Abstract
Neighborhood socioeconomic deprivation is associated with adverse outcomes after pediatric liver transplant. We sought to determine if this relationship varies by transplant center. Using SRTR, we included patients<18 years transplanted 2008–2013 (N=2804). We matched patient ZIP codes to a deprivation index (range [0,1]; higher values indicate increased socioeconomic deprivation). A center-level patient-mix deprivation index was defined by the distribution of patient-level deprivation. Centers (n=66) were classified as high or low deprivation if their patient-mix deprivation index was above or below the median across centers. Center quality was classified as low or high graft failure if graft survival rates were better or worse than the overall 10-year graft survival rate. Primary outcome was patient-level graft survival. We used random-effect Cox models to evaluate center-level covariates on graft failure. We modeled center quality using stratified Cox models. In multivariate analysis, each 0.1 increase in the patient-mix deprivation index was associated with increased hazard of graft failure (HR 1.32; 95%CI: 1.05, 1.66). When stratified by center quality, patient-mix deprivation was no longer significant (HR 1.07, 95%CI: 0.89, 1.28). Some transplant centers care for predominantly high deprivation children and maintain excellent outcomes. Revealing and replicating these centers’ practice patterns should enable more equitable outcomes.
INTRODUCTION
A substantial and compelling body of evidence demonstrates that social determinants of health—where we live, eat, sleep, and play—impact health.1 Social determinants are particularly relevant after transplant because self-management capacity influences medication adherence2 and graft health.3 We previously demonstrated that neighborhood-level socioeconomic deprivation, a composite measure derived from US Census Bureau data, is associated with patient-level post-transplant morbidity and mortality.2,3 An outstanding question remains whether transplant care teams can mitigate the effects of neighborhood socioeconomic deprivation on long-term outcomes for children. To our knowledge, no studies to date have examined differences in transplant centers’ long-term outcomes accounting for the contextual socioeconomic characteristics of the population served.
There is significant center-to-center variation in pediatric liver transplantation protocols and outcomes, including organ offer acceptance patterns, post-transplant length of stay, and likelihood of early graft loss.4–6 One of the benefits of the robust reporting requirements in the transplantation field is that these requirements enable us to identify high performing centers. Recognizing centers as high performers could prompt national quality improvement efforts to identify best practices.7 In this study, we examined center-level characteristics associated with long-term transplant outcomes. We sought to explore whether high center quality, defined by low 10-year death censored graft failure rates, mitigates the adverse effects of neighborhood socioeconomic deprivation. We queried the Scientific Registry of Transplant Recipients (SRTR) database and linked it with a validated measure of neighborhood socioeconomic deprivation matched to a patients’ home ZIP code.2,3,8 Based on our earlier work, we hypothesized that centers caring for children from predominantly high deprivation neighborhoods would have worse outcomes following transplant but that the effect of center performance would diminish the effect of deprivation.
METHODS
Data Source
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
This study was reviewed by and deemed to be exempt by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center. Informed consent requirement was waived.
Study Population
We identified pediatric patients (<18 years of age) who received a liver transplant between January 1, 2008 and December 31, 2013 in the U.S. (N=2804) across 66 pediatric liver transplant centers. We chose this time frame because we were interested in center variation in long-term outcomes. Patients were excluded (N=330) if they did not have a documented home ZIP code within SRTR that could be matched to the neighborhood socioeconomic deprivation index. No adjustment was made for missing data.
Center-Level Exposure
The primary exposure of interest was the neighborhood socioeconomic deprivation index8 for each included patient, aggregated at the level of the transplant center (hereafter referred to as the ‘patient-mix deprivation index’). While there are many deprivation indices9 (e.g. Center for Disease Control’s Social Vulnerability Index10), we chose this particular index because it was specifically designed to measure material economic deprivation, generated using statistically robust techniques, and the methods for development are transparent and open source.8,11 The Social Vulnerability Index, on the other hand, was designed to capture the negative health effects of natural or human-caused disasters, or disease outbreaks. Specifically, it was designed to help emergency response planners and public health officials identify and map communities that most likely need support before, during, and after a hazardous event. In the context of the present study, we considered the below index to be the most appropriate index. Briefly, the deprivation index is a composite measure derived from US Census Bureau data using six American Community Survey variables: 1) % of households with income below the federal poverty line; 2) median household income; 3) % of population ≥25 years with at least high school education; 4) % of the population without health insurance; 5) % of households receiving public assistance; and 6) % of housing units that are vacant. Applying weights, the index is scaled and normalized to a range of [0,1] and values closer to 1 indicate neighborhoods with increased socioeconomic deprivation. The index is now available for 72,057 of all 73,056 (99%) US census tracts enhancing external validity. The index is intended to capture the relative socioeconomic context of a neighborhood; it also has been used to approximate household-level socioeconomic circumstances.8,12–16 The index, underlying measures, and the code used are publicly available.11 Methods for the development and use of the deprivation index are described elsewhere.2,3,8,17 To derive the patient-mix deprivation index, we first matched the neighborhood deprivation index to patient home ZIP codes as reported to SRTR at the time of transplant. We then calculated the median deprivation index for patients within each transplant center and defined that value as the patient-mix deprivation index. We classified centers as above (high patient-mix deprivation) and below (low patient-mix deprivation) the median patient-mix deprivation index across all centers.
