Abstract
Programmed cell death protein 1 (PD-1) is an immune checkpoint modulator and a major target of immunotherapy as anti-PD-1 monoclonal antibodies have demonstrated remarkable efficacy in cancer treatment. Accumulating evidence suggests an important role of PD-1 in the central nervous system (CNS). PD-1 has been implicated in CNS disorders such as brain tumors, Alzheimer’s disease, ischemic stroke, spinal cord injury, multiple sclerosis, cognitive function, and pain. PD-1 signaling suppresses the CNS immune response via resident microglia and infiltrating peripheral immune cells. Notably, PD-1 is also widely expressed in neurons and suppresses neuronal activity via downstream Src homology 2 domain-containing protein tyrosine phosphatase 1 and modulation of ion channel function. An improved understanding of PD-1 signaling in the cross-talk between glial cells, neurons, and peripheral immune cells in the CNS will shed light on immunomodulation, neuromodulation, and novel strategies for treating brain diseases.
Keywords: PD-1, Central nervous system, Immune checkpoint, Immunotherapy, Neurotherapy
Introduction
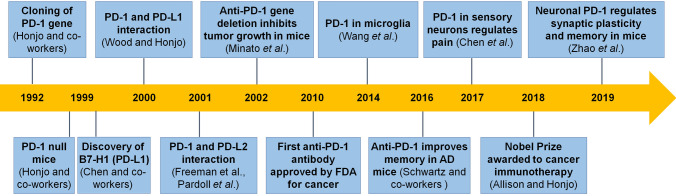
Programmed cell death protein 1 (PD-1, also known as PDCD1 and CD279) is a cell surface receptor which contains 288 amino-acids and is widely expressed in immune cells (T cells, B cells, natural killer cells, dendritic cells, and macrophages) and other cell types (microglia and neurons) (Table 1). In 1992, PD-1 was initially found by the Honjo group at Kyoto University during screening for genes involved in apoptosis [1]. Over the ensuing decades, it has become clear that PD-1 is a negative regulator of immune responses [2, 3] (Fig. 1). PD-1 binds two ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC) [4–7]. PD-L1 is expressed on a variety of hematopoietic and non-hematopoietic cells [8–11]. PD-L2 is mainly restricted to antigen presenting cells (APCs) [8, 12]. Binding to either of these ligands, PD-1 signaling regulates the immune response by down-regulating the immune system and promotes self-tolerance by suppressing T cell inflammatory activity. This inhibitory signaling through the PD-1 pathway is an important mechanism underlying many physiological and pathological conditions. Physiologically, the PD-1 signaling pathway regulates T cell activation, T cell tolerance, and immune hemostasis [13]. Perturbation of the PD-1 pathway can profoundly impact host physiology [14, 15]. Pathologically, PD-1 and its ligands are strongly expressed during many chronic diseases, especially in cancer [16, 17]. In 2002, Minato et al. found that Pdcd1 gene deletion inhibited tumor growth in mice. In 2010, the first clinical trial of anti-PD-1 antibody (BMS-936558/ONO-4538) was launched in Japan for cancer treatment (Fig. 1). Since 2014, several anti-PD-1 monoclonal antibodies such as Nivolumab (Opdivo), Pembrolizumab (Keytruda), and Cemiplimab-rwlc (Libtayo) have been approved by the FDA. Given the success of the emerging immunotherapy with anti-PD-1 and anti-CTLA4 (cytotoxic T-lymphocyte-associated protein 4) monoclonal antibodies in cancer treatment, the 2018 Nobel Prize in Physiology or Medicine was awarded to James P. Allison and Tasuku Honjo for their discovery of cancer therapy by inhibition of negative immune regulation (Fig. 1).
Table 1.
Expression of PD-1 in various tissues and cells
| Tissue or cell with PD-1 expression | Level of expression | Function of expression | References (methods) |
|---|---|---|---|
| B-cells | Low expression in peripheral blood under normal conditions | Inhibits B-cell activation, proliferation, and differentiation |
[102] (FC, RT-PCR) |
| Dendritic cells | Low expression under normal conditions | Restricts T-cell activation and lowers innate immunity |
[103] (FC), [104] (FC, RT-PCR) |
| DRG sensory neurons | Expressed in DRG sensory neurons as well as in axons | Interacts with PD-L1 to modulate pain and lower sensitivity | [19] (IF, ISH, WB), [95, 105] (ISH, IF, PLA, co-IP) |
| Hippocampal neurons | Expressed in hippocampal CA1 and CA3 neurons, low expression in DG neurons | Regulates neuronal excitability, synaptic transmission, plasticity, and memory | [92] (IF, ISH) |
| Macrophages | Low expression under normal conditions | Functions as a control mechanism for systemic immune responses through redirection or delays |
[31] (FC, IF), [106] (FC, RT-PCR), [107] (FC) |
| Microglia | Low expression under normal conditions | Regulates the inflammatory reaction after injury or infection | [23] (RT-PCR, IF) |
| NK cells | Low expression under normal conditions | Prevents NK cell activation and cytotoxicity in specific situations | [108] (FC), [109] (FC, IHC) |
| Retinal ganglion cells | Expressed in almost all adult retinal ganglion cells | Promotes apoptosis, which is necessary for proper maturation | [110] (IF, IHC, WB, RT-PCR), [111] (IF, WB, RT-PCR) |
| Spinal cord | Expressed in spinal neurons, primary afferent terminals, and microglia | Regulates pain, opioid analgesia and tolerance, GABAergic neurotransmission | [95] (IF), [24, 95] (ISH) |
| T-cells | Highly expressed in activated T-cells | Functions as an immune checkpoint receptor | [13, 27, 112] (FC), [113] (IF, FC), [114] (FC, PCR) |
Abbreviations FC, flow cytometry; IHC, immunohistochemical analysis; IF, immunofluorescence; ISH, in situ hybridization; PCR, polymerase chain reaction; RT-PCR, reverse-transcription-polymerase chain reaction; WB, Western blotting; PLA, proximity ligation assay; co-IP, co-immunoprecipitation; DRG, dorsal root ganglion; NK cells, natural killer cells.
Fig. 1.
Timeline for major events leading to the development of PD-1 functions and PD-1-based immunotherapy.
