Abstract
Background & Aims:
A non-endoscopic approach to Barrett’s esophagus (BE) surveillance after radiofrequency ablation (RFA) would offer a less invasive method for monitoring. We assessed the test characteristics and cost-effectiveness of the Cytosponge® in post-RFA patients.
Methods:
We performed a multicenter study of dysplastic BE patients after at least one round of RFA. A positive Cytosponge® before endoscopy was defined as intestinal metaplasia (IM) on cytological assessment and/or TFF3 immunohistochemistry. Sensitivity, specificity, and receiver operator characteristic (ROC) curves were calculated. Multivariable regression was used to estimate the odds of a positive Cytosponge® in BE. A microsimulation cost-effectiveness model was performed to assess outcomes of various surveillance strategies: endoscopy-only, Cytosponge®-only, and alternating endoscopy/Cytosponge®.
Results:
Of 234 patients, Cytosponge® adequately sampled the distal esophagus in 175 (75%). Of the 142 with both endoscopic and histologic data, 19 (13%) had residual/recurrent BE. For detecting any residual Barrett’s, Cytosponge® had a sensitivity of 74%, specificity of 85%, accuracy of 84%, and ROC curve showed an area under the curve of 0.74. The adjusted odds of a positive Cytosponge® in BE were 17.1 (95% CI: 5.2–55.9). Cytosponge®-only surveillance dominated all the surveillance strategies, being both less costly and more effective. Cytosponge®-only surveillance required <1/4th the endoscopies, resulting in only 0.69 additional EAC cases/1,000 patients, and no increase in EAC deaths when compared to currently-practiced endoscopy-only surveillance.
Conclusions:
A positive Cytosponge® test was strongly associated with residual BE after ablation. While the assay needs further refinement in this context, it could serve as a cost-effective surveillance examination.
Keywords: Barrett’s esophagus, dysplasia surveillance, Cytosponge, cost-effectiveness
INTRODUCTION
Current guidelines1,2 recommend endoscopic eradication therapy (EET) for patients with dysplastic Barrett’s esophagus (BE) or early stage esophageal adenocarcinoma (EAC), which is effective in achieving complete eradication of intestinal metaplasia (CEIM).3–5 Following CEIM, patients undergo lifelong endoscopic surveillance exams due to the risk of recurrent disease.3, 6 Surveillance intervals are determined by the baseline degree of dysplasia and occur at an increased frequency in the first two years following CEIM, then annually thereafter.1 Over the course of the first 2 years alone after CEIM, a patient with high-grade dysplasia (HGD) is recommended to undergo 6 surveillance endoscopies.1
These endoscopic surveillance exams can induce anxiety and discomfort for patients and are associated with significant costs, resource use, and some procedure-related risks. Given the number of necessary surveillance exams following CEIM, an alternative, more cost-effective, and less invasive surveillance technique to detect residual or recurrent BE would be of great utility. One candidate method for these surveillance exams is a minimally-invasive esophageal sampling device, the Cytosponge®, which can be coupled to biomarker assays. The Cytosponge® is a sponge sampling device that is enclosed in a tablet-sized, gelatin capsule. The gelatin capsule dissolves in the stomach, allowing the sponge to expand before being withdrawn using an attached thread. As the sponge is retracted, it samples the mucosa of the GEJ and the esophagus. Previous studies have shown the Cytosponge® to be a well-tolerated and safe method for BE screening. When combined with a biomarker TFF3 to detect goblet cells, it has a sensitivity of 94%, after excluding inadequate samples in which the device did not reach the stomach. The specificity of 92% 7,8 for detection of intestinal metaplasia (IM) in BE patients was maintained in a screening population in a large randomised controlled trial (RCT).9
To date, the performance of the Cytosponge® to detect recurrent or residual BE after ablation has not been studied. Given the substantial potential difference in cost and ease of use between endoscopic and Cytosponge® sampling, a Cytosponge®-based surveillance paradigm offers the potential for cost-effective long-term post-treatment monitoring. Specific evaluation of the test performance in a post-ablation setting is required, since radiofrequency ablation (RFA) may alter esophageal motility and passage of the capsule into the stomach, and the cell collection of neo-squamous epithelium and recurrent BE may be different.10 Therefore, the aims of this study were to determine the test characteristics and cost-effectiveness of the Cytosponge® to detect residual or recurrent BE after EET.
