Dear Editor,
Autism spectrum disorders (ASDs) are neurodevelopmental disorders with phenotypic and genetic heterogeneity, and are among the most heritable of neurodevelopmental disorders [1]. Rare single nucleotide variants (SNVs) of genes and/or rare copy number variants (CNVs) involving gene variants and/or genomic imbalances play an important role in ASD, but their molecular pathogenic mechanisms remain indistinct [2]. Over the decades, genetic and neurobiological studies mainly involving severe ASD comorbid with intellectual disability (ID) or developmental delay (DD) have indicated that loss of function affects neural development [3].
GABAergic signaling and impaired GABAA receptor function may contribute to the pathogenesis of multiple neuropsychiatric disorders including ASD [4]. Phospholipase C-like 1 (PLCL1), also named phospholipase C (PLC)-related inactive protein type 1 (PRIP-1), plays a central role in controlling GABAA receptor phospho-dependent modulation, and therefore, the efficacy of synaptic inhibition mediated by these receptors [5]. Several studies have focused on the mouse PLCL1 loss-of-function models. The Plcl1−/− mice exhibit altered behaviors including frequent ambulation and impaired motor coordination [6], or show an epileptic phenotype [7]. While in double knockout of Plcl1 and Plcl2 (compensatory to Plcl1 function) mice, orofacial movements are affected; including reduced vertical jaw movements, tongue protrusions and movements of the head and vibrissae [8]. In humans, a pediatric specific Phenome-Wide Association Study revealed that one single nucleotide polymorphism (rs1595825) in PLCL1 intron 1 is associated with developmental delays of speech and language [9]. Despite the relevant studies on animal models and human, the role of PLCL1 in neural development, particularly, whether ASD patients have rare PLCL1 variants, and whether these variants have harmful effects that may be associated with the development of ASD remains unknown.
To identify rare variants of the PLCL1 gene in ASD, we first screened 437 American patients with ASD using array comparative genomic hybridization (array CGH), and identified 35 pathogenic CNVs (supplementary materials and methods). Among them, a 542,605 bp microdeletion (542 kb deletion) located in chromosome 2q33.1 was identified in one of the 437 patients (Table S1), which covers the exons 2–6 of the PLCL1 gene, LOC101927619 (long intragenic non-protein coding RNA 1923), exons 3 and 4 of LOC105373831, and the whole length of LOC105373830 (two uncharacterized non-coding RNA genes). Neither of the patient’s parents carried the same variant, and no similar loss was found in 8,045 non-neurological/psychiatric patients [the database of Genetic Diagnostics Lab of Boston Children’s Hospital (BCH-GDL database)]. Therefore, we conclude that the 542 kb deletion found in ASD is rare and de novo.
We further searched several publicly available databases of CNVs or SNVs detected/submitted by clinical genetic diagnosis laboratories. In the DECIPHER database (website: decipher.Sanger.ac.uk), we found approximately 45 genomic deletions and duplications that included an overlapped region similar to the 542 kb deletion. Of them, 6 relevant smaller microdeletions were selected to compare with the 542 kb deletion (Table S1). Notably, all 6 deletions (range 2.54–6.94 Mb) were associated with DD, but were not seen in normal controls. Furthermore, the PLCL1 deletion was the only gene shared within all 7 cases (Table S1). Most interestingly, their phenotypes were comorbid within all 7 cases: 6/7 had ID and 4/7 had delayed speech and language development, which were frequently observed in patients with ASD/ID/DD; while the 542 kb deletion only involved one coding gene, PLCL1. Therefore, these results strongly suggest that the 542 kb deletion/PLCL1 gene deletion is likely a pathogenic variant associated with ASD/DD/ID. We further performed functional studies of PLCL1 in mice.
Moreover, we screened 455 Han Chinese patients with ASD, and found four missense variants of PLCL1 in coding sequence (c.82 G>A, c.311 A>G, c.434 G>A, and c.2922 G>T) and two non-coding region variants in the upstream regulatory region of PLCL1 (c.−636 C>A and c.−136_−134 delGCC) by Sanger sequencing (Fig. 1A, Table S2). All the variants were heterozygous (Fig. S1) and existed in unrelated individual samples. Moreover, the variants were not present in 576 control DNA samples or in the dbSNP, Ensembl, and ExAC databases, thus we considered they may be rare variants that only present in ASD patients.