In addition to the prominent role of PD-1 in the immune system, accumulating evidence also suggests an activating role of PD-1 signaling in both the central nervous system (CNS) and the peripheral nervous system (PNS) (Figs. 2–5). PD-1 reduces neuroinflammatory responses and may also regulate neuronal activity in several CNS diseases, such as brain tumors, Alzheimer’s disease, stroke, chronic pain, multiple sclerosis, and cognitive deficits [18, 19]. The mechanisms underlying the actions of PD-1 in these disease conditions are multifaceted. First, the recent progress in demonstrating peripheral immune cell recruitment to the CNS under pathological conditions challenges the historical view of CNS immune privilege. Functional lymphatic vessels in the meninges have recently been discovered that provided a direct drainage pathway for PD-1+ immune cells from the cervical lymph nodes into the brain [20, 21]. Thus, PD-1+ immune cells such as T cells may play a role in the CNS similar to that in the peripheral immune system. Second, PD-1 is expressed by macrophages as well as microglia in the spinal cord and brain [22, 23]. Under CNS disease conditions such as brain trauma and spinal cord injury, brain resident PD-1+ microglia are activated in the spinal cord and brain and PD-1+ macrophages are also recruited to the CNS, where these microglia and macrophages undergo substantial phenotypic changes to regulate neuroinflammation and disease progression [23]. Finally, accumulating evidence demonstrates that PD-1 is also expressed by CNS neurons and that PD-1 signaling in neurons regulates neuronal excitability, synaptic transmission, and plasticity via PD-1/SHP-1 signaling and downstream modulation of ion channels [19, 24].
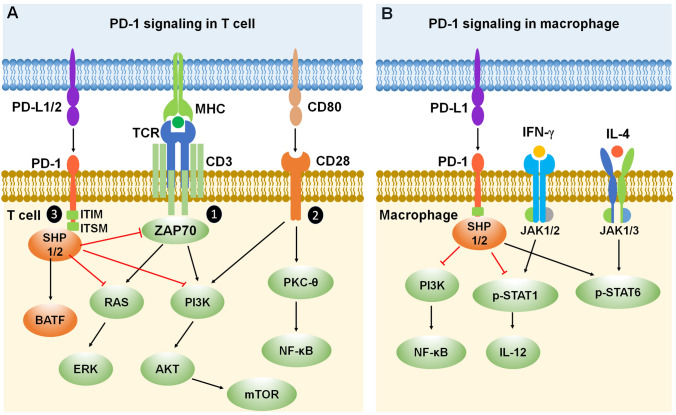
Fig. 2.
PD-1 signaling in T cells and macrophages. A Mechanisms of PD-1 signaling in T cells. PD-1 inhibits T cell function by recruiting phosphatases SHP-1/SHP-2 to the ITIM/ITSM domain in the PD-1 tail and increasing the expression of transcription factor BATF. In addition, PD-1 inhibitory signaling antagonizes positive T cell signaling events triggered by (1) TCR interacting with MHC and (2) CD28 interacting with CD80. (3) PD-1 signaling inhibits ZAP70 and the RAS-ERK and PI3K-AKT-mTOR signaling pathways. B Mechanisms of PD-1 signaling in macrophages. PD-1 inhibits macrophage function by recruiting phosphatases SHP-1/SHP-2 to the ITIM/ITSM domain in the PD-1 tail, leading to inhibition of the PI3K-NF-κB signaling pathway. Moreover, PD-1 signaling suppresses IFN-γ-activated M1 macrophage polarization by reducing the phosphorylation of STAT1 and the secretion of IL-12, while promoting IL-4-activated M2 macrophage polarization by increasing STAT6 phosphorylation. Red lines ending in a bar represent inhibitory signaling, and black arrows indicate positive signaling. Abbreviations: PD-1, programmed cell death protein 1; PD-L1/2, PD-1 ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC); ITIM, immunoreceptor tyrosine-based inhibitory motif; ITSM, immunoreceptor tyrosine-based switch motif; SHP, Src homology 2 domain-containing protein tyrosine phosphatase; BATF, basic leucine zipper ATF-like transcription factor; MHC, major histocompatibility complex; TCR, T cell receptor; CD, cluster of differentiation; ZAP70, zeta-chain-associated protein kinase 70; RAS, a small GTPase encoding RAS (retrovirus-associated DNA sequences); ERK, extracellular signal-regulated kinase; PI3K, type I phosphatidylinositol 3-kinase; AKT, serine/threonine-specific protein kinase; mTOR, mammalian target of rapamycin; PKC-θ, protein kinase C theta; NF-κB, nuclear factor kappa B; p-STAT1/6, phosphorylated signal transducer and activator of transcription 1/6; IL, interleukin; IFN-γ, interferon gamma; JAK, Janus kinase.
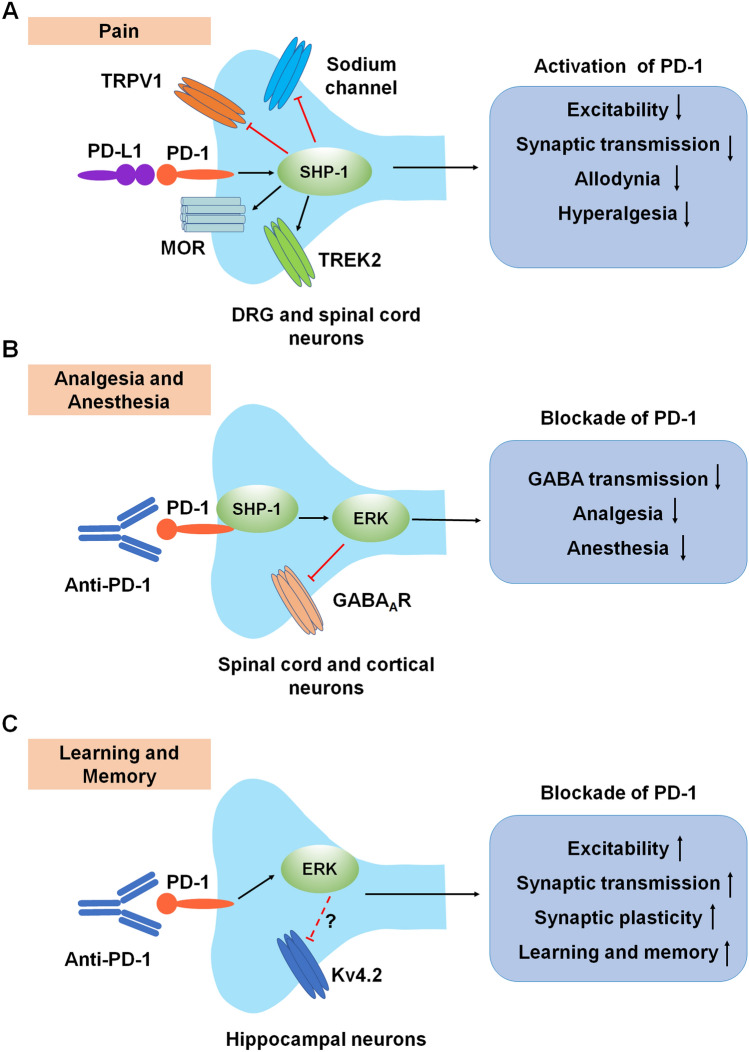
Fig. 5.
