Abstract
In this work, C. testosteroni JLU460ET isolated from animal waste was confirmed to have great degradation capability for 17β-estradiol and testosterone. This bacterium could degrade nearly 90% of 17β-estradiol (5 mg L−1) in 4 days and transform it into estrone for further degradation. One hundred percent testosterone (144 mg L−1) could be completely degraded after 9 h of incubation. This is the first report of C. testosteroni strains with the ability to degrade both estrogens and testosterone. The whole genome sequence of C. testosteroni JLU460ET was obtained and annotated, containing one chromosome (5,497,097 bp) with 61.37% GC content. A total of 4805 protein-coding genes and 134 RNA genes (including 29 rRNA genes, 102 tRNA genes and three ncRNA genes) were identified. Furthermore, the complete genome sequence of C. testosteroni JLU460ET was compared with four other C. testosteroni strains. Altogether, these five C. testosteroni strains contain 3508 core genes and 7616 pan genes. A steroid degradation pathway including 11 steroid degradation genes exists in core genes of five C. testosteroni strains. Twenty-two steroid degradation genes were found in the C. testosteroni JLU460ET genome, which has the most reported steroid degradation genes among the five C. testosteroni genomes. Further functional genomic analysis identified a gene cluster responsible for testosterone degradation in C. testosteroni JLU460ET, as well as a gene encoding 17β-HSD, the key enzyme for transforming 17β-estradiol into estrone. This work could enrich the genome sources of steroid-degrading strains and promote the study of steroid-degradation mechanism in bacteria.
Keywords: C. testosteroni JLU460ET, 17β-estradiol degradation, Testosterone degradation
Introduction
Steroids are a class of natural or synthetic organic compounds characterized by a molecular structure of 17 carbon atoms arranged in four rings. Natural steroids include all sex hormones, sterols of vertebrates, adrenal cortical hormones, bile acids, and molting hormones of insects. Synthetic steroids include growth-stimulating agents, oral contraceptives and anti-inflammatory agents. Testosterone (T), estrone (E1), estradiol (E2), and estriol (E3) are the most important natural steroid hormones in animals. Among the natural steroid hormones, E2 has the highest estrogenic activity (Caldwell et al. 2012; Combalbert and Hernandez-Raquet 2010). Estrogens pollution in the environment are mainly derived from human beings and livestock (Khanal et al. 2006; Combalbert and Hernandez-Raquet 2010; Chen et al. 2010). Levels of E1, E2, and 17α-ethinylestradiol (EE2) released in effluents from wastewater treatment plants (WWTPs), have been estimated to be up to 670, 150, and 70 ng L−1, respectively (Khanal et al. 2006; Wu et al. 2017), and 30–2500 ng L−1 estradiol in animal waste (Chen et al. 2010). Long-term exposure to estrogen pollution in the environment can cause alterations in the development, growth, and reproduction of all animals (Caldwell et al. 2012; Combalbert and Hernandez-Raquet 2010; Kidd et al. 2007; Wang et al. 2019). Increasing estrogen pollution in the environment has become one of the major challenges of the new age (Whitman 2017) and hence the quest for microbes with environmental estrogen-degrading abilities such as Gordonia sp. strain R9 (Liu et al. 2020), Stenotrophomonas maltophilia ZL1 (Li et al. 2012), Pseudomonas putida SJTE1 (Wang et al. 2018). In this article, a new C. testosteroni strain JLU460ET was successfully obtained to degrade estrogens and testosterone from animal waste.
The first C. testosteroni strain, type-strain ATCC11996, was enriched from soil and isolated in 1952 for its ability to degrade testosterone (Weiss et al. 2013). The members of the genus Comamonas are Gram-negative, strict aerobes and frequently occur in diverse habitats, including activated sludge, marshes, marine habitats, and plant and animal tissues (Ma et al. 2009), growing on organic acids, amino acids, and peptones (Ma et al. 2009). In the environment, the genus Comamonas can also degrade a large array of xenobiotic pollutants (especially aromatic compounds), including 3-nitrobenzoate, 4-chloronitrobenzene, 4-toluenesulfonate, 4-sulfobenzoate, chloroaniline, nitrobenzene, lignin–polymer, biphenyl, 4-chlorobiphenyl, dibenzofuran, naphthalene–2–sulfonate, and 4-sulfophenylcarboxylates (Weiss et al. 2013). The genus Comamonas is an important environmental bacterium, so its physiology, biochemistry, and genetics have been elucidated in great detail (Weiss et al. 2013). To date, 11 strains of the genus Comamonas have elucidated the complete genome, and five complete genomes belong to C. testosteroni species in the NCBI genome database.
In general, existing C. testosteroni strains could use androgen as carbon source, but not estrogens. For example, both C. testosteroni strains KF-1 and TA441 are able to utilize testosterone, progesterone, taurocholate, cholate, and taurodeoxycholate as carbon sources, but not ergosterol, 17β-estradiol, and ethinylestradiol (Weiss et al. 2013; Horinouchi et al. 2010, 2012). It is unusal for C. testosteroni JLU460ET to degrade both steroid estrogens and testosterone. The complete genome of C. tesosteroni JLU460ET was obtained and analyzed with four other C. tesosteroni strains to further improve the understanding of the molecular basis for the degradation of steroids in the species C. tesosteroni.
Materials and methods
Chemicals and kits
Estradiol (E2, > 98% purity), estrone (E1, > 98% purity), estriol (E3, > 98% purity), 17–alpha–ethinylestradiol (EE2, > 98% purity), testosterone (T, > 99% purity), and cholesterol (Ch, > 99% purity) were purchased from J&K Scientific Co. (Beijing, China). Ethanol, acetonitrile, and ethyl acetate, all of High Performance Liquid Chromatography (HPLC)-grade, were purchased from Thermo Fisher Scientific (USA). Stock solutions of E1, E2, E3, EE2 and T were prepared in ethanol and stored at 4 °C. LB broth, agar powder, Petri dishes, 96-well plates, antibiotics and other chemicals were obtained from Sangon Biotech (Shanghai, China). A polymerase chain reaction (PCR) mixture kit and bacterial genomic DNA extraction kit were purchased from Tiangen (China).
Isolation and identification of E2-degrading bacterium strain C. testosteroni JLU460ET
A 10-mL leachate of chicken manure was added to a 250-mL flask containing 100 mL M9-G medium (M9 medium without glucose) supplemented with 5 mg L−1 E2 as the sole carbon source to enrich E2-degrading bacteria. One liter M9-G medium includes NaHPO4·7H2O, 12.8 g; KH2PO4, 3.0 g; NaCl, 0.5 g; NH4Cl, 1.0 g; MgSO4, 0.24 g; CaCl2, 0.011 g; and 2 mL trace elements, composed of 0.063 g CuSO4·5H2O, 0.1 g H3BO3, 0.012 g NaMO4·2H2O, 0.0112 g MnSO4·H2O and 0.0534 g ZnSO4·7H2O in 1 L water. The enrichment culture was shaken at 150 rpm for 2 weeks at 30 °C, and then 2 mL of enrichment culture was transferred to 20 mL fresh M9-G + E2 medium and incubated for one more week. The resulting culture was spread onto M9-G + E2 plates and incubated at 30 °C for 2 days. Colonies were selected and inoculated into new fresh M9-G + E2 medium. This process was repeated several times to obtain pure isolates. One colony was selected and designated strain JLU460ET (JLU means Jilin University; 460 means the room number of deposition; E means estrogens; T means testosterone) for further identification (Liu et al. 2020).
