Abstract
MRI is routinely used for rectal cancer staging to evaluate tumor extent and to inform decision-making regarding surgical planning and the need for neoadjuvant and adjuvant therapy. Extramural venous invasion (EMVI), which is intravenous tumor extension beyond the rectal wall on histopathology, is a predictor for worse prognosis. T2-weighted images (T2WI) demonstrate EMVI as a nodular-, bead-, or worm-shaped structure of intermediate T2 signal with irregular margins that arises from the primary tumor. Correlative diffusion-weighted images demonstrate intermediate to high signal corresponding to EMVI, and contrast enhanced T1-weighted images demonstrate tumor signal intensity in or around vessels. Diffusion-weighted and post contrast images may increase diagnostic performance but decrease inter-observer agreement. CT may also demonstrate obvious EMVI and is potentially useful in patients with a contraindication for MRI. This article aims to review the spectrum of imaging findings of EMVI of rectal cancer on MRI and CT, to summarize the diagnostic accuracy and inter-observer agreement of imaging modalities for its presence, to review other rectal neoplasms that may cause EMVI, and to discuss the clinical significance and role of MRI-detected EMVI in staging and restaging clinical scenarios.
Keywords: Extramural venous invasion, Rectal Neoplasms, Prognosis, Disease-free survival, Magnetic resonance imaging
Key points
T2WI demonstrates EMVI as an expanded, irregular vessel with intermediate tumor-signal intensity.
CT can sometimes depict EMVI as a heterogeneously enhancing, serpentine cord-like structure
Inter-observer agreement in assessing the presence or absence of EMVI is variable.
EMVI is a predictor of poor prognosis and indicates biologic aggressiveness.
EMVI may be seen in association with rectal tumors other than adenocarcinoma.
Background
Colorectal cancer is the third most common neoplasm in adults, with approximately 43,000 patients diagnosed with rectal cancer in the United States in 2020 [1]. MRI provides detailed imaging of rectal cancers and relevant pelvic structures and is routinely used to evaluate tumor extension beyond the muscularis propria of the rectal wall as well as involvement of the mesorectal fascia [2–4]. Rectal cancer MRI is routinely used to inform therapeutic decisions, e.g., the use and type of neoadjuvant therapy, oncologic surgical planning, and preoperative assessment after neoadjuvant therapy [5].
Direct tumor invasion into the extramural veins on histopathology, known as extramural venous invasion (EMVI), has been recognized as an indicator of poor prognosis [6, 7]. Brown et al. found that MRI-detected EMVI correlated well with histopathological EMVI [8]. MRI-detected EMVI is now widely accepted as an independent poor prognostic factor for disease-free survival. Consequently, MRI-detected EMVI is an important consideration in therapeutic decision-making similar to histopathological EMVI [9]. Despite the fact that diagnosis can be challenging, radiologists should evaluate rectal cancers for EMVI at pelvic MR as it is an important imaging biomarker for nodal or distant metastasis, unfavorable prognosis, and need for neoadjuvant therapy [10].
This article reviews the imaging findings of EMVI on MRI and CT, summarizes the diagnostic accuracy and inter-observer agreement for detection of EMVI, and reviews the clinical significance and role of MRI-detected EMVI in staging and restaging scenarios with an aim to disseminate a common understanding of EMVI and its varied appearances.
Venous anatomy of the rectum
Understanding the rectal venous plexus anatomy can be helpful when aiming to accurately identify EMVI on MRI. The venous drainage of the anorectum occurs via two interconnected plexuses, the inner submucosal plexus of the anorectum (the internal rectal plexus) and the outer muscularis plexus (the external rectal plexus). Venous drainage of the upper 2/3 of the rectum is via the superior rectal/hemorrhoidal veins, which drain into the inferior mesenteric vein [11]. Venous drainage of the lower 1/3 of the rectum is via the middle and inferior rectal veins, which drain into the internal iliac veins, common iliac veins, and inferior vena cava (Fig. 1). The middle rectal vein is found in 32.6% of individuals, and is unilateral in the majority [12]. Thus, venous return from the rectum enters both the portal and systemic circulation.
Fig. 1.
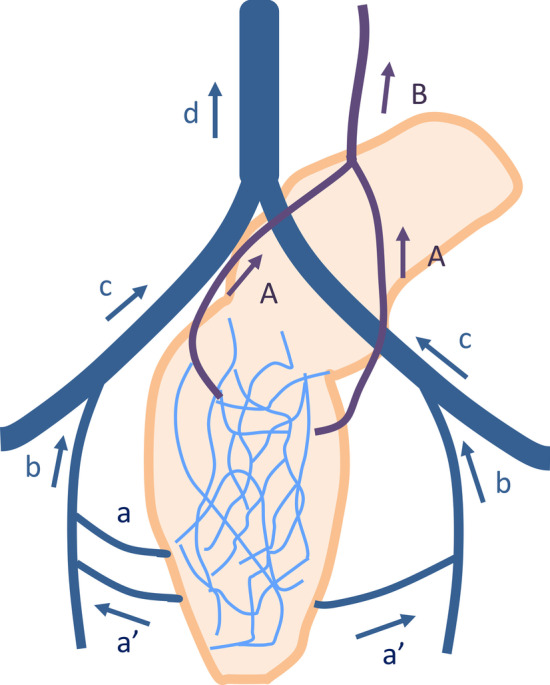
Venous anatomy of the rectum. The upper 2/3 of the rectum drains via the superior rectal vein (A SRV) into the inferior mesenteric vein (B IMV). The lower 1/3 of the rectum drains via the middle (a MRV) and inferior rectal vein (a’ IRV) into the internal iliac vein (b IIV), common iliac vein (c CIV), and inferior vena cava (d IVC)
EMVI in histopathology
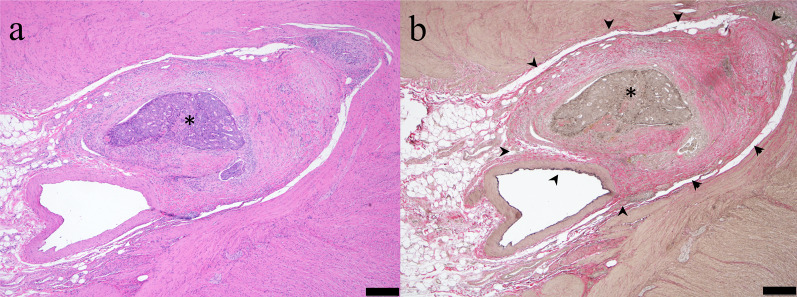
EMVI is defined histopathologically as the presence of tumor cells within blood vessels located beyond the muscularis propria of the rectal wall (Fig. 2a); however, desmoplastic reaction and endothelial destruction induced by tumor invasion destroy the vessel wall and can preclude identification of venous anatomy, making EMVI challenging to detect even at histopathology [13]. An elastin stain highlights elastic fibers present in the adventitia of veins (but not lymphatics), and improves the identification of EMVI compared to routine hematoxylin–eosin stain alone (Fig. 2b) [14, 15]. The detection of venous invasion is dependent on the number of examined tissue blocks or slides [16]; therefore, imaging detection of EMVI can guide the pathologist in selecting the location and number of tissue samples for slides.
Fig. 2.
Extramural venous invasion on histopathology. a The tumor cells (asterisk) are surrounded by a vessel on hematoxylin–eosin stain. b Elastin stain is helpful to depict extramural venous invasion (asterisk) by highlighting elastin fiber (arrowheads) around tumor cells. The scale bar is 500 µm
Invasion of tumor into blood vessels is considered to be an initial step in hematogenous metastasis [17], and histologic venous invasion of colorectal carcinoma was identified as a risk factor for metastatic disease in the 1930s [6]. Venous invasion is categorized as intramural or extramural based on whether the location is within or beyond the bowel wall. Histopathologically-detected EMVI portends a worse 5-year survival rate and an increased risk of hepatic metastasis compared to intramural venous invasion, which demonstrates a worse prognosis than no venous invasion [7, 16]. The presence of EMVI on resection specimens following neoadjuvant therapy is associated with a significantly worse prognosis in patients with rectal cancer [18, 19]. If a patient did not receive neoadjuvant therapy prior to surgery, and the surgical histopathology shows high risk features, including EMVI, chemotherapy is often recommended following surgery.