MATERIALS AND METHODS
Study Design, Setting, and Population
This was a prospective study conducted at four tertiary care referral centers in the United Kingdom and one tertiary care referral center in the United States. Eligible patients were adults (≥18 years) with dysplastic BE (low-grade dysplasia (LGD), HGD or intramucosal adenocarcinoma (IMC), confirmed by a second expert gastrointestinal pathologist, who had undergone at least one round of EET and were scheduled for further ablative therapy or endoscopic surveillance after CEIM. EET consisted of endoscopic mucosal resection (EMR) of any nodular areas followed by RFA. Given the high efficacy of EET at these centers, only including patients who have completed treatment would leave only 5–8% with residual disease. While it would be most desirable to perform a study only on post-CEIM patients, the size of this study would be prohibitive to allow definition of the operating characteristics of the Cytosponge® in this setting.
Study Procedures
Cytosponge® was administered prior to endoscopy by trained research personnel. The capsule was ingested with ~50 ml of water. After a 7-minute period, to allow dissolution of the gelatin capsule and release of the Cytosponge® in the proximal stomach, the sponge was withdrawn using the attached string, sampling the esophageal lining from the stomach to the mouth. After the Cytosponge® was withdrawn, the string was cut, and the sponge was stored in BD SurePath liquid fixative at 4°C.
Cytosponge® sample processing was undertaken in Cambridge, UK as described previously.8 Briefly, the cells were dissociated from the sponge by gentle agitation, and then the solution was centrifuged into a clot preparation, fixed in formalin, and stained with hematoxylin and eosin. Representative sections were examined by a specialist histopathologist (MO’D) who was blinded to the endoscopic findings. H&E combined with immunohistochemical assessment of TFF3 was performed to improve identification of IM from the cytological sample to help distinguish pseudo-goblet cells and respiratory epithelium.11,12 The presence of columnar epithelium (CE) is a quality control metric to assess if Cytosponge® reached the stomach. A sample was considered adequate if at least one gland group of columnar mucosa was present. A positive Cytosponge® was defined as the presence of one or more goblet cells in a gastrointestinal-type columnar cell group on the H&E and/or TFF3 slide.
All patients underwent upper endoscopy approximately 2 hours after Cytosponge® administration to reduce risk of aspiration after ingesting 50 ml of water. Biopsies were obtained from BE segments in those with residual BE undergoing further endoscopic treatment, and from the cardia, GEJ and neosquamous esophagus in post-CEIM patients. A subset of patients (n=33) undergoing ablation, but had not achieved CEIM, only had endoscopic evidence of CE documented, without concurrent biopsies, due to the endoscopist’s concern of biopsies interfering with ablation.
Definitions
For this study, the presence of BE was defined per guidelines1 as CE of ≥1cm in the tubular esophagus, with concurrent IM on biopsies or EMR specimens of that area. Short segment BE was defined as <3cm of esophageal columnar mucosa and long segment BE as ≥3 cm.
Statistical Analysis
We calculated operating characteristics for the Cytosponge® as a diagnostic test for BE, including sensitivity, specificity, and positive and negative predictive value. Receiver operator characteristic (ROC) curves were created to assess the diagnostic utility of the Cytosponge®. Multivariable logistic regression adjusting for age and sex was used to estimate the odds of a positive Cytosponge® test in those with and without residual BE.
Sensitivity Analysis
In an additional sensitivity analysis, subjects with endoscopic data without biopsies were added to those in the primary analysis. Those with ≥1cm of endoscopic CE without biopsies (because they were to undergo ablation at the same exam) were categorized as BE cases (n=19). Subjects with <1 cm CE or no CE without biopsies (n=14) were categorized as controls. All of the above statistical analyses were performed using STATA 13.
Cost-Effectiveness Analysis
We ran 1,000,000 hypothetical male patients through a microsimulation model to assess outcomes of four different BE/EAC surveillance strategies. The microsimulation surveillance model was an extension of a validated natural history model of EAC, calibrated to incidence and mortality data from the Surveillance, Epidemiology, and End Results (SEER 9) registry.13,14 Modelled patients were male, aged 68, and assumed to have achieved CEIM after RFA treatment for dysplastic BE. LGD patients received annual surveillance in the first two years after RFA, and every 3 years thereafter.15 HGD patients received surveillance every 3 months in the first year after RFA, every 6 months in the second year, and every year thereafter.1 We varied surveillance type at each interval. The following strategies were analyzed: 1) endoscopy-only surveillance; 2) alternating Cytosponge® and endoscopy at each surveillance; 3) endoscopy every third surveillance; and 4) Cytosponge®-only surveillance. We assumed 100% adherence to endoscopy and Cytosponge® surveillance. Post-treatment surveillance continued through age 80. Patients still alive after age 80 entered natural history until death or age 100. We used a natural history comparator with no post-treatment surveillance.