Antibiotic susceptibility tests for chloramphenicol (25 μg mL−1), ampicillin (100 μg mL−1), carbenicillin (300 μg mL−1), tetracycline (10 μg mL−1), gentamicin (50 μg mL−1), streptomycin (50 μg mL−1) and kanamycin (30 μg mL−1) were tested by incubation in an LB broth medium with antibiotics. Cultures at different time points were measured at OD600 determination (Liu et al. 2020).
The growth of strain JLU460ET cultured with different steroids (E1, E2, E3, EE2, T, Ch) as the sole carbon source was tested by incubation of 1 mL strain JLU460ET in 30 mL M9 medium without glucose (M9-G) medium supplemented with the individual steroids at 30 °C and pH 7.0, after which the OD600s were determined. Samples were obtained at different time points.
The amplification and identification of 16S rDNA in C. testosteroni JLU460ET was referenced as strain Gordonia sp. R9 (Liu et al. 2020). A total of 16 complete whole genomes in the family Comamonadaceae were chosen to construct the phylogenetic tree based on core pan genes. The phylogenetic tree of the family Comamonadaceae includes 11 genus Comamonas strains, one Delftia acidovorans strain SPH-1, one Variovorax paradoxus strain EPS, one Alicycliphilus denitrificans strain K601, one Ramlibacter tataouinensis strain TTB310, and one Polaromonas naphthalenivorans strain CJ2.
High-performance liquid chromatography method coupled with fluorescence detection (HPLC-FD) for estrogen detection and HPLC for testosterone detection in C. testosteroni JLU460ET
The degradation efficiency of strain JLU460ET against E2 and T was determined according to the loss of substrate. Sample preparation: (1) To analyze the degradation efficiency of strain JLU460ET against E2, two mL of strain JLU460ET was inoculated into 50 mL M9-G medium containing E2 at different concentrations (50 ng L−1, 50 μg L−1, 1 mg L−1, 3 mg L−1, and 5 mg L−1). Temperatures (4–40 °C) and pH (5–9) values were also considered. Samples were collected at various time points up to 168 h post incubation. The collected samples were stored at − 20 °C and extracted by ethyl acetate for HPLC-FD use (Liu et al. 2020). (2) To analyze the degradation efficiency of strain JLU460ET against T, two mL of strain JLU460ET was inoculated into 50 mL M9-G medium containing 144 mg L−1 testosterone and 50 mL LB medium containing 144 mg L−1 testosterone. The collected samples were stored at − 20 °C and extracted by ethyl acetate for HPLC use.
Analytical conditions for E2 by HPLC-FD (Liu et al. 2015b): Chromatographic separations were performed on a reversed-phase C18 column gradient-eluted with a 45:55 v/v mixture of acetonitrile and water with a flow rate of 1.00 mL min−1. In each case, 10 μL samples were injected into the HPLC-FD system. Excitation/emission wavelengths were 288/310 nm. Under these chromatographic conditions, E2 could be determined with retention time at 6 min.
Analyzing conditions for T by HPLC: Chromatographic separations were performed on a reversed-phase C18 column gradient-eluted with a 50:50 v/v mixture of acetonitrile and water with a flow rate of 1.00 mL min−1. In each case, 10 μL samples were injected into the HPLC system. Detection was performed at 242 nm. Under these chromatographic conditions, T could be determined with retention time at 6.5 min.
Genomic DNA extraction and sequencing of C. testosteroni JLU460ET
To obtain insights into the estrogen degradation mechanism of C. testosteroni JLU460ET, its whole genome was sequenced. JLU460ET cells were cultured in LB broth medium containing E2. After overnight incubation, genomic DNA was extracted using a bacterial genomic DNA extraction kit (Tiangen, China). The harvested DNA was detected by agarose gel electrophoresis and quantified. High-quality chromosome DNA was used for whole genome sequencing, using Pacbio RSII and Illumina Hiseq 500.
After quality checks and filtering to obtain quality data (clean data), de novo genome assembly of the reads was constructed using SMRT Analysis v2.3.0, Celera Assembler, SOAPanp, SOAPindel, and GATK software (Istrail et al. 2004; Li et al. 2009). Overlap of contigs, gap close software and complete genomes of other strains were used to close the gaps to obtain the whole genome sequence.
Comparative genomics analysis of C. testosteroni JLU460ET with four other C. testosteroni strains
Comparative genomic analysis included core gene and specific gene analyses in 5 C. testosteroni strains. Core genes and specific genes in 5 C. testosteroni strains were analyzed by CD-HIT rapid clustering of similar proteins software with a threshold of 50% pairwise identity and a 0.7 length difference cutoff in amino acids. Then, a Venn diagram was drawn to show the relationships among the samples. The genome sequences of four other C. testosteroni strains, CNB-1, NCTC10698, TK102, and KF-1, were obtained from the NCBI database (the accession numbers and isolation information are shown in Table 1). These five C. testosteroni strains have complete whole genome sequences. The other two testosterone-degrading strains (TA441 and ATCC11996) (Arai and Ishii 2019; Gong et al. 2012) have only draft sequences, so they are not included for comparative genomics analysis.
Table 1.
General genome features of five C. testosteroni strains
| JLU460 ET | CNB-1 | NCTC10698 | TK102 | KF-1 | |
|---|---|---|---|---|---|
| References | This study | Ma et al. (2009) | NCBI | Fukuda et al. (2014) | Weiss et al. (2013) |
| Isolation source | Animal leachate, China | Industrial sludge, China | Soil, unknown | Soil, Japan | Linear alkylbenzene sulphonate (LAS) degrading lab trickling filter, Switzerland |
| Compounds | E1, E2, E3, Ch, T | 4–chloroni–trobenzene | Polychlorina–ted Biphenyl | Biphenyl, chlorobenzenes | 4-sulfophenylalkane |
| Accession number | CP067086.1 | CP001220.2 | NZ_UFXL01000001.1 | NZ_CP006704.1 | AAUJ02000001.1 |
| Number of contigs | 1 | 1 | 1 | 1 | 1 |
| Genomic size (bp) | 5,497,097 | 5,373,644 | 5,470,499 | 6,062,703 | 6,026,527 |
| GC content (%) | 61.37 | 61.4 | 61.4 | 61.9 | 61.79 |
| Protein-coding genes | 4805 | 4803 | 4825 | 5293 | 5322 |
| RNA genes | 134 | 92 | 123 | 130 | 119 |
| rRNA genes | 29 | 9 | 20 | 27 | 18 |
| tRNA genes | 102 | 80 | 100 | 100 | 98 |
| Other ncRNA genes | 3 | 3 | 3 | 3 | 3 |
| Transposon | 10 | 6 | 10 | 11 | 24 |
NA not available, E1 estrone, E2 17β-estradiol, E3 estriol, Ch cholesterol, T testosterone, JLU460 ET C. testosteroni strain JLU460 ET, CNB-1 C. testosteroni strain CNB-1, NCTC10698 C. testosteroni strain NCTC10698, TK102 C. testosteroni strain TK102, KF-1 C. testosteroni strain KF-1
Identification of putative genes responsible for steroid degradation in the C. testosteroni JLU460ET genome
Since genes responsible for cholesterol and testosterone biodegradation were very clear in C. testosteroni strains (Horinouchi et al. 2012), genes for cholesterol and testosterone degradation in C. testosteroni JLU460ET were analyzed based on the steroid degradation pathway in Kyoto Encyclopedia of Genes and Genomes (KEGG, map 00984).