EMVI in magnetic resonance imaging
The MERCURY study evaluated high-resolution rectal MRI with an in-plane resolution of 0.6 × 0.6 mm and reported that MRI could predict surgical margin status [20] and extramural depth equivalent to that on pathology with a difference less than 0.5 mm [3]. Using this same optimized scan protocol with high-resolution T2WI, Brown and colleagues also first described EMVI in the setting of rectal cancer and noted its presence to be an important prognostic finding, similar to EMVI on histopathology [8]. Rectal MRI is considered capable of depicting EMVI greater than 3 mm despite the spatial resolution of MRI being limited compared with histopathology [8].
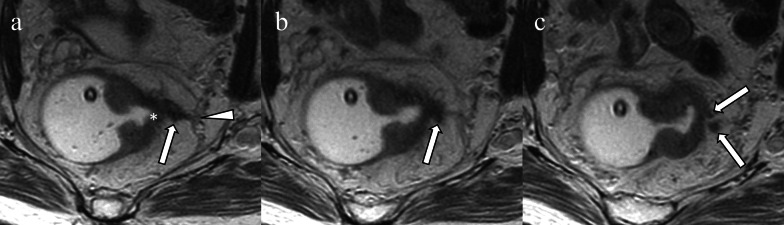
EMVI at MRI is usually associated with primary tumor extending beyond the muscularis propria and into the mesorectal fat. The leading edge of the tumor with such extramural extension and associated EMVI is typically nodular or sawtooth rather than smooth. The infiltrated vessel is contiguous with the primary tumor, may be expanded, and has lost the normal black signal flow void, which is replaced with intermediate signal T2 tumor intensity. The vessel often has irregular margins and may appear beaded or nodular on T2 and post contrast imaging (Fig. 3) [5, 21].
Fig. 3.
Extramural venous invasion on T2-weighted image. A nodular-shaped structure (a arrow) arising from the ulcer crater and leading edge of the primary lesion (a asterisk) invades the mesorectal fascia (a arrowhead). The structure also extends cranially in the mesorectal fascia (b arrow) and is branched (c arrows)
To help in assessing EMVI, Smith et al. developed a 5-point scoring system on T2WI based on the aforementioned MR imaging features [22]. This scoring system stratifies a broad spectrum of findings into five categories and has been used in several research studies [22–32]. Score 0 is defined as tumor extension through the muscle layer, without nodularity, and lacking vessels adjacent to the area of tumor penetration. Score 1 is defined as minimal extramural stranding and nodular extension, but not in the vicinity of any vascular structure. Score 2 is defined as stranding in the perirectal fat in the vicinity of normal diameter extramural vessels. Score 3 is defined by intermediate T2 signal intensity within a slightly expanded vessel with a smooth contour. Score 4 occurs when a perirectal vessel has an irregular contour or nodular expansion with internal intermediate T2 signal intensity [22]. A score of 0 or 1 is predictive of absence of EMVI at histopathology whereas scores of 3 or 4 are suggestive of the presence EMVI (Fig. 4). A score of 2 was initially defined as a negative [22], but subsequent research has shown that one-third of cases with this score can be positive histpathologically, so a score of 2 is often considered equivocal [26].
Fig. 4.

Extramural venous invasion scoring system based on T2-weighted images. a No vessel exists adjacent to the primary tumor (score 0). b Normal diameter vessel adjacent to the primary tumor demonstrates no tumor signal intensity (score 1). c Slightly expanded vessel without abnormal signal intensity (score 2). d Expanded vessel including obvious tumor signal intensity (score 3). e Expanded vessel with irregular or nodular contour containing tumor signal intensity (score 4).
Adapted from Smith NJ, et al. Br J Surg 2008 [22]
Beyond the 5-point grading, additional features of MR detected EMVI have been investigated, including the significance of the number and diameter of involved vessels, the location (e.g., originating from the upper, middle, or lower rectum), and the causes of vascular involvement (i.e., EMVI arising from the primary tumor as opposed to involved lymph nodes or tumor deposits defined as satellite peritumoral nodules of carcinoma in the mesorectal fat remote from the primary tumor, with no sign of residual lymph nodes or identifiable vessels or neural structures [33]). EMVI arising from the upper rectum and larger vessels is associated with a greater risk of poor prognosis or distant metastasis [24, 34]. EMVI arising from lymph nodes or tumor deposits has been shown to carry a prognosis similar to EMVI arising from the primary rectal tumor [34].
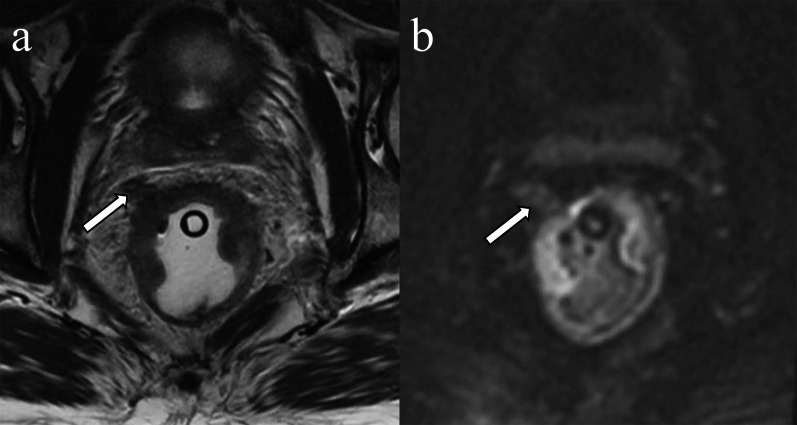
Diffusion-weighted images (DWI) often depict the intravenous tumoral component as intermediate or high tumor-signal intensity within normal or slightly expanded extramural vessels adjacent to the primary tumor (Fig. 5). Despite the potential pitfalls of DWI such as susceptibility artifact, limited spatial resolution, T2 shine through, and fibrosis, DWI can be helpful in identifying EMVI at both initial staging and restaging after neoadjuvant therapy [35, 36].
Fig. 5.
Extramural invasion on diffusion weighted imaging. A 41-year-old man with rectal adenocarcinoma. T2-weighted image demonstrates a distended perirectal vessel with a lack of flow void and central tumor signal intensity which is contiguous with the primary rectal tumor (a arrow). Diffusion-weighted image shows a high signal intensity cord-like structure contiguous with the primary rectal tumor (b arrow)
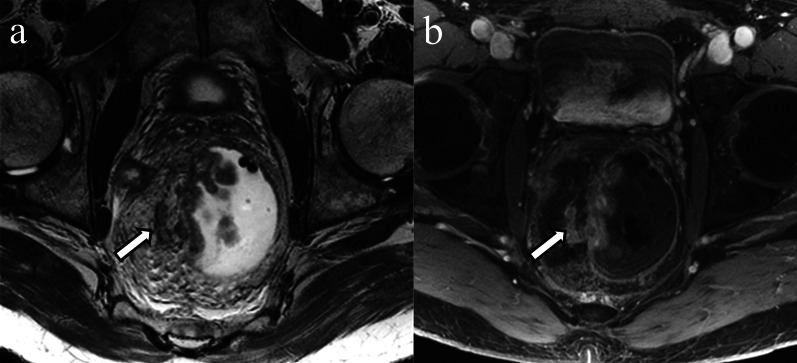
On contrast-enhanced T1 weighted images (CE-T1WI), EMVI can be identified as either enhancing tumor within the vessel or as a non- or hypoenhancing intraluminal filling defect [26] within an expanded vessel (≥ 3 mm), that is contiguous with the primary tumor (Table 1) [30]. The delayed phase of contrast enhancement can be helpful as intravenous mixing of contrast during early phases of enhancement in a normal vein may mimic EMVI [41]. CE-T1WI has not been shown to increase accuracy for rectal cancer staging/restaging, and its routine use remains controversial [37–39] as concluded by expert panels from Europe [40] and North America [41]. While 65% of North American panelists reported that they use gadolinium-based contrast media in MRI examinations for rectal cancer, only 29% of European panelists reported doing so [42]. However, CE-T1WI may be a helpful adjunct when the absence/presence of EMVI is equivocal by T2WI [26] (Fig. 6).
