Abstract
Background/Aims:
This systematic review critically evaluated peer-reviewed publications describing morphological features consistent with, or using terms related to, a ‘neuroma’ or ‘microneuroma’ in the human cornea using laser-scanning in vivo confocal microscopy (IVCM).
Methods:
The review was prospectively registered on PROSPERO (CRD42020160038). Comprehensive literature searches were performed in OVID Medline, OVID Embase and the Cochrane Library in November 2019. The review included primary research studies and reviews that described laser-scanning IVCM for examining human corneal nerves. Papers had to include at least one of a pre-specified set of keyword stems, broadly related to neuromas and microneuromas, to describe a corneal nerve feature.
Results:
Twenty-five papers (20 original studies; five reviews) were eligible. Three original studies evaluated corneal nerve features in healthy eyes. Most papers assessed corneal nerves in ocular and systemic conditions; nine studies did not include a control/comparator group. There was overlap in terminology used to describe nerve features in healthy and diseased corneas (e.g., bulb-like/bulbous, penetration, end/s/ing). Inspection of IVCM images within the papers revealed that features termed ‘neuromas’ and ‘microneuromas’ could potentially be physiological corneal stromal-epithelial nerve penetration sites. We identified inconsistent definitions for terms, and limitations in IVCM image acquisition, sampling and/or reporting that may introduce bias and lead to inaccurate representation of physiological nerve characteristics as pathological.
Conclusion:
These findings identify a need for consistent nomenclature and definitions, and rigorous IVCM scanning and analysis protocols to clarify the prevalence of physiological, as opposed to pathological, corneal nerve features.
INTRODUCTION
In vivo confocal microscopy (IVCM) is a valuable tool for acquiring high-resolution anatomical images of the cornea. [1–3] Cross-sectional and/or volume scans can be acquired non-invasively, and analysed for a range of features, including cell and nerve densities, and morphological characteristics. There is interest in using laser-scanning IVCM to derive information about corneal nerves, particularly those located at the level of the basal epithelial cells, in conditions characterised by corneal neuropathy and/or pain,[4–8] given the inability to reliably visualise these structures clinically using other means.
Corneal neuropathic pain is a relatively ill-defined entity, characterised by symptoms ranging from ocular burning, drying and stinging, through to severe eye ‘aching’ and photophobia.[5] Damage to the corneal nerves, either following trauma during ocular surgery, or secondary to chronic ocular surface disease such as dry eye, can lead to development of neuropathic pain. Whilst the epidemiology of corneal neuropathic pain is unclear, estimates of the prevalence of symptoms potentially relevant to the condition range from 30% (eye discomfort[9]) to 50% (photophobia[10]) in population-based studies. Recent studies have proposed that the presence of corneal microneuromas (sometimes referred to as neuromas) are a pathological feature of corneal neuropathy[11] and ocular surface disease,[12] and thus may serve as diagnostic biomarkers. However, nerve features of similar phenotype, detectable using corneal IVCM, have also been reported in healthy corneas,[13] suggesting there may be inconsistent identification and reporting of microneuromas in the literature.[14]
Using robust IVCM imaging protocols (including suitable scanning modes, and image selection and analysis processes) and ensuring the appropriate interpretation of image features are essential to its utility. Whilst a general approach to evaluating the cornea using laser-scanning IVCM has been described,[15] there is currently no broadly accepted protocol for evaluating corneal microneuromas. It is possible for physiological features, reminiscent of “microneuromas”, to be mistaken for neuro-pathological sites.[14] This is particularly true for corneal nerve injury, where phenomena described as ‘neuromas’ and ‘microneuromas’ share homology in their appearance to physiological corneal stromal-epithelial nerve penetration sites.[14] Misclassifications and/or use of suboptimal analytical approaches to quantify corneal nerve features creates potential for patient misdiagnoses, and inappropriate adoption of these entities as image-based biomarkers to measure therapeutic efficacy in intervention trials.
There has not yet been a systematic evaluation of the literature to consider these factors. The aim of this systematic review was to locate and critically evaluate clinical studies and reviews describing phenotypes consistent with, or using terms related to, a neuroma or microneuroma for features seen in human corneal nerves using laser-scanning IVCM. We sought to assess and synthesise the evidence, within identified papers, for these terms being used to describe pathological phenomena, in contrast to potentially physiological features.
MATERIALS AND METHODS
This review was prospectively registered on PROSPERO (CRD42020160038), conducted in accordance with the principles in the Cochrane Handbook,[16] and reported to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist.[17]
Eligibility criteria
Two stages were adopted to identify relevant citations. In Stage 1, published papers that met the following criteria were identified:
Study designs: Primary research studies that used laser-scanning IVCM to examine the cornea on at least one human, where epithelial nerve plexus parameters were examined. Also included were review papers that referenced primary research studies that met these criteria. Conference abstracts were excluded.
Study scope: Studies that reported on aspects of corneal architecture other than sub-basal nerve parameters (e.g., epithelial thickness, endothelial cell count), and studies describing methods for analysing IVCM images where human participants were not recruited were excluded.
Populations: There was no eligibility restriction based on participant health status.
Language: Only studies published in English were included.