To date, C. testosteroni TA441 and C. testosteroni ATCC 11996 have been two well-known testosterone-degrading strains. However, these two strains only published draft whole genome sequences, and we could not compare the complete whole genome sequence between those testosterone-degrading strains. A cluster of genes responsible for testosterone degradation previously identified in C. testosteroni TA441 has been studied in detail (Horinouchi et al. 2010, 2012; Ibero et al. 2019). In this paper, the testosterone degradation gene cluster from TA441 and JLU460ET was compared specifically.
The genetic basis for estrogen biodegradation in bacteria remains unclear. Only 17 beta-hydroxysteroid dehydrogenase (17beta-HSD) genes were confirmed as the first and key genes for the degradation of E2 (Wang et al. 2019), so 17beta-HSD genes in C. testosteroni JLU460ET were BLASTed against NCBI.
Results and discussion
Isolation and identification of E2-degrading C. tesosteroni strain JLU460ET
A strain capable of degrading E2 and T was isolated from animal waste collected in Shenyang, China. Analysis of 16S rRNA gene sequences showed that strain JLU460ET was identical to C. tesosteroni strains (data not shown). A phylogenetic tree constructed by using whole genomes from 16 family Comamonadaceae strains based on core pan genes showed that strain JLU460ET was grouped with the other four C. tesosteroni strains (Fig. 1). The phylogenetic tree also showed that strain Comamonas sp. lk was also grouped with C. tesosteroni species. The antibiotic results (Fig. 2) showed that strain JLU460ET was able to grow in the presence of ampicillin (100 μg mL−1) and carbenicillin (300 μg mL−1). After 18 h of incubation, strain JLU460ET could also grow in medium with gentamicin (50 μg mL−1), streptomycin (50 μg mL−1) and kanamycin (30 μg mL−1). Strain JLU460ET was found to be sensitive to chloramphenicol (25 μg mL−1) and tetracycline (10 μg mL−1), similar to other C. tesosteroni strains (Oppermann et al. 1996).
Fig. 1.

A phylogenetic tree was constructed by using whole genomes from 16 strains of the family Comamonadaceae based on core-pan genes. The number on the branch indicates the credibility of the branch; the branch length indicates the size of the evolutionary distance; and the evolutionary distance is based on the average replacement of each nucleotide number of times. Delftia acidovorans strain SPH-1, Variovorax paradoxus strain EPS, Alicycliphilus denitrificans strain K601, Ramlibacter tatataouinensis strain TTB310, and Polaromonas naphthalenivorans strain CJ2 were selected as outgroups
Fig. 2.
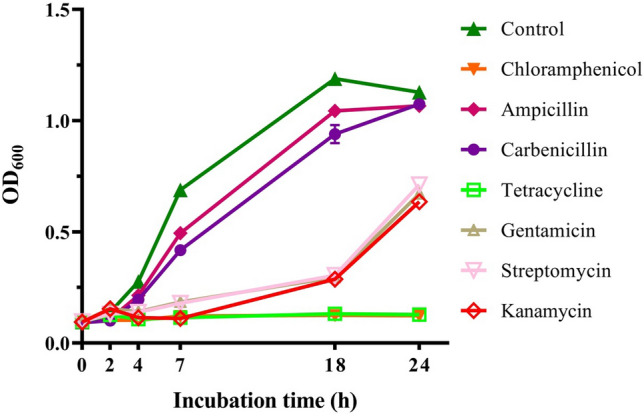
Antibiotic resistance of strain JLU460ET. Strain JLU460ET was cultured at 30 °C in LB broth medium with different antibiotics (control: none; ampicillin: 100 mg mL−1; tetracycline: 10 mg mL−1; carbenicillin: 300 mg mL−1; chloramphenicol: 25 mg mL−1; gentamicin: 50 mg mL−1; streptomycin: 50 mg mL−1; kanamycin: 30 mg mL−1). Bacterial growth was determined at OD600
According to the results above, 16S rDNA identification, the phylogenetic tree and antibiotic results showed that strain JLU460ET is consistent with other C. tesosteroni strains (Liu et al. 2015a). Therefore, strain JLU460ET was named C. tesosteroni JLU460ET. The species of C. tesosteroni can degrade a large array of xenobiotic pollutants in the environment, especially aromatic compounds and testosterone, from which it gets the name. However, reported C. tesosteroni strains could not degrade estrogens (Weiss et al. 2013; Horinouchi et al. 2010, 2012). This is the first reported C. tesosteroni strain that can degrade both estrogens and testosterone.
C. tesosteroni JLU460ET can utilize different steroids as sole carbon and energy sources
Previous studies have reported that most species of C. testosteroni can degrade testosterone but not estrogens. It was therefore necessary to determine the bioavailability of different steroids following treatment with C. tesosteroni JLU460ET. As shown in Fig. 3, C. tesosteroni JLU460ET grew very well in M9-G medium supplemented with E3, Ch and T as sole carbon and energy sources, in which the OD600 value reached 05–0.8. C. tesosteroni JLU460ET also grew well in the medium with E2, especially after 120 h of incubation. In M9-G medium supplemented with E1, strain JLU460ET grew, but the growth was not as good. Finally, strain JLU460ET could not grow in the medium with EE2 (Fig. 3). In conclusion, C. tesosteroni JLU460ET could use all tested steroids as sole carbon and energy sources, except EE2. Compared to other reported C. tesosteroni strains, C. tesosteroni JLU460ET uses more kinds of steroids as carbon and energy sources (Weiss et al. 2013; Horinouchi et al. 2010, 2012).
Fig. 3.
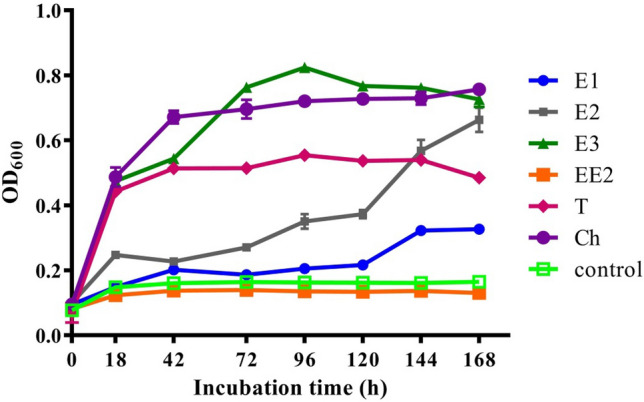
Growth curve of strain JLU460ET in M9-G minimal medium with different substrates (E1: estrone; E2: 17 beta-estradiol; E3: estriol; EE2: 17 alpha-ethynylestradiol; T: testosterone; Control: no steroids). Cells were cultured for 168 h, and samples were detected at different time points. Biomass is shown as OD600
E2 and testosterone can be degraded best by C. tesosteroni JLU460ET at 30 °C and pH = 7
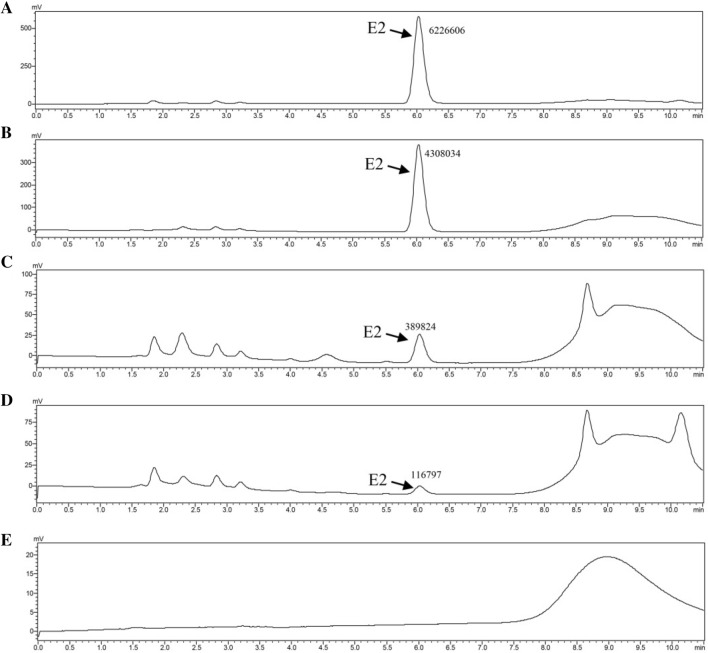
To test the effect of temperature and pH on E2 degradation by C. tesosteroni JLU460ET, cultures were incubated in M9-G medium supplemented with 5 mg mL−1 E2 at 4–40 °C and pH 5.0–9.0. According to the degradation curve of E2 (Fig. 4a), most E2 could be degraded between 20 and 30 °C after 96 h incubation. C. tesosteroni JLU460ET degrade E2 best at 30 °C. E2 could almost not be degraded at temperatures lower than 20 °C or over 30 °C. According to the degradation curve of intermediate E1 (Fig. 4b), E2 could be transformed into E1 rapidly at 30 °C for further degradation. After 96 h of incubation, most of the intermediate E1 could also be degraded at 30 °C. The HPLC-FD chart also showed that JLU460ET could degrade E2 completely (Fig. 5). The transformation from E2 into E1 occurs slowly at 20 °C, possibly the reason C. tesosteroni JLU460ET degrades E2 much faster at 30 °C than at 20 °C. The degradation efficiency of E2 by C. tesosteroni JLU460ET at different initial pH values showed that E2 could be degraded completely at pH 7.0 but not at other pH values (Fig. 6). Results showed that C. tesosteroni JLU460ET has a narrow range of temperature and pH for E2 degradation.