Table 1.
Imaging findings of extramural venous invasion
| Pulse sequences or modality | Findings contiguous with primary tumor |
|---|---|
| T2WI | Intermediate signal intensity with slightly expanded vessels (score 3) or nodular, bead- or worm-shaped structure irregular margin (score 4) |
| DWI | Intermediate to high signal intensity in the expanded vessels at the site consistent with findings on T2WI |
| CE-T1WI |
Filling defect in the vessel, +/- vessel dilation Tumor signal intensity within the expanded vessel (s) |
| CT | Heterogeneously enhancing, serpentine, cord-like expanded structure with irregular margin |
These findings contiguous with the primary lesion invading beyond the muscularis propria
CE-T1WI, contrast enhanced T1-weighted image; DWI, diffusion-weighted image; T2WI, T2-weighted image
Fig. 6.
Extramural invasion on contrast-enhanced T1-weighted image. A 56-year-old male with rectal adenocarcinoma. The dilated vessel which is in continuity with the tumor has an irregular margin and contains intermediate signal intensity rather than flow void on the T2-weighted image (a arrow). The tumor in the vessel enhances on the contrast-enhanced T1-weighted image (b arrow)
Performance of rectal cancer MRI to detect EMVI
Brown et al. showed that rectal cancer MRI revealed 83% of histopatologically-detected EMVI with a diameter greater than 3 mm [8]. A recent meta-analysis by Kim et al. showed that MRI had a pooled sensitivity of 61% and specificity of 87% for detecting EMVI in colorectal cancer using histopathology as the reference standard [43]. Table 2 shows sensitivity, specificity, and inter-observer agreement of MR for detection of EMVI in rectal cancer using T2WI alone or T2 combined with DWI or CE-T1WI before and after neoadjuvant therapy [8, 22–32, 44]. Inter-observer agreement for assessing EMVI employing the 5-point scoring system is variable (κ = 0.372–0.828), even when T2WI is used alone [26–32].
Table 2.
Estimated Performance of MRI for extramural venous invasion
| Authors | Year | No. of patients | No. of readers | Pulse sequences | Sensitivity | Specificity | Inter-observer agreement |
|---|---|---|---|---|---|---|---|
| Before neoadjuvant therapy | |||||||
| Smith [22] | 2008 | 142 | 1 | T2WI | 0.62 | 0.88 | NA |
| Koh [23] | 2008 | 79 | NA | T2WI | 1.00 | 0.89 | NA |
| Sohn [24] | 2015 | 447 | NA | T2WI | 0.282 | 0.94 | NA |
| Jhaveri [26] | 2016 | 49 | 2 | T2WI, CE-T1WI |
T2WI alone: 0.43–0.50 T2WI + CE-T1WI: 0.57 |
T2WI alone: 0.96–1.00 T2WI + CE-T1WI: 0.96 |
T2WI alone: 0.828 T2WI + CE-T1WI: 0.858 |
| Liu [27] | 2016 | 183 | 2 | T2WI | 0.617 | 0.820 | 0.780 |
| Yu [28] | 2016 | 86 | 2 | T2WI | 0.583 | 0.920 | 0.803 |
| Lee [29] | 2018 | 200 | 2 | T2WI | 0.9048 | 0.4114 | 0.801 |
| Liu [30] | 2016 | 59 | 2 | T2WI, CE-T1WI |
T2WI alone: 0.500–0.722 CE-T1WI alone: 0.556 T2WI + CE-T1WI:0.778–0.833 |
T2WI alone: 0.732–0.780 CE-T1WI alone: 0.659–0.683 T2WI + CE-T1WI: 0.732–0.756 |
T2WI alone: 0.603 CE-T1WI alone: 0.216 T2WI + CE-T1WI: 0.413 |
| Ahn [32] | 2019 | 79 | 2 | T2WI, DWI | T2WI alone: 0.80–0.85 | T2WI alone: 0.53–0.75 | T2WI alone: 0.704 |
| T2WI + DWI: 0.90 | T2WI + DWI: 0.54–0.66 | T2WI + DWI: 0.611 | |||||
| Bae [31] | 2019 | 147 | 3 | T2WI, CE-T1WI | T2WI + CE-T1WI: 0.720–0.840 | T2WI + CE-T1WI: 0.454–0.856 | T2WI + CE-T1WI: 0.376–0.693 |
| Fornell-Perez [45] | 2020 | 54 | 3 | T2WI, DWI |
T2WI alone: 0.571 T2WI + DWI: 0.476 |
T2WI alone: 0.759 T2WI + DWI: 0.872 |
T2WI alone: 0.483 T2WI + DWI: 0.438 |
| After neoadjuvant therapy | |||||||
| Jhaveri [26] | 2016 | 20 | 2 | T2WI, CE-T1WI | T2WI alone: 0.29 | T2WI alone: 0.95–1.00 | T2WI alone: 0.781 |
| T2WI + CE-T1WI: 0.43–0.57 | T2WI + CE-T1WI: 0.95 | T2WI + CE-T1WI: 0.604 | |||||
| Lee [29] | 2018 | 200 | 2 | T2WI | 0.7619 | 0.7975 | 0.736 |
| Fornell-Perez [45] | 2020 | 46 | 3 | T2WI, DWI |
T2WI alone: 0.667 T2WI + DWI: 0.778 |
T2WI alone: 0.829 T2WI + DWI: 0.915 |
T2WI alone: 0.372 T2WI + DWI: 0.361 |
| Bae [31] | 2019 | 75 | 3 | T2WI, CE-T1WI | T2WI + CE-T1WI: 0.667–0.792 | T2WI + CE-T1WI: 0.490–0.922 | T2WI + CE-T1WI: 0.383–0.693 |
CE-T1WI, contrast enhanced T1-weighted image; DWI, diffusion-weighted image; T2WI, T2-weighted image
Data regarding the value of DWI in detecting EMVI are conflicting. Ahn et al. reported that the addition of DWI reduced inter-observer agreement with no additional diagnostic benefit [32]. Conversely, Fornell-Perez et al. reported that the addition of DWI improved diagnostic accuracy in detecting EMVI, especially in post chemoradiation therapy patients [45]. Interestingly, Coruh et al. found that the ADC (apparent diffusion coefficient) values of the primary tumor are significantly lower in EMVI positive tumors [46].
Jhaveri et al. showed that the sensitivity and specificity of rectal MRI for EMVI did not significantly change with the addition of contrast-enhancement (for initial or restaging studies), but that interobserver agreement remained good [26]. Similarly the meta-analysis by Kim et al. showed no significant improvement in performance with gadolinium contrast or DWI [43]. In contrast, Lui et al. showed that contrast enhancement may improve sensitivity but at the cost of reduced interobserver agreement [30].
EMVI in computed tomography
Compared with MRI, the role of CT in assessing EMVI is limited because of its lower contrast resolution. However, CT can be an alternative to assess EMVI in patients who have a contraindication to MRI or who are unable to undergo MRI due to claustrophobia. On CT, EMVI is often seen as a heterogeneously enhancing, serpentine cord-like structure connecting veins with the irregular, contiguous margins of the primary tumor (Fig. 7). Ortega et al. investigated the diagnostic accuracy of CT-detected EMVI in rectal cancer using venous distension and intravascular tumor enhancement as the imaging criteria and using MRI-detected EMVI as the reference standard. They reported a high specificity of 100%, but low sensitivity of 14% and NPV of 47% [47]. Routine mention of CT-detected EMVI in clinical reports is not required; however, the presence of CT-detected EMVI can aid in therapeutic decision making in patients who have a contraindication to MRI, so reporting EMVI may be helpful when detected. Further investigation is warranted to determine the clinical significance of CT-detected EMVI.