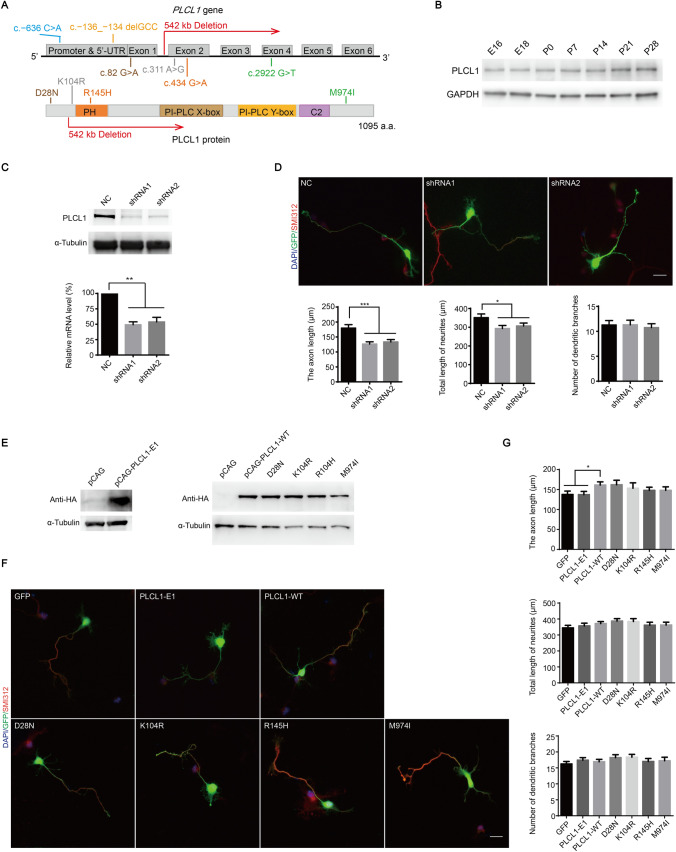
Fig. 1.
The dosage of PLCL1 is crucial for neurite outgrowth of primary cultured mouse cortical neurons, and the deletion of PLCL1 exons 2–6 affects the promotion of neuronal growth, while PLCL1 missense variants have no effect. A The location of PLCL1 rare variants in studied ASD patients on the PLCL1 gene and coding protein. Colored bars represent the PLCL1 protein functional domains. B The PLCL1 expression pattern in mouse cerebral cortex during the embryonic and postnatal period. C The efficiency of endogenous Plcl1 knockdown was verified using shRNA packaged in lentivirus by Western blot (top) or using pFUGW-H1-shRNA plasmids by qRT-PCR (bottom) in primary cultured mouse cortical neurons. D Plcl1 knockdown affects mouse neuronal growth. Upper panel shows the specific cellular morphology of neurons transfected with pFUGW-H1 vector (NC, as a control), Plcl1 shRNA1 and shRNA2. All neurons were co-labeled with DAPI (to identify nuclei), GFP (to identify overall neuronal morphology) and SMI 312 (an axonal marker). Scale bar, 20 μm. Bottom panel shows the statistical results of the axon length, total length of neurites, and the number of dendritic branches of mouse neurons with Plcl1 knockdown. Approximately 90 cells from three independent experiments were counted and the P-value was determined by one-way ANOVA. *P <0.05, **P <0.01, ***P <0.001. E Western blot analysis of ectopic expression of PLCL1-WT, PLCL1-E1, and four missense variants in HEK-293T cells. F The morphology of neurons overexpressing the CAG vector (GFP, as a control), PLCL1-WT, PLCL1-E1, and four missense variants. Scale bar, 20 μm. G The axon length (upper), total length of neurites (middle), and the number of dendritic branches (bottom) were measured and analyzed as described above. Error bars, ± SEM.
By genetic/genomic screening, it seemed that the PLCL1 deletion and variants were not uncommon in ASD/DD/ID, and potential detrimental effects (Tables S1, S2; Fig. S2) of the above PLCL1 variants may exist, so we subsequently investigated the role of PLCL1 in neural development, particularly whether these rare PLCL1 variants have loss- or gain-of-function effects.