Fig. 4.
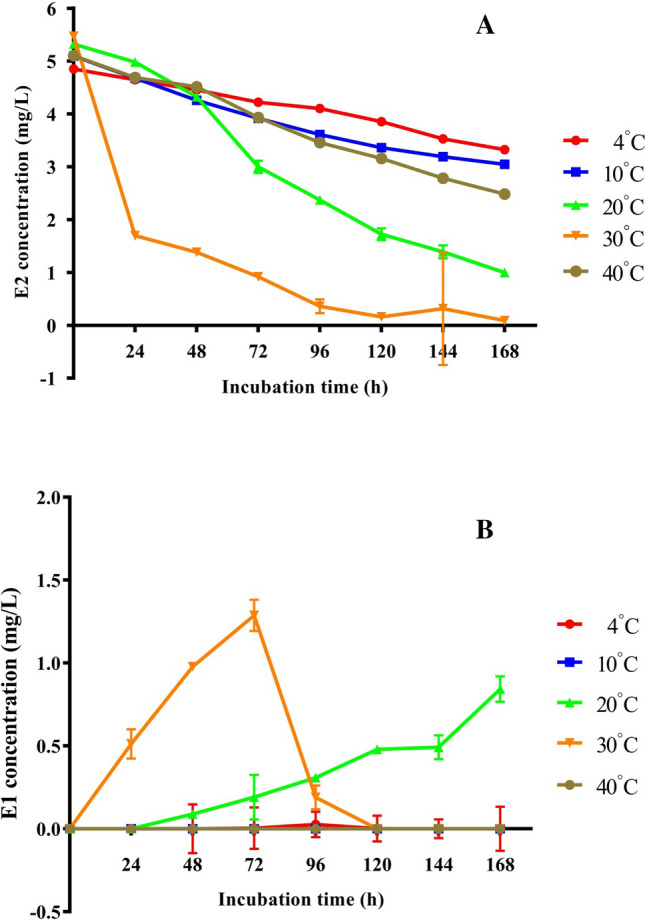
Effect of temperature on the degradation of E2 by strain JLU460ET. Strain JLU460ET was incubated in M9-G medium with 5 mg L−1 E2 at different temperatures, and samples were collected at different time points followed by HPLC-FD detection. a E2 degradation by strain JLU460ET was detected at different temperatures; b E1 as an intermediate for E2 degradation by strain JLU460ET
Fig. 5.
HPLC-FD charts of E2 degradation by strain JLU460ET. C. testosteroni strain JLU460ET was cultured with 5 mg L−1 E2 as the sole carbon source, and samples were collected at different time points. a E2 degradation without incubation (0 h); b–e: E2 degradation over time after incubation with strain JLU460ET. The peak area of E2 was marked
Fig. 6.
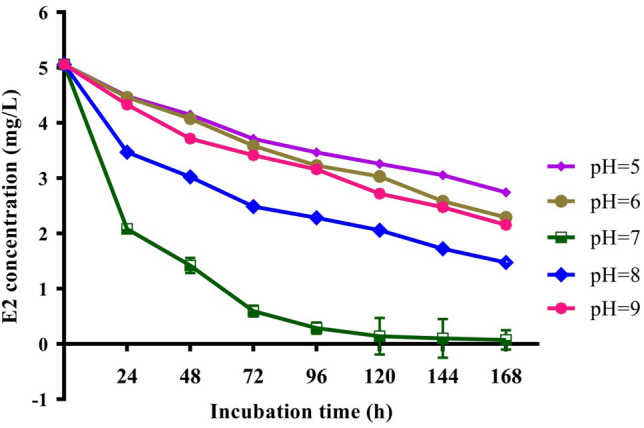
Effect of initial pH on the biodegradation of E2 by strain JLU460ET. Strain JLU460ET was incubated in M9-G medium with 5 mg L−1 E2 at 30 °C under different pH values, and samples were collected at different time points followed by HPLC-FD detection
To test whether C. tesosteroni JLU460ET could degrade trace amounts of E2, concentrations of E2 ranging from 50 ng L−1 to 5 mg L−1 were incubated with the bacterium under the optimal conditions of 30 °C and pH 7.0. The results showed that initial E2 concentrations ranging from 1 to 5 mg L−1 were almost 100% degraded after incubation for 120 h, while a longer time (168 h) was needed to degrade E2 with an initial concentration of 50 ng L−1 to 50 μg L−1 (Fig. 7). Results showed that C. tesosteroni JLU460ET could degrade E2 at the concentrations in the environment.
Fig. 7.

