One of the grand strategies in the development of the nervous system is to overproduce constructive elements and then selectively to prune the excess, for example, by synaptic pruning. Given limited resources, surviving synapses are nurtured and reinforced, while the unsuccessful synapses are eliminated. Synaptic pruning also persists in the mature nervous system via activity-dependent plasticity, which is crucial for learning and memory. To assemble refined mature neuronal circuits and maintain homeostasis, synaptic pruning must proceed in a controlled and timely manner. Aberrant synaptic pruning may lead to neurodevelopmental disorders, such as autism, schizophrenia, and epilepsy [1].
Phagocytosis is defined as the process by which phagocytes identify, engulf, and digest large particles, such as pathogens, dead cells, and cellular debris. In the central nervous system, phagocytosis functions as a defense and clearance mechanism. Glial cells are the critical effectors of synaptic pruning through phagocytosis to eliminate unnecessary synapses. Microglia are considered to be the primary phagocytes [2], which mainly use immune signaling pathways such as the classical complement pathway to remove unwanted synapses [3]. In addition to microglia, astrocytes are regarded as less-efficient phagocytes in the brain through the Multiple Epidermal Growth Factor-Like Domains Protein 10 (Megf10) and MER Proto-Oncogene, Tyrosine Kinase (Mertk) phagocytic pathways [4, 5]. Particularly, the complement component C1q binds to Megf10 in astrocytes to trigger intracellular downstream signals [6], which requires PTB Domain-Containing Engulfment Adapter Protein 1 and ATP-binding cassette subfamily A member 1. Mertk in astrocytes uses the integrin pathway to regulate phagocytosis involving CRKII/DOCK180/Rac1 [7]. Astrocyte-mediated phagocytosis is significantly reduced in Megf10-/- and Mertk-/- mice, and is further reduced in double-knockout mice, indicating that Megf10 and Mertk mediate synapse elimination in parallel [5].
Although astrocytes were initially regarded as secondary phagocytes that back up microglial phagocytic activity in the brain, recent studies indicate that microglia and astrocytes play orchestrated roles: microglia specialize in engulfing cell bodies and proximal dendrites, while astrocytes preferentially engulf distal processes and diffuse neuritic debris [8]. Nevertheless, the precise interplay between microglia and astrocytes in synapse elimination is still unknown. Specifically, the brain contains various types of synapse, including excitatory and inhibitory synapses. And a has synapse two components, the pre- and the post-synaptic compartments. It remains unexplored whether the diversity of synapses is responsible for the distinct phagocytic pathways used by different phagocytes (Fig. 1).
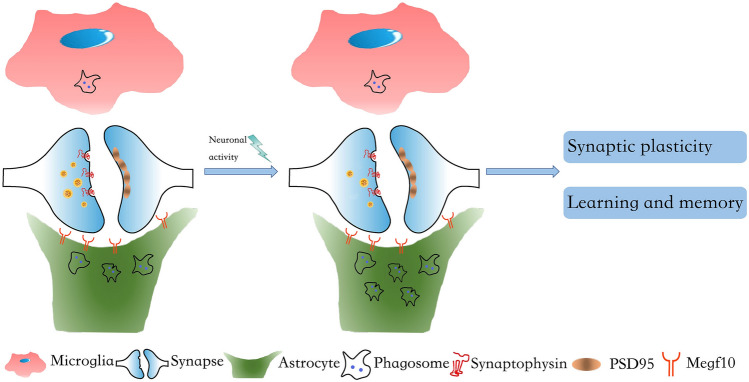
Fig. 1.
Astrocytes play a major role in the neuronal activity-dependent elimination of excitatory synapses. Astrocytes (green) eliminate unwanted excitatory synapses from neurons (blue) by phagocytosing them through Megf10 receptors in adult mouse hippocampus. Neuronal activity selectively increases the Megf10-dependent astrocytic phagocytosis of excitatory synapses, which has important implications for synaptic plasticity and memory formation.