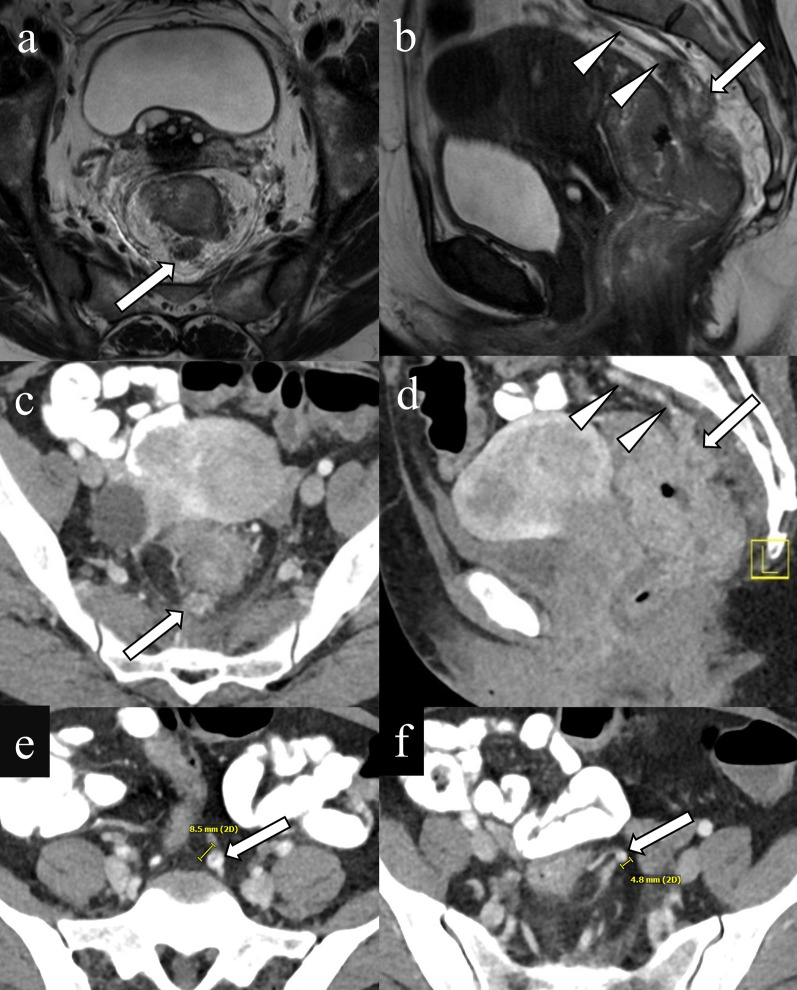
Fig. 7.
Extramural venous invasion on CT. A 47-year-old female with rectal adenocarcinoma. A nodule with an irregular margin containing intermediate signal intensity is observed in the mesorectum on axial T2WI (a arrow). Sagittal T2WI reveals a cord-like structure with tumor signal intensity in the superior rectal vein (b arrow) which drains to the inferior mesenteric veins (b arrowheads) Similarly, contrast-enhanced CT demonstrates an enhancing irregular nodule within the posterior mesorectum on the axial image (c arrow). On the sagittal image a cord-like nodular mass is contiguous with the dilated superior rectal vein (d arrow) and inferior mesenteric veins (d arrow heads) on the sagittal image. The diameters of the inferior mesenteric and superior rectal veins are 8.5 mm (e arrow) and 4.8 mm (f arrow), respectively, which is suggestive of the presence of EMVI
Dilation of the superior rectal and inferior mesenteric veins may help to predict EMVI (Fig. 7). Wu et al. reported that using a cut off value of 3.7 mm for the diameter of the superior hemorrhoidal vein, lymphovascular invasion by rectal cancer could be predicted on CT [48]. Similarly, in a study by Coruh et al., the diameter of the superior rectal and the inferior mesenteric veins were significantly larger in patients with EMVI on CT. Cutoff values of 3.95 mm for the superior rectal vein and 5.95 mm for the inferior mesenteric vein predicted EMVI with 93.3% and 93.3% sensitivity and 67.9% and 71.4% specificity, respectively [46]. It was hypothesized that the presence of an intravenous tumor may result in increased blood flow in the major drainage pathways of the rectum such as the superior rectal and inferior mesenteric veins, and that dilation may be an indirect indicator of EMVI. Likewise, increased venous drainage caused by tumor neoangiogenesis may result in vein dilation.
Clinical significance of MRI-detected EMVI
Multiple studies have demonstrated the clinical significance of EMVI detected on MRI as a predictor of poor prognosis or biologic aggressiveness in rectal cancer. MRI-detected EMVI is generally present in patients with T3 and nodal disease and is consequently, at least in North America, used to determine which patients will benefit from neoadjuvant therapy. Conversely, the overuse of neoadjuvant therapy in rectal cancer patients does not improve survival and can result in bowel and sexual dysfunction [49], and a recent European clinical trial has advocated that rectal cancer MRI demonstrating absence of EMVI may facilitate patient selection for primary surgery [50].
Predicting survival
MRI-detected EMVI is reported to be an independent significant prognostic factor for overall disease-free survival and systemic recurrence in rectal cancer [51]. In locally advanced rectal cancer, MRI-detected EMVI predicted decreased disease-free survival (hazard ratio: 2.46) [52]. In another study, MRI-detected EMVI before neoadjuvant therapy was an independent poor prognostic factor for progression-free survival (hazard ratio: 1.85) [53], disease-free survival (hazard ratio: 1.35–31.33) [29, 54–58] and overall survival (hazard ratio: 1.18–2.90) (Fig. 8) [29, 59]. MRI-detected EMVI after neoadjuvant chemotherapy has also been shown to be a predictor of decreased disease-free survival (hazard ratio: 1.97–2.68) [25, 29], recurrence-free survival (hazard ratio: 2.74) [60] and overall survival (hazard ratio: 1.98–4.23) [29, 59, 60] (Table 3).
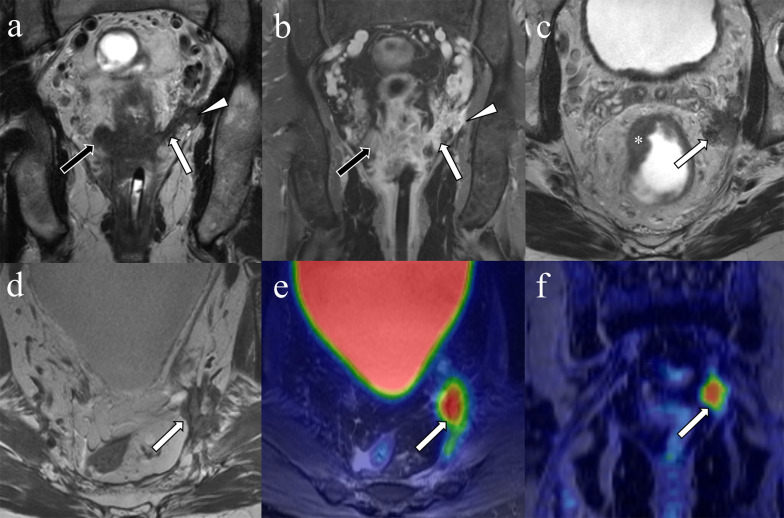
Fig. 8.
Local recurrence after surgery from residual disease associated with positive circumferential margin due to extramural venous invasion. A 58-year-old man with rectal cancer. T2-weighted (a) and postcontrast (b) coronal image before treatment demonstrate nodular-shaped structure (black arrows) on the right extending to the mesorectal fat tissue, and worm-shaped (white arrows) structure on the left with tumor signal intensity arising from the primary lesion, indicating extramural venous invasion (EMVI) extending the mesorectal fascia (arrow heads). Axial T2-weighted image shows a primary tumor (c asterisk) and an irregular tumoral deposit in and abutting the mesorectal facia near the left pelvic sidewall (c arrow). Axial and coronal 18F-FDG-PET/MRI images 23 months after surgery following neoadjuvant therapy show FDG avidity corresponding to a developed nodular recurrence at the same location (d–f arrows)
Table 3.