First, we explored Plcl1 gene expression in the early stage of mouse cerebral cortex. We examined PLCL1 protein levels in embryonic (E) and postnatal (P) cortex of mouse brains and found that PLCL1 was strongly expressed from E16 to P28 (Fig. 1B). Furthermore, human PLCL1 protein has been reported to be distributed specifically in the brain and expressed abundantly in the cerebral cortex and hippocampus [10]. Taken together, the results suggest that PLCL1 may play an important role in brain development and normal neuronal function.
The formation of protrusions and the abnormal elongation of axons and dendrites may be the structural basis for the pathogenesis of ASD [11]. To elucidate the role of PLCL1 in the growth of neuronal axons and dendrites, we specifically knocked down Plcl1 in mouse primary cortical neurons with short hairpin RNAs (shRNAs) (Fig.1C), and found that the length of axons and dendrites were significantly decreased compared with control neurons that were transfected with nonspecific control shRNAs (NC), while the number of dendritic branches was not affected (Fig. 1D).
Since the encoded peptide chain of exon 1 of PLCL1 does not locate in the coding region of any domain of PLCL1 (Fig. 1A), we speculate that the deletion of exons 2–6 of the PLCL1 gene found in ASD patients may cause loss-of-function of PLCL1 protein and affect the proper neurite development, similar to the effect of reducing PLCL1 protein. To explore whether the deletion of exons 2–6 and the four missense variants of PLCL1 detected in ASD patients might affect the normal function of PLCL1 protein, we transfected the plasmids only expressing GFP (as a control), or PLCL1-wild-type (WT), or PLCL1-E1 (the recombinant plasmid only including exon 1), or the four missense variants (Fig. 1E) into mouse primary cortical neurons, considering the PLCL1 is highly conserved between human and mouse (approximately 94.4% according to protein peptide alignment). We found that overexpression of PLCL1-WT significantly increased the axon length compared with control neurons expressing GFP alone (Fig. 1F, G), while the total length of neurites and the branching number of neuronal dendrites did not change. Overexpression of PLCL1-E1 had no effect on the neuronal growth, which resembled that of control (Fig. 1F, G). The result confirmed that PLCL1-E1 lacked the normal function of PLCL1-WT. In addition, the effect of overexpression of the four missense variants in mouse neurons did not significantly differ from that of PLCL1-WT (Fig. 1F, G), indicating that the four variants did not affect the normal function of PLCL1 in neuronal growth. Taken together, the PLCL1 dosage is critical for proper neurite and axonal outgrowth, and the deletion of PLCL1 exons 2–6 is a loss-of-function mutant, while the four missense variants are not.
Dendritic spine abnormalities have been reported to be strongly associated with various neurological and neuropsychiatric disorders [12], and dysfunction of the GABAergic pathway in early development can lead to severe excitatory/inhibitory (E/I) imbalance in the neural circuitry, which may be the cause of behavioral defects in ASD patients [13]. Vesicular glutamate transporter 1 (VGLUT1) and vesicular GABA transporter (VGAT) are excitatory and inhibitory markers of synaptic transmission. To further explore the role of PLCL1 in synaptic development, we examined the function of PLCL1 in spine formation, dendrite development, and excitatory and inhibitory synapse formation by immunostaining in mouse primary cortical neurons. We found that Plcl1 knockdown with shRNA resulted in a decrease in both VGLUT1 and VGAT density on dendrites and spines compared with the control, but not for the spine density, length of dendrites, and brunching number (Fig. 2A, B). Thus, Plcl1 knockdown altered the excitatory and inhibitory synapse formation. Furthermore, PLCL1-WT successfully rescued the suppression of both VGLUT1 and VGAT density in Plcl1 knockdown mouse neurons (Fig. 2C, D). The four missense variants had no significantly different effect compared with PLCL1-WT, indicating that they did not affect the normal function of PLCL1 in excitatory and inhibitory synapse formation (Fig. 2C, D).
