac troponins and DCI7,8. For further research, standardized definitions and approaches are needed in this field and, according to our data, considerably larger sample sizes are warranted in order to draw robust conclusions considering vasospasm and DCI. Further, our work confirms an association between troponin T levels and cardiac as well as pulmonary complications28. Finally, while there was an association in the univariate analysis between troponin T levels and 3 months outcomes at all 14 days of measurement, the relation between troponin T tertiles at admission and death as well as worse outcome was no longer present in the multivariate analysis. This finding is in line with previously published retrospective data and even expands those due to the prospective approach3,5. Moreover, in a multicenter cohort study, regional wall motion abnormalities (RWMA) after SAH were found to be an independent predictor of outcome after SAH while troponin T elevation was not7. Our results confirm the latter observation, but there are also trials suggesting a causal relationship between the early biomarker release such as troponin T and outcome after SAH8,25. Further research in a preferably multicenter setting is warranted to clarify the impact of the elevated biomarker levels on the outcome after SAH.
Our study has several limitations. First, this study does not investigate the mechanisms behind the biomarker elevation nor does it consider other cardiac parameters. Second, it is a single center trial with a limited number of patients. Nevertheless, the data were obtained prospectively and continuously. Besides, troponin T as well as 3 months outcome data were collected in all included patients. Third, our follow-up period ended at 3 months. We cannot preclude that a longer follow-up might have led to different results.
Nevertheless, our results add to previous literature as the high sensitive troponin T essay was used as suggested by the current guidelines29. We were able to show that troponin T measurements are associated with outcome after SAH as well as cardiovascular and pulmonary complications and might be useful in risk stratification of SAH patients. However, our results suggest that troponin T has limited ability to independently predict outcome after SAH. Further studies are needed to test the utility of hsTroponin T measurements in the early phase after SAH.
Acknowledgements
The authors are deeply thankful to all ICU-personnel for their support and their contribution to data collection.
Abbreviations
- CT
Computer tomography
- DCI
Delayed cerebral ischemia
- GCS
Glasgow Coma Scale
- GDHT
Goal directed hemodynamic therapy
- GOS
Glasgow Outcome Scale
- HR
Hazard ratio
- MAP
Mean arterial pressure
- NSM
Neurogenic stunned myocardium
- OR
Odds ratio
- RW
Regional wall motion abnormality
- SAH
Subarachnoid hemorrhage
- SD
Standard deviation
- TCD
Transcranial Doppler
- WFNS
World Federation of Neurosurgical Societies
Author contributions
A.A., B.J., J.G.: study design, data collection, data analysis, manuscript draft; M.B., F.R., R.B.: study design, data collection, critical feedback on the manuscript; M.H., M.W., I.B., M.G., G.S., B.M., L.B.: data interpretation, critical feedback on manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Competing interests
JG: Consulting: BrainLab, Funded Research: Zeiss; MB: grants and personal fees from MSD, personal fees from Grünenthal, and personal fees from GE Healthcare outside the submitted work; BM: Honoraria: Brainlab, Relievant, DepuySynthes, Icotec, Medtronic, Spineart, Ulrich Medical; Consulting/Advisory Board: Relievant, DepuySynthes, Icotec, Medtronic, Spineart; Funded research: Brainlab, Relievant, Icotec, Medtronic, Ulrich Medical, Royalties/Patent: Spineart; all other authors declared no conflicts of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wartenberg KE, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit. Care Med. 2006;3:617–623. doi: 10.1097/01.CCM.0000201903.46435.35. [DOI] [PubMed] [Google Scholar]
- 2.Van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–318. doi: 10.1016/S0140-6736(07)60153-6. [DOI] [PubMed] [Google Scholar]
- 3.Naidech AM, et al. Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage. Circulation. 2005;112:2851–2856. doi: 10.1161/CIRCULATIONAHA.105.533620. [DOI] [PubMed] [Google Scholar]
