Abstract
Objective:
Years of sport participation (YoP) is conventionally-used to estimate cumulative repetitive head impacts experienced by contact sport athletes. The relationship of this measure to other estimates of head impact exposure and the potential associations of these measures with neurobehavioral functioning are unknown. We investigated the association between YoP and the Head Impact Exposure Estimate (HIEE), and whether associations between the two estimates of exposure and neurobehavioral functioning varied.
Methods:
Former American Football players (N=58; age=37.9±1.5 years) completed in-person evaluations approximately 15-years following sport discontinuation. Assessments consisted of neuropsychological assessment and structured interviews of head impact history (i.e., HIEE). General linear models were fit to test the association between YoP and the HIEE, and their associations with neurobehavioral outcomes.
Results:
YoP was weakly correlated with the HIEE, p=.005, R2=.13. Higher YoP was associated with worse performance on the Symbol Digit Modalities Test, p=.004, R2=.14, and Trail Making Test-B, p=.001, R2=.18. The HIEE was associated with worse performance on the Delayed Recall trial of the Hopkins Verbal Learning Test-Revised, p=.020, R2=.09, self-reported cognitive difficulties (Neuro-QoL Cognitive Function), p=.011, R2=.10, psychological distress (Brief Symptom Inventory-18), p=.018, R2=.10, and behavioral regulation (Behavior Rating Inventory of Executive Function for Adults), p=.017, R2=.10.
Conclusion:
YoP was marginally associated with the HIEE, a comprehensive estimate of head impacts sustained over a career. Associations between each exposure estimate and neurobehavioral functioning outcomes differed. Findings have meaningful implications for efforts to accurately quantify the risk of adverse long-term neurobehavioral outcomes potentially associated with repetitive head impacts.
Keywords: contact sport participation, repetitive head impacts, head injury, TBI, RHI
Introduction
It has been suggested that long-term changes in neurobehavioral functioning are more likely associated with repetitive head impacts (RHI) falling below the relative magnitude of clinically diagnosed concussive injury, and less so due to concussion itself (Alosco et al., 2018; McKee, Alosco, & Huber, 2016; Stein, Alvarez, & McKee, 2015; Tagge et al., 2018). Recent studies have reported on adverse long-term neurobehavioral functioning as being associated with lengthier participation in American football, independent of concussion history (Lepage et al., 2018; Mez et al., 2017; Montenigro et al., 2017; Roberts et al., 2019; Schultz et al., 2018; Singh et al., 2014). However, not all studies have observed this association (Bohr, Boardman, & McQueen, 2019; Deshpande et al., 2017; Deshpande, Hasegawa, Weiss, & Small, 2020; Gysland et al., 2012; Willer et al., 2018). One potential reason for the inconsistent findings in the associations between neurobehavioral outcomes and RHI may be due to limitations related to the method utilized to retroactively estimate RHI exposure.
The current convention used to estimate RHI exposure in most studies to date has involved inquiring about total years of contact sport participation, often measured as year started to year stopped (Mez et al., 2019; Mez et al., 2017; Roberts et al., 2019; Schultz et al., 2018; Singh et al., 2014). Years of participation (YoP) is likely limited in as a method for estimating RHI exposure, in that the number and magnitude of head impacts that can occur during sport widely differ based upon a number of factors, such as sport played, position, and participation level (Crisco et al., 2011; Mihalik, Bell, Marshall, & Guskiewicz, 2007; Sandmo, Andersen, Koerte, & Bahr, 2020). Furthermore, gross inquiry of YoP contains potential for errors as an estimate of RHI, as it lacks consideration of time as a starter, time missed due to injury, and years off in between first and last year of participation. For example, two individuals could be considered as having similar levels of “RHI exposure” by playing eight years of football between the years 2000 to 2007. However, this contains potential for error as an estimate for RHI exposure, as one of the two individuals could have missed two years in between that time due to injury, played in 0% of games as a non-starter, sustained fewer impacts at lower levels of play (youth vs. high school), and played a position (i.e., quarterback) with one-fourth as many impacts as other positions (Crisco et al., 2011; Kelley et al., 2017).
Attempts have been made towards improved quantification of RHI exposure estimation beyond YoP by developing metrics that incorporate various aspects of play history, such as percentage of each year played and expected frequency and magnitude of impacts during that time derived from published studies using head impact measurement devices (Karton, Blaine Hoshizaki, & Gilchrist, 2020; Kerr et al., 2015; Montenigro et al., 2017). One of these metrics includes the Head Impact Exposure Estimate (HIEE), which provides a single estimate of the number of head impacts sustained by an individual over the course of their athletic career by incorporating several of the above considerations (e.g., percentage of year played, level of play, football position played, etc.; Kerr et al., 2015). The vast majority of our current knowledge regarding the association between RHI exposure and adverse neurobehavioral outcomes later in life is primarily based upon YoP as the estimate of exposure (Mez et al., 2019; Mez et al., 2017; Roberts et al., 2019; Schultz et al., 2018; Singh et al., 2014). Given this, it is critical to examine the relationship between the conventional method of RHI estimation, YoP, and more detailed estimates of exposure (i.e., HIEE), as well as their independent and mutual associations with neurobehavioral functioning.
Given the importance of further understanding how estimates of RHI exposure are associated with one another, this study incorporated the following aims: Aim 1) examine the association between YoP and a detailed estimate of head impact exposure (i.e., HIEE); Aim 2) identify whether the associations between these two estimates of RHI exposure and neurobehavioral functioning differ; and Aim 3) through exploratory analyses, examine the degree to which various demographic factors and aspects of current functioning were associated with YoP and the HIEE.
Materials and Methods
Participants and Procedure
This study was approved by an Institutional Review Board (IRB) and all participants provided written informed consent prior to study activities. Participants were former collegiate athletes (retired for approximately 15-years) recruited from 30 different National Collegiate Athletic Association (NCAA) member institutions who had previously participated in an online health survey in 2014 (Kerr, Thomas, Simon, McCrea, & Guskiewicz, 2018). Inclusion criteria included at least one year of collegiate football participation and completion of Part I of the study (online Health Survey). Exclusion criteria for the larger parent study (NCAA 15-Year Follow-up Study), in which individuals underwent a comprehensive in-person evaluation (e.g., neurocognitive testing, neuroimaging, saliva/buccal swab collection), included a history or suspicion of psychotic disorder with active symptoms and any contraindication to study procedures (e.g., contraindications to neuroimaging, unable to travel).
Measures
Aim 1 Exposure metrics
Years of football participation
YoP is a common estimate for head impact exposure. In order to maintain consistency with previous studies examining associations between YoP with various neurobiological and neurobehavioral outcomes, YoP was queried by asking participants the years in which they first began and ended playing football (Brett et al., 2020; Schultz et al., 2018; Stern et al., 2019). For example, a participant who reported a starting year of 1995 and ending year of 2002 was recorded as having eight YoP.
Head Impact Exposure Estimate (HIEE)
The HIEE is a structured oral interview, in which individuals provide information related to each year of football participation, beginning with high school and continuing through college and professional football, where applicable (Kerr et al., 2015). For each level of play, participants denote their primary and secondary positions for each year, as well as the average number and length of contact practices per week. It is emphasized to participants to remove any weeks or seasons they did not play due to injury or any other reason. For games, participants are also asked to report the number of games they played and the percentage of time they played from the following choices: 0%, 25%, 50%, 75%, 100%.
The above information generates a “number of contact hours” metric for each individual year of participation. The number of contact hours is converted into a single estimate of the number of head impacts that an individual sustained in the course of those contact hours based on previously published head impact telemetry system (HITS) data (Broglio et al., 2011; Crisco et al., 2010). Specifically, the frequency and magnitude of estimated impacts for each individual year is adjusted through weighting based on level of play and position recorded in published HITS data. Ultimately, the HIEE produces an estimate of head impact exposure for each year played based on the factors above, which are summed to generate a single estimate of head impact exposure over the course of a participant’s career. The HIEE requires approximately 30 minutes to complete.