Neuromodulation by PD-1 in the PNS and CNS. A Modulation of pain in primary sensory neurons and spinal dorsal horn neurons. Activation of PD-1 signaling in DRG neurons decreases neuronal excitability and synaptic transmission and inhibits physiological pain and pathological pain (allodynia and hyperalgesia) through modulation of ion channels. B Modulation of GABA-mediated analgesia and anesthesia in CNS neurons. C Modulation of learning and memory in hippocampal neurons. Anti-PD-1 antibody treatment increases hippocampal neuronal excitability, synaptic transmission, and synaptic plasticity, thereby enhancing learning and memory. Red lines ending in a bar represent inhibitory signaling, and black arrows indicate positive signaling. Abbreviations: PD-1, programmed cell death protein 1; PD-L1, PD-1 ligand; TRPV1, transient receptor potential subtype V1; MOR, mu-opioid receptor; TREK2, TWIK-related K+ channel-2; DRG, dorsal root ganglion; SHP-1, Src homology 2 domain-containing protein tyrosine phosphatase 1; ERK, extracellular signal-regulated kinase; GABAAR, gamma-aminobutyric acid A receptor; Kv4.2, potassium voltage-gated channel subfamily D member 2.
The role of the PD-1 pathway in the immune system has been elegantly discussed in a number of reviews [25, 26]. In this review, we focus on the diverse roles of PD-1 signaling in the context of the CNS, including in physiological cognitive function as well as pathological conditions such as brain tumors, Alzheimer’s disease, stroke, spinal cord injury, multiple sclerosis, and pain (Table 2). We further discuss how this knowledge can be applied to understanding how the PD-1 pathway can modulate the treatment of CNS diseases. And finally, we consider the challenges and opportunities for utilizing the PD-1 signaling pathway for immunotherapies and neurotherapies in CNS disease conditions.
Table 2.
Brain diseases and other conditions influenced by PD-1 signaling
| Disease or condition | Resources | Role of PD-1 | References |
|---|---|---|---|
| Alzheimer’s disease |
Human patients Mice |
Decreased PD-1 expression decreases amyloid plaques in some studies but not others | [64, 65, 67, 115] |
| Glioblastoma |
Human patients Mice |
Tumor cells interact with PD-1 through the PD-l/PD-L1 axis to increase PD-1 expression, which then decreases T-cell production | [116–119] |
| Melanoma |
Human patients Mice |
Metastatic tumor cells interact with PD-1 through the PD-l/PD-L1 axis to increase PD-1 expression, which then decreases T-cell production | [120–123] |
| Multiple sclerosis |
Human patients Mice |
Increased PD-1 expression correlated with disease remission due to the PD-1/PD-L1 pathway limiting the immune response | [84, 85, 124, 125] |
| Memory | Mice | PD-1 deficiency or blockade in brain improves learning and memory | [92] |
| Pain |
Human patients Mice |
The PD-1/PD-L1 axis has an analgesic effect by suppressing peripheral neuronal excitability and spinal synaptic transmission, thereby reducing pain | [19, 95, 98, 126–128] |
| Spinal cord injury | Mice | PD-1 is highly expressed after spinal injury to restrict the inflammatory response | [23, 81] |
| Stroke |
Human patients Mice |
Increased PD-1 expression linked to reduced post-stroke inflammation, but the PD-1 ligands PD-L1 and PD-L2 play distinct roles in stroke | [22, 71–74] |
Immune Modulation of CNS Disorders by PD-1
PD-1 Signaling in Immune T Cells, Macrophages, and Microglia
PD-1 is widely expressed by immune cells and its signaling pathway is best characterized in activated T cells [27]. Activated T cells receive three signals from APCs during cytokine production, proliferation, differentiation, apoptosis, and survival (Fig. 2A). Signal one consists of TCR-CD3 (T cell receptor and cluster of differentiation 3) and its co-receptor (CD4 or CD8) binding to the major histocompatibility complex (MHC), and subsequently activating the co-receptor associated lymphocyte-specific protein tyrosine kinase (LCK). LCK phosphorylates the intracellular portions of the CD3 complex and creates a docking site for zeta-chain-associated protein kinase 70 (ZAP70), which is expressed near the surface membrane of T cells and plays a crucial role in T-cell signaling. ZAP70 starts multiple signaling events through activation of the RAS-ERK (extracellular signal-regulated kinase) and PI3K-AKT (type I phosphatidylinositol 3-kinase to serine/threonine-specific protein kinase) pathways. Signal two is composed of CD80-CD28 interactions between APCs and T cells. LCK phosphorylates the CD28 intracellular domain, providing a docking site for the PI3K complex. PI3K then generates phosphatidylinositol-(3,4,5)-trisphosphate, activating downstream kinases including AKT, which enhances proliferation and survival through the mammalian/mechanistic target of rapamycin (mTOR) pathway. CD28 activation further activates protein kinase C theta (PKC-θ) and subsequent activation of the nuclear factor kappa B (NF-κB) pathway. Signal three is a result of PD-1 signaling which serves as an antagonist of the two activated pathways noted above. PD-1 has two tyrosine-based signaling motifs in its cytoplasmic domain: an immunoreceptor tyrosine-based inhibitory motif (ITIM) and an immunoreceptor tyrosine-based switch motif (ITSM), both of which are essential for PD-1 function. When engaged with PD-L1, PD-1 counteracts TCR-CD3 signal transduction and terminates ZAP70 and PI3K phosphorylation by recruiting SHP-1 or/and SHP-2 phosphatases to its tyrosine phosphorylated ITIM and ITSM motifs, affecting downstream signaling pathways including those involving PI3K-AKT and RAS-ERK [28]. In addition, PD-1 inhibits T cell functions by increasing the expression of transcription factors such as basic leucine zipper ATF-like transcription factor (BATF), which further counters effector transcriptional programs [29]. The functional outcome of these effects is decreased T cell activation, proliferation, survival, and cytokine production as well as altered metabolism.
Subsequent studies have shown that, in addition to T cells, PD-1 is also expressed by macrophages and microglia (Figs. 2B and 3A), especially under pathological conditions [30, 31]. Macrophages and microglia have different phenotypes such as M1 and M2, which show nearly opposite functionality in the immune system and CNS. M1 macrophages and microglia are highly pro-inflammatory and effective killer cells. M2 macrophages and microglia, on the other hand, are induced by a variety of stimuli, including interleukin-4 (IL-4) [32]. In addition to M1 and M2 phenotypes, macrophages and microglia must have additional phenotypes for maintaining homeostasis and promoting resolution [33–35]. PD-1+ macrophages play the main role in the peripheral immune system, and brain-resident PD-1+ microglia may serve an analogous function in the CNS. In cancer, there is a higher proportion of M2 macrophages among PD-1+ tumor-associated macrophages versus M1 macrophages [31]. Anti-PD-1 therapy stimulates macrophage infiltration into tumors and increases the proportion of M1 over M2 macrophages in tumors. Thus, PD-1 signaling alters the function of macrophages and microglia by affecting the M1/M2 phenotypes during pathological conditions.