Using novel fluorescent phagocytosis reporters, Hyungju Park, Won-Suk Chung, and colleagues have demonstrated that astrocytes, not microglia, play a major role in constantly eliminating unnecessary adult excitatory synapses in response to brain activity. To monitor glial phagocytosis, they generated a novel molecular sensor. In a neutral pH environment, the sensor on the cell membrane has intact fluorescent intensity of both mCherry and eGFP, while under acidic conditions, for instance after phagocytosis in the lysosome, the sensor preserves only the mCherry signal. Consequently, investigators were able to localize and quantify phagosomes by tracing the puncta with a mCherry but not an eGFP signal. Then the authors targeted the sensor into different synaptic terminals via linking it to pre- or post-synaptic markers driven by cell-type-specific promotors, through which they were able to separately quantify the phagocytic events of one type of synapse. Moreover, by co-localizing the phagocytic signals with microglia or astrocytic markers, they measured how often and by which type of glial cell synapses were phagocytosed. Their results revealed that in the CA1 region of the adult mouse hippocampus, although both excitatory and inhibitory synaptic terminals can be eliminated by glial phagocytosis, remarkably more excitatory synapses than inhibitory synapses were phagocytosed. Strikingly, they also found that astrocytes had more phagocytic puncta than microglia regardless of the type of synapse. Further investigation showed that only astrocytic phagocytosis of excitatory synapses increased with neuronal activity induced by environmental enrichment, while neither the uptake of inhibitory synapses by astrocytes nor the uptake of synapses by microglia were affected.
Recently, Megf10 and Mertk have been reported to be major phagocytic pathways in astrocytes. To investigate the mechanisms underlying the synapse elimination by astrocytic phagocytosis, the authors also deployed the fluorescent phagocytosis reporters in a mouse model in which Megf10 was inducibly deleted in adult astrocytes. They found that astrocytes use Megf10 specifically to engulf excitatory synapses in the adult hippocampus. Further experiments showed that astrocytic deletion of Megf10 resulted in significantly more total synapses and excitatory synapses, as well as causing abnormal synaptic plasticity. In addition, mice with hippocampus-specific astrocyte conditional Megf10-knockout displayed a significant deficit in novel object recognition and novel object location tests, suggesting a critical role of Megf10-mediated astrocytic phagocytosis in learning and memory.
Previously, astrocytes were reported to participate in synapse elimination in the developing visual system in mice [5, 9]. And human astrocytes have been demonstrated to be able to phagocytose synapses in dissociated cultures and cerebral organoids [10, 11], indicating that astrocytes prune synapses in the human CNS. Nevertheless, astrocytes were regarded as a secondary or back-up phagocyte in the CNS. Contrary to this consensus, Hyungju Park, Won-Suk Chung and their colleagues have shown that astrocytes are the dominant phagocytes. Compared with previous findings in developing brains, synapse pruning in adults may have quite distinct significance in physiological functions. This study showed that astrocytes control excitatory synapse elimination in adult mouse hippocampal CA3–CA1 circuits, and that Megf10-mediated astrocytic phagocytosis is functionally relevant to synaptic plasticity and memory formation [12]. Thus, this study proposes a possible mechanism for controlling synaptic turnover and re-patterning connectivity, which may contribute to the rapid renewal of memory traces in the adult hippocampus.