The HIEE has been observed as effectively differentiating between cohorts of athletes from different levels of play (i.e., collegiate versus professional; Kerr et al., 2015). While investigation into the reliability of the measure is somewhat limited, the published development study of the HIEE observed that player reports and public records of online professional games differed to a very small degree (maximum discrepancy of 1 game). High school and collegiate games could not be verified. Additionally, the measure has yet to be validated against prospectively collected HITS data. Please see Kerr et al. (2015) for further details involving the HIEE.
Aim 2 Neurobehavioral functioning
Self-report psychological functioning and cognitive functioning
Psychological self-report symptom measures included the Brief Symptom Inventory-18 (BSI-18) Global Severity Index (GSI), a measure of internalizing psychopathology and somatic symptoms, as well as the Beck Depression Inventory (BDI) and Beck Anxiety Inventory (BAI; Beck, Epstein, Brown, & Steer, 1988; Beck, Steer, & Brown, 1996; Derogatis, 2001). Self-report measures of cognitive and executive functioning included the Behavioral Regulation (BRI) and Metacognition (MI) indices on the Behavior Rating Inventory of Executive Function-Adult (BRIEF-A) and Quality of Life in Neurological Disorders (Neuro-QoL) Cognitive Functioning Short-Form (National Institute of Neurological Disorders and Stroke User Manual for the Quality of Life in Neurological Disorders (Neuro-QoL) Measures Version 2.0., 2015; Roth, Isquith, & Gioia, 2005). Scores on self-report measures of psychological and cognitive/executive functioning were converted to normative-based T-scores (mean=50 and standard deviation, SD=10) in order to better approximate a normal distribution. For self-report measures in which standardized scores are not available (i.e., BDI and BAI), sensitivity analyses were performed to verify that associations between RHI exposure estimates and outcomes did not meaningfully differ when modeling theses associations as having a negative binomial or normal distribution. Higher scores on all measures indicate greater dysfunction within the construct being measured, with the exception of Neuro-QoL Cognitive Functioning, in which higher T-scores represent better self-rated cognitive function.
Objectively measured neurocognitive functioning
A well-validated paper & pencil neurocognitive test battery was used to assess cognitive domains (e.g., memory, executive function, processing speed) commonly affected by various neurologic disorders. Neurocognitive functioning measures included the Trail Making Test A and B (TMT-A and TMT-B; Reitan & Wolfson, 1985), Symbol Digit Modalities Test (SDMT; A. Smith, 2007), Hopkins Verbal Learning Test-Revised (HVLT-R; Total Immediate and Delayed Recall; Brandt & Benedict, 2001), and Verbal Fluency (F-A-S; Spreen & Benton, 1977). Raw score performances, as opposed to standardized scores, for neurocognitive measures more reliably met statistical assumptions for the planned analyses and were included as outcomes of interest. For most measures, higher performance indicates a better performance, with the exception of the TMT-A and TMT-B, where higher scores (i.e., slower completion times) represent worse performance.
Aim 3 Exploratory factors to differentiate YoP and HIEE
Several exploratory variables were examined in order to identify factors that may be uniquely associated with each exposure estimate. Exploratory factors/constructs and their respective measures included: pain intensity and interference (Brief Pain Inventory and Pain subscale from the Veteran’s Rand-36 Item Health Survey [VR-36]; Cleeland & Ryan, 1994; Jones et al., 2001), current life stressors (Life Events Scale; Holmes & Rahe, 1967), current physical functioning (Physical Functioning, Role Limitations due to Physical Health, Energy/Fatigue, and General Health indices from the VR-36), social functioning (VR-36 Social Functioning index), and various demographic or history factors such as history of psychiatric diagnosis (depression or anxiety), estimated premorbid intelligence (Wechsler Test of Adult reading [WTAR]; Wechsler, 2001), self-identified race (White and non-White), age, highest level of education (BA/BS versus graduate degree or greater), and division of collegiate football play (Division I versus Division III).
Statistical Analysis
Statistical analyses were performed using SPSS version 25 and the RLM Macro for calculating robust standard error estimates for select models (Darlington & Hayes, 2016). Statistical significance was evaluated at the 0.05 level.
Aim 1 analysis
A general linear model (GLM) was fit to estimate the association between YoP and the HIEE. Specifically, YoP was entered as the predictor variable within the model to assess the degree to which YoP predicted estimation of head impact exposure in the more detailed HIEE metric. R2 indicated the degree of variance of head impact estimation via the HIEE accounted for by YoP.
Aim 2 analyses
The effects of YoP and HIEE on clinical measures of neurobehavioral functioning were assessed independently for each outcome of interest within separate GLMs. Subsequently, models including both exposure estimates as predictors were also conducted for each outcome. For instances in which only one particular exposure estimate was identified as being significantly predictive of an outcome, a step-wise linear regression was performed with that exposure estimate entered first, and the other estimate entered as a second block entry, in order to statistically assess the additional increase in variance explained by the second metric (i.e., change in R2). Otherwise, both exposure metrics were entered into the GLMs simultaneously. Standardized beta coefficients for YoP and HIEE were calculated. Zero-order, partial, and part correlations were computed and inspected to determine the unique contribution of each exposure estimate on outcomes. Multicollinearity between the two exposure estimates was assessed using the variance inflation factor (i.e., <5 deemed as acceptable; Allison, 1999)
Statistical assumptions were examined and met, with the exception of models involving three outcomes (HVLT-R Delayed Recall, BSI-GSI, and BAI), which were observed as violating the assumption of homoscedasticity (based on Breusch-Pagan and Koenker tests; (Breusch & Pagan, 1979; Koenker, 1981) and robust standard errors were calculated for the models involving these select outcomes (Hayes & Cai, 2007). Given that the HIEE considers only published head impact telemetry data available in high school and higher levels of competition athletics, analyses included YoP from high school and later for equivalent comparison to the HIEE. Sensitivity analyses involving all models were performed with total YoP (i.e., including pre-high school) in order to ensure consistency of results. Additional sensitivity analyses included the number of prior self-reported concussions in all models as a covariate in the first block entry of the above models to control for effects of concussion history on neurobehavioral outcomes. Concussion history was binned into four categories including 0–1, 2–4, 5–7, 8+ (Table 1).
Table 1.
Demographic, sport, and medical history
| M ± SD / N (%) | |
|---|---|
| Age | 37.9 ± 1.47 |
| Race | |
| White/Non-Hi spanic | 48 (82.8%) |
| Non-White | 7 (12.1%) |
| Other | 3 (5.2%) |
| WTAR | 111.8 (7.7) |
| NIH Picture Vocabulary | 113.2 (10.3) |
| Education | |
| Bachelor’s degree | 34 (58.6%) |
| Graduate/ Professional Degree | 24 (41.4%) |
| Years of Football Participation | 12.1 ± 3.2 |
| Years of Football Participation | 8.1 ± 1.6 |
| from High School and Beyond | |
| NCAA Level of Play | |
| Division I | 42 (72.4%) |
| Division III | 16 (27.6%) |
| Concussion History | |
| 0 to 1 | 23 (39.7%) |
| 2 to 4 | 10 (17.2%) |
| 5 to 7 | 12 (20.7%) |
| 8+ | 13 (22.4%) |
| Alcohol Use Disorders | 6.2 ± 4.9 |
| Identification Test * | |
| Drug Abuse Screening Test ^ | 0.02 ± 0.86 |
| Self-Reported Medical History | |
| Anxiety | 8 (13.8%) |
| Attention-deficit/hyperactivity disorder | 3 (5.2%) |
| Chronic headache syndrome | 2 (3.4%) |
| Chronic Obstructive Pulmonary Disease | 0 (0.0%) |
| Coronary artery disease/Myocardial Infarction | 0 (0.0%) |
| Depression | 7 (12.1%) |
| Heart Attack/Myocardial Infarction | 0 (0.0%) |
| Hyperlipidemia | 9 (15.5%) |
| Hypertension | 8 (13.8%) |
| Inflammatory Bowel Disease | 0 (0.0%) |
| Learning Disability | 1 (1.7%) |
| Migraine | 3 (5.2%) |
| Osteoarthritis/Degenerative arthritis | 7 (12.1%) |
| Rheumatoid arthritis | 1 (1.7%) |
| Stroke | 0 (0.0%) |
WTAR SS = Wechsler Test of Adult Reading Standard Score
Approximately 19% of the sample exceeded a cut-off score indicative of harmful or hazardous drinking (≥ 8)
No participants exceeded cut-off scores for commonly used to identify the presence of substance use disorders (>11).