In Stage 2, papers identified as eligible in Stage 1 were searched for keyword stems that needed to be used to describe a corneal nerve feature, seen using IVCM, to be included. At least one of the following keyword stem terms needed to be included: ‘neurom’, ‘microneuroma’, ‘microneuroma’, ‘stump’, ‘swell’, ‘swoll’, ‘sprout’, ‘branch poi’, ‘hyperreflectiv’, ‘hyper-reflectiv’, ‘bifurc’, ‘perforat’, ‘penetr’, ‘bulb’, ‘bulbar end’, ‘entry poi’, ‘blunt’, ‘abrupt’, ‘anomal’, ‘abnormalit’, ‘injur’, ‘tangl’, ‘bulge’, ‘ending’, ‘protru’ or ‘projecti’. If a word was used only in a general context, such as in the Introduction or Discussion (e.g., “abnormality in corneal nerves”, “corneal nerve injury”), the paper was excluded.
Literature searches
Comprehensive searches were performed in: Ovid MEDLINE(R) (Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to search date), Ovid EMBASE (Embase Classic+Embase, 1947 to search date) and the Cochrane Library. Search strategies were formulated with assistance from an experienced systematic review health informatician and are provided as Supplementary Material. Databases were searched from inception to 5th November 2019. To ensure literature saturation, we scanned reference lists of included studies and relevant reviews identified by the search, and also searched the first and senior authors’ personal bibliographic reference databases to identify potential additional studies.
Study record management and selection
Citation results from each database were imported into EndNote, and duplicate entries were removed. Covidence[18] systematic review software was used for study screening. Two review authors (two of: ACZ, MEDS, EM and LED) independently assessed titles/abstracts of study records and excluded those not meeting the eligibility criteria. For records considered eligible or potentially eligible, full texts were sourced and independently evaluated by two review authors (two of: ACZ, MEDS, EM and LED). Classification disagreements were resolved by consensus.
Information extraction
Information from eligible studies was independently extracted by two review authors (two of: HRC, RR, HJ, MW, ACZ, MEDS, EM and LED). Discrepancies were resolved by discussion and consensus. Extracted information comprised:
Publication details: year, journal;
Paper details: type of publication, research question (i.e., intervention, diagnostic-test accuracy, aetiology, prognosis or screening intervention, based upon the National Health and Medical Research Council classification,[19] study design (e.g., randomised controlled trial (RCT), pseudo-RCT, etc.) participant health status (e.g., healthy, diabetes);
IVCM methods: whether a representative IVCM image of the corneal sub-basal nerves was provided (dichotomous classification: yes/no); number of images analysed per participant; region corneal nerve feature(s) noted; device scan mode (section/sequence/volume); masking of image selector and/or outcome assessor to participant health status/intervention group, if appropriate (forced-choice classification: yes/no/not applicable);
Keywords: which keyword(s) of interest (as detailed in the ‘eligibility criteria’ section) were identified; evidence for appropriateness of use of the terminology.
Outcomes
The main outcome was identification of papers using terms describing the appearance of, or related to, a ‘neuroma’ or ‘microneuroma’ in human corneal nerves, visible on laser-scanning IVCM images. We also evaluated the consistency of terminology used to describe these nerve features, focussing on the identification of pathological versus physiological characteristics.
Risk of bias assessment
As the aim was to capture and synthesise the landscape of terminology used in the field (rather than to evaluate the quality of studies relating to a specific research question), formal risk of bias assessments were deemed to not be appropriate. However, risk of bias related to laser-scanning IVCM methods was assessed using the items defined in the ‘IVCM methods’ section of the data extraction (detailed above), based on the tool developed by De Silva et al.[20]
Information synthesis
We have undertaken a systematic narrative synthesis, with relevant information summarised in text, tables and figures.
RESULTS
Search results
The electronic searches yielded 1740 non-duplicate citations and three additional reviews were identified from the authors’ bibliographic databases. Full texts were obtained for 567 records deemed to meet, or potentially meet, the Stage 1 eligibility criteria. Of these, 342 met these criteria and proceeded to the Stage 2 keyword evaluation. A PRISMA flow diagram of the study selection process is provided in Supplementary Material (Figure S1).