Center-level Covariates
To better understand how the effect of patient-mix deprivation on outcome varied by center quality, we classified patients by their transplant center’s graft failure rate. To calculate this, we first determined the estimated graft failure rate for the entire cohort (22.3%). Next, we determined the 10-year graft failure rate for each center within the cohort. Finally, we classified centers as “low graft failure” or “high graft failure” if they had graft survival rates above or below the estimated 10-year death censored graft survival rates for the entire cohort, respectively. We did not risk-adjust these graft failure rates because of power limitations. The SRTR does not risk-adjust 3-year outcomes for pediatric transplant centers in the program-specific reports.18 Volume was taken as the average annual number of transplants performed at the center over the study period. We categorized centers into three categories based on transplants performed per year: <5, 5–15, and >15 because previous work has shown sub-optimal outcomes for patients at centers that perform fever than 5 transplants annually.19 We classified UNOS region into 4 distinct U.S. regions based on previous research.20 Urban-rural status was determined using the Rural-Urban Commuting Area codes from the US Department of Agriculture.21
Outcome
The primary outcome was patient-level death censored graft survival. Graft survival was defined as the time from liver transplant to graft failure or death, whichever occurred first. For patients without documented graft failure or death, graft survival was censored at the last date of follow up. We applied administrative censoring at 10 years post-transplant for those who were followed longer than 10 years. We evaluated this measure as a time-to-event occurrence.
Statistical Analysis
Patients were grouped by patient-mix deprivation (above or below centers’ median patient-mix deprivation) and by center graft failure. Thus, patients were classified by their transplant center’s characteristics: low-graft failure, high-deprivation centers; high-graft failure, high-deprivation centers; low-graft failure, low-deprivation centers; and high-graft failure, low-deprivation centers. Descriptive statistics were calculated for patient demographic, allocation, and transplant characteristics. Patient characteristics were compared across these four groups using the appropriate statistical tests. The relationship between center-level deprivation and time-to-event were visualized with Kaplan-Meier curves by center performance status. To model the relationship between patient-mix deprivation and outcome, we analyzed patient-mix deprivation continuously in all of our Cox proportional hazard models. For the sake of data depiction and ease of interpretation, we dichotomized this variable (dichotomized at the median). We chose a 10 year follow up period to meet the dual goals of examining long-term outcomes and reflecting the modern era of pediatric liver transplantation. Additionally, our deprivation index data were derived from 2015 US Census Bureau data, which further restricted our timeframe. We used random-effect Cox proportional hazards models to evaluate the relationship between center characteristics and the outcome measure. Because we were interested in center-level effects, no individual level characteristics were included in the Cox proportional hazard models. Models included a center-level random intercept to account for unmeasured center-level characteristics that might contribute to correlations among outcomes within each center.22 We conducted all analyses at the patient-level using center-level covariates because of power limitations when analyzing the sample at the center level (indeed, there were only 66 transplant centers in our sample). In developing the multivariable models, we first used univariate random-effect Cox proportional hazard models to evaluate the relationship between center-level patient-mix deprivation and center-level co-variables on the primary outcome. We used backwards selection procedures, including only pre-screened co-variables with a p<0.15, to sequentially remove variables until all variables in the final model were p<0.05. Since we wanted to model how center quality influenced relationships between deprivation and outcome, we stratified Cox proportional hazards models by center quality (high- and low-graft failure rate centers). We conceptualized that patients from high and low graft failure centers have different baseline risks of graft failure. Stratified Cox proportional hazards models assume different baseline hazards of each stratum. To assess for potentially different effects of deprivation in performance stratum, we also modeled the interaction between center quality and patient-mix deprivation. All analyses were performed in R v4.0.2 (The R Foundation for Statistical Computing).