Aim 3 analyses
Exploratory analyses were performed to examine the association between the two exposure estimates and various demographic, psychosocial, and physical factors. Pearson correlations examined the association between estimates of exposure and continuous outcomes/demographic factors (e.g., age). Independent t-tests examined group differences in demographic variables, such as presence of psychiatric disorder (Yes versus No) and highest level of education (BA/BS versus graduate degree or greater) across estimates of RHI exposure.
Results
Demographic and Contact Sport History
Demographic information and medical history of the sample (N=58) are provided in Table 1. The sample had a mean age of 37.9 (SD=1.5) and predominantly identified as White (82.8%). Athletes reported participating in 12.1 (SD=3.2; range= 6 to 19 years) years of football throughout their lifetime, on average, and retired from sport for approximately 15.39 years (SD=1.7). YoP from high school and through higher levels of play was a mean of 8.1 years (SD=1.6; range = 4 to 12 years). Of the sample, approximately 15.5% briefly played football professionally (five participants played professionally for one year and four played professionally for two years).
Aim 1. YoP and the HIEE
YoP significantly predicted head impact exposure estimates from the HIEE (β=.12, p=.005, R2=.13). Results from the GLM showed that YoP accounted for only approximately 13% of the variance of head impact exposure estimation from the HIEE. This suggests that 87% of variation in head impact estimation from the detailed metric is accounted for by factors other than the number of years in which an athlete participates in football. Neither exposure metric was significantly associated with concussion history (HIEE ρ=.19, p=.16; YoP ρ=−.23, p=.16).
Aim 2. YoP, HIEE, and neurobehavioral functioning
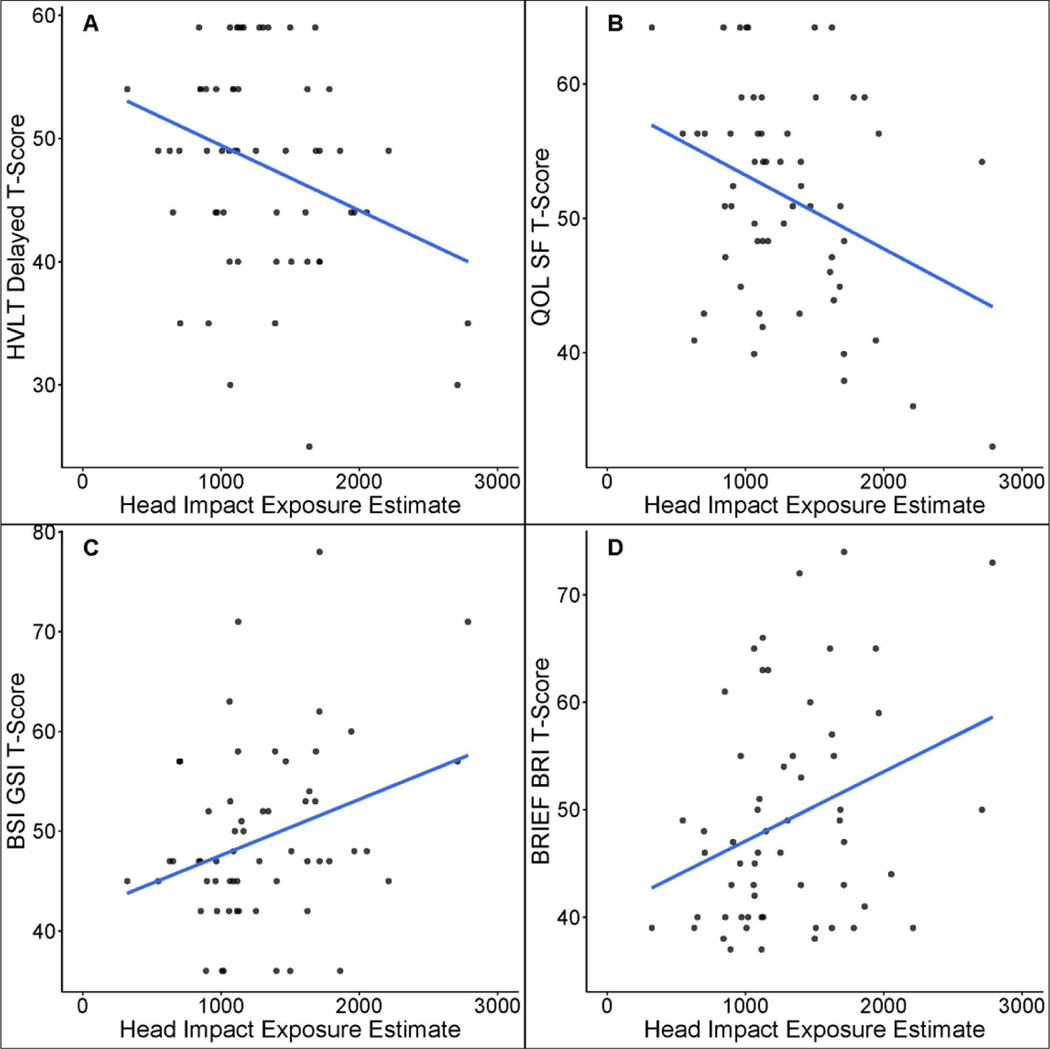
A measure of processing speed and a measure of timed executive functioning were significantly associated with YoP (Table 2). Specifically, greater YoP was significantly predictive of worse performance on the SDMT, β= −.37, p=.004, R2 = .14, and TMT-B, β= .42, p=.001, R2= 0.18 (Figure 1). The HIEE was significantly predictive of worse performance of delayed memory recall on a measure of verbal episodic memory (HVLT-R; β=- .30, p=.020, R2=.09) and processing speed (SDMT; β=−.26, p=.046, R2=.07). Greater self-reported difficulties with general cognition (Neuro-QoL Cognitive Function; β=−.33, p=.011, R2=.11), general psychological distress (BSI-GSI; β =.311, p =.018, R2 =.10), and a select aspect of executive functioning (i.e., behavioral regulation; BREIF-A BRI; β=31, p=.017, R2 =.10) were also significantly associated with the HIEE (Figure 2).
Table 2.