Characteristics of included studies
Twenty-five papers, published between 2005 and 2019, were included. Of these, 20 were original research studies and five were review articles. The key characteristics of included studies are summarised in Table S1 (Supplementary Material). The papers described using laser-scanning IVCM to investigate corneal nerve parameters in a variety of conditions, including healthy controls,[13 21] keratoconus,[22–24] atopic keratoconjunctivitis,[25] polyneuropathy,[26] post-phototherapeutic keratectomy (PTK),[27] Stevens-Johnson syndrome and toxic epidermal necrolysis,[28] neurotrophic keratopathy,[29 30] bullous keratopathy,[31] pseudoexfoliation syndrome (PXF),[32] ocular surface disease,[12] post-laser in situ keratomileusis (LASIK),[3 33] photoallodynia,[11 34] herpes zoster ophthalmicus[35] and neuropathic corneal pain.[5 7]
The study designs included methodological, observational and interventional studies (Table S1, Supplementary Material). Many studies did not include a control/comparator group.[12 22 27–30 33] A range of IVCM scanning protocols were adopted, comprising “section scans” (single cross-sectional images in one plane),[13 22 23 27 33] “sequence scans” (sequential capture of section scans at 15 frames/second for ~7 seconds), [4 11 12 24 25 29 32 35] and “volume/depth scans” (multiple, typically 40, cross-sectional images at varying corneal depths, typically with 2μm axial spacing between images).[7 8 26 30 31 36] The IVCM scanning mode was not reported in one paper.[28]
Most studies examined one corneal location, typically the central region[4 7 11 13 23–26 29 30 32 35], with a few also scanning para-central,[8 31] mid-peripheral[13 27 33] and/or peripheral[28] areas. To quantify corneal nerve features, most studies analysed three to four images per participant, often selected visually as “most representative”.[4 7 11 12 23–26 29 32 36] Other studies used large numbers of montaged images,[13] multiple images per participant,[27] or did not explicitly report the number used per participant.[8 22 28 30 31 33] Of the 15 original research studies where masking of the participant group/intervention allocation was considered important to minimise outcome bias, eight studies[4 7 11 12 24–26 35] described masking of the person who selected the IVCM images for analysis, and nine studies[4 7 11 12 24 26 32 35 36] reported the image outcome assessor to be masked.
Keyword identification
(a). Papers describing “physiological” nerve features
Three papers used at least one keyword to describe features of corneal nerves in healthy individuals.[13 21 31] Terms used to describe physiological features were: bifurcation, bulb-like termination, bulbous termination, ending, penetration point and perforation.
Referring to a representative image showing hyper-reflective and dysmorphic nerve features (Figure 1A), Patel and McGhee (2005) identified “probable sites of perforation of nerves through Bowman’s layer” in the mid-peripheral cornea.[13] Al-Aqaba et al. (2011) described “sub-basal nerves with bulbous terminations” in a healthy (control) eye, and “perforation sites” indicated by “bulb-like structures just above the Bowman zone”; corneal eccentricity was not reported. A clinical review (2019) by the same first author described nerve ‘perforation sites’ from the stroma through Bowman’s layer, with a predominant mid-peripheral and few such sites in the central cornea.[21] An IVCM image from this paper (Figure 1B) shows a “bulb-like termination of sub-basal nerves.”[21]
Figure 1. Laser-scanning IVCM images of corneal nerve features, reproduced with permission from papers included in the review.
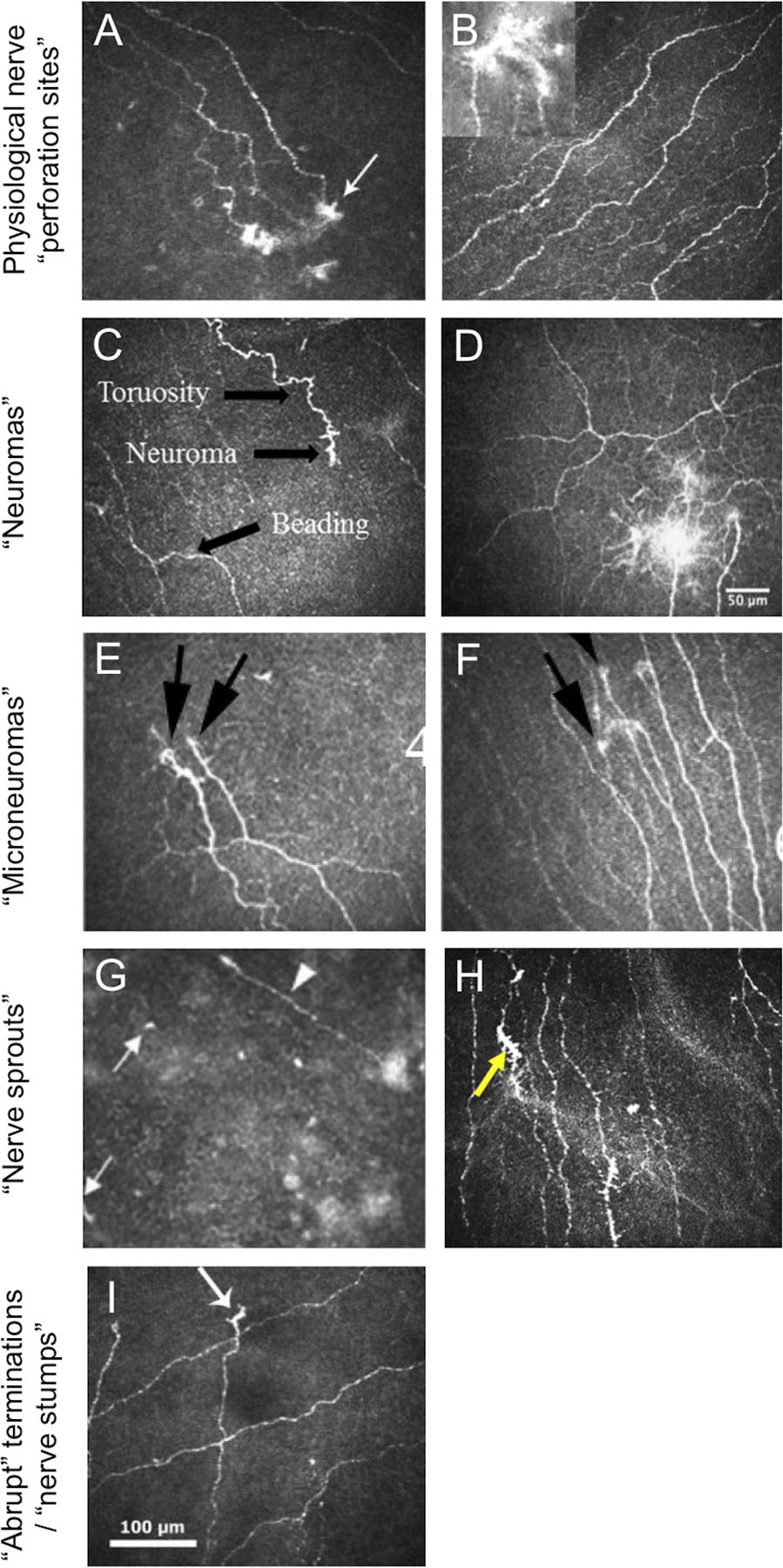