RESULTS
Study Population
A total of 2474 children who underwent liver transplantation in the US during our study period were included in these analyses. Baseline characteristics by patient-mix deprivation and center quality are shown in Table 1. Overall, the median age of our cohort was 2.2 (IQR 0.8, 8.6) years, and 50.9% of the cohort were female. A majority of the cohort (77.4%) were non-Hispanic, and 73.5% of the cohort were white. The most common indication for transplant was biliary atresia (32.1%). The median deprivation index for the cohort was 0.38 (IQR 0.30, 0.46). The median patient-mix deprivation index was 0.37 (IQR 0.31, 0.43).
Table 1.
Demographic characteristics of cohort by center case-mix and performance status
| Low Patient-Mix Deprivation | High Patient-Mix Deprivation | |||||
|---|---|---|---|---|---|---|
| Low Graft Failure | High Graft Failure | Low Graft Failure | High Graft Failure | |||
| N=745 | N=261 | N=1028 | N=440 | |||
| N (%) or median [IQR] | ||||||
| p-value | p-value | |||||
| Deprivation Index | 0.32 [0.27, 0.40] | 0.35 [0.28, 0.45] | <0.001 | 0.40 [0.33, 0.47] | 0.43 [0.35, 0.50] | <0.001 |
| Age at Transplant | 2.1 [0.8, 8.6] | 2.5 [0.9, 10.6] | 0.06 | 2.1 [0.8, 7.6] | 2.3 [0.9, 9.1] | 0.16 |
| Female | 381 (51.1) | 124 (47.5) | 0.35 | 529 (51.4) | 225 (51.1) | 0.96 |
| Ethnicity | ||||||
| Hispanic | 157 (21.1) | 45 (17.2) | 0.21 | 234 (22.8) | 124 (28.2) | 0.03 |
| Non-Hispanic | 588 (78.9) | 216 (82.8) | 794 (77.2) | 316 (71.8) | ||
| Race | ||||||
| White | 549 (73.7) | 195 (74.7) | 0.74 | 771 (75.0) | 304 (69.1) | 0.045 |
| Black | 106 (14.2) | 39 (14.9) | 185 (18.0) | 103 (23.4) | ||
| Other | 90 (12.1) | 27 (10.3) | 72 (7.0) | 33 (7.5) | ||
| Organ Type | ||||||
| Deceased donor | 637 (85.5) | 241 (92.3) | 0.01 | 925 (90.0) | 403 (91.6) | 0.39 |
| Living donor | 108 (14.5) | 20 (7.7) | 103 (10.0) | 37 (8.4) | ||
| Cold Ischemia Time (hr) | 6.6 [4.5, 8.5] | 7.5 [6.1, 9.0] | <0.001 | 6.5 [5.0, 8.2] | 6.4 [4.9, 7.9] | 0.07 |
| Underlying Liver Dx | ||||||
| Biliary atresia | 249 (33.4) | 55 (21.1) | <0.001 | 364 (35.4) | 126 (28.6) | <0.001 |
| Other Cholestasis | 133 (17.9) | 89 (34.1) | 157 (15.3) | 102 (23.2) | ||
| Acute Liver Failure | 78 (10.5) | 24 (9.2) | 115 (11.2) | 40 (9.1) | ||
| Metabolic | 88 (11.8) | 9 (3.4) | 94 (9.1) | 30 (6.8) | ||
| Tumor | 60 (8.1) | 13 (5.0) | 108 (10.5) | 30 (6.8) | ||
| Autoimmune | 30 (4.0) | 11 (4.2) | 40 (3.9) | 22 (5.0) | ||
| Other | 107 (14.4) | 60 (23.0) | 150 (14.6) | 90 (20.5) | ||
| Insurance Type | ||||||
| Private | 391 (52.5) | 111 (42.5) | 0.003 | 416 (40.5) | 152 (34.5) | 0.048 |
| Public | 336 (45.1) | 148 (56.7) | 590 (57.4) | 282 (64.1) | ||
| Other | 18 (2.4) | 2 (0.08) | 22 (2.1) | 6 (1.4) | ||
| Laboratory MELD/PELD | 16 [5, 25] | 15 [6, 23] | 0.48 | 13 [3, 24] | 16 [6, 26] | 0.01 |
| Allocation MELD/PELD | 26 [16, 33] | 22 [13, 30] | 0.02 | 26 [18, 30] | 24 [15, 32] | 0.09 |
| Status 1A/1B | 231 (31.0) | 64 (24.5) | 0.06 | 291 (28.3) | 134 (30.5) | 0.44 |
| Center Transplant Volume | ||||||
| <5 per year | 64 (8.6) | 21 (8.0) | 0.21 | 37 (3.6) | 73 (16.6) | <0.001 |
| 5–15 per year | 333 (44.7) | 133 (51.0) | 326 (31.7) | 130 (29.5) | ||
| >15 per year | 348 (46.7) | 107 (41.0) | 665 (64.7) | 237 (53.9) | ||
| Geographical Region | ||||||
| Northeast | 307 (41.2) | 26 (10.0) | <0.001 | 219 (21.3) | 66 (15.0) | <0.001 |
| Southeast | 10 (1.3) | 0 (0.0) | 507 (49.3) | 193 (43.9) | ||
| Midwest | 168 (22.6) | 169 (64.8) | 186 (18.1) | 55 (12.5) | ||
| West | 260 (34.9) | 66 (25.3) | 116 (11.3) | 126 (28.6) | ||
| Rurality | ||||||
| Urban | 622 (83.5) | 205 (78.5) | 0.03 | 836 (81.3) | 396 (90.0) | <0.001 |
| Rural | 108 (14.5) | 54 (20.7) | 186 (18.1) | 43 (9.8) | ||
In the low patient-mix deprivation groups (Table 1), patients from high graft failure centers were less likely to receive a living donor transplant, have a longer cold ischemia time, more likely to have “cholestasis” or “other” as the indication for transplant, more likely to have public insurance, have a lower allocation MELD/PELD at transplant, more likely to be from the Midwest, and more likely to live in a rural area. In the high patient-mix deprivation groups (Table 1), patients from high graft failure centers were more likely to be Hispanic ethnicity, more likely to be Black race, more likely to have “cholestasis” or “other” as the indication for transplant, more likely to have public insurance, more likely to be at a low-volume transplant center, more likely to live in the West region, and more likely to have be from an urban environment.