Degradation efficiency of strain JLU460ET at different initial E2 concentrations. Strain JLU460ET was incubated in M9-G medium with 5 × 10−5 mg L−1, 5 × 10−2 mg L−1, 1 mg L−1, 3 mg L−1, and 5 mg L−1 E2 at 30 °C and pH 7.0, and samples were collected at different time points followed by HPLC-FD detection
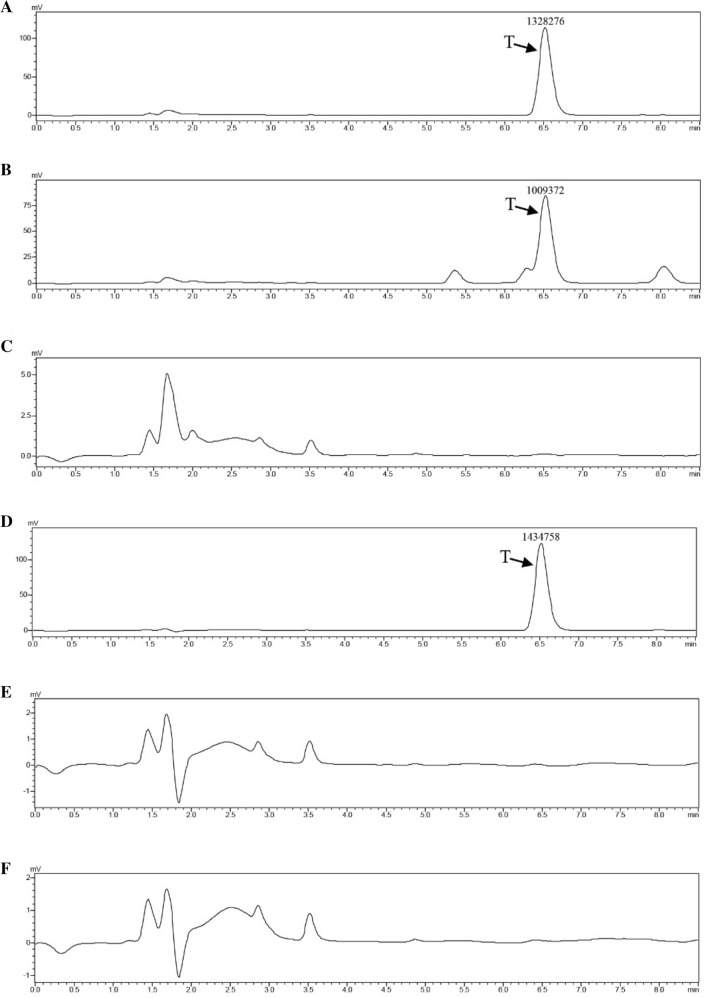
To test testosterone degradation by C. tesosteroni JLU460ET, it was cultured in LB broth medium and M9-G inorganic salt medium containing 144 mg L−1 testosterone. The results (Fig. 8) showed that in the M9-G medium, testosterone was completely degraded after incubation for 9 h. It took approximately 21 h to completely degrade 144 mg L−1 testosterone in LB broth. Thus, although C. tesosteroni JLU460ET could use testosterone as the sole carbon and energy source very well, C. tesosteroni JLU460ET uses carbons in LB broth medium first. After carbons in LB broth medium were consumed, C. tesosteroni JLU460ET started to utilize testosterone. This sequence of events could be demonstrated by the HPLC chart (Fig. 9). In an M9-G inorganic salt medium containing testosterone, testosterone was the only carbon and energy source, so it was degraded very rapidly, and no intermediates could be detected after 9 h of incubation. In LB broth medium containing testosterone, testosterone was not the only carbon and energy source, so it was degraded slowly, and several intermediates were detected after 9 h of incubation. Compared to other C. tesosteroni strains, the testosterone degradation efficiency in C. tesosteroni JLU460ET is similar to the degradation efficiency in other strains (Horinouchi et al. 2012).
Fig. 8.
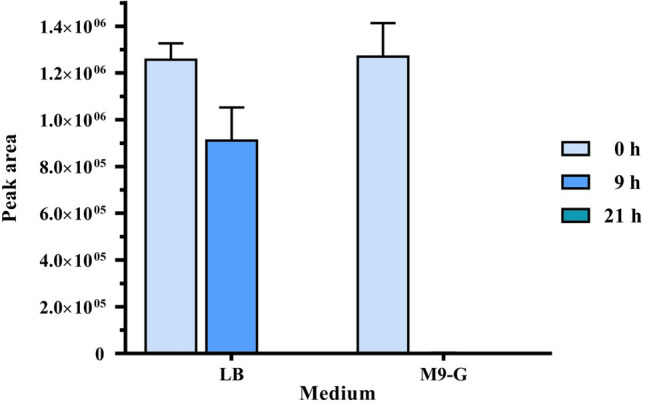
Degradation of testosterone by strain JLU460ET. Strain JLU460ET was cultured at 30 °C in LB broth medium or M9-G with 144 mg L−1 testosterone for 22 h. Samples were obtained at 9 h or 21 h and then extracted with ethyl acetate, and samples without incubation were used as controls. Testosterone was determined by HPLC detection
Fig. 9.
HPLC charts of T degradation by strain JLU460ET. C. testosteroni strain JLU460ET was cultured with 144 mg L−1 testosterone as the sole carbon source, and samples were collected at different time points. a T degradation in LB medium without incubation (0 h); b, c T degradation in LB medium over time after incubation with strain JLU460ET; d T degradation in M9-G medium without incubation (0 h); e, f T degradation in M9-G medium over time after incubation with strain JLU460ET. The peak area of T was marked
Complete genome sequencing and general genome features of C. tesosteroni JLU460ET
To investigate the steroid degradation mechanism in C. tesosteroni JLU460ET, chromosomal DNA was extracted and sequenced. The raw whole-genome sequence data were 955 Mb from Illumina HiSeq 500 and 1,749,171,537 bp of polymerase reads base from PacBio RSII. The complete genome of C. tesosteroni JLU460ET is composed of one circular chromosome of 5,497,097 bp with an average GC content of 61.37%. The genome is predicted to contain 4805 coding genes. The coding gene length was 4,726,128 bp with a GC content of 62.59%, which was higher than the complete genome average GC content. The internal length of the gene is 770,869 bp, with a GC content of 53.96%, which is lower than the complete genome average GC content. The genomic characteristics of C. tesosteroni JLU460ET were compared with four other C. tesosteroni strains, including C. testosteroni strain CNB-1, C. testosteroni strain NCTC10698, C. testosteroni strain TK102, and C. testosteroni strain KF-1. The genome sizes of these five C. tesosteroni strains ranged from 5.3 to 6.0 M bp (Table 1). The number of coding genes in all the genomes was greater than 4800, and the number of RNA genes was between 92 and 134. All five strains had high G + C contents, which were above 61%. The number of transposon genes was between 6 and 24, and other feature information of the five C. tesosteroni strains is listed in Table 1.
Genomic bases of steroid utilization in C. testosteroni JLU460ET
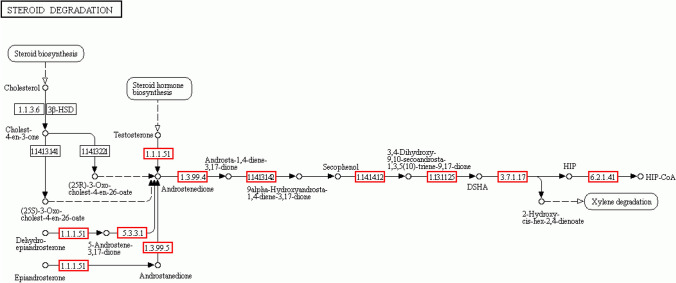
To date, the mechanism of steroid degradation has been based mainly on cholesterol and testosterone degradation. In KEGG steroid degradation (map 00984), seven enzymes are necessary for testosterone degradation. All of these genes responsible for testosterone degradation were also found in the JLU460ET genome (Fig. 10). They are 3(17)–beta–hydroxysteroid dehydrogenase [EC:1.1.1.51], 3–oxosteroid 1–dehydrogenase [EC:1.3.99.4], 3–ketosteroid 9–alpha–monooxygenase subunit A [EC:1.14.15.30], 3–hydroxy–9,10–secoandrosta–1,3,5(10)–triene–9,17–dione monooxygenase [EC:1.14.14.12], 3,4–dihydroxy–9,10–secoandrosta–1,3,5(10)–triene–9,17–dione–4,5–dioxygenase [EC:1.13.11.25], 4,5:9,10–diseco–3–hydroxy–5,9,17–trioxoandrosta–1(10),2–diene–4–oate hydrolase [EC:3.7.1.17], HIP-CoA ligase [EC:6.2.1.41]. As shown in Fig. 10, cholesterol oxidase [EC:1.1.3.6] and cholest–4–en–3–one–26–monooxygenase [EC:1.14.15.29] were needed for cholesterol degradation. Both enzymes were not found in JLU460ET, but JLU460ET indeed could use cholesterol as the sole carbon and energy source, as previously shown in Fig. 3, indicating that some other enzymes might be responsible for cholesterol degradation in JLU460ET. Nine transporters belonging to the ABC transporter family were found in JLU460ET to transport cholesterol. More work is needed to identify cholesterol degradation genes in JLU460ET.