Just as it gave several answers for synapse elimination, this investigation raised more interesting questions. The authors proposed a predominant role of astrocytic phagocytosis in synapse elimination under physiological conditions. Few studies have examined astrocytic phagocytosis in pathological synaptic dysfunction following injury or nervous system degeneration in adults. In mouse models of Alzheimer’s disease (AD), astrocytes have been suggested to have the capacity to clear Aβ [13]. APOE4, a strong genetic risk factor for AD, suppresses astrocytic phagocytosis, and the protective APOE allele for AD, APOE2, remarkably promotes phagocytosis by astrocytes [14]. Overall, the phagocytic capacity of astrocytes appears to decrease during reactive astrogliosis [15, 16]. On the contrary, several publications have shown that phagocytosis by microglia is strongly implicated in neurological pathologies [17]. Inhibition or knockout of C1q, complement C3, or the microglia-specific C3 receptor (CR3), which are required for microglial phagocytosis, reduce the synapse loss in AD [18]. Similarly, mice with depleted microglia or deficiency in C3 or CR3a are protected from virus-induced synaptic loss [19]. There are other reports of microglial phagocytosis involved in the synaptic loss induced by HIV [20], by ageing [21], and in schizophrenia [22]. Given this evidence, it would be interesting to further define the possible divergent roles of astrocytic and microglial phagocytosis under pathological conditions.
Generally, all phagocytosis can be divided into 3 major steps: “find me”, “eat me”, and “digest me”. This study left black boxes in each step. For example, what guides the astrocytes to phagocytose the unnecessary excitatory synapses after neuronal activity? This question can be addressed how neuronal activity induces unnecessary excitatory synapses to recruit astrocytes or how neuronal activity protects necessary excitatory synapses from elimination. In the brain, some examples of synapse pruning may give hints to answer this question. For instance, astrocytes secrete the glycoprotein hevin (SPARCL1), which modulates the formation of excitatory inputs in the visual cortex and regulates the elimination of these connections [23]. Accumulation of C1q and C3 on certain subsets of synapses promote microglia to engulf them. On the other hand, CD47 on the neuronal cell membrane, and its receptor in microglia, SIRPα, suppress the phagocytosis of synaptic structures [24]. Consequently, one possible mechanism is that neuronal activity triggers the relocation or regulates the expression of these “eat-me” or “don’t-eat-me” signals to mediate phagocytosis by astrocytes.
In addition to the above topics, it seems that in the brain, each region has different rates of synapse elimination by microglia and astrocytes. It will be of great interest to elucidate the internal and external factors controlling these rates. Another intriguing finding of this study is that both astrocytes and microglia have significantly different levels of phagocytosis between presynaptic and postsynaptic structures. It is reasonable to speculate that there are important mechanisms of eliminating synapses other than phagocytosis, and these occur more often in postsynaptic than presynaptic structures, such as miRNA in extracellular vesicles proposed by Prada et al. [25]. Last but not least, since Megf10 and Mertk are major phagocytic pathways in astrocytes, the results of this investigation arouse curiosity about the role of Mertk-mediated astrocytic phagocytosis in synapse elimination. Overall, this study emphasizes the astrocyte-mediated synapse turnover in learning and memory and provides novel insight into treating various brain disorders by modulating astrocytic phagocytosis to restore synaptic connectivity.
Acknowledgements
This Research Highlight was supported by Grants from the National Natural Science Foundation of China (31830033 and 82090032), the Program for Changjiang Scholars and Innovative Research Teams in University (IRT_16R37), and the Key-Area Research and Development Program of Guangdong Province, China (2018B030334001).