- 4.Kilbourn KJ, Levy S, Staff I, Kureshi I, McCullough L. Clinical characteristics and outcomes of neurogenic stress cadiomyopathy in aneurysmal subarachnoid hemorrhage. Clin. Neurol. Neurosurg. 2013;115:909–914. doi: 10.1016/j.clineuro.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Norberg E, Odenstedt-Herges H, Rydenhag B, Oras J. Impact of acute cardiac complications after subarachnoid hemorrhage on long-term mortality and cardiovascular events. Neurocrit. Care. 2018;29:404–412. doi: 10.1007/s12028-018-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Megjhani M, et al. Heart rate variability as a biomarker of neurocardiogenic injury after subarachnoid hemorrhage. Neurocrit. Care. 2020;32:162–171. doi: 10.1007/s12028-019-00734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Bilt I, et al. Cardiac dysfunction after aneurysmal subarachnoid hemorrhage: relationship with outcome. Neurology. 2014;82:351–358. doi: 10.1212/WNL.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 8.Oras J, Grivans C, Bartley A, Rydenhag B, Ricksten SE, Seeman-Lodding H. Elevated high-sensitive troponin T on admission is an indicator of poor long-term outcome in patients with subarachnoid haemorrhage: a prospective observational study. Crit. Care. 2016;20:11. doi: 10.1186/s13054-015-1181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anetsberger A, et al. Impact of goal-directed therapy on delayed ischemia after aneurysmal subarachnoid hemorrhage: randomized controlled trial. Stroke. 2020;51:2287–2296. doi: 10.1161/STROKEAHA.120.029279. [DOI] [PubMed] [Google Scholar]
- 10.Connolly ES, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage. Stroke. 2012;43:1711–1737. doi: 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- 11.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/S0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 12.Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J. Neurosurg. 1968;28:14–20. doi: 10.3171/jns.1968.28.1.0014. [DOI] [PubMed] [Google Scholar]
- 13.Drake C. Report of world federation of neurological surgeons committee on a universal subarachnoid hemorrhage grading scale. J. Neurosurg. 1988;68:985–986. doi: 10.3171/jns.1988.68.6.0985. [DOI] [PubMed] [Google Scholar]
- 14.Frontera JA, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery. 2006;59:21–27. doi: 10.1227/01.NEU.0000218821.34014.1B. [DOI] [PubMed] [Google Scholar]
- 15.Thygesen K, the Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction et al. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2533. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 16.Grosset DG, Straiton J, du Trevou M, Bullock R. Prediction of symptomatic vasospasm after subarachnoid hemorrhage by rapidly increasing transcranial Doppler velocity and cerebral blood flow changes. Stroke. 1992;23:674–679. doi: 10.1161/01.STR.23.5.674. [DOI] [PubMed] [Google Scholar]
- 17.Rosengart AJ, Schultheiss KE, Tolentino J, Macdonald RL. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:2315–2321. doi: 10.1161/STROKEAHA.107.484360. [DOI] [PubMed] [Google Scholar]
- 18.Vergouwen MD, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41:2391–2395. doi: 10.1161/STROKEAHA.110.589275. [DOI] [PubMed] [Google Scholar]
- 19.Jaja BNR, et al. Development and validation of outcome prediction models for aneurysmal subarachnoid haemorrhage: the SAHIT multinational cohort study. BMJ. 2018;360:j5745. doi: 10.1136/bmj.j5745. [DOI] [PubMed] [Google Scholar]
- 20.Bruder N, Rabinstein A, Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage Cardiovascular and pulmonary complications of aneurysmal subarachnoid hemorrhage. Neurocrit. Care. 2011;15:257–269. doi: 10.1007/s12028-011-9598-4. [DOI] [PubMed] [Google Scholar]
- 21.Zaroff JG, et al. Cardiovascular predictors of long-term outcomes after non-traumatic subarachnoid hemorrhage. Neurocrit. Care. 2012;17:374–381. doi: 10.1007/s12028-011-9592-x. [DOI] [PubMed] [Google Scholar]
- 22.Yarlagadda S, et al. Cardiovascular predictors of in-patient mortality after subarachnoid hemorrhage. Neurocrit. Care. 2006;5:102–107. doi: 10.1385/NCC:5:2:102. [DOI] [PubMed] [Google Scholar]
- 23.van der Bilt IA, et al. Impact of cardiac complications on outcome after aneurysmal subarachnoid hemorrhage: a meta-analysis. Neurology. 2009;72:635–642. doi: 10.1212/01.wnl.0000342471.07290.07. [DOI] [PubMed] [Google Scholar]
- 24.Banki NM, et al. Acute neurocardiogenic injury after subarachnoid hemorrhage. Circulation. 2005;112:3314–3319. doi: 10.1161/CIRCULATIONAHA.105.558239. [DOI] [PubMed] [Google Scholar]
- 25.Guette P, et al. Prognostic value of high-sensitivity troponin T in aneurysmal subarachnoid hemorrhage: a prospective observational study. Brain Inj. 2019;33:1372–1378. doi: 10.1080/02699052.2019.1641742. [DOI] [PubMed] [Google Scholar]
- 26.Duello KM, Nagel JP, Thomas CS, Blackshear JL, Freeman WD. Relationship of troponin T and age- and sex-adjusted BNP elevation following subarachnoid hemorrhage with 30-day mortality. Neurocrit. Care. 2015;23:59–65. doi: 10.1007/s12028-014-0105-6. [DOI] [PubMed] [Google Scholar]
- 27.Parekh N, et al. Cardiac troponin I predicts myocardial dysfunction in aneurysmal subarachnoid hemorrhage. J. Am. Coll. Cardiol. 2000;36:1328–1335. doi: 10.1016/S0735-1097(00)00857-3. [DOI] [PubMed] [Google Scholar]
- 28.Naidech AM, et al. Cardiac troponin I and acute lung injury after subarachnoid hemorrhage. Neurocrit. Care. 2009;11:177–182. doi: 10.1007/s12028-009-9223-y. [DOI] [PubMed] [Google Scholar]
- 29.Roffi M, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]