Univariate models of exposure estimates in predicting neurobehavioral outcomes
| Years of Participation | Head Impact Exposure Est. | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Measure | Raw Score M ± SD | Standardized Score M ± SD | β | p | R2 | β | p | R2 |
| Performance Based | ||||||||
| HVLT-R Immediate Recall* | 27.33 ± 3.28 | 47.91 ± 7.86 | −.107 | .425 | .011 | −0.183 | 0.169 | 0.034 |
| HVLT-R Delayed Recall* | 9.72 ± 1.78 | 47.86 ± 8.53 | −.107 | .425 | .011 | −0.304 | 0.020 | 0.092 |
| F-A-S* | 44.26 ± 9.75 | 47.76 ± 8.63 | −.058 | .668 | .003 | −.176 | 0.187 | 0.031 |
| SDMT^ | 56.62 ± 8.31 | 103.34 ± 13.84 | −.374 | .004 | .140 | −0.263 | 0.046 | 0.069 |
| TMT-A* | 22.10 ± 6.48 | 52.71 ± 11.03 | .219 | .099 | .048 | 0.078 | 0.559 | 0.006 |
| TMT-B* | 47.45 ± 13.99 | 54.88 ± 10.45 | .423 | .001 | .179 | 0.011 | 0.936 | 0.000 |
| Self-Report | ||||||||
|
| ||||||||
| Neuro-QoL Cognition* | 34.21± 5.45 | 51.64 ± 7.94 | −.029 | .831 | .001 | −0.333 | 0.011 | 0.111 |
| BRIEF-A BRI* | 42.86 ± 9.97 | 49.02 ± 10.11 | .086 | .519 | .007 | 0.312 | 0.017 | 0.097 |
| BRIEF-A MI* | 58.47 ± 14.11 | 50.29 ± 11.00 | .110 | .412 | .012 | 0.253 | 0.055 | 0.064 |
| BSI-GSI* | 6.47 ± 8.82 | 49.26 ± 8.82 | .151 | .258 | .023 | 0.311 | 0.018 | 0.097 |
| Beck Depression Inventory | 7.29 ±7.26 | N/A | .028 | .838 | .001 | 0.193 | 0.146 | 0.037 |
| Beck Anxiety Inventory | 6.24 ±7.77 | N/A | .192 | .149 | .037 | 0.216 | 0.103 | 0.047 |
M=Mean; SD=Standard deviation; β=standardized beta estimate; p= p-value; R2 = r-squared (coefficient of determination)
T-score
Standard score; HVLT-R= Hopkins Verbal Learning Test-Revised; F-A-S= Verbal Fluency, F-A-S; SDMT= Symbol Digit Modalities Test; TMT= Trail Making Test; Neuro-QoL Cognition= Quality of Life in Neurological Disorders Cognitive Functioning Short-form; BRIEF-A= Behavior Rating Inventory of Executive Function – Adult; BRI= Behavioral Regulation Index; MI= Metacognition Index; BDI= Beck Depression Inventory; BAI= Beck Anxiety Inventory; BSI-GSI= Brief Symptom Inventory-18 Global Severity Index; N/A= Not applicable
Figure 1.
Scatterplot of two cognitive measures, Symbol Digit Modalities Test (SDMT) and Trail Making Test B (TMT-B), that were significantly associated with years of participation (YoP). Associations of these measures with the Head Impact Exposure Estimate (HIEE) are shown in the bottom quadrants for comparative purposes. The Y-axis represents raw score performance on measures and x-axis denotes cumulative YoP (from high school and beyond) or the HIEE. Higher performance on the TMT-B indicates worse performance, whereas higher performances on the SDMT represents better performance. Of note, the HIEE was no longer significantly associated with SDMT performance once YoP was included within the model.
Figure 2.
Scatterplots of measures significantly associated with the Head Impact Exposure Estimate (HIEE). The Y-axis represents standardized t-scores (mean= 50 and standard deviation, SD= 10) and x-axis denotes the HIEE. HVLT-R Delay= Hopkins Verbal Learning Test-Revised Delayed Recall; QoL SF= Quality of Life in Neurological Disorders (Neuro-QoL) Cognitive Functioning Short-form; BSI-GSI= Brief Symptom Inventory-18 Global Severity Index; BRIEF-A= Behavior Rating Inventory of Executive Function – Adult; BRI= Behavioral Regulation Index; higher ratings on the BRIEF-A and BSI-GSI indicate greater dysfunction, whereas higher ratings on the Neuro-QoL Cognitive Functioning indicate higher (better) self-reported cognitive functioning; higher performance on the HVLT-R Delayed Recall indicates better performance.
When YoP and the HIEE were both included within models, YoP maintained significant associations with SDMT and TMT-B, (ps<0.05; Table 3). The HIEE only contributed an additional 2% of variance (R2=.02) for both the SDMT and TMT-B performance, which was not a statistically significant increase in variance explained, ps>.05. Similarly, the associations between the HIEE and the aforementioned outcomes (HVLT-R Delayed Recall, Neuro-QoL Cognitive Functioning, BSI-GSI, and BREIF-A BRI) remained statistically significant when YoP was entered secondarily into models, ps<.05. YoP did not significantly increase the amount of variance explained in scores on the HVLT-R Delayed Recall, Neuro-QoL Cognitive Functioning, BSI-GSI, or the BRIEF-A BRI (R2 increases <.01; ps>.05). Conversely, when YoP and the HIEE were simultaneously included within the model, the HIEE was no longer significantly associated with SDMT performance (β= −.15, p=.27). The difference in the zero-order (−.263) and part correlation (−.137) of the HIEE with SDMT performance within the model including both exposure metrics suggests that the amount of variance in SDMT scores explained by the HIEE reduced from 7% to less than 2% when the effect YoP was also considered.
Table 3.
Multivariable model involving both estimates of exposure simultaneously
| Years of Football Participation | Head Impact Exposure Est. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Measure | β | Zero- order Correl. | Partial Correl. | Part Correl. | p | β | Zero- order Correl. | Partial Correl. | Part Correl. | p | R2 |
| HVLT-R IR | −.047 | −.107 | −.044 | −.044 | .743 | −.166 | −.183 | −.156 | −.155 | .247 | .035 |
| HVLT DR | .004 | −.107 | .003 | .003 | .980 | −.305 | −.304 | −.286 | −.285 | .031 | .092 |
| F-A-S | .007 | −.058 | .006 | .006 | .962 | −.178 | −.176 | −.166 | −.166 | .216 | .031 |
| SDMT | −.321 | −.374 | −.310 | −.299 | .019 | −.147 | −.263 | −.148 | −.137 | .273 | .159 |
| TMT-A | .219 | .219 | .205 | .204 | .126 | −.001 | .078 | −.001 | −.001 | .996 | .048 |
| TMT-B | .482 | .423 | .450 | .450 | <.001 | −.163 | .011 | −.168 | −.152 | .211 | .202 |
| Neuro-QoL | .085 | −.029 | .086 | .081 | .530 | −.006 | −.333 | −.342 | −.342 | .010 | .118 |
| Cognition | |||||||||||
| BRIEF-A BRI | −.030 | .086 | −.030 | −.028 | .827 | .323 | .312 | .302 | .301 | .022 | .098 |
| BRIEF-A MI | .021 | .110 | .020 | .020 | .880 | .246 | .253 | .231 | .229 | .084 | .065 |
| BSI-GSI | .045 | .151 | .044 | .042 | .746 | .295 | .311 | .278 | .275 | .036 | .098 |
| BDI | .049 | .028 | −.046 | −.045 | .733 | .211 | .193 | .197 | .197 | .142 | .039 |
| BAI | .131 | .192 | .125 | .122 | .335 | .169 | .216 | .161 | .158 | .232 | .062 |
β=standardized beta estimated; partial correlation= correlation between exposure metric and outcome when controlling for the influence of the other exposure metric on both the first exposure metric and the neurobehavioral outcome; part correlation= correlation between exposure metric and outcome when controlling for the influence of the other exposure metric on only the first exposure metric and not the neurobehavioral outcome; Correl.=Correlation; p= p-value; R2= r-squared (coefficient of determination); HVLT-R= Hopkins Verbal Learning Test-Revised; IR= Immediate Recall; DR= Delayed Recall; F-A-S= Verbal Fluency, F-A-S; SDMT= Symbol Digit Modalities Test; TMT= Trail Making Test; Neuro-QoL Cognition= Quality of Life in Neurological Disorders Cognitive Functioning Short-form; BRIEF-A= Behavior Rating Inventory of Executive Function – Adult; BRI= Behavioral Regulation Index; MI= Metacognition Index; BDI= Beck Depression Inventory; BAI= Beck Anxiety Inventory; BSI-GSI= Brief Symptom Inventory-18 Global Severity Index
Sensitivity analyses assessing whether inclusion of concussion history as part of the above models influenced statistically significant associations revealed that all associations between YoP (SDMT and TMT-B) and the HIEE (HVLT-R Delayed Recall, BSI-GSI, Neuro-QoL Cognitive Function, and BRIEF-A BRI) remained broadly unchanged when concussion history was added to the univariate models. Additionally, sensitivity analyses were performed in order to ensure the above associations remained generally comparable when considering YoP as total years of football played versus YoP from high-school and later. The sole difference was observed for TMT-B, in which the effect of YoP on performance was weakened when total YoP (rather than YoP from high school) were included in the univariate model (β= .42, p=.001 to β= .23, p=.080), the model including the HIEE (β= .48, p=.001 to β= .27, p=.062), and the model with both the HIEE and concussion history (β= .53, p<.001 to β= .32, p=.035).