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Neniskyte U, Gross CT. Errant gardeners: glial-cell-dependent synaptic pruning and neurodevelopmental disorders. Nat Rev Neurosci. 2017;18:658–670. doi: 10.1038/nrn.2017.110. [DOI] [PubMed] [Google Scholar]
- 2.Galloway DA, Phillips AEM, Owen DRJ, Moore CS. Phagocytosis in the Brain: Homeostasis and Disease. Front Immunol. 2019;10:790. doi: 10.3389/fimmu.2019.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- 4.Magnus T, Chan A, Linker RA, Toyka KV, Gold R. Astrocytes are less efficient in the removal of apoptotic lymphocytes than microglia cells: implications for the role of glial cells in the inflamed central nervous system. J Neuropathol Exp Neurol. 2002;61:760–766. doi: 10.1093/jnen/61.9.760. [DOI] [PubMed] [Google Scholar]
- 5.Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iram T, Ramirez-Ortiz Z, Byrne MH, Coleman UA, Kingery ND, Means TK, et al. Megf10 is a receptor for C1Q that mediates clearance of apoptotic cells by astrocytes. J Neurosci. 2016;36:5185–5192. doi: 10.1523/JNEUROSCI.3850-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung YJ, Chung WS. Phagocytic roles of glial cells in healthy and diseased brains. Biomol Ther (Seoul) 2018;26:350–357. doi: 10.4062/biomolther.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damisah EC, Hill RA, Rai A, Chen F, Rothlin CV, Ghosh S, et al. Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo. Sci Adv 2020, 6: eaba3239. [DOI] [PMC free article] [PubMed]
- 9.Chung WS, Allen NJ, Eroglu C. Astrocytes control synapse formation, function, and elimination. Cold Spring Harb Perspect Biol. 2015;7:a020370. doi: 10.1101/cshperspect.a020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron. 2016;89:37–53. doi: 10.1016/j.neuron.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sloan SA, Darmanis S, Huber N, Khan TA, Birey F, Caneda C, et al. Human astrocyte maturation captured in 3d cerebral cortical spheroids derived from pluripotent stem cells. Neuron. 2017;95(779–790):e776. doi: 10.1016/j.neuron.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JH, Kim JY, Noh S, Lee H, Lee SY, Mun JY, et al. Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature 2020. [DOI] [PubMed]
- 13.Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, et al. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- 14.Chung WS, Verghese PB, Chakraborty C, Joung J, Hyman BT, Ulrich JD, et al. Novel allele-dependent role for APOE in controlling the rate of synapse pruning by astrocytes. Proc Natl Acad Sci U S A. 2016;113:10186–10191. doi: 10.1073/pnas.1609896113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin C, Zhou LQ, Ma XT, Hu ZW, Yang S, Chen M, et al. Dual functions of microglia in ischemic stroke. Neurosci Bull. 2019;35:921–933. doi: 10.1007/s12264-019-00388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vilalta A, Brown GC. Neurophagy, the phagocytosis of live neurons and synapses by glia, contributes to brain development and disease. FEBS J. 2018;285:3566–3575. doi: 10.1111/febs.14323. [DOI] [PubMed] [Google Scholar]
- 19.Vasek MJ, Garber C, Dorsey D, Durrant DM, Bollman B, Soung A, et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature. 2016;534:538–543. doi: 10.1038/nature18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tremblay ME, Marker DF, Puccini JM, Muly EC, Lu SM, Gelbard HA. Ultrastructure of microglia-synapse interactions in the HIV-1 Tat-injected murine central nervous system. Commun Integr Biol. 2013;6:e27670. doi: 10.4161/cib.27670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Q, Colodner KJ, Matousek SB, Merry K, Hong S, Kenison JE, et al. Complement C3-deficient mice fail to display age-related hippocampal decline. J Neurosci. 2015;35:13029–13042. doi: 10.1523/JNEUROSCI.1698-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh SK, Stogsdill JA, Pulimood NS, Dingsdale H, Kim YH, Pilaz LJ, et al. Astrocytes assemble thalamocortical synapses by bridging NRX1alpha and NL1 via hevin. Cell. 2016;164:183–196. doi: 10.1016/j.cell.2015.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehrman EK, Wilton DK, Litvina EY, Welsh CA, Chang ST, Frouin A, et al. CD47 protects synapses from excess microglia-mediated pruning during development. Neuron. 2018;100(120–134):e126. doi: 10.1016/j.neuron.2018.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prada I, Gabrielli M, Turola E, Iorio A, D'Arrigo G, Parolisi R, et al. Glia-to-neuron transfer of miRNAs via extracellular vesicles: a new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol. 2018;135:529–550. doi: 10.1007/s00401-017-1803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]