Supplementary Table 1 contains all model inputs. We assumed no disutility for Cytosponge® surveillance. At each surveillance interval, the patient was tested for recurrence with either endoscopy or Cytosponge® based on the strategies above. Probabilities of misdiagnosis by endoscopy were obtained from prior literature6 (Supplementary Table 2). Cytosponge® false positive and false negative rates were obtained from the current study. A one-way sensitivity analysis was performed addressing the uncertainty in the performance characteristics of the Cytosponge® using a sensitivity and specificity of 50% as a lower threshold or 100% as a higher threshold for both. We assumed that the same Cytosponge® false negative rate for non-dysplastic BE (NDBE), LGD, HGD, and EAC. If test results were negative, patients would receive no further treatment or surveillance until the next interval. Patients received a confirmation endoscopy two months after a positive Cytosponge®. Touch-up RFA treatment performed at the same session as a positive endoscopy result, reset the surveillance schedule. A patient could receive a maximum of three RFA touch-ups.
RESULTS
Of 234 patients, 175 (75%) had an adequate Cytosponge® sample. Of the 175 patients with adequate sponge samples, mean age was 71 ± 9 years, 83% were men, and median time from first ablation was 20 months (Table 1). On endoscopy, 65% (n=114) had no CE, 25% (n=43) had short segments, and 10% (n=18) had long segments of CE. Among those with an inadequate sponge sample, 92% (n=54) had either no BE (n=36) or short segment BE (n=18). There was some variability amongst the four centers in the proportion of patients with an inadequate sample (Supplementary Table 3). There were no differences between the groups with and without an adequate Cytosponge® sample, (Table 1).
Table 1:
Sample Characteristics
| Patients with adequate sponge sample (n=175) | Patients with inadequate sponge sample (59) | p | |
|---|---|---|---|
|
| |||
| Age, mean±SD | 70.9±8.7 | 68.8±8.5 | 0.12 |
| Male, n(%) | 146 (83) | 51 (86) | 0.58 |
| BE length on endoscopy, n(%) | 0.55 | ||
| None | 114 (65) | 36 (61) | |
| Short (<3cm) | 43 (25) | 18 (31) | |
| Long (≥3cm) | 18 (10) | 5 (8) | |
| Intestinal metaplasia on biopsy * , n(%) | 33 (23) | 14 (30) | 0.37 |
| History of EMR, n(%) | 114 (65) | 36 (61) | 0.57 |
| Months since first ablation, median (IQR) | 20 (2–113) | 22 (2–166) | 0.73 |
| Months since last ablation, median (IQR) | 10 (1–111) | 10 (2–88) | 0.74 |
Of the 142 with biopsies
The 175 patients with an adequate Cytosponge® sample were included in the primary analysis, among whom 142 (81%) had both endoscopic and histologic data available (Figure 1). Among the 142 subjects, 87% (n=123) were categorized as non-BE controls and 13% (n=19) as BE cases. The remaining 33 of the 175 patients had endoscopic CE but did not have biopsies performed (7 had no CE on endoscopy, 8 had long segment CE and 18 had short segment CE). These 33 subjects with unknown histology were excluded in the primary analysis.