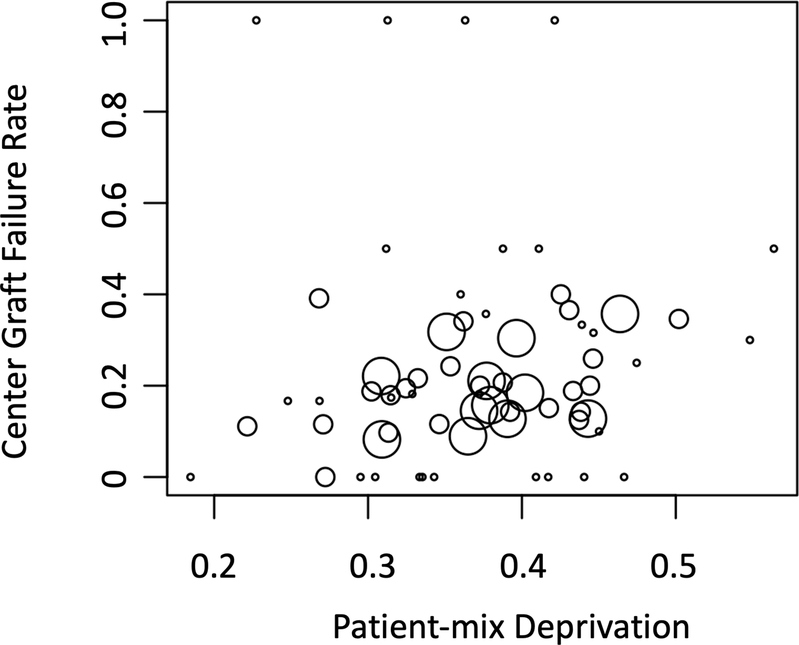
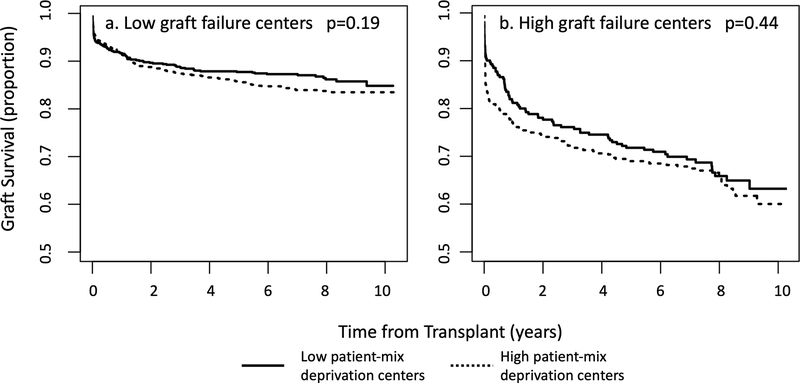
Figure 1 depicts graft failure rates by patient-mix deprivation. The overall graft survival for the cohort at 1, 5, and 10 years were 87.8%, 81.9%, and 77.7% respectively. Figure 2 depicts graft survival for patients by patient-mix deprivation at a) high graft failure institutions and b) low graft failure institutions. For patients cared for in centers with low patient-mix deprivation scores, 10-year event-free survival was 84.8% (95% CI: 81.7%, 88.1%) and 63.2% (95% CI: 56.6%, 70.7%) for patients at low graft failure and high graft failure centers, respectively. For patients at high-deprivation centers, 10-year event-free survival was 83.5% (95% CI: 81.2%, 85.9%) and 60.0% (95% CI: 54.1%, 66.6%) for patients at low graft failure and high graft failure centers, respectively. For centers with low graft failure rates, there was no significant difference in graft survival at 10 years for patients from low deprivation and high deprivation centers (84.8% vs. 83.5%, respectively, p=0.19). For centers with high graft failure rates, there was no difference in survival for patients from low deprivation and high deprivation centers (63.2% vs. 60.0%, respectively, p=0.44).
Figure 1. Bubble plot of center-level median deprivation and 10 year center-level graft failure.
Each dot is a transplant institution. Dot size is based on transplant center volume over the study period.
Figure 2.
Event-free survival by patient-mix deprivation at a) low graft failure transplant centers and b) high graft failure transplant centers.
Random-effect Cox proportional hazards models
Table 2 depicts results of univariate analysis of center-level characteristics on graft survival with a center-specific random effect. Each 0.1 increase in the patient-mix deprivation index was associated with a 28% (HR 1.28; 95% CI: 0.99, 1.65) increased hazard of graft failure. Patients from centers with a higher proportion of ‘other’ diagnoses for liver transplant indication and those from centers with higher median MELD/PELD at transplant had increased hazard of graft failure. Patients from centers with a higher prevalence of patients with tumor as the indication for transplant had a lower hazard of graft failure.
Table 2.
Descriptive statistics of center-level covariables and associated univariate Cox proportional hazards analysis of co-variables on graft survival
| Center Level Median [IQR] or N (%) | Hazard of Event | |||
|---|---|---|---|---|
| N = 66 | HR a, b | 95% CI | p-value | |
| Patient-mix Deprivation Index | 0.37 [0.31, 0.43] | 1.28 | 0.99, 1.65 | 0.06 |
| Median Age at Transplant | 2.4 [1.7, 7.2] | 1.03 | 0.97, 1.08 | 0.33 |
| % Female | 0.50 [0.38, 0.56] | 1.07 | 0.95, 1.21 | 0.29 |
| Ethnicity | ||||
| % Hispanic | 0.11 [0.00, 0.25] | 0.96 | 0.87, 1.06 | 0.42 |
| Race | ||||
| % White | 0.67 [0.50, 0.80] | 0.97 | 0.89, 1.06 | 0.50 |
| % Black | 0.10 [0.03, 0.26] | 1.06 | 0.95, 1.17 | 0.29 |
| % Other | 0.04 [0.00, 0.09] | 1.07 | 0.91, 1.26 | 0.40 |
| Organ Type | ||||
| % Living donor | 0.02 [0.00, 0.13] | 0.94 | 0.83, 1.06 | 0.29 |
| Median Cold Ischemia Time (hr) | 6.1 [5.0, 7.4] | 0.99 | 0.89, 1.10 | 0.88 |
| Underlying Liver Dx | ||||
| % Biliary atresia | 0.30 [0.14, 0.37] | 0.91 | 0.79, 1.04 | 0.16 |
| % Other Cholestasis | 0.11 [0.00, 0.17] | 1.08 | 0.96, 1.22 | 0.21 |
| % Acute Liver Failure | 0.08 [0.02, 0.16] | 0.86 | 0.70, 1.05 | 0.14 |
| % Metabolic | 0.03 [0.00, 0.10] | 0.84 | 0.65, 1.09 | 0.18 |
| % Tumor | 0.03 [0.00, 0.09] | 0.77 | 0.60, 1.01 | 0.05 |
| % Autoimmune | 0.02 [0.00, 0.06] | 1.11 | 0.96, 1.28 | 0.14 |
| % Other | 0.14 [0.05, 0.22] | 1.13 | 1.02, 1.26 | 0.02 |
| Insurance Type | ||||
| % Private | 0.42 [0.22, 0.53] | 1.02 | 0.93, 1.13 | 0.64 |
| % Public | 0.43 [0.34, 0.64] | 1.01 | 0.93, 1.10 | 0.77 |
| % Other | 0.00 [0.00, 0.01] | 0.77 | 0.44, 1.34 | 0.35 |
| Median Laboratory MELD/PELD | 16 [13, 19] | 1.04 | 1.00, 1.08 | 0.05 |
| Median Allocation MELD/PELD | 22 [20, 25] | 1.02 | 0.92, 1.12 | 0.77 |
| % Status 1A/1B | 0.23 [0.10, 0.35] | 1.10 | 0.98, 1.23 | 0.11 |
| Center Transplant Volume (3 Categories) | ||||
| <5 per year | 30 (45.5) | REF | ||
| 5–15 per year | 24 (36.4) | 0.80 | 0.54, 1.19 | 0.28 |
| >15 per year | 12 (18.2) | 0.71 | 0.48, 1.06 | 0.10 |
| Geographical Region | ||||
| Northeast | 15 (22.7) | REF | ||
| Southeast | 23 (34.8) | 0.97 | 0.63, 1.49 | 0.89 |
| Midwest | 18 (27.3) | 1.1 | 0.71, 1.72 | 0.67 |
| West | 10 (15.2) | 0.82 | 0.50, 1.36 | 0.44 |
| Rurality | ||||
| % Rural | 0.13 [0.00, 0.25] | 0.96 | 0.84, 1.09 | 0.50 |
For continuous proportion variables, hazard ratios are scaled to represent a 0.1 increase in the proportion.