Fig. 10.
Steroid degradation pathway in C. testosteroni JLU460ET. Numbers are EC number of enzymes. The red box indicates that steroid degradation enzymes were found in C. testosteroni JLU460ET
C. testosteroni was named based on its ability to metabolize testosterone. A cluster of genes similar to the previously identified genes responsible for testosterone degradation in C. testosteroni TA441 was found in the strain JLU460ET genome. The comparison of these steroid degradation genes is shown in Fig. 11. Except for the testosterone degradation gene cluster, more testosterone degradation genes were found in other places in the JLU460ET genome (Table 2). Key genes involved in degradation of testosterone were identified in the JLU460ET genome: Hydroxysteroid 11–beta–dehydrogenase 1 (hsaB); 3–ketosteroid 9alpha-monooxygenase subunit B (kshB); 3–oxo–5alpha–steroid 4-dehydrogenase (tesI); 3-oxosteroid 1-dehydrogenase (kstD); 3–hydroxy–9,10–secoandrosta–1,3,5(10)–triene–9,17–dione monooxygenase (hsaA); 3–hydroxy–9,10–secoandrosta–1,3,5(10)–triene–9,17–dione monooxygenase reductase component (hsaB); 4,5:9,10–diseco–3–hydroxy–5,9,17–trioxoandrosta–1(10), 2–diene–4–oate hydrolase (hsaD); Ketosteroid isomerase; 3alpha–hydroxysteroid 3–dehydrogenase (hsdA); 7–alpha–hydroxysteroid dehydrogenase (hdhA); 3–ketosteroid 9alpha–monooxygenase subunit A (kshA); 3,4–dihydroxy–9,10–secoandrosta–1,3,5(10)–triene–9,17–dione 4,5–dioxygenase (hsaC).
Fig. 11.
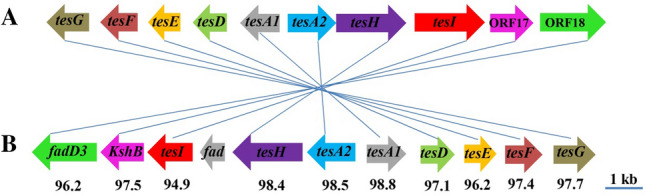
Steroid degradation genes present in C. testosteroni JLU460ET. a Steroid degradation genes from C. testosteroni TA441 (accession number LC010134). b Putative steroid degradation gene cluster of C. testosteroni JLU460ET (accession number CP067086). Genes encoding the same function are pictured in the same color and connected with a line. Numbers indicate the percent identities of two connected genes. Detailed information on these steroid degradation genes in C. testosteroni JLU460ET is listed in Table 2
Table 2.
Detailed information of putative steroid-degrading genes in C. testosteroni JLU460ET
| ORF | Homologous protein (function identified) | |||||
|---|---|---|---|---|---|---|
| Name | Length (aa) | Name and function | Accession no. | Length (aa) | Host organism | Identity (aa%) |
| G1GL000049 | 270 | Hydroxysteroid 11β-dehydrogenase 1 (11β-HSD) | ELX12334.1 | 268 | Janthinobacterium sp. HH01 | 73.5 |
| G1GL001253 | 165 | 3–hydroxy–9,10–secoandrosta–1,3,5(10)–triene–9,17–dione monooxygenase reductase component | SFA82401.1 | 333 | Collimonas sp. OK607 | 44.5 |
| G1GL001553 | 259 | 3α, 20β-hydroxysteroid dehydrogenase | KXB51592.1 | 256 | Corynebacterium kroppenstedtii | 43.3 |
| G1GL002594 | 219 | 3β-hydroxysteroid dehydrogenase | KGG82547.1 | 219 | C. thiooxydans | 97.7 |
| G1GL004479 | 254 | 3(or 17) β-hydroxysteroid dehydrogenase | CAA44977.1 | 254 | C. testosteroni | 98.4 |
| G1GL004516 | 266 | SDR family NAD(P)-dependent oxidoreductase | WP_034369301.1 | 266 | C. testosteroni | 87.6 |
| G1GL004519 | 504 | Long-chain-fatty-acid–CoA ligase | EHN63427.1 | 504 | C. testosteroni ATCC 11996 | 99.8 |
| G1GL004919* | 532 | HIP: CoA ligase | Q7WSH3.1 | 540 | C. testosteroni | 96.2 |
| G1GL004920* | 355 | 3–ketosteroid–9α–hydroxylase reductase subunit (kshB) | KGH25271.1 | 341 | C. testosteroni | 97.1 |
| G1GL004921* | 373 | 3–oxo–5α–steroid 4–dehydrogenase (tesI) | Q59327.1 | 530 | C. testosteroni | 80.9 |
| G1GL004922* | 206 | 3–oxo–5α–steroid 4–dehydrogenase (tesI) | Q59327.1 | 530 | C. testosteroni | 87 |
| G1GL004923* | 576 | 3–oxosteroid 1–dehydrogenase (kstD) (tesH) | Q06401.1 | 573 | C. testosteroni | 83.6 |
| G1GL004924* | 398 | 3–hydroxy–9,10–secoandrosta–1,3,5(10)–triene–9,17–dione monooxygenase (hsaA) (tesA2) | RDI10018.1 | 398 | Comamonas sp. AG1104 | 99.8 |
| G1GL004925* | 325 | 3–hydroxy–9,10–secoandrosta–1,3,5(10)–triene–9,17–dione monooxygenase reductase component (hasB) | SFA82401.1 | 333 | Collimonas sp. OK607 | 60.8 |
| G1GL004926* | 279 | Hydrolase for 4, 5–9, 10–diseco–3–hydroxy–5, 9, 17–trioxoandrosta–1(10), 2–dien–4–oic acid (hsaD) (tesD) | LC010134.1 | 279 | C. testosteroni TA441 | 97.1 |
| G1GL004927* | 264 | 2–hydroxyhexa–2,4–dienoate hydratase (tesE) | LC010134.1 | 264 | C. testosteroni TA441 | 96.2 |
| G1GL004928* | 307 | Acetaldehyde dehydrogenase (tesF) | LC010134.1 | 307 | C. testosteroni TA441 | 97.4 |
| G1GL004929* | 346 | 4–hyroxy–2–oxovalerate aldolase (tesG) | LC010134.1 | 350 | C. testosteroni TA441 | 97.7 |
| G1GL004958 | 119 | Steroid Delta-isomerase | GAW78733.1 | 119 | C. testosteroni | 95.7 |
| G1GL004960 | 273 | 3α-hydroxysteroid dehydrogenase/carbonyl reductase | AAC79849.1 | 257 | C. testosteroni | 100 |
| G1GL004967 | 130 | Ketosteroid isomerase | BAP91373.1 | 133 | C. testosteroni | 99.2 |
| G1GL004971 | 256 | 7α-hydroxysteroid dehydrogenase | SUY76515.1 | 256 | C. testosteroni | 100 |
| G1GL004975 | 369 | 3–ketosteroid 9α–monooxygenase subunit A (kshA) | ASM54765.1 | 345 | P. nigrifaciens | 64.6 |
| G1GL005008 | 284 | 3,4–dihydroxy–9,10–secoandrosta-1,3,5(10)–triene–9,17–dione 4,5–dioxygenase (hsaC) (tesB) | NYJ56904.1 | 284 | Comamonas sp. B9U6D | 97.2 |
*Indicates that similar steroid-degrading genes in C. testosteroni strain TA441 also exist in C. testosteroni JLU460ET
Nonetheless, the mechanism of estrogen degradation remains unclear (Yu et al. 2013; Liu et al. 2020). 17–beta–HSD was shown to be the key enzyme to transform 17–beta–estradiol (E2) to estrone (E1) (Ye et al. 2017). This step is considered the first key step for the degradation of E2. One copy of the 17–beta–HSD genes was found in JLU460ET, which has > 98% identity based on BLAST results from NCBI. Wang et al. (Wang et al. 2018) showed that 3–oxoacyl–(acyl–carrier–protein) reductase functions as 17–β–hydroxysteroid dehydrogenase in the estrogen-degrading Pseudomonas putida SJTE-1. Four copies of 3–oxoacyl–(acyl–carrier–protein) were found in JLU460ET, but their function in estrogen degradation in JLU460ET needs to be proven in the future. The other genetic basis for estrogen biodegradation in bacteria remains unclear, especially for estrogen-degradation genes.