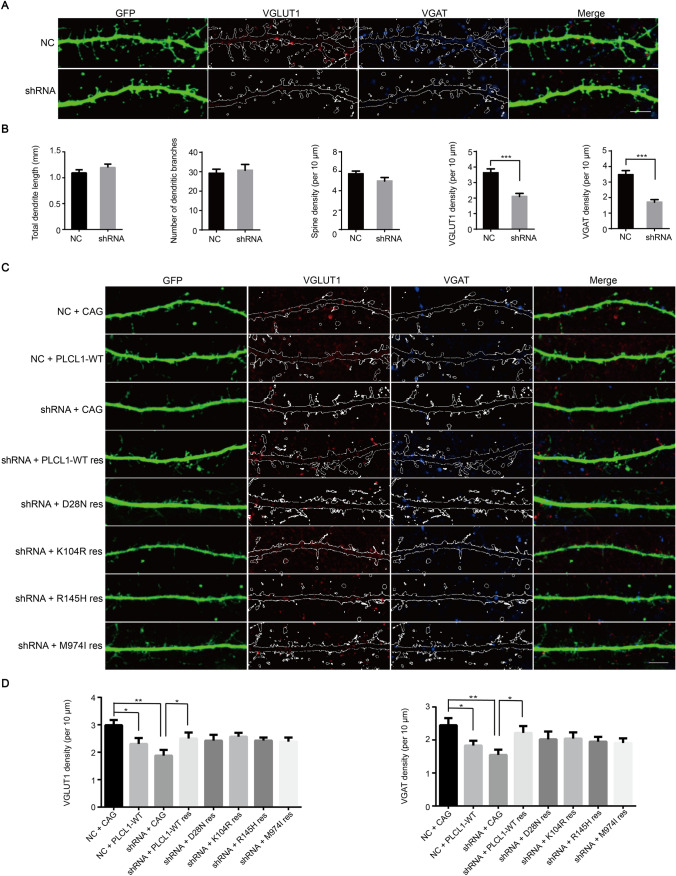
Fig. 2.
PLCL1 is crucial in neuronal excitability and its dosage imbalance affects glutamatergic and GABAergic synapse development in vitro. A Decreased VGLUT1 and VGAT density caused by Plcl1 knockdown. Morphology of the VGLUT1 and VGAT density of neurons transfected with Plcl1 shRNA and control NC vector. The skeleton represents dendrite segments and dendritic spines. Scale bar, 5 μm. B Statistical analysis of the total dendrite length, branching number, spine density (per 10 μm), VGLUT1 density (red puncta number per 10 μm), and VGAT density (blue puncta number per 10 μm) between NC and shRNA groups. Forty neurons from three independent experiments were measured and counted. P-value is determined by unpaired t-test. ***P <0.001. C PLCL1-WT and mutant variants of PLCL1 rescues (res) the phenotype of decreased VGLUT1 and VGAT density caused by Plcl1 knockdown. The specific morphology of neurons in each condition expressing the indicated vectors. All neurons were co-labeled with VGLUT1, VGAT, and GFP. Scale bar, 5 μm. D Statistical results for the VGLUT1 density and VGAT density between each group. Approximately 30 cells from three independent experiments were randomly selected and counted. P-value was determined by one-way ANOVA. Error bars, ± SEM. *P <0.05; **P <0.01.
In addition to PLCL1 depletion, overexpression of PLCL1 also reduced VGLUT1 and VGAT density (Fig. 2C, D), while the total dendrite length, branch number, and spine density were unaffected (Fig. S3). Thus, the dosage of PLCL1 is crucial for glutamatergic and GABAergic synapse development. We speculated that the variant with deletion of exons 2–6, a loss-of-function mutant as we mentioned above, may affect glutamatergic and GABAergic synapse development as well.
According to the predicted transcription factors that bind to the PLCL1 promoter (Table S2, Fig. S4A), we selected four relatively high-scoring transcription factors E2F4, E2F6, SP1, and GATA1 to assess the effects of the two promoter variants c.−636 C>A and c.−136_−134 delGCC on PLCL1 transcription activity and protein expression. We ectopically expressed E2F4, E2F6, SP1, and GATA1 (Fig. S4B), and co-transfected the expression plasmids with wild-type PLCL1 promoter (pGL3-WT, c.−1 to c.−901), mutant type c.−636 C>A (pGL3−636), c.−136_−134 delGCC (pGL3−136), and the empty vector into HEK-293T cells, and used promoter luciferase assays to determine the transcription activity of the promoters. We found that the activation of pGL3−136 by ectopic expression of GATA1 was significantly lower than that of pGL3-WT (Fig. S4C), while GATA1 had a similar strong activation effect on pGL3-WT and pGL3−636. E2F4, E2F6, and SP1 had similar effects on pGL3-WT and the two mutants (Fig. S4D). Therefore, we speculated that the mutant-type pGL3−136 may affect the transcriptional activation of PLCL1 promoter by GATA1.