Figure 1:
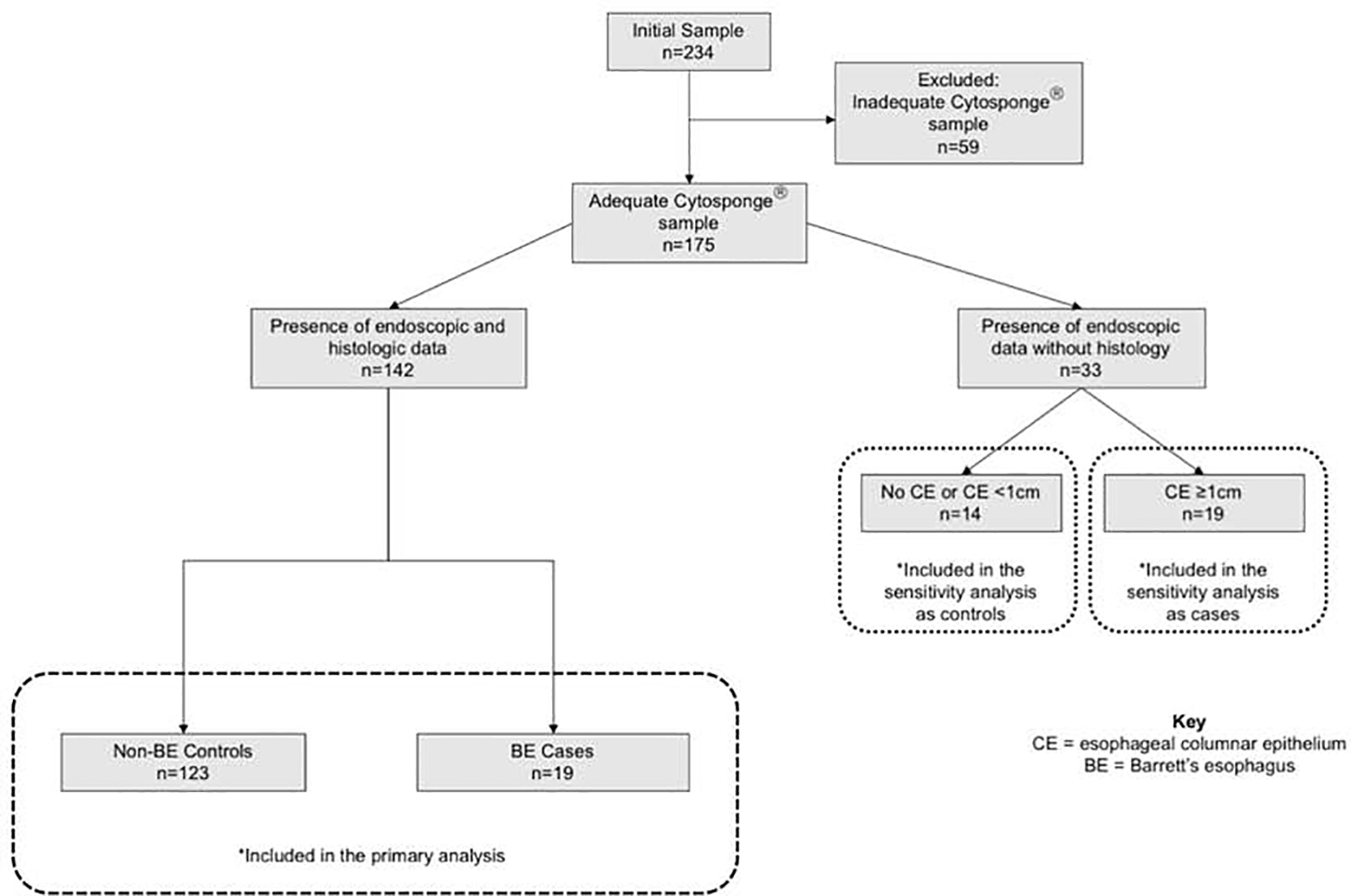
Study flow diagram
The Cytosponge® test was positive in 74% (n=14) of BE cases vs. 15% (n=18) without BE, p<0.01 (Table 2). The Cytosponge® test was negative in 85% (n=105) of those without BE vs. 26% (n=5) with BE. The Cytosponge® assay had a sensitivity of 74% (95% confidence interval (CI): 49%,91%), specificity of 85% (95% CI: 78%,91%) for BE detection, and overall accuracy of 84% (95% CI: 77%,89%). The ROC curve showed an area under the curve (AUC) of 0.74 (Figure 2).
Table 2:
Operating Characteristics of the Cytosponge® in detecting presence of Barrett’s esophagus (BE)
| Total (n=142) | No BE (n=123) | BEb (n=19) | |
|---|---|---|---|
|
| |||
| Positive Cytosponge a , n (%) | 32 (23) | 18 (15) | 14 (74) |
| Negative Cytosponge, n (%) | 110 (77) | 105 (85) | 5 (26) |
Positive if presence of IM and/or TFF3
Presence of endoscopic and histologic evidence of BE
Figure 2:

ROC curve for Cytosponge® Assay for Detection of Barrett’s esophagus after Endoscopic Eradication Therapy
Of the 5 BE cases that Cytosponge® did not detect, 4 had long segment BE (mean segment length [range]: 7.8 [4–11] cm) and 1 had short segment BE. None of these 5 cases had atypia on the sponge or dysplasia on biopsy. Of the BE cases Cytosponge® accurately detected, none had dysplasia on biopsies and 4 had atypia on the sponge. Of the 18 patients (15%) who had a positive Cytosponge and no BE, 28% (n=5) had <1cm endoscopic CE that did not meet criteria for the diagnosis of BE, and 17% (n=3) had an endoscopically normal esophagus with concurrent cardia biopsies showing IM. For the remainder 55% (n=10), the etiology of the false positive result was obscure. In multivariable logistic regression, the adjusted odds of a positive Cytosponge® in BE cases were markedly higher compared to those without BE (OR: 17.1; 95% CI: 5.2, 55.9).