Relationship between MRI-detected extramural venous invasion and patient outcome
| Author | Year | No. of patients | Neoadjuvant therapy | Endpoint | Hazard ratio (95% CI) |
|---|---|---|---|---|---|
| EMVI before neoadjuvant therapy | |||||
| Chand [54] | 2014 | 478 | CRT (69.2%: 331/478) | DSF (3 year) | 2.08 (1.10–3.07) |
| Sclafani [53] | 2014 | 269 | CRT | PFS (5 year) | 1.85 (1.12–3.05) |
| Jalil [59] | 2016 | 56 | CRT | OS | 2.90 (1.00–8.37) |
| Patel [55] | 2017 | 46 | Chemotherapy | DSF (3 year) | 31.33 (2.31–425.4) |
| Lee [29] | 2018 | 200 | CRT | DSF (3 year) | 1.35 (0.64–2.82) |
| OS | 1.18 (0.51–2.77) | ||||
| Jia [52] | 2018 | 185 | No | DSF (3 year) | 2.46 (1.28–4.74) |
| Meng [56] | 2019 | 115 | CRT | DSF (3 year) | 2.50 (1.24–5.01) |
| Gu [57] | 2019 | 146 | short course CRT | DSF (3 year) | 3.56 (2.03–13.32) |
| Meng [58] | 2019 | 171 | CRT | DSF (3 year) | 2.59 (1.40–4.80) |
| EMVI after neoadjuvant therapy | |||||
| Chand [25] | 2015 | 188 | CRT | DSF (3 year) | 1.97 (1.01–3.90) |
| Jalil [59] | 2016 | 56 | CRT | OS | 4.23 (1.41–12.69) |
| Lee [29] | 2018 | 200 | CRT | DSF (3 year) | 2.68 (1.37–5.27) |
| OS | 1.98 (0.88–4.42) | ||||
| Shiraishi [60] | 2019 | 102 | Chemotherapy | RFS (5 year) | 2.74 (1.36–5.50) |
| OS | 3.15 (0.91–10.89) | ||||
CRT, chemoradiation therapy; DSF, disease-free survival; OS, overall survival; PFS, progression-free survival; RFS, recurrence-free survival
Rectal cancer MRI can also demonstrate in situ evidence of response or progression of EMVI after neoadjuvant therapy. Also, a change in MRI-detected EMVI status from positive to negative has been shown to predict histopathologic response [61]. Additionally, patients who have significant response of MRI-detected EMVI after neoadjuvant therapy, defined as more than 50% of the intravascular tumor content converted to low signal intensity fibrosis (i.e., signal intensity as low as the muscularis propria), have improved disease-free survival [62]. Regarding surgery, EMVI at initial staging MRI was a risk factor of failure to convert from positive to negative circumferential resection margin by neoadjuvant chemoradiotherapy [63]. Even when considering patients with positive resection margins, patients with EMVI have decreased survival compared to those without EMVI [64].
Predicting lymph node and distant metastases
In one study, EMVI score correlated with histologic lymph node stage [28]. In other studies, MRI-detected EMVI was found to be present in about a quarter of patients with rectal cancer, and had a specificity of about 81% for predicting N2 [23] and 88% for regional lymph node metastases [23, 27]. MRI detected-EMVI is also associated with a significantly higher risk for both synchronous [24, 65] and metachronous metastasis [66]. Approximately 25% of rectal cancer patients with EMVI on MRI developed subsequent liver and lung metastases at 1-year, compared to about 7% of patients without EMVI (Relative risk: 3.70) [66]. These findings are in keeping with the concept that EMVI may be the first step in hematogenous metastasis [17]. Table 4 summarizes the results of the studies correlating MRI-detected EMVI with lymph node and distant metastases.
Table 4.
Risk of lymph node and distant metastasis with MRI-detected extramural venous invasion
| Authors | Year | No. of patients | Treatment | MRI timing | Endopoint | Summary of results |
|---|---|---|---|---|---|---|
| Koh [23] | 2008 | 79 | No | Pre surgery | Nodal disease | Sv: 0.56 (0.79–0.96) Sp: 0.81 (0.69–0.90) |
| Liu [27] | 2016 | 183 | No | Pre surgery | Nodal disease | OR: 4.22 (1.79–9.95) |
| Bugg [66] | 2014 | 202 | No data | Pre surgery | Metachronous metastasis | RR: 3.70 (95%CI: NA) |
| Sohn [24] | 2015 | 447 | nCRT (9.2%: 41/447) | Pre nCRT | Synchronous metastasis | OR:3.02 (1.65–5.51) |
nCRT, neoadjuvant chemoradiation therapy; R, odds ratio; RR, relative risk; Sp, specificity; Sv, sensitivity
Extramural venous invasion of other rectal tumors
Rectal neoplasms other than usual adenocarcinomas such as squamous cell carcinoma, mucinous adenocarcinoma, and neuroendocrine tumor also can invade perirectal vessels (Figs. 9, 10, 11).
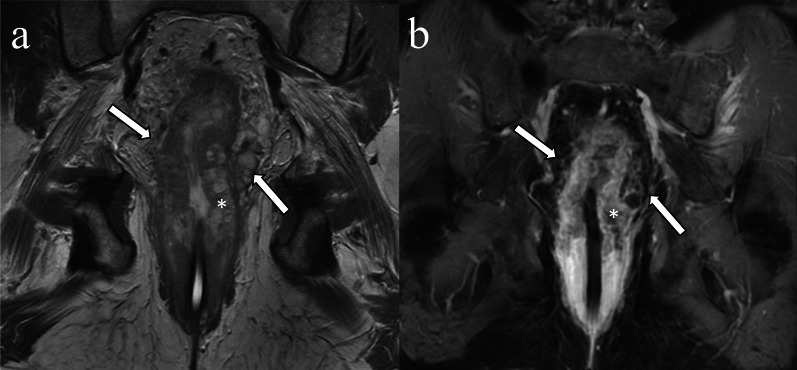
Fig. 9.
Extramural venous invasion in mucinous adenocarcinoma. A 53-year-old man with rectal mucinous adenocarcinoma. The circumferential rectal tumor shows high signal intensity on coronal T2WI (a asterisk). Peripheral heterogeneous enhancement is observed on contrast enhanced T1WI (b asterisk). The intravenous component demonstrates signal intensity and enhancement similar to the primary lesion (a, b arrows)
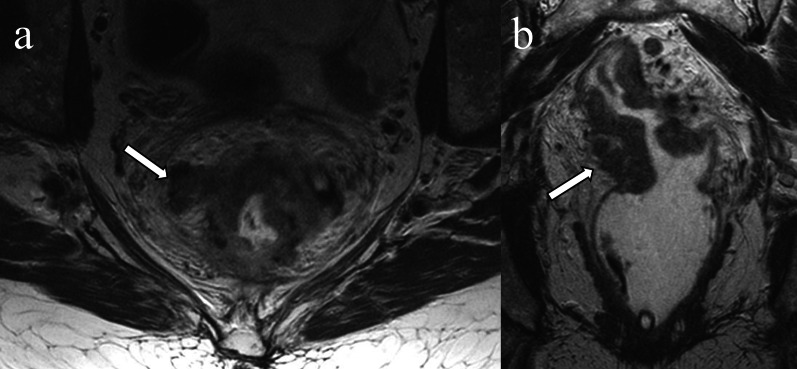
Fig. 10.
Extramural invasion in squamous cell carcinoma. A 58-year-old male with rectal squamous cell carcinoma. A nodular, elongated structure extends cranially from the right side of the circumferential rectal tumor (a, b arrow)
Fig. 11.
Extramural venous invasion in neuroendocrine tumor. A 50-year-old man with poorly differentiated neuroendocrine tumor, large-cell type. On the T2WI a tubular structure with irregular margins and signal intensity similar to the tumor (a arrow) extends from the rectal mass (a asterisk). The primary lesion (b asterisk) and extramural venous invasion (b arrow) are more conspicuous on DWI. Mesorectal lymph nodes which were histopathologically proven to be nodal metastases are depicted on T2WI and DWI (a, b arrowheads)
Mucinous rectal carcinoma is a distinct pathologic subtype of rectal cancer defined as a tumor composed of greater than 50% extracellular mucin and neoplastic epithelium surrounded by extracellular mucin lakes on histopathology [13]. The primary tumor is markedly hyperintense on T2WI owing to extracellular mucin [67]. Following administration of IV contrast material, the mucinous tumor has peripheral and heterogeneous enhancement. Mucinous rectal adenocarcinomas commonly have lower signal on higher b value DWI and a higher mean apparent diffusion coefficient compared to non-mucinous rectal cancers [68]. When EMVI is present, the intravenous tumor component shows the same signal characteristics as the primary tumor (Fig. 9). MRI is considered superior to biopsy in identifying mucinous malignancy secondary to sampling errors with biopsy [69]. Metachronous metastases are seen more often in mucinous carcinoma than non-mucinous carcinoma regardless of whether EMVI is present or absent [70].