All random-effect Cox proportional hazards models include a center-specific random effect to account for correlation among patients from the same center.
In the final multivariate random-effect Cox model (Table 3), each 0.1 increase in the patient-mix deprivation index was associated with a 32% increased hazard of graft failure (HR 1.32; 95% CI: 1.05, 1.66) after liver transplant, after adjusting for the following center level characteristics: proportion of patients with acute liver failure, proportion of patients with a tumor, and proportion of patients listed as Status 1A/1B. In the random-effect Cox models stratified by center quality, the effect of patient-mix deprivation was no longer significant (HR 1.07 95%CI: 0.89, 1.28). There was no interaction between patient-mix deprivation and performance status.
Table 3.
Final multivariable Cox proportional hazard models without and with center performance status
| Multivariable Models | ||||||
|---|---|---|---|---|---|---|
| without Center Quality | Stratified by Center Quality | Stratified by Center Quality, including interaction | ||||
| Variable | HR [95%CI] | p-value | HR (95%CI) | p-value | HR (95%CI) | p-value |
| Center Deprivation | 1.32 [1.05, 1.66] | 0.02 | 1.07 [0.89, 1.28] | 0.49 | 1.06 (0.80, 1.40) | 0.67 |
| % Acute Liver Failure | 0.81 [0.67, 0.99] | 0.04 | 0.91 [0.78, 1.07] | 0.25 | 0.91 (0.78, 1.07) | 0.26 |
| % Tumor | 0.74 [0.58, 0.93] | 0.01 | 0.95 [0.79, 1.15] | 0.61 | 0.95 (0.79, 1.15) | 0.62 |
| % Status 1a/1b | 1.15 [1.04, 1.28] | 0.01 | 1.09 [1.00, 1.19] | 0.05 | 1.09 (1.00, 1.19) | 0.047 |
| Center Deprivation*Center Quality Interaction | 1.01 (0.70, 1.45) | 0.97 | ||||
HR: Hazard ratio, Prop: proportion; REF: reference
For continuous proportion variables, hazard ratios are scaled to represent a 0.1 increase in the proportion.
All random-effect Cox proportional hazards models include a center-specific random effect to account for correlation among patients from the same center.
DISCUSSION
To our knowledge, this is the first study to evaluate the effects of a transplant center’s patient-mix with regards to neighborhood socioeconomic deprivation on long-term pediatric liver transplantation outcomes. Our findings demonstrate that post-transplant, patients at centers caring for predominantly high deprivation children have an increased likelihood of adverse outcome after transplant. However, the effect of patient-mix deprivation was diminished when stratified by center quality. There were centers able to achieve outcomes comparable with those serving primarily low deprivation populations. This suggests that there are center-specific practices that may mitigate the impacts of neighborhood socioeconomic deprivation on transplant outcomes. The gap in outcomes between high performance and low performance centers is wide and underscores the need to identify modifiable center practices that contribute to improved outcomes.
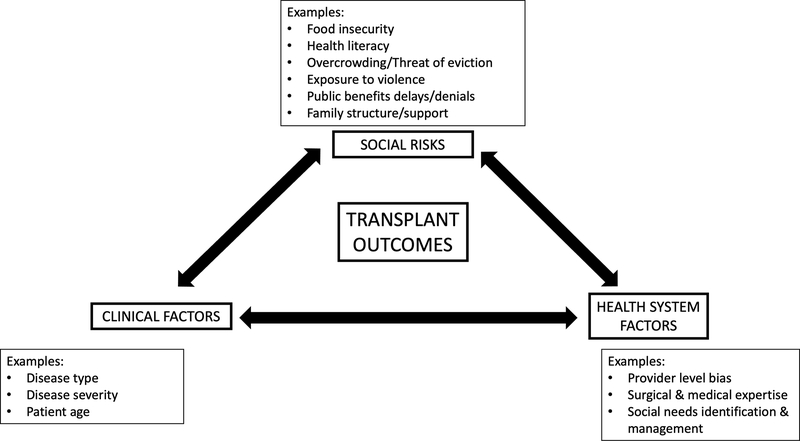
Based on these findings, we developed a conceptual framework (Figure 3) for how adverse social determinants (such as neighborhood deprivation) interplay with center-level factors and disease factors to influence transplant outcomes. A 2019 National Academy of Medicine report23 the authors identified 5 activities to integrate social care into medical care. These activities include adverse social determinant awareness, adjustment, assistance, alignment, and advocacy. This frameworks can help to ground future studies exploring center-level practices that can affect outcomes for socioeconomically deprived children.
Figure 3. Conceptual framework for the confluence of social, medical, and health-system factors on post-transplant outcomes.