A. From Patel and McGhee[13] showing “probable sites of perforation of nerves through Bowman’s layer (white arrow) in the infero-temporal mid-periphery”. B. From Al-Aqaba et al.[21] showing “Normal appearance of the sub-basal nerve plexus seen in a healthy control. Bulb-like termination of sub-basal nerves is shown in the inset.” C. From Aggarwal et al.[11] showing a “neuroma” from a patient with neuropathy-induced severe photoallodynia. D. From Cruzat et al.[2] of “multiple neuromas” in a patient with corneal allodynia. E. and F. Both from Dieckmann (2017)[34] from individuals with neuropathic corneal pain showing “presence of micro-neuromas (black arrows)”. G. From Lagali et al.[27] identifying “presumed sprouting subbasal nerves (white arrows) and a regenerating subbasal nerve (arrowhead)” after phototherapeutic keratectomy. H. From Shen et al.[36] showing “nerve sprouts” (yellow arrow) in an individual with episodic migraine. I. From Patel et al.[22] showing “apparent abrupt terminations (arrow) of sub-basal nerve fiber bundles within the region of the cone in severe keratoconus.
Parissi et al.[24] described corneal epithelial nerves “emerging from penetration points” in patients who had previously undergone corneal collagen cross-linking treatment for keratoconus. The IVCM images in this paper appear similar to the appearance of the nerve entry points described in healthy individuals.
(b). Papers describing “pathological” nerve features
Nerve features viewed as ‘pathological’ by study authors were frequently described by terms including: abrupt, bulbous, end/s/ing, microneuroma, micro-neuroma, neuroma, sprout/ing/, stump/s and swelling. A synthesis of the most frequently used terms follows.
(i). Neuroma/s
The term neuroma was first used to describe a corneal nerve feature in a laser-scanning IVCM image in 2015, in a retrospective case-control study by Aggarwal et al. in individuals with photoallodynia without concurrent ocular surface disease.[11] In this study, a neuroma was defined to “represent stumps of severed nerves… identified as abrupt endings of a nerve fiber on confocal images.” Since this publication, three original studies[8 12 35] and two reviews[2 5] have described ‘neuromas’ in the corneal sub-basal and/or stromal nerve plexus in IVCM images from diseased eyes. Of these original articles, one study[12] included the same definition as Aggarwal et al., and two studies did not define the term.[8 35] Studies by Aggarwal et al.[11] and Cavalcanti et al.[35] included healthy (control) eyes, but neither explicitly stated whether the neuroma-like features were observed in this population. In a review of corneal neuropathic pain, Goyal et al.[5] described these phenomena as “sprouts (neuroma) manifesting (as) regenerative attempts, all of which become sources of ectopic spontaneous pain.” Representative IVCM images showing examples of ‘neuromas’ from these papers identify that the term has been used to describe a heterogeneous range of nerve features, ranging from an enlarged ending[35] (Figure 1C) to a hyperfluorescent nerve entanglement, ~70μm in radial diameter (Figure 1D).[2]
(ii). Microneuroma/s
The term microneuroma, sometimes written ‘micro-neuroma’, has also emerged in the literature to describe nerve features associated with corneal neuropathy.[4 7 8 21 35] Cruzat et al.[2] defined microneuromas as “abrupt swelling(s) of injured nerve endings and neurite sprouting”. This definition aligns with that of Morkin et al.[7], and Dieckmann et al.[34] who identified these features to “reflect sudden swelling of injured nerves at their terminal endings and have been shown to be specific for neuropathic corneal pain,[6] and thus potentially diagnostic.” Aggarwal et al.[4] noted that “with axonal injury, the damaged axons seal the injured stump and forms terminal bulbs with small fine branches in an attempt to regenerate. These stumps are called micro-neuromas.[37 38]” One study reported microneuromas to be absent from control (healthy) eyes, based on sampling and analysis of “3 images most representative of the subbasal nerve plexus”, from the central cornea, per participant.[4]
Currently, there are no criteria to distinguish corneal neuromas from microneuromas. In some instances the terms have been adopted interchangeably in the same report.[2 35] Representative IVCM images showing examples of microneuromas from included papers (Figures 1E and 1F) suggest a similar phenotype to neuromas; there is no obvious classification based on location, size, reflectivity, shape or morphology.