When using a definition of BE which included patients with endoscopic CE of any length with concurrent biopsies showing IM, the sensitivity of Cytosponge® was lower at 63%, specificity was 87%, accuracy was 82%, and ROC showed an AUC of 0.75 (Supplementary Figure 1).
Sensitivity Analysis
In this sample of 175 subjects (36 BE cases and 139 controls), Cytosponge® was positive in 69% (n=36) of BE cases vs. 16% (n=22) of controls. Cytosponge® was negative in 84% (n=117) of controls vs. 31% (n=11) of BE cases, p<0.01 for both. The assay had a sensitivity of 69%, specificity of 84% and accuracy of 81% for BE detection. AUC for ROC curve was 0.75 (Supplementary Figure 2).
Cost-Effectiveness Analysis Model
Figure 3 depicts EAC incidence and mortality from each post-CEIM surveillance strategy. As expected, post-treatment surveillance substantially decreased EAC incidence and mortality. However, differences among the four post-treatment surveillance strategies were very small in terms of EAC incidence and mortality. The Cytosponge®-only strategy led to only 0.69 additional EAC cases and no increase in EAC deaths per 1000 patients compared to the endoscopy-only strategy. Importantly, the endoscopy-only strategy required more than four times as much endoscopy per surveyed person when compared to the Cytosponge®-only strategy (5,974 vs 1,442 per thousand patients surveyed).
Figure 3:

Incidence and Mortality of Esophageal Adenocarcinoma (EAC) among all tested surveillance strategies after Endoscopic Eradication Therapy (EET)
As there were only marginal differences in EAC incidence and mortality, there were also very marginal differences in quality adjusted life years (QALYs) among the post-treatment surveillance strategies (Figure 4). The endoscopy-only surveillance strategy resulted in 11,839 QALYs/1,000 patients and the Cytosponge®-only strategy with 11,844 QALYs/1,000 patients surveyed. The QALYs of the other two mixed modality surveillance strategies fell in between. Therefore, the increased costs and disutilities associated with endoscopy drove the results (Table 3). The Cytosponge®-only strategy resulted in the lowest cost and highest QALYs among the post-treatment surveillance strategies (Figure 4), and therefore dominated all of the other surveillance strategies. The Cytosponge®-only strategy resulted in an incremental cost-effectiveness ratio (ICER) of $13,259/QALY compared to no surveillance.
Figure 4:

Cost/benefit curves for the four post-CEIM surveillance strategies: endoscopy-only, alternating Cytosponge® and endoscopy at each surveillance interval, endoscopy every third surveillance, and Cytosponge®-only surveillance. All numbers are reported per 1000 patients.
Table 3.
Cost and QALY Breakdown per 1000 Patients
| No Surveillance | Endoscopy Only | Alternating Cytosponge and Endoscopy | Endoscopy Every Third Surveillance | Cytosponge Only | |
|---|---|---|---|---|---|
|
| |||||
| Endoscopies | — | 5974 | 3511 | 2625 | 1442 |
| Cytosponge | — | — | 3171 | 4276 | 5858 |
| RFA touch-ups | — | 796.35 | 756 | 744 | 709 |
| Cost ($1000) | $8792.07 | $12,364.20 | $11,192.56 | $10,778.01 | $10,245.32 |
| Cancer care | $2652.66 | $1534.98 | $1535.68 | $1547.26 | $1564.93 |
| Endoscopies | — | $3923.12 | $2283.99 | $1691.39 | $934.60 |
| Cytosponge | — | — | $507.43 | $684.64 | $927.56 |
| EET/touch-ups | $6139.42 | $6906.10 | $6865.46 | $6854.72 | $6818.24 |
| Life-years | 11,778.59 | 11,879.03 | 11,879.78 | 11,879.88 | 11,880.30 |
| QALYs | 11,734.45 | 11,839.12 | 11,841.86 | 11,842.61 | 11,844.05 |
| Cancer disutility | 18.48 | 5.72 | 5.75 | 5.80 | 5.81 |
| Endoscopy disutility | — | 4.31 | 2.51 | 1.86 | 1.03 |
| EET disutility | 7.83 | 12.06 | 11.83 | 11.77 | 11.57 |
EET, endoscopic eradication therapy; QALY, quality-adjusted life-year; RFA, radiofrequency ablation.