Aim 3. Potential confounding factors and exposure metrics
A number of demographic, health, and physical indices were associated with both YoP and the HIEE, including premorbid intellectual functioning, multiple metrics of pain, and role limitations due to physical difficulties (Table 4). The largest difference observed between the two exposure estimates was for age (YoP, r=.26, p=.005; HIEE, r=.07, p=.612), in which YoP was more strongly associated with older age. The greatest discrepancy observed for the HIEE involved social functioning, in which lower social functioning was positively associated with the HIEE, but not YoP (HIEE, r=−.26, p=.045; YoP, r=−.14, p=.308).
Table 4.
Exploratory associations between exposure estimates and various demographic, health, and psychosocial factors
| Years of Participation | Head Impact Exposure Est. | |||
|---|---|---|---|---|
| Pearson r/t-value | p-value | Pearson r/t-value | p-value | |
| Age | .26 | .005 | .07 | .612 |
| Education (BA/BS or above) | 1.30 | .198 | 1.67 | .100 |
| Race (White/non-white) | −.86 | .393 | −.18 | .119 |
| WTAR | −.39 | .003 | −.28 | .033 |
| Collegiate Division (I or III) | 1.10 | .277 | .77 | .446 |
| Psychiatric History (y/n) | −1.34 | .185 | −.07 | .946 |
| VR-36 Physical Function* | −.18 | .178 | −.197 | .149 |
| VR-36 Role Limitation* | −.34 | .008 | −.29 | .026 |
| VR-36 Energy/Fatigue* | −.13 | .336 | −.14 | .307 |
| VR-36 General Health* | .03 | .814 | −.03 | .807 |
| VR-36 Social Function* | −.14 | .308 | −.26 | .045 |
| VR-36 Pain* | −.31 | .018 | −.33 | .012 |
| BPI Pain Severity | .30 | .024 | .24 | .066 |
| BPI Pain Interference | .30 | .020 | .26 | .047 |
| Life Events Scale | .24 | .076 | .06 | .658 |
WTAR= Wechsler Test of Adult Reading Standard Score; VR-36= Veteran’s Rand-36 Item Health Survey; BPI= Brief Pain Inventory
Higher scores indicate better functioning the respective area
Discussion
In this study of former collegiate football players 15-years removed from sport, YoP was only mildly associated with the HIEE, a comprehensive estimate of an athlete’s cumulative volume of head impacts sustained over a career, accounting for only 13% of the variance. Though there was some degree of overlap, each exposure metric was associated with disparate sets of neurobehavioral functioning outcomes. Thus, the mode of exposure estimation used for a given study should be strongly considered when attempting to quantify the risk of adverse long-term neurobehavioral outcomes as the result of RHI from collision sports participation. Ideally, a more comprehensive estimate of head impact exposure, such as the HIEE, should be used when attempting to investigate the association between cumulative head impact exposure and long-term neurobehavioral outcomes; however, in the absence of opportunity to collect such information, YoP can be utilized as an estimate of head impact exposure given its modest relationship with the HIEE, as well as some degree of overlap with neurobehavioral outcomes.
Aim1. YoP, HIEE, and estimating exposure
There was small association between YoP and the HIEE. This finding has implications for research investigating the association between exposure to RHI and neurologic health later in life when using YoP, in that it indicates that some degree of error in estimation (i.e., either over- or under-estimation) is occurring while attempting to capture the exposure of interest. Error in estimation contains potential to produce biased estimates of risk, resulting in inaccurate conclusions regarding risk and outcome relationships, and possibly giving rise to faulty public policy initiatives (Edwards & Keil, 2017). It is assumed that the HIEE is most optimal and more accurate in estimating RHI exposure due to the greater level of detail incorporated into the final exposure estimate. However, it cannot be ruled out that the reason for the small association between YoP and the HIEE, and discrepant associations with outcome measures, could be due to error involved in the recall of specific practices or games participated in at least 15-years prior as part of the HIEE. While the initial development study of the HIEE reported that player reports and public records of online professional games differed by a maximum discrepancy of 1 game, there is potential for greater error in recall of games or details around specific practice involvement at younger ages.
The modest association between YoP and the HIEE, a more comprehensive estimate of head impact exposure, is particularly important, as several studies have reported on significant associations between years of football/contact sport participation with the presence of neuropathology (Mez et al., 2019; Mez et al., 2017), lower cognitive performance (i.e., reaction time/visual memory; Schultz et al., 2018; Singh et al., 2014), brain morphometry (i.e., thalamic volumes; Brett et al., 2020) and depression (Roberts et al., 2019). These adverse outcomes associated with YoP are attributed to RHI. Current results showing potential error when using YoP as an estimate of RHI suggest that other factors may also be influencing these associations.
Aim 2. YoP, HIEE, and neurobehavioral functioning
Prior studies have reported similar associations between contact sport exposure and poorer memory (verbal and visual) performance in older adults (Alosco et al., 2017; Lepage et al., 2018). Most studies to date have been limited by use of group comparisons (i.e., former football players compared to controls) to investigate the association between RHI exposure and adverse neurobehavioral outcomes later in life, making it difficult to decompose the differential effects of head impact exposure, cumulative concussions, and other factors (Alosco et al., 2017; Alosco et al., 2020; Goswami et al., 2016; Lepage et al., 2018; Schultz et al., 2018; Singh et al., 2014; Terpstra et al., 2019). Results from this study revealed that performance on metrics of processing speed (SDMT) and delayed verbal memory recall (HVLT-R) were associated with the HIEE, a comprehensive estimate of head impact exposure that captures multiple dimensions of exposure history within a single metric. When YoP was taken into account, the association between the HIEE and SDMT was no longer significant and the amount of variance in SDMT performance explained by the HIEE was reduced from 7% to less than 2%. These findings further reinforce the notion that while these two exposure estimates are measuring aspects of the same construct (i.e., RHI), they each are also capturing different factors that may also influence neurobehavioral functioning.
Regarding psychological functioning, the current study observed a significant association between the HIEE with general psychological distress (BSI-GSI), but not with measures intended to specifically assess depression (BDI-II) and anxiety (BAI). A fundamental difference between these measures is that one-third of the items on the measure of general distress are dedicated to capturing somatic symptoms of distress. Results from the exploratory analyses of the current study showed that the HIEE was significantly associated with a number of measures reflecting pain and physical difficulties, reinforcing the notion that the HIEE may have been significantly associated with only the measure of general distress because of the strong emphasis on physical symptoms. Interestingly, YoP was also significantly associated with similar measures reflecting pain and physical difficulties in the exploratory analyses, but not related to the measure of general psychological distress (BSI-GSI) in the primary analyses.