To determine whether steroid-degrading genes are located in the core genes of the genus C. tesosteroni, the whole genome sequences of five C. tesosteroni strains were compared (Fig. 12). The five C. tesosteroni strains contained 25,556 genes in all, including 7616 pan genes, 3508 core genes and 4108 dispensable genes. Strain JLU460ET has 392 specific genes, strain CNB-1 has 615 specific genes, strain TK102 has 534 specific genes, strain KF-1 has 728 specific genes, and strain NCTC10698 has 160 specific genes.
Fig. 12.
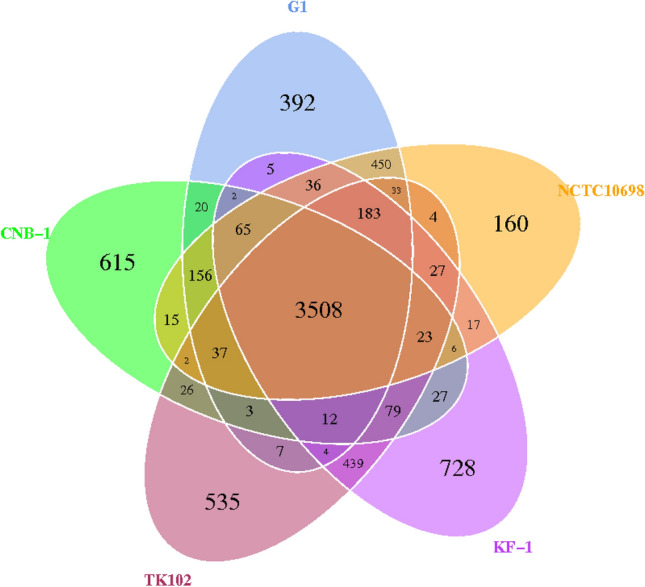
Comparison among the five C. testosteroni strains. Numbers of orthologous gene families and unique genes. The Venn diagram shows the number of orthologous gene families of the core genome (the center part) and the numbers of unique genes of each genome. The different colors indicated different strains
Core and pan genome analyses showed that 11 steroid-degrading genes existed in the core genes of these five C. tesosteroni strains. They are ketosteroid isomerase-related protein, 3-beta hydroxysteroid dehydrogenase, 3(17)–beta–hydroxysteroid dehydrogenase, 3–ketosteroid 9–alpha–monooxygenase subunit B (kshB), 3–oxosteroid 1–dehydrogenase (kstD), 3–hydroxy–9,10–secoandrosta–1,3,5(10)–triene–9,17–dione monooxygenase (hsaA), 3–hydroxy–9,10–secoandrosta–1,3,5(10)–triene–9,17–dione monooxygenase reductase component (hsaB), 4,5:9,10–diseco–3–hydroxy–5,9,17–trioxoandrosta–1(10), 2–diene–4–oate hydrolase (hsaD), 3–alpha–hydroxysteroid 3–dehydrogenase (hsdA), 7–alpha–hydroxysteroid dehydrogenase (hdhA) and 3–ketosteroid 9alpha–monooxygenase subunit A (kshA).
According to specific gene analysis of the five genus C. tesosteroni strains, no steroid degradation genes were found in NCTC10698 strain-specific genes. One steroid degradation gene, 3-beta hydroxysteroid dehydrogenase/isomerase (lcl| AAUJ02000001.1_prot_EED65428.1_374), exists in KF-1-specific genes. Four steroid degradation genes existed in CNB-1-specific genes: 3–beta–hydroxy–delta5–steroid dehydrogenase (CP001220.2_prot_ACY32670.1_1924), 3-beta hydroxysteroid dehydrogenase/isomerase (CP001220.2_prot_ACY34125.1_3378), sterol methyltransferase (CP001220.2_prot_ACY34320.1_3573) and aslA arylsulfatase (CP001220.2_prot_ACY32668.1_1922). Six steroid degradation genes existed in TK102-specific genes: 5 copies of 3-beta hydroxysteroid dehydrogenase/isomerase (WP_034379727.1; WP_043371569.1; WP_043373426.1; WP_051962144.1; WP_051962237.1) and 2-hydroxyacid dehydrogenase (WP_144244970.1). Altogether, C. testosteroni JLU460ET had the most reported steroid degradation genes among the 5 C. testosteroni strains, and 86.5% of the specific genes in strain JLU460ET were unidentified genes, possibly the reason C. testosteroni JLU460ET had the power to degrade both testosterone and estrogens very well.
In conclusion, the characterization of C. testosteroni JLU460ET, which exhibited high degradation capability for E2 and E1, was performed, as well as genomic analysis. In addition to the previously identified genes involved in the degradation of testosterone and E2, several putative steroid-degrading enzyme genes were present in C. testosteroni JLU460ET; thus, the present study contributes to the genome database of steroid-degrading microorganisms and provides new insights into steroid degradation mechanisms.
Author contributions
TZ designed the experiments and wrote the manuscript. NL performed the experiments. YS, JL and MZ assisted the experiments. All the authors discussed the results and commented on the manuscript.
Funding
This study was supported by the National Science Foundation of China (Grant no. 32072924 and 31702299).
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval and consent to participate
This article does not contain data from any study with human participants or animals.