In the sensitivity analysis, even at operating characteristics well below the worst reported in the literature, a Cytosponge®-only strategy remained dominant. At 50% sensitivity and specificity, the Cytosponge®- only strategy remained the most cost-effective, but resulted in an increased EAC incidence of 27.66 and increased EAC-associated mortality of 9.73 per 1000 patients. Costs increased and QUALYs decreased to 11,842 QALYs/1,000 patients, which increased the ICER to $22,036. At 100% sensitivity and specificity, the results changed similarly in the opposite direction with a decreased EAC incidence of 24.92 and mortality of 8.88, and the ICER decreased to $9,696.
DISCUSSION
Currently, there are no clinically reliable non-endoscopic methods of disease surveillance in the post-treatment BE population, obligating such patients to the costs, inconvenience, and risks of recurrent endoscopy. In this multicenter prospective study of BE patients who have undergone at least one round of EET, the Cytosponge® assay was able to detect residual BE in the tubular esophagus with a sensitivity of 74% and specificity of 85%. The ROC curve showed an AUC of 0.74. When compared to the current practice of post-ablation endoscopic surveillance, the Cytosponge®-only surveillance strategy resulted in a negligible increase of EAC cases and no increase in EAC-associated mortality, while being more cost-effective.
The lower sensitivity of the Cytosponge® than previously reported8 in an untreated population with >50% with long segment BE is perhaps not surprising in a post-treatment group, given the smaller area of mucosa harboring BE. This can decrease the chance that the sponge would successfully sample goblet cells, and it is also possible that cell shedding rates of residual IM differ from neo-squamous cells. Recurrent or residual BE in post-treatment patients is typically small islands or short segments, which can be easily missed even on endoscopy. Therefore, the sensitivity of the assay at 74% in this study, while lower than that in treatment naïve patients, is promising to detect BE in post-ablation patients. The specificity is also lower than previously reported in BE screening studies using Cytosponge®,8, 16, 17 and may reflect focal cardia IM, or minute islands of CE identified on the sponge but not on biopsies due to sampling error; although, we are unable to demonstrate this with the current study.
The cost-utility analysis showed that a Cytosponge®-only surveillance strategy was preferred over strategies incorporating endoscopy, dominating the endoscopy-only as well as the alternating endoscopy-Cytosponge® strategies. Even though Cytosponge® failed to detect about a quarter of patients with BE, the relatively benign outcome associated with these lesions, combined with the recurrent applications of surveillance exams (allowing for subsequent detection), resulted in very small increases in cancer incidence and death in the Cytosponge®-only arm, compared to large increases in cost and disutility in the strategies incorporating endoscopy. On the other hand, the endoscopy-only strategy utilized over 4 times as much endoscopy. Given the costs and inconvenience of endoscopy, as well as the trivial changes in clinical outcomes between the strategies, Cytosponge®-only was the preferred approach.
There is potential for further optimization of Cytosponge® with use of adjunct biomarkers to improve the test sensitivity in post-CEIM surveillance. Expression of microRNAs (miRNAs) when combined with TFF3 have been shown to improve the testing characteristics of Cytosponge® in differentiating between BE cases and controls with an AUC of 0.93, 93% sensitivity, and 94% specificity.18 Therefore, identifying miRNA expression profiles in post-ablation esophageal tissue may offer an adjunctive biomarker to increase the diagnostic accuracy of Cytosponge®. Additionally, methylation panels used in conjunction with Cytosponge® have also shown to have some promise for detection of BE.19 One viable strategy might be to combine these markers with standard histology to improve the sensitivity of the assay in post-ablation patients, given their small burden of disease.
This study has multiple strengths. It is the first study to assess both the testing characteristics and cost-effectiveness of Cytosponge® in a post-treatment BE population. The study was prospectively conducted at multiple centers making the results more generalizable and used standardized data collection protocols. The approach it assesses, if adopted, may allow for reallocation of scarce healthcare resources without diminishing patient outcomes.