Similar to investigations of cognitive functioning described above, many prior studies examining the unique contributions of RHI exposure to greater psychological symptoms in former athletes are limited and have not investigated RHI exposure independent from concussion history. Previous studies utilizing group comparisons (football versus control group) have also reported on higher self-reported levels of psychological distress (e.g., depression or apathy) among former football players later in life as compared to controls, but they have not explicitly controlled for the effects of concussion (Alosco et al., 2017; Alosco et al., 2020; Lepage et al., 2018; Multani et al., 2016; Strain et al., 2013). Controlling for the effects of prior concussions is vital, as the association between cumulative concussion history and psychiatric outcomes has been well documented in the literature (Brett, Huber, Wild, Nelson, & McCrea, 2019; Brett, Mummareddy, Kuhn, Yengo-Kahn, & Zuckerman, 2019; Didehbani, Cullum, Mansinghani, Conover, & Hart, 2013; Guskiewicz et al., 2007). Using a different comprehensive estimate of head impact exposure (Cumulative Head Impact Index), one prior study controlled for concussion history using instrumental variable modeling and reported a significant association between greater head impact exposure with depression symptoms, apathy scores, and self-reported executive function difficulties on the same measure as the current study (i.e., BRIEF-A; Montenigro et al., 2017).
Based on the previous findings demonstrating a positive relationship between psychological distress and subjective cognitive difficulties, the concurrently observed association between the HIEE with both general psychological distress and self-reported global and executive function difficulties is unsurprising (Crumley, Stetler, & Horhota, 2014; Smith, Petersen, Ivnik, Malec, & Tangalos, 1996). Self-reported cognitive and psychological difficulties have been independently recorded as risk factors for longitudinal decline in objectively assessed cognitive functioning among individuals at mid-life (Mitchell, Beaumont, Ferguson, Yadegarfar, & Stubbs, 2014; Wilson et al., 2003). This is particularly important as it relates to the current sample (mean age of 37.9), who represent an important population with opportunity for prophylactic intervention (e.g., mental health treatment, increased mental activity, improved health-promoting behaviors, etc.) to mitigate potential long-term adverse outcomes (Roberts et al., 2020; Yao et al., 2020). Longitudinal follow-up is required to further investigate this possibility.
Aim 3. YoP, HIEE, and exploratory factors
Within the current study, YoP explained only a select proportion of variance (13%) in the HIEE; therefore, 87% of the variation in head impact estimation was accounted for by other factors. Given this, the observed associations between YoP and performance on objectively measured tests of processing speed and executive functioning suggest that other factors related to YoP, and not cumulative head impact exposure, may be related to cognitive test performance. Within the current study, exploratory analyses failed to identify potential factors (i.e., pain, sleep disturbance) that may be uniquely associated with YoP and not the HIEE, however.
Further studies are required to better understand the additional factors underlying the associations among YoP and these adverse outcomes beyond RHI. For example, prior commentaries have suggested that developmental or other intrinsic factors prior to sport enrollment may account for the unmeasured effects related to greater contact sport participation. Scheier (2019) hypothesized a potential phenomenon in which children exhibiting greater externalizing behaviors may be more likely to be enrolled in physical contact sports earlier in development as a means to help provide an outlet for these behaviors (Gerstorf, Siedlecki, Tucker-Drob, & Salthouse, 2008). This could potentially account for executive functioning differences within the current study, as one possibility (Palermo et al., 2006). Social and cultural factors may also influence sport enrollment and choice, as one study reported that those who are culturally disadvantaged (e.g., SES, academic resources, etc.) exhibited greater interest and rely more strongly on basketball and football in order to obtain social capital (e.g., development of effective functional social relationships, interactions, and interpersonal sense of identity; Eitle & Eitle, 2002).
Limitations
Though the HIEE is an extremely thorough estimate of head impact exposure that involves a guided structured interview to ensure the utmost accuracy in recall of all aspects of football play, this instrument is still reliant on retrospective recall. As such, there is a possibility of bias due to faulty recall. Further validation of this comprehensive metric is also required, including measurement of how the recall of seasons long activities may align with recorded data from prospectively collected HITs data. Relatedly, the HIEE does not consider pre-high school football participation as part of the head impact exposure estimate due to the fact that robust head telemetry data for youth football had not yet been published at the time of the instrument’s development. Given that the objective of this study was to examine the association between YoP and the HIEE, as well as their associations with neurobehavioral outcomes, YoP from high school and after was a more appropriate measure for this aim. Sensitivity analyses using total years of football participation revealed that the observed findings were relatively robust.
The current study included predominantly White-identifying former players with estimated intellectual abilities in the high average range and the generalizability of the current findings beyond this sample requires further investigation. Relatedly, the current sample was comprised of football players who participated in sport at least at the collegiate level and the findings cannot be generalized to those who only play at lower levels or other sports. This is noteworthy given that frequency and magnitude of head impacts can vary depending on level of play (Crisco et al., 2011; Daniel, Rowson, & Duma, 2012; Mihalik et al., 2007; Sandmo et al., 2020). At this time, comprehensive metrics of head impact exposure solely exist for football only and further efforts should be made toward development of comprehensive metrics for other contact sports (Karton et al., 2020; Kerr et al., 2015; Montenigro et al., 2017). Finally, the association between estimates of head impact exposure and visual memory or attention could not be determined, as the neuropsychological battery in the current study did not include tests representative of these cognitive domains.
Conclusion
YoP was marginally associated with a more comprehensive estimate of cumulative head impact exposure over a career, and each estimate of exposure was related to disparate neurobehavioral outcomes in former football players. Associations between both exposure estimates and neurobehavioral outcomes yielded small effect sizes and RHI must be considered alongside other intrinsic, social, behavioral, and environmental factors when examining neurobehavioral functioning in former collision-sport athletes. Studies attempting to investigate the long-term effects of RHI should make efforts to better estimate exposure to head impacts beyond years of primary sport participation in order to more accurately assess the association between exposure and long-term neurologic health outcomes among former athletes.
ACKNOWLEDGEMENT
The authors would like to thank Robyn Furger (Department of Neurosurgery at the Medical College of Wisconsin), Candice Goerger (Center for the Study of Retired Athletes at the University of North Carolina at Chapel Hill), and Leah Thomas (Center for the Study of Retired Athletes at the University of North Carolina at Chapel Hill) for their study coordination and management efforts. We are grateful for the participation of the athletes, without whom this research would not be possible.
FUNDING
This research was supported through funding from the National Collegiate Athletic Association, and institutional support from the University of North Carolina at Chapel Hill and Medical College of Wisconsin. The REDCap electronic database used for this project were supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number UL1TR001436. BLB acknowledges support from the National Institute of Neurological Disorders and Stroke under the National Institutes of Health under the award NO L30NS113158.