Consistent with the luciferase assay results, overexpression of E2F4, E2F6, and SP1 had no significant effect on PLCL1 expression in HEK-293T cells, while overexpression of GATA1 up-regulated PLCL1 protein (Fig. S4E). Moreover, GATA1-induced PLCL1 protein expression was weakened after ectopic GATA1 knockdown (Fig. S4F). Therefore, we further verified that GATA1 has a positive regulatory effect on the expression of PLCL1 protein. Taken together, the rare variant c.−136_−134 delGCC of the PLCL1 promoter in ASD patients suppressed the transcription-activating effect of GATA1 on PLCL1, and subsequently decreased PLCL1 expression. As abnormal expression of PLCL1 leads to abnormal neurite outgrowth and glutamatergic and GABAergic synapse development, we believe that the variant may be related to the pathogenesis of ASD.
In this study, we identified one de novo PLCL1 variant, exons 2–6 deletion (within the 542 kb genomic deletion located on chromosome 2q33.1) in American ASD cohorts, and one PLCL1 promoter rare variant c.−136_−134 delGCC in Chinese ASD patients, which may relate to the onset of ASD. A 778 kb duplication including PLCL1 exons 2–6 was reported in one patient diagnosed with agenesis of the corpus callosum with ASD, DD, and seizures [14], which corroborated our observation that overexpression of PLCL1 reduced VGLUT1 and VGAT density, implying that the 542 kb deletion is likely related to neuronal development. Furthermore, whether the absence of three non-coding RNAs in this 542 kb region is involved in the pathogenesis of ASD warrants further research.
The four missense variants behaved similarly to PLCL1-WT when overexpressed in regard to neurite and axon outgrowth, and all could rescue the decreased VGLUT1 and VGAT density, that was caused by Plcl1 knockdown. However, we cannot exclude that they may affect other functions of PLCL1, such as its function in the inositol 1,4,5-trisphosphate [Ins(1,4,5)P3]-mediated Ca2+ signaling pathway, which was not assessed by this study. According to a “bilineal two-hit model” [15], that is, even rare SNVs are not a direct cause of ASD, they can increase the risk of ASD, thus the possibility that variants existing on other genes may be involved in the pathogenesis of ASD in cooperation with PLCL1 variants should not be excluded.
In summary, we report and describe rare variants of the PLCL1 gene in American and Chinese cohorts with ASD, and provided preliminary clues for further elucidating the role of PLCL1 in neuron development. Further research is needed to identify other essential genes which are required either independently or collaboratively with PLCL1 for the phosphorylation or clustering of GABAA receptors, and to better understand the role of PLCL1 in GABAergic signaling as well as in the pathogenesis of central nervous system diseases including ASD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31671461, 31301162, 31625013, and 81941405), 973 Program from the Ministry of Science and Technology of China (2013CB945404 and 2010CB529601), Shanghai Brain-Intelligence Project from STCSM (16JC1420501), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDBS01060200 and XDA16010310), and the Shanghai Municipal Science and Technology Major Project (2018SHZDZX05). We are grateful to Dr. Yasong Du of Shanghai Mental Health Center and to Dr. Xiu Xu of Children’s Hospital of Fudan University for the collection of patients’ samples and clinical data. We thank Dr. Yiping Shen and Hong Shao of the Genetics Diagnostic Lab at Boston Children’s Hospital for their assistance.
Conflict of interest
The authors declare no conflict of interest in this work.
Contributor Information
Guoyuan Liu, Email: gyliu@fudan.edu.cn.
Zilong Qiu, Email: zqiu@ion.ac.cn.
Bai-Lin Wu, Email: Bai-Lin.Wu@childrens.harvard.edu.