Rectal squamous cell carcinoma is a rare tumor, which accounts for less than 1% of colorectal malignancies [71]. Risk factors include chronic infection, smoking, human immunodeficiency virus, and human papillomavirus [72]. Owing to its low prevalence, the imaging characteristics of squamous cell carcinoma are not well-documented, and the frequency and clinical significance of squamous cell carcinoma-associated EMVI are unknown (Fig. 10).
The rectum is a common site of neuroendocrine neoplasms. Rectal neuroendocrine tumor usually appears as a submucosal nodule or focal area of plaque-like wall-thickening; however, less commonly it can present as a large ulcerating, avidly enhancing, invasive mass [73]. The incidence of rectal neuroendocrine tumor has been increasing due to incidental detection, especially for small neoplasms. Large rectal neuroendocrine tumors can be difficult to differentiate from adenocarcinoma. EMVI of rectal neuroendocrine tumors in cross-sectional imaging has also not been well-described (Fig. 11). Neuroendocrine neoplasms smaller than 1.0 cm can be treated with resection; however, lymphatic and venous invasion are predictors of metastasis [74], and salvage surgery is recommended in patients with lymphovascular invasion [75].
Conclusion
MRI-detected EMVI correlates closely with histopathological EMVI and is a predictor of lymph node and distant metastases, tumor recurrence, and poor prognosis. Therefore, it is important to evaluate for the presence of EMVI on rectal cancer MRI examinations before and after neoadjuvant therapy to determine risk-stratification and therapeutic options. Despite the clinical significance of MRI-detected EMVI, inter-observer variability in assessing its presence or absence is problematic both at initial staging and after neoadjuvant treatment. Radiologists should therefore be familiar with the imaging features of EMVI and its implications for patient management. Findings of EMVI include expanded vessel caliber adjacent to the primary tumor, intermediate tumor signal intensity in the vessel, and irregular vessel margin. DWI and contrast enhanced T1 weighted images may be helpful adjuncts to T2WI and may help improve reader confidence in select cases. Further investigation is necessary to determine if multi-parametric MRI improves diagnostic performance without compromising interobserver agreement. In addition, further investigation is needed to assess the clinical importance of CT-detected EMVI and to determine whether detailed features of EMVI, including location, vessel diameter, and the number of involved vessels can improve risk-stratification.
Abbreviations
- CE-T1WI
Contrast enhanced T1-weighted image
- CT
Computed tomography
- DWI
Diffusion-weighted image
- EMVI
Extramural venous invasion
- MERCURY
Magnetic resonance imaging and rectal cancer European equivalence
- MRI
Magnetic resonance imaging
- T2WI
T2-weighted image
Authors' contributions
AI has contributed the conception, acquisition of radiology images, analysis and interpretation data and drafting the manuscript. JGF contributed conception of the study and acquisition of radiology images. SPS, JPH, and JLF contributed conception of the study. RPG contributed acquisition of pathology images. All authors read and approved the final manuscript.
Declarations
Ethics approval and consent to participate
Our Institutional Review Board approved that informed consent was waived as long as the patients did not decline that their data is used for any purpose except for patient care.
Consent for publication
Not applicable.
Competing interests
All authors have no conflict interest to be declared.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Inoue A, Ohta S, Nitta N, et al. Ex vivo MR imaging of colorectal carcinoma before and after formalin fixation: correlation with histopathologic findings. Abdom Radiol (NY) 2018;43:1524–1530. doi: 10.1007/s00261-018-1538-8. [DOI] [PubMed] [Google Scholar]
- 3.MERCURY Study Group Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology. 2007;243:132–139. doi: 10.1148/radiol.2431051825. [DOI] [PubMed] [Google Scholar]
- 4.Gollub MJ, Arya S, Beets-Tan RG, et al. Use of magnetic resonance imaging in rectal cancer patients: Society of Abdominal Radiology (SAR) rectal cancer disease-focused panel (DFP) recommendations 2017. Abdom Radiol (NY) 2018;43:2893–2902. doi: 10.1007/s00261-018-1642-9. [DOI] [PubMed] [Google Scholar]
- 5.Horvat N, Carlos Tavares Rocha C, Clemente Oliveira B, Petkovska I, Gollub MJ. MRI of rectal cancer: tumor staging, imaging techniques, and management. Radiographics. 2019;39:367–387. doi: 10.1148/rg.2019180114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talbot IC, Ritchie S, Leighton M, Hughes AO, Bussey HJ, Morson BC. Invasion of veins by carcinoma of rectum: method of detection, histological features and significance. Histopathology. 1981;5:141–163. doi: 10.1111/j.1365-2559.1981.tb01774.x. [DOI] [PubMed] [Google Scholar]
- 7.Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. The clinical significance of invasion of veins by rectal cancer. Br J Surg. 1980;67:439–442. doi: 10.1002/bjs.1800670619. [DOI] [PubMed] [Google Scholar]
- 8.Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003;90:355–364. doi: 10.1002/bjs.4034. [DOI] [PubMed] [Google Scholar]
- 9.Chand M, Swift RI, Chau I, Heald RJ, Tekkis PP, Brown G. Adjuvant therapy decisions based on magnetic resonance imaging of extramural venous invasion and other prognostic factors in colorectal cancer. Ann R Coll Surg Engl. 2014;96:543–546. doi: 10.1308/003588414X13814021678835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ale Ali H, Kirsch R, Razaz S, et al. Extramural venous invasion in rectal cancer: overview of imaging, histopathology, and clinical implications. Abdom Radiol (NY) 2019;44:1–10. doi: 10.1007/s00261-018-1673-2. [DOI] [PubMed] [Google Scholar]
- 11.Santiago I, Figueiredo N, Pares O, Matos C. MRI of rectal cancer-relevant anatomy and staging key points. Insights Imaging. 2020;11:100. doi: 10.1186/s13244-020-00890-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nuikolouzakis TK, Mariolis-Sapsakos T, Triantopoulou C, et al. Detailed and applied anatomy for improved rectal cancer treatment. Ann Gastroenterol. 2019;32:431–440. doi: 10.20524/aog.2019.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Compton CC. Pathologic prognostic factors in the recurrence of rectal cancer. Clin Colorectal Cancer. 2002;2:149–160. doi: 10.3816/CCC.2002.n.020. [DOI] [PubMed] [Google Scholar]
- 14.Howlett CJ, Tweedie EJ, Driman DK. Use of an elastic stain to show venous invasion in colorectal carcinoma: a simple technique for detection of an important prognostic factor. J Clin Pathol. 2009;62:1021–1025. doi: 10.1136/jcp.2009.065615. [DOI] [PubMed] [Google Scholar]
- 15.Sternberg A, Amar M, Alfici R, Groisman G. Conclusions from a study of venous invasion in stage IV colorectal adenocarcinoma. J Clin Pathol. 2002;55:17–21. doi: 10.1136/jcp.55.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Betge J, Pollheimer MJ, Lindtner RA, et al. Intramural and extramural vascular invasion in colorectal cancer: prognostic significance and quality of pathology reporting. Cancer. 2012;118:628–638. doi: 10.1002/cncr.26310. [DOI] [PubMed] [Google Scholar]
- 17.van Zijl F, Krupitza G, Mikulits W. Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res. 2011;728:23–34. doi: 10.1016/j.mrrev.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merkel S, Weber K, Schellerer V, et al. Prognostic subdivision of ypT3 rectal tumours according to extension beyond the muscularis propria. Br J Surg. 2014;101:566–572. doi: 10.1002/bjs.9419. [DOI] [PubMed] [Google Scholar]