Footnotes
Potential Competing Interests
Nothing to report
References
- Allison PD (1999). Multiple Regression: A Primer. . Thousand Oaks, CA: Pine Forge Press. [Google Scholar]
- Alosco ML, Jarnagin J, Tripodis Y, Platt M, Martin B, Chaisson CE, . . . Stern RA (2017). Olfactory Function and Associated Clinical Correlates in Former National Football League Players. J Neurotrauma, 34(4), 772–780. doi: 10.1089/neu.2016.4536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Koerte IK, Tripodis Y, Mariani M, Chua AS, Jarnagin J, . . . Stern RA (2018). White matter signal abnormalities in former National Football League players. Alzheimers Dement (Amst), 10, 56–65. doi: 10.1016/j.dadm.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Tripodis Y, Baucom ZH, Mez J, Stein TD, Martin B, . . . Stern RA (2020). The Late Contributions of Repetitive Head Impacts and TBI to Depression Symptoms and Cognition. Neurology. doi: 10.1212/WNL.0000000000010040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, & Steer RA (1988). An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol, 56(6), 893–897. doi: 10.1037//0022-006x.56.6.893 [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Bohr AD, Boardman JD, & McQueen MB (2019). Association of Adolescent Sport Participation With Cognition and Depressive Symptoms in Early Adulthood. Orthop J Sports Med, 7(9), 2325967119868658. doi: 10.1177/2325967119868658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, & Benedict RHB (2001). Hopkins verbal learning test – Revised. Administration manual. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Brett BL, Bobholz SA, Espana L, Huber DL, Mayer AR, Harezlak J, . . . Meier TB (2020). Cumulative Effects of Prior Concussion and Primary Sport Participation on Brain Morphometry in Collegiate Athletes: A Study From the NCAA–DoD CARE Consortium. Frontiers in Neurology: Dementia and Neurodegenerative Diseases, Advanced online publication. doi: 10.3389/fneur.2020.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett BL, Huber DL, Wild A, Nelson LD, & McCrea MA (2019). Age of First Exposure to American Football and Behavioral, Cognitive, Psychological, and Physical Outcomes in High School and Collegiate Football Players. Sports Health, 1941738119849076. doi: 10.1177/1941738119849076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett BL, Mummareddy N, Kuhn AW, Yengo-Kahn AM, & Zuckerman SL (2019). The Relationship Between Prior Concussions and Depression Is Modified by Somatic Symptomatology in Retired NFL Athletes. J Neuropsychiatry Clin Neurosci, 31(1), 17–24. doi: 10.1176/appi.neuropsych.18040080 [DOI] [PubMed] [Google Scholar]
- Breusch TS, & Pagan AR (1979). A Simple Test for Heteroskedasticity and Random Coefficient Variation. Econometrica, 47(5), 1287–1294. [Google Scholar]
- Broglio SP, Eckner JT, Martini D, Sosnoff JJ, Kutcher JS, & Randolph C. (2011). Cumulative head impact burden in high school football. J Neurotrauma, 28(10), 2069–2078. doi: 10.1089/neu.2011.1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeland CS, & Ryan KM (1994). Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap, 23(2), 129–138. [PubMed] [Google Scholar]
- Crisco JJ, Fiore R, Beckwith JG, Chu JJ, Brolinson PG, Duma S, . . . Greenwald RM (2010). Frequency and location of head impact exposures in individual collegiate football players. J Athl Train, 45(6), 549–559. doi: 10.4085/1062-6050-45.6.549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisco JJ, Wilcox BJ, Beckwith JG, Chu JJ, Duhaime AC, Rowson S, . . . Greenwald RM (2011). Head impact exposure in collegiate football players. J Biomech, 44(15), 2673–2678. doi: 10.1016/j.jbiomech.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumley JJ, Stetler CA, & Horhota M. (2014). Examining the relationship between subjective and objective memory performance in older adults: a meta-analysis. Psychol Aging, 29(2), 250–263. doi: 10.1037/a0035908 [DOI] [PubMed] [Google Scholar]
- Daniel RW, Rowson S, & Duma SM (2012). Head impact exposure in youth football. Ann Biomed Eng, 40(4), 976–981. doi: 10.1007/s10439-012-0530-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington RB, & Hayes AF (2016). Regression Analysis and Linear Models: Concepts, Applications, and Implementation. New York, NY: Guilford Press. [Google Scholar]
- Derogatis L. (2001). Brief Symptom Inventory 18 (BSI-18): Administration, Scoring, and Procedures Manual. Bloomington, MN: Pearson. [Google Scholar]
- Deshpande SK, Hasegawa RB, Rabinowitz AR, Whyte J, Roan CL, Tabatabaei A, . . . Small DS (2017). Association of Playing High School Football With Cognition and Mental Health Later in Life. JAMA Neurol. doi: 10.1001/jamaneurol.2017.1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande SK, Hasegawa RB, Weiss J, & Small DS (2020). The association between adolescent football participation and early adulthood depression. PLoS One, 15(3), e0229978. doi: 10.1371/journal.pone.0229978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didehbani N, Munro Cullum C, Mansinghani S, Conover H, & Hart J. (2013). Depressive symptoms and concussions in aging retired NFL players. Arch Clin Neuropsychol, 28(5), 418–424. doi: 10.1093/arclin/act028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JK, & Keil AP (2017). Measurement Error and Environmental Epidemiology: a Policy Perspective. Curr Environ Health Rep, 4(1), 79–88. doi: 10.1007/s40572-017-0125-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitle TM, & Eitle DJ (2002). Race, Cultural Capital, and the Educational Effects of Participation in Sports. Sociology of Education, 75(2), 123–146. [Google Scholar]
- Gerstorf D, Siedlecki KL, Tucker-Drob EM, & Salthouse TA (2008). Executive dysfunctions across adulthood: measurement properties and correlates of the DEX self-report questionnaire. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn, 15(4), 424–445. doi: 10.1080/13825580701640374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami R, Dufort P, Tartaglia MC, Green RE, Crawley A, Tator CH, . . . Davis KD (2016). Frontotemporal correlates of impulsivity and machine learning in retired professional athletes with a history of multiple concussions. Brain Struct Funct, 221(4), 1911–1925. doi: 10.1007/s00429-015-1012-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Harding HP, Matthews A, . . . Cantu RC (2007). Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc, 39(6), 903–909. doi: 10.1249/mss.0b013e3180383da5 [DOI] [PubMed] [Google Scholar]
- Gysland SM, Mihalik JP, Register-Mihalik JK, Trulock SC, Shields EW, & Guskiewicz KM (2012). The relationship between subconcussive impacts and concussion history on clinical measures of neurologic function in collegiate football players. Ann Biomed Eng, 40(1), 14–22. doi: 10.1007/s10439-011-0421-3 [DOI] [PubMed] [Google Scholar]
- Hayes AF, & Cai L. (2007). Using heteroskedasticity-consistent standard error estimators in OLS regression: an introduction and software implementation. Behav Res Methods, 39(4), 709–722. doi: 10.3758/bf03192961 [DOI] [PubMed] [Google Scholar]
- Holmes TH, & Rahe TH (1967). The Social Readjustment Rating Scale. Journal of Psychosomatic Research, 11, 213. [DOI] [PubMed] [Google Scholar]
- Jones D, Kazis L, Lee A, Rogers W, Skinner K, Cassar L, . . . Hendricks A. (2001). Health status assessments using the Veterans SF-12 and SF-36: methods for evaluating otucomes in the Veterans Health Administration. J Ambul Care Manage, 24(3), 68–86. doi: 10.1097/00004479-200107000-00011 [DOI] [PubMed] [Google Scholar]
- Karton C, Blaine Hoshizaki T, & Gilchrist MD (2020). A novel repetitive head impact exposure measurement tool differentiates player position in National Football League. Sci Rep, 10(1), 1200. doi: 10.1038/s41598-019-54874-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley ME, Urban JE, Miller LE, Jones DA, Espeland MA, Davenport EM, . . . Stitzel, J. D. (2017). Head Impact Exposure in Youth Football: Comparing Age- and Weight-Based Levels of Play. J Neurotrauma, 34(11), 1939–1947. doi: 10.1089/neu.2016.4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr ZY, Littleton AC, Cox LM, DeFreese JD, Varangis E, Lynall RC, . . . Guskiewicz KM (2015). Estimating Contact Exposure in Football Using the Head Impact Exposure Estimate. J Neurotrauma, 32(14), 1083–1089. doi: 10.1089/neu.2014.3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr ZY, Thomas LC, Simon JE, McCrea M, & Guskiewicz KM (2018). Association Between History of Multiple Concussions and Health Outcomes Among Former College Football Players: 15-Year Follow-up From the NCAA Concussion Study (1999–2001). Am J Sports Med, 46(7), 1733–1741. doi: 10.1177/0363546518765121 [DOI] [PubMed] [Google Scholar]