References
- 1.Iakoucheva LM, Muotri AR, Sebat J. Getting to the cores of autism. Cell. 2019;178:1287–1298. doi: 10.1016/j.cell.2019.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, et al. Association between microdeletion at 16p11.2 and autism. N Engl J Med. 2008;358:667–675. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- 3.Qiu Z, Yuan B. Towards the framework of understanding autism spectrum disorders. Neurosci Bull. 2019;35:1110–1112. doi: 10.1007/s12264-019-00443-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang JQ, Barnes G. A common susceptibility factor of both autism and epilepsy: functional deficiency of GABAA receptors. J Autism Dev Disord. 2013;43:68–79. doi: 10.1007/s10803-012-1543-7. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimura K, Takeuchi H, Sato O, Hidaka K, Doira N, Terunuma M, et al. Interaction of p130 with, and consequent inhibition of, the catalytic subunit of protein phosphatase 1alpha. J Biol Chem. 2001;276:17908–17913. doi: 10.1074/jbc.M009677200. [DOI] [PubMed] [Google Scholar]
- 6.Kanematsu T, Jang IS, Yamaguchi T, Nagahama H, Yoshimura K, Hidaka K, et al. Role of the PLC-related, catalytically inactive protein p130 in GABAA receptor function. EMBO J. 2002;21:1004–1011. doi: 10.1093/emboj/21.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu G, Yoshida S, Migita K, Yamada J, Mori F, Tomiyama M, et al. Dysfunction of extrasynaptic GABAergic transmission in phospholipase C-related, but catalytically inactive protein 1 knockout mice is associated with an epilepsy phenotype. J Pharmacol Exp Ther. 2012;340:520–528. doi: 10.1124/jpet.111.182386. [DOI] [PubMed] [Google Scholar]
- 8.Tomiyama K, Song L, Kobayashi M, Kinsella A, Kanematsu T, Hirata M, et al. Orofacial movements in phospholipase C-related catalytically inactive protein-1/2 double knockout mice: Effect of the GABAergic agent diazepam and the D1 dopamine receptor agonist SKF 83959. Synapse. 2010;64:714–720. doi: 10.1002/syn.20798. [DOI] [PubMed] [Google Scholar]
- 9.Namjou B, Marsolo K, Caroll RJ, Denny JC, Ritchie MD, Verma SS, et al. Phenome-wide association study (PheWAS) in EMR-linked pediatric cohorts, genetically links PLCL1 to speech language development and IL5-IL13 to Eosinophilic Esophagitis. Front Genet. 2014;5:401. doi: 10.3389/fgene.2014.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murakami A, Matsuda M, Nakasima A, Hirata M. Characterization of the human PRIP-1 gene structure and transcriptional regulation. Gene. 2006;382:129–139. doi: 10.1016/j.gene.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Bakos J, Bacova Z, Grant SG, Castejon AM, Ostatnikova D. Are molecules involved in neuritogenesis and axon guidance related to autism pathogenesis? Neuromolecular Med. 2015;17:297–304. doi: 10.1007/s12017-015-8357-7. [DOI] [PubMed] [Google Scholar]
- 12.Dang T, Duan WY, Yu B, Tong DL, Cheng C, Zhang YF, et al. Autism-associated Dyrk1a truncation mutants impair neuronal dendritic and spine growth and interfere with postnatal cortical development. Mol Psychiatry. 2018;23:747–758. doi: 10.1038/mp.2016.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fanelli F, Marino R, Keller F. Focusing on the interactions between the GABAergic system and neurosteroids in neurodevelopmental disorders. Curr Pharm Des. 2013;19:6491–6498. doi: 10.2174/1381612811319360009. [DOI] [PubMed] [Google Scholar]
- 14.Sajan SA, Fernandez L, Nieh SE, Rider E, Bukshpun P, Wakahiro M, et al. Both rare and de novo copy number variants are prevalent in agenesis of the corpus callosum but not in cerebellar hypoplasia or polymicrogyria. PLoS Genet. 2013;9:e1003823. doi: 10.1371/journal.pgen.1003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandler WM, Antaki D, Gujral M, Kleiber ML, Whitney J, Maile MS, et al. Paternally inherited cis-regulatory structural variants are associated with autism. Science. 2018;360:327–331. doi: 10.1126/science.aan2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.