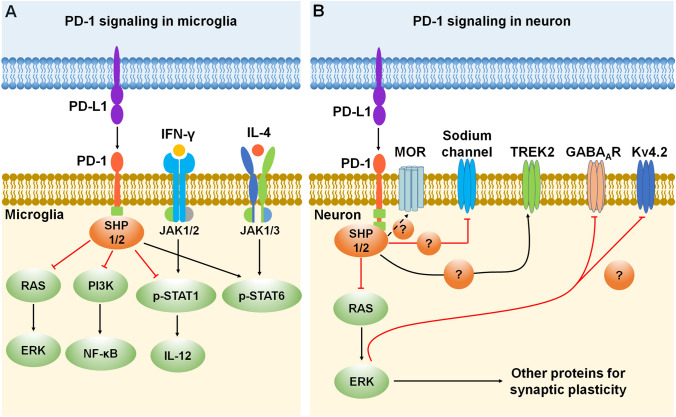
Fig. 3.
PD-1 signaling and expression in microglia and neurons. A Mechanisms of PD-1 signaling in microglia. PD-1 inhibits microglial function by recruiting phosphatases SHP-1/SHP-2 to the ITIM/ITSM domain in the PD-1 tail and then inhibiting the RAS-ERK and PI3K-NF-κB signaling pathways. Moreover, PD-1 signaling suppresses IFN-γ-activated M1 microglia polarization by reducing the phosphorylation of STAT1 and the secretion of IL-12, while promoting IL-4-activated M2 microglia polarization by increasing STAT6 phosphorylation. B Mechanisms of PD-1 signaling in neurons. Activation of the PD-1 pathway dampens neuronal excitation via activation of the phosphatase SHP-1/2 and resulting in the downstream modulation of sodium and potassium channels (TREK2 and Kv4.2), as well as GABAA receptors. Moreover, PD-1 signaling regulates mu-opioid receptor (MOR) function through activation of the phosphatase SHP-1. Red lines ending in a bar represent inhibitory signaling, and black arrows indicate positive signaling. Abbreviations: PD-1, programmed cell death protein 1; PD-L1, PD-1 ligand; SHP, Src homology 2 domain-containing protein tyrosine phosphatase; RAS, a small GTPase encoding RAS (retrovirus-associated DNA sequences); ERK, extracellular signal-regulated kinase; PI3K, type I phosphatidylinositol 3-kinase; NF-κB, nuclear factor kappa B; p-STAT 1/6, phosphorylated signal transducer and activator of transcription 1/6; IL, interleukin; IFN-γ, interferon gamma; JAK, Janus kinase; MOR, mu-opioid receptor; TREK2, TWIK-related K+ channel-2; GABAAR, gamma-aminobutyric acid A receptor; Kv4.2, potassium voltage-gated channel subfamily D member 2.
The canonical interferon (IFN) regulatory factor/STAT (signal transducer and activator of transcription) signaling pathways activated by IFN-γ promote the formation of M1 macrophages via STAT1 activation. In contrast, IL-4 promotes the M2 phenotype via STAT6 activation [36]. PD-1 activation induces M2 polarization of macrophages and microglia through decreased STAT1 phosphorylation and increased STAT6 phosphorylation, as well as the down-regulation of crucial downstream NF-κB signaling, which otherwise could be activated by PI3K (Figs. 2B and 3A). Activation of PD-1 also reduces the production of cytokine IL-12 by macrophages and microglia, which further regulates the function of immune cells and affects pathological conditions [37] (Figs. 2B and 3A). In addition, microglial ERK activation is a critical regulator of pro-inflammatory immune responses in many pathological conditions including neuropathic pain, and PD-1 signaling may regulate the microglial ERK signaling pathway in these conditions [38–41] (Fig. 3A). Finally, PD-1 signaling in neurons shares similarities with that in immune cells and glial cells but also shows clear differences by functional interactions with ion channels (Fig. 3B).
Anti-PD-1 Immunotherapy for Brain Tumors and Brain Metastases
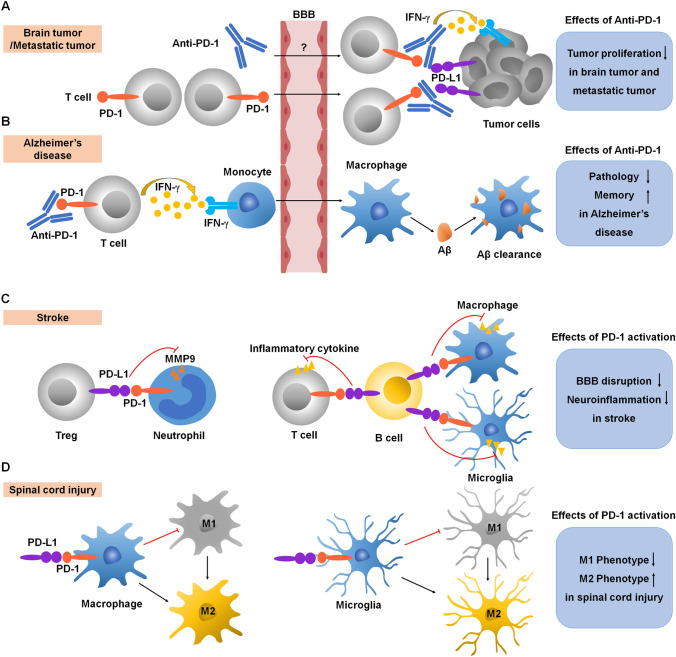
Anti-PD-1 immunotherapies have been used as clinical treatments for brain tumors [42–44]. Anti-PD-1 blocking antibodies have been shown by various studies to cause an increase of T cells in the brain and anti-tumor immunity by mobilizing the immune system (Fig. 4A). Although PD-1 blockade has shown positive effects for treating brain tumors and metastases in cancer patients, the clinical efficacy of PD-1 blockades has shown certain limitations and unpredictability. The characteristics of certain brain tumors can make effective treatment difficult. Glioblastoma (GBM) is the most common and aggressive brain tumor diagnosed in adults but <10% of GBM patients show a long-term response to anti-PD-1 treatment [45]. One of the limiting factors is the blood-brain barrier (BBB), which makes it difficult for anti-PD1 antibodies such as nivolumab to access the tumor. Combining anti-PD-1 antibodies with BBB peptide shuttles enhances delivery of the drug to the brain and efficiently eliminates brain tumor cells [46]. Another limitation of PD-1 blockade to treat brain tumors and metastases is the low immunogenic response and immunosuppressive microenvironment in brain tumors [47]. Immunosuppression mediated by the CNS-native myeloid cells in the tumor microenvironment has been linked to poor outcomes in cancer and a reduced response to immunotherapies. A recent study showed that loss of Cx3cr1 (C-X3-C motif chemokine receptor 1) in CNS-myeloid triggers a Cxcl10 (C-X3-C motif chemokine receptor 10)-mediated vicious cycle, promoting brain metastases and immunosuppression [48]. TREM2 (triggering receptor expressed on myeloid cells 2)-positive myeloid cells have also been shown to mediate immunosuppression in the tumor microenvironment, and TREM2 deficiency or administration of a TREM2 antibody increases the efficacy of anti-PD-1 immunotherapy [49, 50]. Thus, it will be necessary to identify potential biomarkers in patients who could obtain the greatest benefit from anti-PD-1 treatment. A recent study in GBM patients treated with anti-PD-1 immunotherapy showed a significant enrichment of PTEN (phosphatase and tensin homolog deleted on chromosome ten) mutations associated with immunosuppressive expression signatures in non-responders and an enrichment of mitogen-activated protein kinase pathway