In this adapted conceptual framework,34 patient social risks, clinical risk factors, and health system factors interact with one another to drive outcomes. Social and political determinants of health, such as structural racism, government policies, housing quality, and employment opportunities, are upstream and may have an impact on all of the factors depicted in this conceptual framework.
Notably, we did not observe that increased center-level proportions of Hispanic or Black patients were associated with increased risk of graft failure. As we have previously described,3 we conceptualize race to be a social construct that captures overlapping but distinct social determinants to neighborhood deprivation. Such determinants include structural and interpersonal racism, bias, segregation, and mistrust in the healthcare system. In our previous study,3 we found that the effect of Black race on adverse outcomes was diminished when neighborhood socioeconomic deprivation was included in the models—suggesting that this relationship is in part mediated by neighborhood deprivation. This study lends support to the theory that more direct measures of adverse social determinants (such as neighborhood deprivation) may help to explain persistent disparities seen in transplant outcomes. Indeed, the COVID-19 pandemic has brought to the national spotlight how systemic racism contributes to excess disease burden for Black Americans.24,25 Such disparities may reflect the increased burden of essential jobs, overcrowding, and racial segregation on Black Americans. Uncovering the key underlying adverse social determinants that contribute to the racial disparities observed in pediatric liver transplant could lead to the development of interventions that result in more equitable outcomes.
Currently, regulatory agencies (e.g. SRTR and OPTN) track waitlist, and both 1- and 3-year mortality outcomes after transplant. They do not currently include any long-term (>3 years) post-transplant morbidity or mortality measures. While one-year survival after transplant is above 90%, outcomes are less hopeful beyond 3 years. Only a third of pediatric transplant recipients have the ‘Ideal Outcome’ 10 years post-transplant—defined as normal graft function in the absence of immunosuppression morbidities.26,27 Children facing socioeconomic deprivation are even less likely to achieve these ideal outcomes.2,3,28 A priority in the transplant community, therefore, should include identifying health system interventions that can improve both long-term outcomes—and achieve equitable outcomes—to ensure that all children can survive and thrive well into adulthood. Regulatory agencies should invest in developing and reporting long-term and equity outcome metrics to incentivize transplant centers to accelerate center-level attention to these topics. Such measures must extend past simple survival metrics and incorporate measures of morbidity and quality of life.29
Future work should explore the practices in transplant institutions that more consistently achieve positive outcomes, regardless of patients’ socioeconomic status. Research to characterize the individual journeys patients endure across the transplant course might explore patient-level factors (e.g. prevalence of health-related social risks like food insecurity and housing instability), health system factors (e.g. how the health system does or does not address such social risks), and state level factors (e.g. public insurance quality) that may affect transplant outcomes (Table 4). Such studies might purposively sample transplant centers across patient-mix deprivation and center quality to surface potential provider-level and site-level factors that contribute to disparate outcomes. Specifically, these studies should explore how care should be adjusted to accommodate the unique challenges faced by socioeconomically disadvantaged children23 and how centers screen for and address medical complications—particularly for children from socioeconomically deprived neighborhoods. Previous studies have demonstrated that ‘failure to rescue’ (that is, failing to act on a complication) affects short term survival.6 Future studies explore center variation in ‘failure to rescue’ for long term outcomes to identify quality improvement tools that can contribute to improved outcomes. Such studies would ideally surface specific care practices that could be incorporated into national transplant-related process quality measures.
Table 4.
Potential strategies by level to address adverse social determinants in children undergoing liver transplant
| Level | Knowledge Gaps | Potential Strategies |
|---|---|---|
|
| ||
| Patient | •Prevalence and impact of health-related social risks on children undergoing liver transplant | •Quantitative research |
| •Unique barriers and facilitators that children with social risks encounter on transplant journey | •Qualitative research | |
| •Patient/caregiver comfort with social needs screening by liver transplant providers | •Qualitative research | |
|
| ||
| Health System | •Systematic approach to integrating social care into transplant medicine | •Systems level implementation |
|
| ||
| Regulatory Bodies (e.g. UNOS) | •Public reporting of equity and long-term outcomes | •More robust social needs data collection |
| •Development and reporting of a ‘health equity’ quality metric | ||
| •Reporting long-term outcomes | ||
|
| ||
| State/Federal Level | •Public insurance quality and outcomes for children after liver transplant | •Quantitative and qualitative research |
| •Roll of Affordable Care Act/Medicaid expansion policies on outcomes for children after liver transplant | ||
In other pediatric chronic diseases, increasing the clinical team’s awareness of social needs has facilitated care plan customization and increased health systems’ activities to strengthen social services referrals. For example, in pediatric asthma, a multi-modal quality improvement initiative that includes discharge huddles for patients that live in high risk neighborhoods, partnerships with Medicaid managed care organizations, medical legal partnerships, and greater connections to community resources resulted in decreased readmissions for asthma exacerbations.30–32 As we increase awareness of the unique challenges faced by families receiving transplant services, similar innovations could be more systematically included in pediatric liver transplant care. These types of interventions could be initially tested and refined across networks such as the Society of Pediatric Liver Transplant (SPLIT) or Starzl Network for Excellence in Pediatric Transplantation (SNEPT).