This study has limitations. First, 34% (n=59) of the subjects had an inadequate Cytosponge® sample, but given that many patients come from long distances, we did not repeat the Cytosponge® in these subjects. The higher proportion of inadequate sponges that previously reported,17 might be especially common in this post-ablation group, who tend to have substantial hiatal hernias, diminished lower esophageal sphincter tone, and possibly some altered motility. Since a majority had no residual disease, it is likely that these “inadequate” sponges never came into contact with columnar mucosa. However, other explanations for suboptimal sampling include administration techniques, despite standardized training sessions for personnel administering Cytosponge®, as 67% of the inadequate samples were from two centers. Second, the Cytosponge® was less sensitive for detecting miniscule islands of residual IM. While the clinical significance of minute islands of IM after RFA is unclear, it is a common clinical practice to eradicate them. Finally, for the cost-effectiveness analysis we assumed 100% adherence to both endoscopy and Cytosponge® which, while likely not reflective of real-world adherence, can be considered appropriate for this initial study. Further work using real world data on adherence to Cytosponge surveillance programs can be used when available.
In summary, Cytosponge® was able to detect residual BE, often minute in amount, in patients who have undergone EET, with promising test characteristics. Comparative modelling of endoscopy and Cytosponge® based surveillance strategies showed that a Cytosponge®-only surveillance method was cost-effective, with only a negligible increase in EAC incidence and no increase in associated mortality, when compared to endoscopy-based surveillance. While further optimization would be desirable to improve the testing characteristics before using this assay as a surveillance tool, the ability of Cytosponge® to detect BE in the post-treatment population holds promise for it as either an adjunct tool or as a stand-alone method for post-EET surveillance. In the interim, the potential utility of the assay in a paradigm including Cytosponge® alternating with endoscopy deserves further consideration.
Supplementary Material
What you need to know?
Background:
Following successful treatment of Barrett’s esophagus (BE) with radiofrequency ablation (RFA), patients require lifelong endoscopic surveillance to monitor for disease recurrence, which is associated with significant costs, resource utilization, patient discomfort, and some procedure-related risks. An alternate, more cost-effective, and less invasive techniques for post-ablation surveillance would be of great utility.
Findings:
This is the first study to assess both the testing characteristics and cost-effectiveness of Cytosponge® to detect residual or recurrent BE after RFA. The Cytosponge® assay was able to detect residual BE in the tubular esophagus with a sensitivity of 74% and specificity of 85% in a post-ablation population, and was both less costly and more effective than endoscopy according to formal cost-effectiveness analysis.
Implications for Patient Care:
While further optimization of the Cytosponge® assay to improve the testing characteristics is needed for the assay to be reliably used for surveillance in the post-ablation setting for BE patients, it holds promise to be utilized as either an adjunct or stand-alone method for post-treatment surveillance.
Acknowledgments
Grant support: The study was supported in part by NIH award numbers K24DK100548 (NJS), UL1TR002489 (NJS), NIH/NCI U01CA199336 (CH), P30 DK034987, and the American Gastroenterological Association Research Scholar Award (SE).
Disclosures: Dr. Shaheen reports research funding from CSA Medical, Pentax, Medtronic, Interpace Diagnostics, Lucid Diagnostics and CDx Medical. He is a consultant for Cernostics, Cook Medical and Boston Scientific. Dr. Eluri reports research funding from Interpace Diagnostics and Takeda. Prof Fitzgerald and Dr Maria O’Donovan are named on patents related to Cytosponge and related assays licensed by the Medical Research Council to Covidien GI Solutions (now Medtronic). RCF reports grant support from Medtronic (BEST3 trial). Maria O’Donovan is a consultant for Medtronic. RCF and MoD are founders and shareholders of Cyted Ltd.