alterations (PTPN11 and BRAF) in responders [51]. Notably, anti-PD-1 immunotherapy aims to induce a pro-inflammatory environment characterized by increased immune infiltrates into tumors. When this immune checkpoint inhibitor is targeted to treat peripheral tumors, the systemic immune activation may cause central neuroinflammation and associated behavioral and cognitive side-effects. Early clinical studies described some behavioral and cognitive outcomes following anti-PD-1 immunotherapy, including headache, cerebellar ataxia, and transient cognitive dysfunction [52–56]. However, there remains a gap in how these therapies modulate behavioral and cognitive changes. Therefore, pharmacological strategies to cross the BBB and elucidating the mechanisms underlying the immunosuppressive microenvironment, combined with measurement of potential biomarkers that favor anti-PD-1 treatment will improve the efficacy of immunotherapy for clinical brain tumors and metastases, and furthermore, predict adverse CNS events in the treatment of peripheral tumors.
Fig. 4.
Immunomodulation by PD-1 in CNS diseases. A Anti-PD-1 antibody treatment induces IFN-γ–dependent activity and promotes T cell recruitment to the brain for anti-tumor immunotherapy. B Anti-PD-1 antibody treatment evokes a systemic IFN-γ–dependent immune response that enables the mobilization of monocyte-derived macrophages to the brain, thereby reducing pathology and improving memory in Alzheimer’s disease. C Activation of PD-1 signaling suppresses (1) the release of MMPs from neutrophils, protecting the BBB and (2) the release of inflammatory cytokines from T cell and microglia and macrophages, reducing neuroinflammation in stroke. D Activation of PD-1 signaling suppresses microglia and macrophage M1 polarization and promotes M2 polarization in spinal cord injury. Red lines ending in a bar represent inhibitory signaling, and black arrows indicate positive signaling. Abbreviations: PD-1, programmed cell death protein 1; PD-L1, PD-1 ligand; IFN-γ, interferon gamma; BBB, blood-brain barrier; Aβ, β-amyloid; MMP, matrix metallopeptidases.
Anti-PD-1 Immunotherapy in Alzheimer's Disease
Alzheimer's disease (AD) is an age-related neurodegenerative disease and the most common cause of dementia [57]. The pathological hallmarks of AD are the extracellular accumulation of β-amyloid (Aβ) plaques which leads to chronic neuroinflammation in the brain [58]. Expression of PD-1 on T cells and PD-L1 on monocytes and macrophages significantly decreases in AD patients and in patients with mild cognitive impairment compared with age- and sex-matched healthy controls, underscoring the importance of PD-1 signaling in AD [59]. Decreased cytokine IL-10 production has been reported in AD patients [60]. Impairment in PD-1 signaling is associated with inhibition of IL-10 production, suggesting that positive PD-1 signaling boosts IL-10 production. IL-10, in turn, has been shown to limit inflammatory responses and ameliorate AD pathology in animal models [61, 62]. Therefore, activation of PD-1 signaling-related immunoregulatory mechanisms during the progression of AD may help re-establish immune homeostasis. In contrast, in mouse models of AD, the trafficking of blood-borne myeloid cells (monocyte-derived macrophages) to the CNS has also been shown to be neuroprotective. PD-1 blockade evokes a systemic IFN-γ-dependent immune response that enables the mobilization of monocyte-derived macrophages to the brain [63]. In additional studies in rodent AD models, PD-1 blockade reduced cerebral Aβ plaque loads and repeated anti-PD-1 treatment confers long-lasting beneficial effects on AD pathology [64, 65] (Fig. 4b). However, follow-up studies from other groups have shown that inhibition of PD-1 signaling is not sufficient to reduce amyloid pathology in a variety of transgenic AD models [66, 67]. These studies suggest that anti-PD-1 treatments may improve dementia via different mechanisms.
PD-1 Signaling in Stroke
Stroke is a devastating CNS condition in which a sudden interruption of blood flow to the brain results in cell death and clinical symptoms such as trouble understanding speech or speaking, paralysis or numbness of the face, arm, or leg, blurred vision, headache, and loss of coordination [68]. Stroke is associated with strong and persistent neuroinflammation. In stroke, the damaged areas of the brain have massive increases in inflammatory factors, activated local microglia, and disruptions of the BBB. There is also major infiltration into the brain of peripheral immune cells, including macrophages, T-cells, and B-cells. PD-1 signaling in T cells and B cells, as well as microphages and microglia, is involved in post-stroke neuroinflammation [69, 70].
Animal models of stroke have shown increased PD-1 expression in activated microglia and macrophages, and that PD-1 deficiency leads to larger brain infarcts and exacerbated neurological deficits. Thus, activation of the PD-1 inhibitory pathway in microglia and macrophages provides a protective effect after stroke (Fig. 4C) [22, 35]. PD-1 expression on B cells leads to inhibition of inflammatory responses in other immune effector cells, and B cells also produce IL-10 and increase the PD-1 expression by T cells, providing neuroprotection against stroke [22, 69, 70].