References
- Arai H, Ishii M. Draft genome sequence of Comamonas testosteroni TA441, a bacterium that has a cryptic phenol degradation gene cluster. Microbiol Resour Announc. 2019;8:e00946–e1019. doi: 10.1128/MRA.00946-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell DJ, Mastrocco F, Anderson PD, Lange R, Sumpter JP. Predicted-no-effect concentrations for the steroid estrogens estrone, 17β-estradiol, estriol, and 17α-ethinylestradiol. Environ Toxicol Chem. 2012;31:1396–1406. doi: 10.1002/etc.1825. [DOI] [PubMed] [Google Scholar]
- Chen TS, Chen TC, Yeh KJC, Chao HR, Liaw ET, Hsieh CY, Chen KC, Hsieh LT, Yeh YL. High estrogen concentrations in receiving river discharge from a concentrated livestock feedlot. Sci Total Environ. 2010;408:3223–3230. doi: 10.1016/j.scitotenv.2010.03.054. [DOI] [PubMed] [Google Scholar]
- Combalbert S, Hernandez-Raquet G. Occurrence, fate, and biodegradation of estrogens in sewage and manure. Appl Microbiol Biotechnol. 2010;86:1671–1692. doi: 10.1007/s00253-010-2547-x. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Hosoyama A, Tsuchikane K, Ohji S, Yamazoe A, Fujita N, Shintani M, Kimbara K (2014) Complete genome sequence of polychlorinated biphenyl degrader Comamonas testosteroni TK102 (NBRC 109938). Genome Announc 2(5):e00865–14 [DOI] [PMC free article] [PubMed]
- Gong W, Kisiela M, Schilhabel M, Xiong G, Maser E. Genome sequence of Comamonas testosteroni ATCC 11996, a representative strain involved in steroid degradation. J Bacteriol. 2012;194:1633–1634. doi: 10.1128/JB.06795-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi M, Kurita T, Hayashi T, Kudo T. Steroid degradation genes in Comamonas testosteroni TA441: isolation of genes encoding a delta 4(5)-isomerase and 3 alpha- and 3 beta-dehydrogenases and evidence for a 100 kb steroid degradation gene hot spot. J Steroid Biochem Mol Biol. 2010;122:253–263. doi: 10.1016/j.jsbmb.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Horinouchi M, Hayashia T, Kudo T. Steroid degradation in Comamonas testosteroni. J Steroid Biochem Mol Biol. 2012;129:4–14. doi: 10.1016/j.jsbmb.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Ibero J, Galán B, Díaz E, García JL. Testosterone degradative pathway of Novosphingobium tardaugens. Genes. 2019;10:871. doi: 10.3390/genes10110871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istrail S, Sutton GG, Florea L, Halpern AL, Mobarry CM, Lippert R, Walenz B, Shatkay H, Dew I, Miller JR, Flanigan MJ, Edwards NJ, Bolanos R, Fasulo D, Halldorsson BV, Hannenhalli S, Turner R, Yooseph S, Lu F, Nusskern DR, Shue BC, Zheng XH, Zhong F, Delcher AL, Huson DH, Kravitz SA, Mouchard L, Reinert K, Remington KA, Clark AG, Waterman MS, Eichler EE, Adams MD, Hunkapiller MW, Myers EW, Venter JC. Whole-genome shotgun assembly and comparison of human genome assemblies. Proc Natl Acad Sci USA. 2004;101:1916–1921. doi: 10.1073/pnas.0307971100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanal SK, Xie B, Thompson ML, Sung S, Ong SK, Leeuwe J. Fate, transport, and biodegradation of natural estrogens in the environment and engineered systems. Environ Sci Technol. 2006;40:6537–6546. doi: 10.1021/es0607739. [DOI] [PubMed] [Google Scholar]
- Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, Flick RW. Collapse of a fish population after exposure to a synthetic estrogen. Proc Natl Acad Sci USA. 2007;104:8897–8901. doi: 10.1073/pnas.0609568104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Li Y, Fang X, Yang H, Wang J, Kristiansen K, Wang J. SNP detection for massively parallel whole-genome resequencing. Genome Res. 2009;19:1124–1132. doi: 10.1101/gr.088013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Nandakumar R, Madayiputhiya N, Li X. Proteomic analysis of 17β-estradiol degradation by Stenotrophomonas maltophilia. Environ Sci Technol. 2012;46:5947–5955. doi: 10.1021/es300273k. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhu W, Cao Z, Xu B, Wang G, Luo M. High correlation between genotypes and phenotypes of environmental bacteria Comamonas testosteroni strains. BMC Genomics. 2015;16:110. doi: 10.1186/s12864-015-1314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Shi Y, Li M, Zhang Td, Gao S. Simultaneous determination of four trace estrogens in feces, leachate, tap and groundwater using solid-liquid extraction/auto solid-phase extraction and high-performance liquid chromatography with fluorescence detection. J Sep Sci. 2015;38:3494–3501. doi: 10.1002/jssc.201500443. [DOI] [PubMed] [Google Scholar]
- Liu N, Shi Y, Li J, Zhu M, Zhang T. Isolation and characterization of a new highly effective 17β-estradiol-degrading Gordonia sp. strain R9. 3 Biotech. 2020;10:174. doi: 10.1007/s13205-020-2156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YF, Zhang Y, Zhang JY, Chen DW, Zhu Y, Zheng H, Wang SY, Jiang CY, Zhao GP, Liu SJ. The complete genome of Comamonas testosteroni reveals its genetic adaptations to changing environments. Appl Environ Microbiol. 2009;75:6812–6819. doi: 10.1128/AEM.00933-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppermann UCT, Belai I, Maser E. Antibiotic resistance and enhanced insecticide catabolism as consequences of steroid induction in the Gram-negative bacterium Comamonas testosteroni. J Steroid Biochem Mol Biol. 1996;58:217–223. doi: 10.1016/0960-0760(96)00021-0. [DOI] [PubMed] [Google Scholar]
- Wang P, Zheng D, Wang Y, Liang R. One 3-oxoacyl-(acyl-Carrier-protein) reductase functions as 17β-hydroxysteroid dehydrogenase in the estrogen-degrading Pseudomonas putida SJTE-1. Biochem Biophys Res Commun. 2018;505:910–916. doi: 10.1016/j.bbrc.2018.10.005. [DOI] [PubMed] [Google Scholar]
- Wang YQ, Li YW, Chen QL, Liu ZH. Long-term exposure of xenoestrogens with environmental relevant concentrations disrupted spermatogenesis of zebra fish through altering sex hormone balance, stimulating germ cell proliferation, meiosis and enhancing apoptosis. Environ Pollut. 2019;244:486–494. doi: 10.1016/j.envpol.2018.10.079. [DOI] [PubMed] [Google Scholar]
- Weiss M, Kesberg AI, Labutti KM, Pitluck S, Bruce D, Hauser L, Copeland A, Woyke T, Lowry S, Lucas S, Land M, Goodwin L, Kjelleberg S, Cook AM, Buhmann M, Thomas T, Schleheck D. Permanent draft genome sequence of Comamonas testosteroni KF-1. Stand Genomic Sci. 2013;8:239–254. doi: 10.4056/sigs.3847890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman WB. Bacteria and the fate of estrogen in the environment. Cell Chem Biol. 2017;24:652–653. doi: 10.1016/j.chembiol.2017.05.028. [DOI] [PubMed] [Google Scholar]
- Wu Q, Lam JCW, Kwok KY, Tsui MMP, Lam PKS. Occurrence and fate of endogenous steroid hormones, alkylphenol ethoxylates, bisphenol A and phthalates in municipal sewage treatment systems. J Environ Sci. 2017;61:49–58. doi: 10.1016/j.jes.2017.02.021. [DOI] [PubMed] [Google Scholar]
- Ye X, Wang H, Kan J, Li J, Huang T, Xiong G. A novel 17β-hydroxysteroid dehydrogenase in Rhodococcus sp. P14 for transforming 17 β-estradiol to estrone. Chem Biol Interact. 2017;276:105–112. doi: 10.1016/j.cbi.2017.06.010. [DOI] [PubMed] [Google Scholar]
- Yu CP, Deeb RA, Chu KH. Microbial degradation of steroidal estrogens. Chemosphere. 2013;91:1225–1235. doi: 10.1016/j.chemosphere.2013.01.112. [DOI] [PubMed] [Google Scholar]