We acknowledge several important limitations of this analysis. First, the neighborhood deprivation index was matched to patient’s home ZIP code. While ZIP codes are convenient, they are not the ideal spatial unit because they can be re-drawn by the US Postal Service to improve mail delivery efficiency. However, ZIP codes are readily available within the SRTR database and define relatively small geographical areas. As more evidence accumulates that neighborhood context is important and relevant to transplant outcomes,2,3,17 regulatory bodies should consider incorporating fixed geographical units (e.g. census blocks or census tracts) into the SRTR database. Second, the focus of this study was on the confluence of transplant center quality, neighborhood deprivation, and long-term outcomes. Therefore, we did not directly address regional variation in outcome (e.g. state/federal policies). This study lays the groundwork for future studies to evaluate the impact of state and federal policies on outcomes for these children. Third, our measure of center quality (center-level graft failure rates) also contributes to our outcome measure of patient-level graft failure. To model the effect of center quality, we used stratified Cox models. Such models assume different baseline hazard functions for children at low and high quality centers and allowed us to evaluate how center quality modifies the relationship between patient-mix deprivation and graft failure. Despite this limitation, the large gap between high and low performing centers (20% difference in graft survival for children) is concerning and highlights the need for collaborative efforts to improve care across centers and decrease inter-center variability in long-term outcomes. Fourth, our measure of patient-mix deprivation does not incorporate the range of socioeconomic deprivation for patients at a particular center. This study lays the groundwork for future studies to better understand how centers caring for relatively wider or narrower range of deprivation differ with regards to outcomes. Fifth, because of small numbers of patients in each racial category in this study, we classified non-white, non-Black children as ‘Other’. This might have limited our ability to detect additional variation across groups. Designing care to meet the needs of every child will require more nuanced data on both race and other socio-demographics. Lastly, registry study limitations, including data quality and robustness, are relevant to our dataset. In this case, our measures were confined to socioeconomic deprivation and graft failure; morbidity data might reveal opportunities for intervention before graft failure and death occur. However, the SRTR database is currently the most comprehensive transplant database in the U.S. and enables us to examine center variation in post-transplant morbidity.
Our findings surface large variations in long-term outcomes after pediatric liver transplantation that are likely to reflect underlying variation in care practices. Socioeconomically deprived children are at risk for adverse long-term outcomes following liver transplantation. Applying the principles of ‘steal[ing] shamelessly and shar[ing] seamlessly,’33 new studies should now examine how select transplant centers overcome deprivation to achieve equitable outcomes29 for children facing these socioeconomic barriers.
ACKNOWLEDGMENTS
The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the NIH under Award Number KL2TR001870 (SIW). The content is solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
UCSF Liver Center P30 DK026743
NIH T32 DK 7727-24 (PI Lee Denson, support for SIW)
ABBREVIATIONS
- HRSA
Health Resources and Services Administration
- OPTN
Organ Procurement and Transplantation Network
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
American Association for the Study of Liver Disease Advanced/Transplant Hepatology Award (SIW)
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by The American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
Data are not shared.
REFERENCES
- 1.Schroeder SA. We Can Do Better — Improving the Health of the American People. http://dx.doi.org/101056/NEJMsa073350.Published online October 9, 2009.http://www.nejm.org/doi/full/10.1056/NEJMsa073350
- 2.Wadhwani SI, Bucuvalas JC, Brokamp C, et al. Association between neighborhood-level socioeconomic deprivation and the Medication Level Variability Index for children following liver transplantation. Transplantation. Published online January 19, 2020. doi: 10.1097/TP.0000000000003157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wadhwani SI, Beck AF, Bucuvalas J, Gottlieb L, Kotagal U, Lai JC. Neighborhood socioeconomic deprivation is associated with worse patient and graft survival following pediatric liver transplantation. Am J Transplant. Published online January 20, 2020:ajt.15786. doi: 10.1111/ajt.15786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell E, Loomes KM, Squires RH, Goldberg D. Variability in acceptance of organ offers by pediatric transplant centers and its impact on wait-list mortality: Mitchell et al. Liver Transpl. 2018;24(6):803–809. doi: 10.1002/lt.25048 [DOI] [PubMed] [Google Scholar]
- 5.Covarrubias K, Luo X, Massie A, et al. Determinants of length of stay after pediatric liver transplantation. Pediatr Transplant. Published online March 25, 2020:e13702. doi: 10.1111/petr.13702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cramm SL, Waits SA, Englesbe MJ, et al. Failure to Rescue as a Quality Improvement Approach in Transplantation: A First Effort to Evaluate This Tool in Pediatric Liver Transplantation. Transplantation. 2016;100(4):801–807. doi: 10.1097/TP.0000000000001121 [DOI] [PubMed] [Google Scholar]
- 7.Englesbe MJ, Kelly B, Goss J, et al. Reducing pediatric liver transplant complications: a potential roadmap for transplant quality improvement initiatives within North America. Am J Transplant. 2012;12(9):2301–2306. doi: 10.1111/j.1600-6143.2012.04204.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brokamp C, Beck AF, Goyal NK, Ryan P, Greenberg JM, Hall ES. Material community deprivation and hospital utilization during the first year of life: an urban population–based cohort study. Annals of Epidemiology. Published online November 29, 2018. doi: 10.1016/j.annepidem.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allik M, Leyland A, Ichihara MYT, Dundas R. Creating small-area deprivation indices: a guide for stages and options. J Epidemiol Community Health. 2020;74(1):20–25. doi: 10.1136/jech-2019-213255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CDC’s Social Vulnerability Index (SVI). Published October 15, 2020.Accessed January 14, 2021. https://www.atsdr.cdc.gov/placeandhealth/svi/index.html