Another study showed that T regulatory cells mediate the inhibition of neutrophils through PD-1/PD-L1 signaling, and this interaction protects against BBB disruption by suppressing the expression of matrix metalloproteinase-9 (MMP-9) (Fig. 4C) [71]. However, the particular role of PD-L1 in stroke remains controversial. Some studies have shown that PD-L1 exacerbates inflammation in stroke, and treatment with anti-PD-L1 antibodies can control CNS inflammation. Conversely, other studies have demonstrated that PD-L1 significantly attenuates neurological deficits and provides neuroprotection in stroke [72–74]. These opposing results indicate a dual effect of PD-L1/PD-1 signaling in CNS inflammatory conditions. Notably, MMP-9 inhibition is beneficial in the early phase of stroke but detrimental in the late phase of stroke [75]. Time-dependent modulation of neuroinflammation by different MMPs has also been shown in neuropathic pain after nerve injury [76]. Thus, PD-L1/PD-1 signaling in stroke may lead to positive or negative outcomes depending on the different phases and stages of stroke.
PD-1 Signaling in Spinal Cord Injury
Spinal cord injury is a severe CNS condition in which damage to the spinal cord results in paradoxical loss-of-function (e.g., mobility) and gain-of-function (e.g., neuropathic pain) [77–79]. The inflammatory response plays an important role in its pathogenesis and excessive neuroinflammation aggravates the neurological damage after such injury [79, 80]. PD-1 signaling in T cells as well as macrophages and microglia are involved in neuroinflammation after spinal cord injury. One study has shown that the injury impairs T cell cytokine production, and this T cell dysfunction is a result of increased expression of PD-1. Thus, blocking PD-1 signaling can rescue the T cell functionality in spinal cord injury [81]. In addition, PD-1 signaling modulates macrophage and microglial phenotypes after injury. Specifically, PD-1 signaling has been shown to suppress the M1 polarization/phenotype and promote the M2 polarization/phenotype, thereby mitigating neuroinflammation from microglia and macrophages after spinal cord injury (Fig. 4D) [23, 82]. However, the particular molecular mechanism that connects PD-1 signaling to the M1/M2 phenotypic change needs to be further investigated.
PD-1 Signaling in Multiple Sclerosis
Multiple sclerosis is a chronic inflammatory disorder of the brain and spinal cord characterized by focal lymphocytic infiltration leading to the damage of myelin and axons [83]. Expression of PD-1 on T cells and PD-L1 on APCs is increased in multiple sclerosis patients and an impairment of PD-1 inhibitory signaling on T cells as a result of a PD-1 polymorphism is associated with progression of the disease [84, 85]. Preclinical studies of experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis, also showed that genetic deletion of Pdcd1 or pharmacological blockade of PD-1 enhances the activation and expansion of T-cells and aggravates the pathology in the CNS [86, 87]. Mechanistically, IFN-β has been used to alleviate multiple sclerosis through up-regulation of PD-L1 and inhibition of CNS neuroinflammation [88]. PD-L1/PD-1 signaling regulates cytokine expression in T cells (IFN-γ and IL-17) and B cells (IL-10) [89, 90]. In addition, IL-12, which is mainly produced by APCs, has also been shown to suppress the development of multiple sclerosis through stimulating IFN-γ production in APCs and enhancing downstream PD-1 signaling [91]. Thus, enhancement of PD-L1/PD-1 inhibitory signaling holds promise as a therapeutic strategy for patients suffering from multiple sclerosis.
Neuromodulation by PD-1 in the PNS and CNS
PD-1 has been studied extensively thus far in non-neuronal cells, and now, PD-1 signaling in neurons is gaining increasing attention. Recent studies have shown that PD-1 is expressed in various neuronal populations including dorsal root ganglion (DRG) sensory neurons, spinal cord neurons, and neurons in specific brain regions, such as the hippocampus [19, 24, 92]. In neurons, PD-1 acts as an inhibitor as it does in immune cells. More specifically, PD-1 expression in neurons affects neuronal excitability, synaptic transmission, and synaptic plasticity (Fig. 5).
Intriguingly, PD-1 is involved in diverse neuronal signaling pathways, four of which are further detailed in this section (Figs. 3B and 5). (1) Activation of PD-1 signaling dampens the excitation of neurons, which occurs via the activation of phosphatase SHP-1 and downstream modulation of sodium and potassium channels. PD-1 also affects the characteristics of the neuronal membrane through TWIK-related K+ channel-2 (TREK2), which is a potassium channel involved in the regulation of the resting membrane potential of sensory neurons [19] (Fig. 5A). SHP-1 has also been shown to dephosphorylate transient receptor potential subtype V1 (TRPV1) in DRG neurons and alleviate inflammatory pain in rats [93]. Furthermore, a recent study has shown that conditioned deletion of SHP-1 in NaV1.8+ neurons facilitates bone cancer pain [94]. These studies strongly suggest that the phosphatase SHP-1 in nociceptors acts as a pain suppressant. (2) The co-localization of PD-1 with the mu-opioid receptor (MOR) in DRG neurons regulates opioid antinociception [95] (Fig. 5A). (3) PD-1 regulates GABAergic neurotransmission and GABA-mediated analgesia and anesthesia [24] (Fig. 5B). (4) PD-1 in hippocampal CA1 neurons regulates neuronal excitability and synaptic plasticity [92] (Fig. 5C). Due to the important role of PD-1 in neuronal regulation, PD-1 inhibitors may have both beneficial effects (e.g., learning and memory) and detrimental effects (e.g., pain) under different pathological conditions.
PD-1 is broadly expressed by DRG sensory neurons, as well as spinal cord dorsal horn and ventral horn neurons. PD-1 in mouse and human DRG neurons is further transported by axons to their peripheral and central terminals in the skin and spinal cord, respectively [19]. PD-L1 has an analgesic effect that is mediated by PD-1 in naïve mice, as well as in mouse models of inflammatory, neuropathic, and cancer pain [19, 94, 96]. Activation of the PD-L1/PD-1 pathway suppresses action potentials in mouse and human DRG sensory neurons through the modulation of sodium and potassium channels. Furthermore, the TREK2 potassium channel, which regulates the resting membrane potential in C-fiber nociceptors [97], is potentiated by PD-1/PD-L1 signaling in DRG neurons. These modifications of sodium channels and TREK2 potassium channels are regulated by SHP-1, which is activated by PD-L1 in DRG nociceptive neurons via phosphorylation (Fig. 5A) [19]. PD-L1 also activates SHP-1 to down-regulate TRPV1 in DRG neurons and delay the development of bone cancer pain in mice [94]. While the PD-L1/PD-1 axis produces acute antinociception through neuromodulation, the delayed effects of this pathway may also depend on immunomodulation. In a mouse model of bone cancer pain, anti-PD-1 treatment with Nivolumab initially increased bone cancer pain through neuronal modulation [98]. In contrast, Nivolumab reduced bone cancer pain in the late phase through modulation of osteoclasts and protection against bone destruction. Thus, anti-PD-1 treatment initially increases cancer pain before reducing it at later time points as a result of both neuromodulation and immunomodulation [98].