- 19.Swets M, Kuppen PJK, Blok EJ, Gelderblom H, van de Velde CJH, Nagtegaal ID. Are pathological high-risk features in locally advanced rectal cancer a useful selection tool for adjuvant chemotherapy? Eur J Cancer. 2018;89:1–8. doi: 10.1016/j.ejca.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 20.MERCURY Study Group Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333:779. doi: 10.1136/bmj.38937.646400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith NJ, Shihab O, Arnaout A, Swift RI, Brown G. MRI for detection of extramural vascular invasion in rectal cancer. AJR Am J Roentgenol. 2008;191:1517–1522. doi: 10.2214/AJR.08.1298. [DOI] [PubMed] [Google Scholar]
- 22.Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95:229–236. doi: 10.1002/bjs.5917. [DOI] [PubMed] [Google Scholar]
- 23.Koh D, Smith N, Swift R, Brown G. The Relationship Between MR Demonstration of Extramural Venous Invasion and Nodal Disease in Rectal Cancer. Clin Med Oncol. 2008;2:267–273. doi: 10.4137/cmo.s370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sohn B, Lim JS, Kim H, et al. MRI-detected extramural vascular invasion is an independent prognostic factor for synchronous metastasis in patients with rectal cancer. Eur Radiol. 2015;25:1347–1355. doi: 10.1007/s00330-014-3527-9. [DOI] [PubMed] [Google Scholar]
- 25.Chand M, Evans J, Swift RI, et al. The prognostic significance of postchemoradiotherapy high-resolution MRI and histopathology detected extramural venous invasion in rectal cancer. Ann Surg. 2015;261:473–479. doi: 10.1097/SLA.0000000000000848. [DOI] [PubMed] [Google Scholar]
- 26.Jhaveri KS, Hosseini-Nik H, Thipphavong S, et al. MRI detection of extramural venous invasion in rectal cancer: correlation with histopathology using elastin stain. AJR Am J Roentgenol. 2016;206:747–755. doi: 10.2214/AJR.15.15568. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Liu M, Yang Z, He W, Wang Z, Jin E. Correlation of MRI-detected extramural vascular invasion with regional lymph node metastasis in rectal cancer. Clin Imaging. 2016;40:456–460. doi: 10.1016/j.clinimag.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Yu J, Huang DY, Xu HX, Li Y, Xu Q. Correlation between magnetic resonance imaging-based evaluation of extramural vascular invasion and prognostic parameters of T3 stage rectal cancer. J Comput Assist Tomogr. 2016;40:537–542. doi: 10.1097/RCT.0000000000000397. [DOI] [PubMed] [Google Scholar]
- 29.Lee ES, Kim MJ, Park SC, et al. Magnetic resonance imaging-detected extramural venous invasion in rectal cancer before and after preoperative chemoradiotherapy: diagnostic performance and prognostic significance. Eur Radiol. 2018;28:496–505. doi: 10.1007/s00330-017-4978-6. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Yang L, Jin E, Wang Z, Yang Z. Effect of gadolinium contrast-enhanced T1-weighted magnetic resonance imaging for detecting extramural venous invasion in rectal cancer. Abdom Radiol (NY) 2016;41:1736–1743. doi: 10.1007/s00261-016-0740-9. [DOI] [PubMed] [Google Scholar]
- 31.Bae JS, Kim SH, Hur BY, et al. Prognostic value of MRI in assessing extramural venous invasion in rectal cancer: multi-readers' diagnostic performance. Eur Radiol. 2019;29:4379–4388. doi: 10.1007/s00330-018-5926-9. [DOI] [PubMed] [Google Scholar]
- 32.Ahn JH, Kim SH, Son JH, Jo SJ. Added value of diffusion-weighted imaging for evaluation of extramural venous invasion in patients with primary rectal cancer. Br J Radiol. 2019;92:20180821. doi: 10.1259/bjr.20180821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng K, Zheng N, Xin C, et al. The prognostic significance of tumor deposit count for colorectal cancer patients after radical surgery. Gastroenterol Res Pract. 2020;2020:2052561. doi: 10.1155/2020/2052561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang XY, Wang S, Li XT, et al. MRI of extramural venous invasion in locally advanced rectal cancer: relationship to tumor recurrence and overall survival. Radiology. 2018;289:677–685. doi: 10.1148/radiol.2018172889. [DOI] [PubMed] [Google Scholar]
- 35.Lambregts DMJ, van Heeswijk MM, Delli Pizzi A, et al. Diffusion-weighted MRI to assess response to chemoradiotherapy in rectal cancer: main interpretation pitfalls and their use for teaching. Eur Radiol. 2017;27:4445–4454. doi: 10.1007/s00330-017-4830-z. [DOI] [PubMed] [Google Scholar]
- 36.Napoletano M, Mazzucca D, Prosperi E, et al. Locally advanced rectal cancer: qualitative and quantitative evaluation of diffusion-weighted magnetic resonance imaging in restaging after neoadjuvant chemo-radiotherapy. Abdom Radiol (NY) 2019;44:3664–3673. doi: 10.1007/s00261-019-02012-4. [DOI] [PubMed] [Google Scholar]
- 37.Vliegen RFA, Beets GL, von Meyenfeldt MF, Kessels AG, Lemaire EE, van Engelshoven JM, Beets-Tan RG. Rectal cancer: MR imaging in local staging–is gadolinium-based contrast material helpful? Radiology. 2005;234:179–188. doi: 10.1148/radiol.2341031403. [DOI] [PubMed] [Google Scholar]
- 38.Gollub MJ, Lakhman Y, McGinty K, et al. Does gadolinium-based contrast material improve diagnostic accuracy of local invasion in rectal cancer MRI? A multireader study. AJR Am J Roentgenol. 2015;204:W160–167. doi: 10.2214/AJR.14.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inoue A, Ohta S, Nitta N, et al. MRI can be used to assess advanced T-stage colon carcinoma as well as rectal carcinoma. Jpn J Radiol. 2016;34:809–819. doi: 10.1007/s11604-016-0591-x. [DOI] [PubMed] [Google Scholar]
- 40.Beets-Tan RGH, Lambregts DMJ, Maas M, et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28:1465–1475. doi: 10.1007/s00330-017-5026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Expert Panel on Gastrointestinal Imaging ACR appropriateness criteria((R)) pretreatment staging of colorectal cancer. J Am Coll Radiol. 2017;14:S234–S244. doi: 10.1016/j.jacr.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Krdzalic J, Maas M, Gollub MJ, Beets-Tan RGH. Guidelines for MR imaging in rectal cancer: Europe versus United States. Abdom Radiol (NY) 2019;44:3498–3507. doi: 10.1007/s00261-019-02251-5. [DOI] [PubMed] [Google Scholar]
- 43.Kim TH, Woo S, Han S, Suh CH, Vargas HA. The diagnostic performance of mri for detection of extramural venous invasion in colorectal cancer: a systematic review and meta-analysis of the literature. AJR Am J Roentgenol. 2019;213:575–585. doi: 10.2214/AJR.19.21112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poulsen LO, Yilmaz MK, Oddershede L, et al. Is the accuracy of preoperative MRI stage in rectal adenocarcinoma influenced by tumour height? Acta Oncol. 2018;57:728–734. doi: 10.1080/0284186X.2018.1433319. [DOI] [PubMed] [Google Scholar]
- 45.Fornell-Perez R, Vivas-Escalona V, Aranda-Sanchez J, et al. Primary and post-chemoradiotherapy MRI detection of extramural venous invasion in rectal cancer: the role of diffusion-weighted imaging. Radiol Med. 2020;125:522–530. doi: 10.1007/s11547-020-01137-7. [DOI] [PubMed] [Google Scholar]
- 46.Coruh AG, Peker E, Elhan A, Erden I, Erden A. Evaluation of extramural venous invasion by diffusion-weighted magnetic resonance imaging and computed tomography in rectal adenocarcinoma. Can Assoc Radiol J. 2019;70:457–465. doi: 10.1016/j.carj.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Ortega CD, Rocha MS. CT Staging To Triage Selection Of Patients With Poor-Prognosis Rectal Cancer For Neoadjuvant Treatment. AJR Am J Roentgenol. 2019;213:358–364. doi: 10.2214/AJR.18.20929. [DOI] [PubMed] [Google Scholar]