Ross et al.[8] sub-classified microneuromas in corneal stromal nerves, based upon IVCM appearance, into three groups: (i) ‘spindle’ microneuromas (“hyper-reflective fusiform enlargement of a stromal nerve trunk without axonal sprouting”); (ii) ‘lateral’ microneuromas (“localised hyper-reflective enlargements of a stromal nerve from which single or multiple tortuous nerves arose”); and (iii) ‘stump’ microneuromas (“abrupt and swollen termination of the stromal nerves”). In contrast to earlier papers,[2] this classification does make nerve ‘sprouts’ a prerequisite for the classification of a microneuroma.
(iii). Nerve sprout/s/ing
Corneal nerve ‘sprouts’ and/or ‘sprouting’ was described in nine papers,[25–32 36] in the absence of the terms neuroma or microneuroma. These papers examined corneal nerves in a variety of conditions, including atopic keratoconjunctivitis,[25] polyneuropathy,[26] post-PTK,[27] Stevens-Johnson syndrome and toxic epidermal necrolysis,[28] neurotrophic keratopathy,[29 30] bullous keratopathy,[31] PXF[32] and episodic migraine.[36] Not all of these conditions are characterised by corneal neuropathic pain, despite previous reports that the sprouting of corneal nerve endings (consistent with a neuroma[5]) is a sign that is “specific for neuropathic corneal pain.”[6 34]
Rao et al.[29] identified nerve sprouts in individuals with neurotrophic keratitis who had received topical autologous plasma therapy, and considered these features to indicate nerve regeneration. The authors[29] described the sprouts as “flower like” and “resembled dendritic cells frequently seen in the subbasal layer; however, these nerve sprouts had a mean length of 120.5 ± 20.0 μm compared with dendritic cells, which have been reported to have a diameter of up to 15 μm.”[39] Whilst this distinction is made in the text of their report, the included representative IVCM image in the original paper[29] of a ‘nerve sprout’ shows a feature of ~25 μm diameter that, in our view, has the distinctive appearance of a corneal immune cell.[40] Studies by Hu et al.[25] and Lagali et al.[27] also described “presumed sprouts” with a similar short-length phenotype (Figure 1G). Representative IVCM images of nerve sprouts in the papers by Zheng et al.,[32] Fung et al.,[30] and Zhao et al.,[26] are broadly consistent with the larger mean length described by Rao et al.[29] In contrast to these dendritic-like sprout morphologies, two papers[28 36] used this term to describe IVCM nerve features with substantial homology to a neuroma/microneuroma, as evident from apparent swollen nerve endings with hyperfluorescent terminal bulbs (Figure 1H).
Al-Aqaba et al.[31] correlated laser-scanning IVCM images, taken prior to penetrating keratoplasty procedures, with whole-mount ex vivo staining of the removed corneal buttons, in individuals with bullous keratopathy. These authors reported evidence of nerve sprouting in each of five examined corneas, and a correspondence between areas of apparent ‘nerve sprouting’ seen using IVCM, with the histologic analyses. Corneal stromal nerves were noted to have “excrescences or thickenings suggestive of early sprouting”.
(iv). Abrupt nerve terminations and stumps
Abrupt terminations of sub-basal nerve fibers were described in populations with keratoconus.[22 23] An IVCM image in Patel et al.[22] is described to show “apparent abrupt terminations of sub-basal nerve fiber bundles” (Figure 1I). This structure appears similar to “nerve sprouts” described in other papers and the “short nerve stumps” evident in eyes with neurotrophic keratopathy[29 30] and post-LASIK.[33]
DISCUSSION
This systematic review identified and synthesised information from clinical reports that have reported phenotypes consistent with, or used terms related to, a ‘neuroma’ or ‘microneuroma’ to describe corneal nerve features from laser-scanning IVCM images. This comprehensive analysis was inspired by our team’s recent article, which raised the notion that, due to their similar appearance, physiological nerve anatomical features may be mistaken for neuro-pathological signs in IVCM images.[14]
We identified 25 relevant papers, of which almost half were published in the preceding four years. Information within these reports confirms that physiological sites where stromal nerves penetrate through to the epithelium appear strikingly similar to nerve features that have been associated with corneal disease and injury. Whilst corneal neuromas and microneuromas are considered markers of neuropathy,[34] there is potential for physiological nerve penetration points to be inadvertently misclassified as pathological entities. We identify inconsistencies in adoption of the terms neuroma and microneuroma, including their interchangeable use in some papers.[2 35] Furthermore, corneal nerve sprouts and stumps, which have been used to define neuromas in some contexts,[11] have been inconsistently used to describe a range of nerve features. These findings highlight a need for a standardised approach to identify, define and classify both physiological and pathological corneal nerve anatomical parameters in IVCM images. Developing and adopting a consistent approach is essential to ensure both the accuracy of patient assessment and diagnosis, and interpretation of clinical efficacy when treating corneal neuropathic pain using changes in neuroma and microneuroma density as surrogate ‘biomarkers’ of therapeutic efficacy.