PD-1 is co-localized with MOR in DRG sensory neurons and their axons in mouse and human (Fig. 5A). Through interaction with MOR, PD-1 regulates the function of opioid receptors in sensory neurons and plays a crucial role in MOR signaling. PD-1 deficiency or blockade impairs morphine-mediated analgesia in mice and nonhuman primates [95]. Morphine produces antinociception via suppression of calcium currents in DRG neurons, inhibition of excitatory synaptic transmission in spinal cord neurons, and induction of outward currents in spinal neurons. But all of these antinociceptive mechanisms are impaired after loss of PD-1 function in Pd1 (or Pdcd1) knockout mice. In addition, loss of PD-1 signaling enhances opioid-induced hyperalgesia and tolerance and potentiates opioid-induced long-term potentiation in the spinal cord [95]. Future studies are warranted to determine how PD-1 interacts with MOR at the molecular level.
Apart from spinal cord neurons, Pd1 mRNA and PD-1 protein are also widely expressed in neurons of many brain regions, including cortical, thalamic, hypothalamic, and hippocampal neurons. Despite low expression levels, PD-1 in CNS neurons is fully functional. Interestingly, PD-1 is required for GABAergic neurotransmission, especially the actions of GABAARs (Fig. 5B). PD-1 blockade with Nivolumab causes a profound reduction (50%) of GABA-currents across the CNS, including lamina IIo and lamina I in the spinal dorsal horn, S1 sensory cortex, the ventral posterior medial and ventral lateral nuclei of the thalamus, hypothalamus, and the hippocampus. GABAergic neurotransmission is known to mediate analgesia and anesthesia, but strikingly, GABA-mediated analgesia and anesthesia are compromised in Pd1−/− mice [24]. Thus, in CNS neurons, PD-1 is coupled to two inhibitory signaling pathways, mediated by opioid receptors and GABA receptors. Strikingly, PD-1 deficiency or blockade leads to enhanced hippocampal learning and memory [92]. Patch-clamp recording has demonstrated that loss of PD-1 increases neuronal excitability, excitatory synaptic transmission, and synaptic plasticity in hippocampal neurons. Because PD-1 suppresses ERK activation, and ERK phosphorylates the Kv4.2 potassium channel to suppress its activity in hippocampal neurons [99], we postulate that PD-1 signaling plays an important role as a neuronal inhibitor in learning and memory by regulating the ERK pathway and Kv4.2 potassium channel activity (Fig. 5C). Anti-PD-1 antibodies may potentially serve as a neurotherapy to improve memory function and counteract cognitive decline.
Conclusions and Future Directions
The expression of PD-1 in immune cells, glial cells, and neurons allows for multiple tiers of immunomodulation and neuromodulation in the CNS. PD-1 acts as an inhibitory receptor in various types of cells. Increasing evidence suggests a critical role of PD-1 signaling in CNS resident microglia and peripherally recruited immune cells, as well as neurons under physiological and pathological conditions. Because PD-1 not only regulates immune responses but also neuronal function, PD-1 modulation can exert a range of neuroimmune effects. Thus, the function of PD-1 signaling in the cross-talk between immune cells, glial cells, and neurons in the CNS needs to be further investigated.
To guide rational PD-1-based immunotherapy and neurotherapy in the CNS, several key issues need to be addressed. (1) PD-1-based therapies used for CNS conditions must overcome the obstacles of the BBB. What is the ideal carrier to deliver anti-PD-1 drugs into the brain? (2) The role of PD-1 signaling in restricting local neuroinflammation in the CNS has not been examined. It will be important to determine whether modulation of the PD-1 signaling pathway during CNS injury or neurodegeneration influences the balance between debris clearance, brain repair, and inflammatory damage. (3) PD-1 ligands (e.g., PD-L1 and PD-L2) are expressed by various cell types. What are the specific contributions of these ligands to glial and neuronal functions in CNS disease conditions? (4) Intracellular signaling of neuronal PD-1 must be distinct from that of immune and glial PD-1 due to unique coupling to ion channels. What are the precise molecular mechanisms underlying PD-1 signaling in microglia and neurons in different CNS disease conditions? It is also important to know how PD-1 is coupled to neuron-specific ion channels. (5) To uncover the precise role of PD-1 signaling in immune cells, glial cells, and neurons, conditional-knockout mice will need to be generated to enable specific PD-1 deletions in different cell types and subtypes (e.g., excitatory versus inhibitory neurons).
Finally, it is important to point out that PD-1 signaling in the CNS can act like a double-edged sword, producing both beneficial and detrimental effects. A temporary PD-1 signaling blockade, for instance, may cause excitatory effects in microglia and neurons providing beneficial effects under physiological conditions, while a more persistent PD-1 signaling blockade may lead to adverse over-excitatory effects. Thus, the challenge for developing new strategies in using PD-1 signaling therapeutically, will be determining which precise neuronal circuits and cell types need to be tuned for different CNS conditions.
Acknowledgements
The work related to this review was partially supported by Duke University Fund.
Conflict of interest
The authors claim that there are no conflicts of interest.
Contributor Information
Junli Zhao, Email: junli.zhao@duke.edu.
Ru-Rong Ji, Email: ru-rong.ji@duke.edu.