- 48.Wu CC, Lee RC, Chang CY. Prediction of lymphovascular invasion in rectal cancer by preoperative CT. AJR Am J Roentgenol. 2013;201:985–992. doi: 10.2214/AJR.12.9657. [DOI] [PubMed] [Google Scholar]
- 49.Marijnen CA, van de Velde CJ, Putter H, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2005;23:1847–1858. doi: 10.1200/JCO.2005.05.256. [DOI] [PubMed] [Google Scholar]
- 50.Kennedy ED, Simunovic M, Jhaveri K, et al. Safety and feasibility of using magnetic resonance imaging criteria to identify patients with "good prognosis" rectal cancer eligible for primary surgery: the phase 2 nonrandomized QuickSilver clinical trial. JAMA Oncol. 2019;5:961–966. doi: 10.1001/jamaoncol.2019.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho MS, Park YY, Yoon J, et al. MRI-based EMVI positivity predicts systemic recurrence in rectal cancer patients with a good tumor response to chemoradiotherapy followed by surgery. J Surg Oncol. 2018;117:1823–1832. doi: 10.1002/jso.25064. [DOI] [PubMed] [Google Scholar]
- 52.Jia XX, Wang Y, Cheng J, et al. Low- versus high-risk rectal cancer based on mri features: outcomes in patients treated without neoadjuvant chemoradiotherapy. AJR Am J Roentgenol. 2018;211:327–334. doi: 10.2214/AJR.17.18980. [DOI] [PubMed] [Google Scholar]
- 53.Sclafani F, Brown G, Cunningham D, et al. PAN-EX: a pooled analysis of two trials of neoadjuvant chemotherapy followed by chemoradiotherapy in MRI-defined, locally advanced rectal cancer. Ann Oncol. 2016;27:1557–1565. doi: 10.1093/annonc/mdw215. [DOI] [PubMed] [Google Scholar]
- 54.Chand M, Bhangu A, Wotherspoon A, et al. EMVI-positive stage II rectal cancer has similar clinical outcomes as stage III disease following pre-operative chemoradiotherapy. Ann Oncol. 2014;25:858–863. doi: 10.1093/annonc/mdu029. [DOI] [PubMed] [Google Scholar]
- 55.Patel UB, Brown G, Machado I, et al. MRI assessment and outcomes in patients receiving neoadjuvant chemotherapy only for primary rectal cancer: long-term results from the GEMCAD 0801 trial. Ann Oncol. 2017;28:344–353. doi: 10.1093/annonc/mdw616. [DOI] [PubMed] [Google Scholar]
- 56.Meng Y, Wan L, Ye F, et al. MRI morphologic and clinicopathologic characteristics for predicting outcomes in patients with locally advanced rectal cancer. Abdom Radiol (NY) 2019;44:3652–3663. doi: 10.1007/s00261-018-1828-1. [DOI] [PubMed] [Google Scholar]
- 57.Gu C, Yang X, Zhang X, et al. The prognostic significance of MRI-detected extramural venous invasion, mesorectal extension, and lymph node status in clinical T3 mid-low rectal cancer. Sci Rep. 2019;9:12523. doi: 10.1038/s41598-019-47466-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meng Y, Wan L, Zhang C, et al. The predictive value of pre-/postneoadjuvant chemoradiotherapy MRI characteristics for patient outcomes in locally advanced rectal cancer. Acad Radiol. 2020;27:e233–e243. doi: 10.1016/j.acra.2019.10.021. [DOI] [PubMed] [Google Scholar]
- 59.Jalil O, Afaq A, Ganeshan B, et al. Magnetic resonance based texture parameters as potential imaging biomarkers for predicting long-term survival in locally advanced rectal cancer treated by chemoradiotherapy. Colorectal Dis. 2017;19:349–362. doi: 10.1111/codi.13496. [DOI] [PubMed] [Google Scholar]
- 60.Shiraishi T, Sasaki T, Ikeda K, Tsukada Y, Nishizawa Y, Ito M. Predicting prognosis according to preoperative chemotherapy response in patients with locally advanced lower rectal cancer. BMC Cancer. 2019;19:1222. doi: 10.1186/s12885-019-6424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu SKT, Tait D, Chau I, Brown G. MRI predictive factors for tumor response in rectal cancer following neoadjuvant chemoradiation therapy–implications for induction chemotherapy? Int J Radiat Oncol Biol Phys. 2013;87:505–511. doi: 10.1016/j.ijrobp.2013.06.2052. [DOI] [PubMed] [Google Scholar]
- 62.Chand M, Swift RI, Tekkis PP, Chau I, Brown G. Extramural venous invasion is a potential imaging predictive biomarker of neoadjuvant treatment in rectal cancer. Br J Cancer. 2014;110:19–25. doi: 10.1038/bjc.2013.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim CH, Yeom S-S, Kwak H-D, et al. Clinical outcomes of patients with locally advanced rectal cancer with persistent circumferential resection margin invasion after preoperative chemoradiotherapy. Ann Coloproctol. 2019;35:72–82. doi: 10.3393/ac.2019.04.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ormsby NM, Bermingham HN, Joshi HM, et al. The significance of extramural venous invasion in R1 positive rectal cancer. Int J Colorectal Dis. 2017;32:119–124. doi: 10.1007/s00384-016-2658-7. [DOI] [PubMed] [Google Scholar]
- 65.Hunter CJ, Garant A, Vuong T, et al. Adverse features on rectal mri identify a high-risk group that may benefit from more intensive preoperative staging and treatment. Ann Surg Oncol. 2011;19:1199–1205. doi: 10.1245/s10434-011-2036-1. [DOI] [PubMed] [Google Scholar]
- 66.Bugg WG, Andreou AK, Biswas D, Toms AP, Williams SM. The prognostic significance of MRI-detected extramural venous invasion in rectal carcinoma. Clin Radiol. 2014;69:619–623. doi: 10.1016/j.crad.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 67.Kim MJ, Park JS, Park SI, et al. Accuracy in differentiation of mucinous and nonmucinous rectal carcinoma on MR imaging. J Comput Assist Tomogr. 2003;1:48–55. doi: 10.1097/00004728-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 68.Wnorowski AM, Menias CO, Pickhardt PJ, Kim DH, Hara AK, Lubner MG. Mucin-containing rectal carcinomas: overview of unique clinical and imaging features. AJR Am J Roentgenol. 2019 doi: 10.2214/AJR.18.20864:1-9. [DOI] [PubMed] [Google Scholar]
- 69.Yu SKT, Chand M, Tait DM, Brown G. Magnetic resonance imaging defined mucinous rectal carcinoma is an independent imaging biomarker for poor prognosis and poor response to preoperative chemoradiotherapy. Eur J Cancer. 2014;50:920–927. doi: 10.1016/j.ejca.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 70.Barbaro B, Leccisotti L, Vecchio FM, et al. The potential predictive value of MRI and PET-CT in mucinous and nonmucinous rectal cancer to identify patients at high risk of metastatic disease. Br J Radiol. 2016;90:20150836. doi: 10.1259/bjr.20150836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ozuner G, Aytac E, Gorgun E, Bennett A. Colorectal squamous cell carcinoma: a rare tumor with poor prognosis. Int J Colorectal Dis. 2015;30:127–130. doi: 10.1007/s00384-014-2058-9. [DOI] [PubMed] [Google Scholar]
- 72.Nahas CS, Shia J, Joseph R, et al. Squamous-cell carcinoma of the rectum: a rare but curable tumor. Dis Colon Rectum. 2007;50:1393–1400. doi: 10.1007/s10350-007-0256-z. [DOI] [PubMed] [Google Scholar]
- 73.Baxi AJCK, Katkar A, Restrepo CS, Betancourt SL, Sunnapwar A. Multimodality imaging findings in carcinoid tumors: a head-to-toe spectrum. Radiographics. 2017;37:516–536. doi: 10.1148/rg.2017160113. [DOI] [PubMed] [Google Scholar]
- 74.Sugimoto S, Hotta K, Shimoda T, et al. The Ki-67 labeling index and lymphatic/venous permeation predict the metastatic potential of rectal neuroendocrine tumors. Surg Endosc. 2016;30:4239–4248. doi: 10.1007/s00464-015-4735-3. [DOI] [PubMed] [Google Scholar]
- 75.Malla S, Kumar P, Madhusudhan KS. Radiology of the neuroendocrine neoplasms of the gastrointestinal tract: a comprehensive review. Abdom Radiol (NY) 2020 doi: 10.1007/s00261-020-02773-3. [DOI] [PubMed] [Google Scholar]