Corneal sensory nerves derive from the ophthalmic division of the trigeminal nerve. Nerve trunks, arising from the limbal plexus, enter the peripheral corneal stroma and exit by penetrating the anterior limiting lamina to form a plexus within the basal corneal epithelium. This plexus is often referred to as the ‘sub-basal nerve plexus’ in the clinical literature, although the nerve plexus anatomically forms amongst the basal epithelia rather than beneath it.[14] Stromal-epithelial nerve penetration points have complex morphologies, which can result in hyper-reflective structures in corneal IVCM images.[13 21]
This review raises an important question concerning the pathological significance of corneal nerve features that have been described as neuromas and microneuromas (or similar). Many of the included primary research studies lacked a relevant control/comparator group.[8 12 22 28–30 33] The only study that reported no control participants to have corneal microneuromas[4] analysed “three images (judged) most representative of the subbasal nerve plexus” per participant. Using a standard IVCM image frame (400 × 400 μm), this equates to a 0.48mm2 sampling area, equivalent to 0.4% of the total corneal area (based on a surface area of 132mm2)[41]. With ~185 stromal-epithelial nerve penetration points in the human cornea,[42] and for simplicity assuming a relatively equal distribution across this tissue (which gives a best-case scenario as most studies examine the central cornea and nerve entry points are predominantly in the mid-periphery[13]), at a minimum, ~0.71mm2 of corneal area might need to be imaged to potentially observe a single physiological penetration point. This equates to at least five non-overlapping image frames per eye. The number of IVCM images analysed per participant (i.e., sampling level) affects the confidence of estimates for quantitative corneal nerve parameters. At least eight images, with <20% image overlap (or approximately six, non-overlapping 400 × 400 μm images), should be analysed for a reliable estimate of corneal nerve density;[43] this is similar to the above estimate for the number of images required to potentially identify a single stromal-epithelial nerve penetration point. To minimise risks of sampling bias, the image selection method should be random, rather than subjective.[20] It is thus problematic that >85% of original studies in this review used four or fewer IVCM images (with most selected subjectively), or did not report the number analysed. In addition to recommending that investigators of IVCM studies perform analyses of corneal neuromas and microneuromas in a masked manner, it would be prudent for these features to be quantified in all studies, rather than reported in a qualitative or quasi-quantitative manner (i.e., presence/absence). There is a need to ensure future research studies adopt appropriate controls, imaging methods and analytical techniques to permit reliable comparisons between healthy and diseased corneas.
Another important finding is the use of inconsistent definitions for corneal neuromas and microneuromas. Whilst some definitions have included the need for nerve sprouting at the blunt end of an injured nerve,[2 4 5] other definitions have not specified this feature.[8 11] The word ‘neuroma’ was first used to define “a tumor growing from a nerve and consisting of fibers”.[44] The term is no longer only used to describe tumors, and in the context of neuropathic pain is defined in the Encyclopaedia of Pain (2013) as “the structure that develops on the proximal cut end of a peripheral nerve branch or nerve fascicle. Severed axons form swollen terminal end bulbs, and there is usually initiation of sprouting. Regenerative sprouts are not able to elongate, they often form a tangled mass at the nerve end, a nerve end neuroma. Transection of small groups of axons scattered throughout a nerve trunk, or of tiny nerve fascicles or tributaries yields microneuromas.”[45] Using this definition, regenerative nerve sprouts are a common, but not necessarily a requisite feature, of neuromas. A microneuroma is defined based upon the same process occurring in smaller nerve axons.
In conclusion, this systematic review identifies limitations in many clinical studies that have used laser-scanning IVCM to describe corneal nerve morphologies associated with neuromas and microneuromas. We demonstrate inconsistencies in the language used to describe human corneal nerves features, a lack of consistent definitions for specific terminology, and limitations in image acquisition and sampling that can introduce bias. To obtain greater clarity about the prevalence and features of physiological versus pathological corneal nerve phenomena, we provide recommendations for study procedures and protocols to support enhanced differentiation of non-pathological nerve entry points from anomalous features resulting from corneal nerve disease or injury (Table 1). Using these recommendations may provide greater clarity relating to the appropriate and standardised interpretation of corneal nerve features from laser-scanning IVCM images. We propose an international consensus to be of value for improving the classification of features indicative of corneal pathology.
Table 1 –
Recommendations for future laser-scanning IVCM studies investigating morphologic features of corneal nerves with a phenotype similar to, or consistent with, neuromas or microneuromas
| Characteristic | Recommendation |
|---|---|
| Terminology and features |
|
| Study design and reporting |
|
| Corneal IVCM sampling and selection |
|
Abbreviation; IVCM, in vivo confocal microscopy.
Supplementary Material
SYNOPSIS/PRECIS.
We identify a need for consistent nomenclature and definitions, and rigorous laser-scanning in vivo confocal microscopy methods to clarify the prevalence and significance of features referred to as corneal “neuromas” and “microneuromas” in the literature.
Funding:
National Health and Medical Research Council of Australia APP1126540 (HRC); Rebecca L Cooper Medical Foundation (LED); NIH/NEI EY08512 (MAS); National Health and Medical Research Council of Australia, APP1101078 and APP1156944 (ND). The funding organisations had no role in the design or conduct of this work.