Abbreviations:
- AUC
area under the curve
- BE
Barrett’s esophagus
- CE
columnar epithelium
- CEIM
complete eradication of intestinal metaplasia
- CED
complete eradication of dysplasia
- CI
Confidence interval
- EAC
esophageal adenocarcinoma
- EET
endoscopic eradication therapy
- EMR
endoscopic mucosal resection
- HGD
high-grade dysplasia
- ICER
incremental cost-effectiveness ratio
- IM
intestinal metaplasia
- IMC
intramucosal adenocarcinoma
- LGD
low-grade dysplasia
- miRNAs
micro RNAs
- NDBE
non-dysplastic Barrett’s esophagus
- OR
odds ratio
- RFA
radiofrequency ablation
- RCT
Randomized controlled trial
- ROC
receiver operator characteristic
- QUALYs
quality adjusted life years
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Shaheen NJ, Falk GW, Iyer PG, et al. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol. 2016;111(1 ):30–50. Epub 2015/11/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63(1):7–42. Epub 2013/10/30. [DOI] [PubMed] [Google Scholar]
- 3.Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett’s Esophagus: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11(10):1245–55. Epub 2013/05/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360(22):2277–88. Epub 2009/05/29. [DOI] [PubMed] [Google Scholar]
- 5.Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA. 2014;311(12):1209–17. Epub 2014/03/29. [DOI] [PubMed] [Google Scholar]
- 6.Pasricha S, Bulsiewicz WJ, Hathorn KE, et al. Durability and predictors of successful radiofrequency ablation for Barrett’s esophagus. Clin Gastroenterol Hepatol. 2014;12(11):1840–7.e1. Epub 2014/05/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadri SR, Lao-Sirieix P, O’Donovan M, et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. BMJ. 2010;341:c4372. Epub 2010/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross-Innes CS, Debiram-Beecham I, O’Donovan M, et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett’s esophagus: a multi-center case-control study. PLoS Med. 2015;12(1):e1001780. Epub 2015/01/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rebecca C Fitzgerald MDP, Maria O’Donovan, Roberta Maroni, Beth Muldrew, Irene Debiram-Beecham,, Marcel Gehrung JO, Monika Tripathi, Sam G Smith, Benoit Aigret, Fiona M Walter, Greg Rubin, on behalf of the BEST3 Trial team Peter Sasieni. Cytosponge-trefoil factor 3 versus usual care to identify Barrett’s oesophagus in a primary care setting: a prospective, multicentre, pragmatic, randomised controlled trial Lancet. 2020. Epub 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemmink GJ, Alvarez Herrero L, Bogte A, et al. Esophageal motility and impedance characteristics in patients with Barrett’s esophagus before and after radiofrequency ablation. Eur J Gastroenterol Hepatol. 2013;25(9):1024–32. Epub 2013/05/28. [DOI] [PubMed] [Google Scholar]
- 11.Lao-Sirieix P, Rous B, O’Donovan M, et al. Non-endoscopic immunocytological screening test for Barrett’s oesophagus. Gut. 2007;56(7):1033–4. Epub 2007/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paterson AL, Gehrung M, Fitzgerald RC, et al. Role of TFF3 as an adjunct in the diagnosis of Barrett’s esophagus using a minimally invasive esophageal sampling device-The Cytosponge(TM). Diagn Cytopathol. 2020;48(3):253–64. Epub 2019/12/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hur C, Hayeck TJ, Yeh JM, et al. Development, calibration, and validation of a U.S. white male population-based simulation model of esophageal adenocarcinoma. PLoS One. 2010;5(3):e9483. Epub 2010/03/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong CY, Kroep S, Curtius K, et al. Exploring the recent trend in esophageal adenocarcinoma incidence and mortality using comparative simulation modeling. Cancer Epidemiol Biomarkers Prev. 2014;23(6):997–1006. Epub 2014/04/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wani S, Rubenstein JH, Vieth M, et al. Diagnosis and Management of Low-Grade Dysplasia in Barrett’s Esophagus: Expert Review From the Clinical Practice Updates Committee of the American Gastroenterological Association. Gastroenterology. 2016;151(5):822–35. Epub 2016/10/06. [DOI] [PubMed] [Google Scholar]
- 16.Ross-Innes CS, Chettouh H, Achilleos A, et al. Risk stratification of Barrett’s oesophagus using a non-endoscopic sampling method coupled with a biomarker panel: a cohort study. The lancet Gastroenterology & hepatology. 2017;2(1):23–31. Epub 2017/04/14. [DOI] [PubMed] [Google Scholar]
- 17.Offman J, Muldrew B, O’Donovan M, et al. Barrett’s oESophagus trial 3 (BEST3): study protocol for a randomised controlled trial comparing the Cytosponge-TFF3 test with usual care to facilitate the diagnosis of oesophageal pre-cancer in primary care patients with chronic acid reflux. BMC Cancer. 2018;18(1):784. Epub 2018/08/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Kleeman S, Coburn SB, et al. Selection and Application of Tissue microRNAs for Nonendoscopic Diagnosis of Barrett’s Esophagus. Gastroenterology. 2018; 155(3):771–83.e3. Epub 2018/06/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chettouh H, Mowforth O, Galeano-Dalmau N, et al. Methylation panel is a diagnostic biomarker for Barrett’s oesophagus in endoscopic biopsies and non-endoscopic cytology specimens. Gut. 2018;67(11):1942–9. Epub 2017/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