- Koenker R. (1981). A Note on Studentizing a Test for Heteroscedasticity. Journal of Econometrics, 17, 107–112. [Google Scholar]
- Lepage C, Muehlmann M, Tripodis Y, Hufschmidt J, Stamm J, Green K, . . . Koerte IK (2018). Limbic system structure volumes and associated neurocognitive functioning in former NFL players. Brain Imaging Behav. doi: 10.1007/s11682-018-9895-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Alosco ML, & Huber BR (2016). Repetitive Head Impacts and Chronic Traumatic Encephalopathy. Neurosurg Clin N Am, 27(4), 529–535. doi: 10.1016/j.nec.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mez J, Daneshvar DH, Abdolmohammadi B, Chua AS, Alosco ML, Kiernan PT, . . . McKee AC (2019). Duration of American Football Play and Chronic Traumatic Encephalopathy. Ann Neurol. doi: 10.1002/ana.25611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mez J, Daneshvar DH, Kiernan PT, Abdolmohammadi B, Alvarez VE, Huber BR, . . . McKee AC (2017). Clinicopathological Evaluation of Chronic Traumatic Encephalopathy in Players of American Football. JAMA, 318(4), 360–370. doi: 10.1001/jama.2017.8334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalik JP, Bell DR, Marshall SW, & Guskiewicz KM (2007). Measurement of head impacts in collegiate football players: an investigation of positional and event-type differences. Neurosurgery, 61(6), 1229–1235; discussion 1235. doi: 10.1227/01.neu.0000306101.83882.c8 [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, & Stubbs B. (2014). Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand, 130(6), 439–451. doi: 10.1111/acps.12336 [DOI] [PubMed] [Google Scholar]
- Montenigro PH, Alosco ML, Martin BM, Daneshvar DH, Mez J, Chaisson CE, . . . Tripodis Y. (2017). Cumulative Head Impact Exposure Predicts Later-Life Depression, Apathy, Executive Dysfunction, and Cognitive Impairment in Former High School and College Football Players. J Neurotrauma, 34(2), 328–340. doi: 10.1089/neu.2016.4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multani N, Goswami R, Khodadadi M, Ebraheem A, Davis KD, Tator CH, . . . Tartaglia MC (2016). The association between white-matter tract abnormalities, and neuropsychiatric and cognitive symptoms in retired professional football players with multiple concussions. J Neurol, 263(7), 1332–1341. doi: 10.1007/s00415-016-8141-0 [DOI] [PubMed] [Google Scholar]
- National Institute of Neurological Disorders and Stroke (NINDS) User Manual for the Quality of Life in Neurological Disorders (Neuro-QoL) Measures, Version 2.0. . (2015). Retrieved from http://www.neuroqol.org/Resources/Resourcesdocuments/Neuro-QOL_UserManualv2_24Mar2015.pdf.
- Palermo MT, Di Luigi M, Dal Forno G, Dominici C, Vicomandi D, Sambucioni A, . . . Pasqualetti P. (2006). Externalizing and oppositional behaviors and karate-do: the way of crime prevention. A pilot study. Int J Offender Ther Comp Criminol, 50(6), 654–660. doi: 10.1177/0306624X06293522 [DOI] [PubMed] [Google Scholar]
- Reitan RM, & Wolfson D. (1985). The Halstead-Reitan Neuropsychological Test Battery: Therapy and clinical interpretation. Tucson, AZ: Neuropsychological Press. [Google Scholar]
- Roberts AL, Pascual-Leone A, Speizer FE, Zafonte RD, Baggish AL, Taylor H Jr., . . . Weisskopf MG (2019). Exposure to American Football and Neuropsychiatric Health in Former National Football League Players: Findings From the Football Players Health Study. Am J Sports Med, 47(12), 2871–2880. doi: 10.1177/0363546519868989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Zafonte RD, Speizer F, Baggish A, Taylor H, Nadler L, & Weisskopf M. (2020). Modifiable risk factors for poor cognitive function in former American-style football players: Findings from the Harvard Football Players Health Study. J Neurotrauma. doi: 10.1089/neu.2020.7070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RM, Isquith PK, & Gioia GA (2005). Behavior Rating Inventory of Executive Function-Adult Version (BRIEF-A). Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Sandmo SB, Andersen TE, Koerte IK, & Bahr R. (2020). Head impact exposure in youth football-Are current interventions hitting the target? Scand J Med Sci Sports, 30(1), 193–198. doi: 10.1111/sms.13562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz V, Stern RA, Tripodis Y, Stamm J, Wrobel P, Lepage C, . . . Koerte IK (2018). Age at First Exposure to Repetitive Head Impacts Is Associated with Smaller Thalamic Volumes in Former Professional American Football Players. J Neurotrauma, 35(2), 278–285. doi: 10.1089/neu.2017.5145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Meier TB, Kuplicki R, Savitz J, Mukai I, Cavanagh L, . . . Bellgowan PS (2014). Relationship of collegiate football experience and concussion with hippocampal volume and cognitive outcomes. JAMA, 311(18), 1883–1888. doi: 10.1001/jama.2014.3313 [DOI] [PubMed] [Google Scholar]
- Smith A. (2007). Symbol Digits Modalities Test: Manual. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Smith GE, Petersen RC, Ivnik RJ, Malec JF, & Tangalos EG (1996). Subjective memory complaints, psychological distress, and longitudinal change in objective memory performance. Psychol Aging, 11(2), 272–279. doi: 10.1037//0882-7974.11.2.272 [DOI] [PubMed] [Google Scholar]
- Spreen O, & Benton AL (1977). Neurosensory Center Comprehensive Examination for Aphasia. . Victoria, B.C.: University of Victoria Neuropsychology Laboratory. [Google Scholar]
- Stein TD, Alvarez VE, & McKee AC (2015). Concussion in Chronic Traumatic Encephalopathy. Curr Pain Headache Rep, 19(10), 47. doi: 10.1007/s11916-015-0522-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RA, Adler CH, Chen K, Navitsky M, Luo J, Dodick DW, . . . Reiman EM (2019). Tau Positron-Emission Tomography in Former National Football League Players. N Engl J Med. doi: 10.1056/NEJMoa1900757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain J, Didehbani N, Cullum CM, Mansinghani S, Conover H, Kraut MA, . . . Womack KB (2013). Depressive symptoms and white matter dysfunction in retired NFL players with concussion history. Neurology, 81(1), 25–32. doi: 10.1212/WNL.0b013e318299ccf8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagge CA, Fisher AM, Minaeva OV, Gaudreau-Balderrama A, Moncaster JA, Zhang XL, . . . Goldstein LE (2018). Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain, 141(2), 422–458. doi: 10.1093/brain/awx350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra AR, Vasquez BP, Colella B, Tartaglia MC, Tator CH, Mikulis D, . . . Green REA (2019). Comprehensive Neuropsychiatric and Cognitive Characterization of Former Professional Football Players: Implications for Neurorehabilitation. Front Neurol, 10, 712. doi: 10.3389/fneur.2019.00712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. (2001). Wechsler Test of Adult Reading: WTAR. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Willer BS, Tiso MR, Haider MN, Hinds AL, Baker JG, Miecznikowski JC, & Leddy JJ (2018). Evaluation of Executive Function and Mental Health in Retired Contact Sport Athletes. J Head Trauma Rehabil, 33(5), E9–E15. doi: 10.1097/HTR.0000000000000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, & Bennett DA (2003). Proneness to psychological distress is associated with risk of Alzheimer’s disease. Neurology, 61(11), 1479–1485. doi: 10.1212/01.wnl.0000096167.56734.59 [DOI] [PubMed] [Google Scholar]
- Yao S, Liu Y, Zheng X, Zhang Y, Cui S, Tang C, . . . Xu N. (2020). Do nonpharmacological interventions prevent cognitive decline? a systematic review and meta-analysis. Transl Psychiatry, 10(1), 19. doi: 10.1038/s41398-020-0690-4 [DOI] [PMC free article] [PubMed] [Google Scholar]