Abbreviations:
- IVCM
in vivo confocal microscopy
- LASIK
laser in situ keratomileusis
- PTK
phototherapeutic keratectomy
- PXF
pseudoexfoliation syndrome
- RCT
randomized controlled trial
Footnotes
Conflict of interest: No conflicting interest exists for any author.
REFERENCES
- 1.Petroll WM, Robertson DM. In Vivo Confocal Microscopy of the Cornea: New Developments in Image Acquisition, Reconstruction, and Analysis Using the HRT-Rostock Corneal Module. Ocul Surf 2015;13(3):187–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cruzat A, Qazi Y, Hamrah P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul Surf 2017;15(1):15–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol 2014;59(3):263–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aggarwal S, Colon C, Kheirkhah A, Hamrah P. Efficacy of autologous serum tears for treatment of neuropathic corneal pain. Ocul Surf 2019;17(3):532–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goyal S, Hamrah P. Understanding Neuropathic Corneal Pain--Gaps and Current Therapeutic Approaches. Semin Ophthalmol 2016;31(1–2):59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moein H-R, Dieckmann G, Abbouda A, et al. In Vivo Confocal Microscopy Demonstrates the Presence of Microneuromas and may Allow Differentiation of Patients with Corneal Neuropathic Pain from Dry Eye Disease. Invest Ophthalmol Vis Sci 2017;58(8):2656–56. [Google Scholar]
- 7.Morkin MI, Hamrah P. Efficacy of self-retained cryopreserved amniotic membrane for treatment of neuropathic corneal pain. Ocul Surf 2018;16(1):132–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross AR, Al-Aqaba MA, Almaazmi A, et al. Clinical and in vivo confocal microscopic features of neuropathic corneal pain. Br J Ophthalmol 2020;104(6):768–75. [DOI] [PubMed] [Google Scholar]
- 9.Chia EM, Mitchell P, Rochtchina E, Lee AJ, Maroun R, Wang JJ. Prevalence and associations of dry eye syndrome in an older population: the Blue Mountains Eye Study. Clin Exp Ophthalmol 2003;31(3):229–32. [DOI] [PubMed] [Google Scholar]
- 10.McCarty CA, Bansal AK, Livingston PM, Stanislavsky YL, Taylor HR. The epidemiology of dry eye in Melbourne, Australia. Ophthalmology 1998;105(6):1114–9. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal S, Kheirkhah A, Cavalcanti BM, et al. Autologous Serum Tears for Treatment of Photoallodynia in Patients with Corneal Neuropathy: Efficacy and Evaluation with In Vivo Confocal Microscopy. Ocul Surf 2015;13(3):250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giannaccare G, Buzzi M, Fresina M, Velati C, Versura P. Efficacy of 2-Month Treatment With Cord Blood Serum Eye Drops in Ocular Surface Disease: An In Vivo Confocal Microscopy Study. Cornea 2017;36(8):915–21. [DOI] [PubMed] [Google Scholar]
- 13.Patel DV, McGhee CN. Mapping of the normal human corneal sub-Basal nerve plexus by in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci 2005;46(12):4485–8. [DOI] [PubMed] [Google Scholar]
- 14.Stepp MA, Pal-Ghosh S, Downie LE, et al. Corneal Epithelial “Neuromas”: A Case of Mistaken Identity? Cornea 2020;39(7):930–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tavakoli M, Malik RA. Corneal confocal microscopy: a novel non-invasive technique to quantify small fibre pathology in peripheral neuropathies. J Vis Exp 2011(47):2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT Chandler J. TJ, Cumpston M, Li T, Page M, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chicester (UK): John Wiley & Sons, 2019. [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation. [Google Scholar]
- 19.Health National and Medical Research Council (NHMRC). NHMRC additional levels of evidence and grades for recommendations for developers guidelines. NHMRC Guidelines. 2009. [Google Scholar]
- 20.De Silva MEH, Zhang AC, Karahalios A, Chinnery HR, Downie LE. Laser scanning in vivo confocal microscopy (IVCM) for evaluating human corneal sub-basal nerve plexus parameters: protocol for a systematic review. BMJ open 2017;7(11):e018646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Aqaba MA, Dhillon VK, Mohammed I, Said DG, Dua HS. Corneal nerves in health and disease. Prog Ret Eye Res 2019;73:100762. [DOI] [PubMed] [Google Scholar]
- 22.Patel DV, McGhee CN. Mapping the corneal sub-basal nerve plexus in keratoconus by in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci 2006;47(4):1348–51. [DOI] [PubMed] [Google Scholar]
- 23.Niederer RL, Perumal D, Sherwin T, McGhee CN. Laser scanning in vivo confocal microscopy reveals reduced innervation and reduction in cell density in all layers of the keratoconic cornea. Invest Ophthalmol Vis Sci 2008;49(7):2964–70. [DOI] [PubMed] [Google Scholar]