- 11.Brokamp C A Nationwide Community Deprivation Index. doi: 10.5281/zenodo.1134946 [DOI] [Google Scholar]
- 12.Schuurman N, Bell N, Dunn JR, Oliver L. Deprivation indices, population health and geography: an evaluation of the spatial effectiveness of indices at multiple scales. Journal of urban health : bulletin of the New York Academy of Medicine. 2007;84(4):591–603. doi: 10.1007/s11524-007-9193-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subramanian SV, Chen JT, Rehkopf DH, Waterman PD, Krieger N. Comparing individual- and area-based socioeconomic measures for the surveillance of health disparities: A multilevel analysis of Massachusetts births, 1989–1991. Am J Epidemiol. 2006;164(9):823–834. doi: 10.1093/aje/kwj313 [DOI] [PubMed] [Google Scholar]
- 14.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health. 2005;95(2):312–323. doi: 10.2105/AJPH.2003.032482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeLano K, Folger AT, Ding L, et al. Associations Between Maternal Community Deprivation and Infant DNA Methylation of the SLC6A4 Gene. Front Public Health. 2020;8. doi: 10.3389/fpubh.2020.557195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheon C, Lin Y, Harding DJ, Wang W, Small DS. Neighborhood Racial Composition and Gun Homicides. JAMA Netw Open. 2020;3(11):e2027591. doi: 10.1001/jamanetworkopen.2020.27591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wadhwani SI, Brokamp C, Rasnick E, Bucuvalas JC, Lai JC, Beck AF. Neighborhood socioeconomic deprivation, racial segregation, and organ donation across 5 states. Am J Transplant. Published online July 12, 2020. doi: 10.1111/ajt.16186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.SRTR Risk Adjustment Model Documentation: Posttransplant Outcomes. Scientific Registry of Transplant Recipients. Accessed May 14, 2020. https://www.srtr.org/reports-tools/posttransplant-outcomes/
- 19.Rana A, Pallister Z, Halazun K, et al. Pediatric Liver Transplant Center Volume and the Likelihood of Transplantation. Pediatrics. 2015;136(1):e99–e107. doi: 10.1542/peds.2014-3016 [DOI] [PubMed] [Google Scholar]
- 20.Moylan CA, Brady CW, Johnson JL, Smith AD, Tuttle-Newhall JE, Muir AJ. Disparities in liver transplantation before and after introduction of the MELD score. JAMA. 2008;300(20):2371–2378. doi: 10.1001/jama.2008.720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart LG, Larson EH, Lishner DM. Rural definitions for health policy and research. Am J Public Health. 2005;95(7):1149–1155. doi: 10.2105/AJPH.2004.042432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberg DS, French B, Lewis JD, et al. Liver transplant center variability in accepting organ offers and its impact on patient survival. Journal of Hepatology. 2016;64(4):843–851. doi: 10.1016/j.jhep.2015.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Committee on Integrating Social Needs Care into the Delivery of Health Care to Improve the Nation’s Health, Board on Health Care Services, Health and Medicine Division, National Academies of Sciences, Engineering, and Medicine. Integrating Social Care into the Delivery of Health Care: Moving Upstream to Improve the Nation’s Health. National Academies Press; 2019:25467. doi: 10.17226/25467 [DOI] [PubMed] [Google Scholar]
- 24.Chin T, Kahn R, Li R, et al. US-county level variation in intersecting individual, household and community characteristics relevant to COVID-19 and planning an equitable response: a cross-sectional analysis. BMJ Open. 2020;10(9):e039886. doi: 10.1136/bmjopen-2020-039886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans MK. Covid’s Color Line — Infectious Disease, Inequity, and Racial Justice. N Engl J Med. 2020;383(5):408–410. doi: 10.1056/NEJMp2019445 [DOI] [PubMed] [Google Scholar]
- 26.Wadhwani SI, Hsu EK, Shaffer ML, Anand R, Ng VL, Bucuvalas JC. Predicting ideal outcome after pediatric liver transplantation: An exploratory study using machine learning analyses to leverage Studies of Pediatric Liver Transplantation Data. Pediatr Transplant. Published online July 22, 2019. doi: 10.1111/petr.13554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng VL, Alonso EM, Bucuvalas JC, et al. Health status of children alive 10 years after pediatric liver transplantation performed in the US and Canada: report of the studies of pediatric liver transplantation experience. The Journal of pediatrics. 2012;160(5):820–6 e3. doi: 10.1016/j.jpeds.2011.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thammana RV, Knechtle SJ, Romero R, Heffron TG, Daniels CT, Patzer RE. Racial and socioeconomic disparities in pediatric and young adult liver transplant outcomes. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2014;20(1):100–115. doi: 10.1002/lt.23769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477–2481. doi: 10.1056/NEJMp1011024 [DOI] [PubMed] [Google Scholar]
- 30.Kercsmar CM, Beck AF, Sauers-Ford H, et al. Association of an Asthma Improvement Collaborative With Health Care Utilization in Medicaid-Insured Pediatric Patients in an Urban Community. JAMA Pediatr. 2017;171(11):1072–1080. doi: 10.1001/jamapediatrics.2017.2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beck AF, Sandel MT, Ryan PH, Kahn RS. Mapping Neighborhood Health Geomarkers To Clinical Care Decisions To Promote Equity In Child Health. Health Aff (Millwood). 2017;36(6):999–1005. doi: 10.1377/hlthaff.2016.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck AF, Anderson KL, Rich K, et al. Cooling The Hot Spots Where Child Hospitalization Rates Are High: A Neighborhood Approach To Population Health. Health Affairs. 2019;38(9):1433–1441. doi: 10.1377/hlthaff.2018.05496 [DOI] [PubMed] [Google Scholar]
- 33.Forrest CB, Margolis P, Seid M, Colletti RB. PEDSnet: how a prototype pediatric learning health system is being expanded into a national network. Health Aff (Millwood). 2014;33(7):1171–1177. doi: 10.1377/hlthaff.2014.0127 [DOI] [PubMed] [Google Scholar]
- 34.Alvidrez J, Castille D, Laude-Sharp M, Rosario A, Tabor D. The National Institute on Minority Health and Health Disparities Research Framework. Am J Public Health. 2019;109(S1):S16–S20. doi: 10.2105/AJPH.2018.304883 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are not shared.