- 24.Parissi M, Randjelovic S, Poletti E, et al. Corneal Nerve Regeneration After Collagen Cross-Linking Treatment of Keratoconus: A 5-Year Longitudinal Study. JAMA Ophthalmol 2016;134(1):70–8. [DOI] [PubMed] [Google Scholar]
- 25.Hu Y, Matsumoto Y, Adan ES, et al. Corneal in vivo confocal scanning laser microscopy in patients with atopic keratoconjunctivitis. Ophthalmology 2008;115(11):2004–12. [DOI] [PubMed] [Google Scholar]
- 26.Zhao C, Lu S, Truffert A, et al. Corneal nerves alterations in various types of systemic polyneuropathy, identified by in vivo confocal microscopy. Klin Monbl Augenheilkd 2008;225(5):413–7. [DOI] [PubMed] [Google Scholar]
- 27.Lagali N, Germundsson J, Fagerholm P. The role of Bowman’s layer in corneal regeneration after phototherapeutic keratectomy: a prospective study using in vivo confocal microscopy. Invest Ophthalmol Vis Sci 2009;50(9):4192–8. [DOI] [PubMed] [Google Scholar]
- 28.Vera LS, Gueudry J, Delcampe A, Roujeau JC, Brasseur G, Muraine M. In vivo confocal microscopic evaluation of corneal changes in chronic Stevens-Johnson syndrome and toxic epidermal necrolysis. Cornea 2009;28(4):401–7. [DOI] [PubMed] [Google Scholar]
- 29.Rao K, Leveque C, Pflugfelder SC. Corneal nerve regeneration in neurotrophic keratopathy following autologous plasma therapy. Br J Ophthalmol 2010;94(5):584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fung SSM, Catapano J, Elbaz U, Zuker RM, Borschel GH, Ali A. In Vivo Confocal Microscopy Reveals Corneal Reinnervation After Treatment of Neurotrophic Keratopathy With Corneal Neurotization. Cornea 2018;37(1):109–12. [DOI] [PubMed] [Google Scholar]
- 31.Al-Aqaba M, Alomar T, Lowe J, Dua HS. Corneal Nerve Aberrations in Bullous Keratopathy. Am J Ophthalmol 2011;151(5):840–49.e1. [DOI] [PubMed] [Google Scholar]
- 32.Zheng X, Shiraishi A, Okuma S, et al. In vivo confocal microscopic evidence of keratopathy in patients with pseudoexfoliation syndrome. Invest Ophthalmol Vis Sci 2011;52(3):1755–61. [DOI] [PubMed] [Google Scholar]
- 33.Deng S, Wang M, Zhang F, Sun X, Hou W, Guo N. Corneal subbasal nerve fiber regeneration in myopic patients after laser in situ keratomileusis. Neural Regen Res 2012;7(20):1556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dieckmann G, Goyal S, Hamrah P. Neuropathic Corneal Pain: Approaches for Management. Ophthalmology 2017;124(11s):S34–s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavalcanti BM, Cruzat A, Sahin A, Pavan-Langston D, Samayoa E, Hamrah P. In vivo confocal microscopy detects bilateral changes of corneal immune cells and nerves in unilateral herpes zoster ophthalmicus. Ocul Surf 2018;16(1):101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen F, Dong X, Zhou X, Yan L, Wan Q. Corneal subbasal nerve plexus changes in patients with episodic migraine: an in vivo confocal microscopy study. J Pain Res 2019;12:1489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beuerman RW, Schimmelpfennig B. Sensory denervation of the rabbit cornea affects epithelial properties. Exp Neurol 1980;69(1):196–201. [DOI] [PubMed] [Google Scholar]
- 38.Lee BH, McLaren JW, Erie JC, Hodge DO, Bourne WM. Reinnervation in the cornea after LASIK. Invest Ophthalmol Vis Sci 2002;43(12):3660–4 [PubMed] [Google Scholar]
- 39.Zhivov A, Stave J, Vollmar B, Guthoff R. In vivo confocal microscopic evaluation of Langerhans cell density and distribution in the normal human corneal epithelium. Graefe’s Arch Ophthalmol 2005;243(10):1056–61. [DOI] [PubMed] [Google Scholar]
- 40.Kamel JT, Zhang AC, Downie LE. Corneal Epithelial Dendritic Cell Response as a Putative Marker of Neuro-inflammation in Small Fiber Neuropathy. Oc Immunol Inflamm 2019:1–4. [DOI] [PubMed] [Google Scholar]
- 41.Kwok LS. Calculation and application of the anterior surface area of a model human cornea. J Theor Biol 1984;108(2):295–313. [DOI] [PubMed] [Google Scholar]
- 42.Al-Aqaba MA, Fares U, Suleman H, Lowe J, Dua HS. Architecture and distribution of human corneal nerves. Br J Ophthalmol 2010;94(6):784–9. [DOI] [PubMed] [Google Scholar]
- 43.Vagenas D, Pritchard N, Edwards K, et al. Optimal image sample size for corneal nerve morphometry. Optom Vis Sci 2012;89(5):812–7. [DOI] [PubMed] [Google Scholar]
- 44.Meriam Webster Dictionary. Secondary Meriam Webster Dictionary. Available at: https://www.merriam-webster.com/dictionary/neuroma. Accessed July 1, 2020.
- 45.Microneuroma. In: Gebhart GF, Schmidt RF, eds. Encyclopedia of Pain. Berlin, Heidelberg: Springer Berlin Heidelberg, 2013:1850–50. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