References
- 1.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 3.Bardhan K, Anagnostou T, Boussiotis VA. The PD1:PD-L1/2 pathway from discovery to clinical implementation. Front Immunol. 2016;7:550. doi: 10.3389/fimmu.2016.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong HD, Zhu GF, Tamada K, Chen LP. B7–H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 5.Freeman GJ, Long AJ, Iwai Y, Latchman Y, Bourque K, Brown JA, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7-family member leads to negative regulation of lymphocyte activation. Blood. 2000;96:810a–811a. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latchman Y, Wood C, Chemova T, Iwai Y, Malenkovich N, Long A, et al. PD-L2, a novel B7 homologue, is a second ligand for PD-1 and inhibits T cell activation. FASEB J. 2001;15:A345–A345. [Google Scholar]
- 7.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–845. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 9.Sugita S, Usui Y, Horie S, Futagami Y, Aburatani H, Okazaki T, et al. T-cell suppression by programmed cell death 1 ligand 1 on retinal pigment epithelium during inflammatory conditions. Invest Ophthalmol Vis Sci. 2009;50:2862–2870. doi: 10.1167/iovs.08-2846. [DOI] [PubMed] [Google Scholar]
- 10.Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 11.Hu J, He H, Yang Z, Zhu G, Kang L, Jing X, et al. Programmed death ligand-1 on microglia regulates Th1 differentiation via nitric oxide in experimental autoimmune encephalomyelitis. Neurosci Bull. 2016;32:70–82. doi: 10.1007/s12264-015-0010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci U S A. 2003;100:5336–5341. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19:309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/S1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 16.Dong HD, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7–H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 17.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong HD, Webster WS, et al. Costimulatory B7–H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. J Urol. 2005;173:169–169. doi: 10.1016/S0022-5347(18)34858-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao S, Li F, Leak RK, Chen J, Hu X. Regulation of neuroinflammation through programed death-1/programed death ligand signaling in neurological disorders. Front Cell Neurosci. 2014;8:271. doi: 10.3389/fncel.2014.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen G, Kim YH, Li H, Luo H, Liu DL, Zhang ZJ, et al. PD-L1 inhibits acute and chronic pain by suppressing nociceptive neuron activity via PD-1. Nat Neurosci. 2017;20:917–926. doi: 10.1038/nn.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alves de Lima K, Rustenhoven J, Kipnis J. Meningeal immunity and its function in maintenance of the central nervous system in health and disease. Annu Rev Immunol. 2020;38:597–620. doi: 10.1146/annurev-immunol-102319-103410. [DOI] [PubMed] [Google Scholar]
- 22.Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H. Programmed death-1 pathway limits central nervous system inflammation and neurologic deficits in murine experimental stroke. Stroke. 2011;42:2578–2583. doi: 10.1161/STROKEAHA.111.613182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao A, Liu F, Chen K, Tang L, Liu L, Zhang K, et al. Programmed death 1 deficiency induces the polarization of macrophages/microglia to the M1 phenotype after spinal cord injury in mice. Neurotherapeutics. 2014;11:636–650. doi: 10.1007/s13311-013-0254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang C, Wang Z, Donnelly CR, Wang K, Andriessen AS, Tao X, et al. PD-1 regulates GABAergic neurotransmission and GABA-mediated analgesia and anesthesia. iScience. 2020;23:101570. doi: 10.1016/j.isci.2020.101570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 29.Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med. 2010;16:1147–1151. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu D, Ni Z, Liu X, Feng S, Dong X, Shi X, et al. Beyond T Cells: understanding the role of PD-1/PD-L1 in tumor-associated macrophages. J Immunol Res. 2019;2019:1919082. doi: 10.1155/2019/1919082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–499. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci. 2016;19:987–991. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- 34.Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron. 2018;100:1292–1311. doi: 10.1016/j.neuron.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin C, Zhou LQ, Ma XT, Hu ZW, Yang S, Chen M, et al. Dual functions of microglia in ischemic stroke. Neurosci Bull. 2019;35:921–933. doi: 10.1007/s12264-019-00388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohmori Y, Hamilton TA. IL-4-induced STAT6 suppresses IFN-gamma-stimulated STAT1-dependent transcription in mouse macrophages. J Immunol. 1997;159:5474–5482. [PubMed] [Google Scholar]
- 37.Zhang Y, Ma CJ, Ni L, Zhang CL, Wu XY, Kumaraguru U, et al. Cross-talk between programmed death-1 and suppressor of cytokine signaling-1 in inhibition of IL-12 production by monocytes/macrophages in hepatitis C virus infection. J Immunol. 2011;186:3093–3103. doi: 10.4049/jimmunol.1002006. [DOI] [PubMed] [Google Scholar]
- 38.Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 39.Katsura H, Obata K, Mizushima T, Sakurai J, Kobayashi K, Yamanaka H, et al. Activation of Src-family kinases in spinal microglia contributes to mechanical hypersensitivity after nerve injury. J Neurosci. 2006;26:8680–8690. doi: 10.1523/JNEUROSCI.1771-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen G, Luo X, Qadri MY, Berta T, Ji RR. Sex-dependent glial signaling in pathological pain: distinct roles of spinal microglia and astrocytes. Neurosci Bull. 2018;34:98–108. doi: 10.1007/s12264-017-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Q, Xu LX, Du TJ, Hou YX, Fan WJ, Wu QL, et al. Enhanced expression of PD-L1 on microglia after surgical brain injury exerts self-protection from inflammation and promotes neurological repair. Neurochem Res. 2019;44:2470–2481. doi: 10.1007/s11064-019-02864-8. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Guo G, Guan H, Yu Y, Lu J, Yu J. Challenges and potential of PD-1/PD-L1 checkpoint blockade immunotherapy for glioblastoma. J Exp Clin Cancer Res. 2019;38:87. doi: 10.1186/s13046-019-1085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xue S, Hu M, Iyer V, Yu J. Blocking the PD-1/PD-L1 pathway in glioma: a potential new treatment strategy. J Hematol Oncol. 2017;10:81. doi: 10.1186/s13045-017-0455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johanns T, Waqar SN, Morgensztern D. Immune checkpoint inhibition in patients with brain metastases. Ann Transl Med. 2016;4:S9. doi: 10.21037/atm.2016.09.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Filley AC, Henriquez M, Dey M. Recurrent glioma clinical trial, CheckMate-143: the game is not over yet. Oncotarget. 2017;8:91779–91794. doi: 10.18632/oncotarget.21586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cavaco M, Gaspar D, Arb Castanho M, Neves V. Antibodies for the treatment of brain metastases, a dream or a reality? Pharmaceutics 2020, 12. [DOI] [PMC free article] [PubMed]
- 47.Sampson JH, Gunn MD, Fecci PE, Ashley DM. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. 2020;20:12–25. doi: 10.1038/s41568-019-0224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guldner IH, Wang Q, Yang L, Golomb SM, Zhao Z, Lopez JA, et al. CNS-native myeloid cells drive immune suppression in the brain metastatic niche through Cxcl10. Cell. 2020;183:1234–1248. doi: 10.1016/j.cell.2020.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Molgora M, Esaulova E, Vermi W, Hou J, Chen Y, Luo J, et al. TREM2 modulation remodels the tumor myeloid landscape enhancing anti-PD-1 immunotherapy. Cell. 2020;182:886–900. doi: 10.1016/j.cell.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katzenelenbogen Y, Sheban F, Yalin A, Yofe I, Svetlichnyy D, Jaitin DA, et al. Coupled scRNA-Seq and intracellular protein activity reveal an immunosuppressive role of TREM2 in cancer. Cell. 2020;182:872–885. doi: 10.1016/j.cell.2020.06.032. [DOI] [PubMed] [Google Scholar]
- 51.Zhao, Chen AX, Gartrell RD, Silverman AM, Aparicio L, Chu T, et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med. 2019;25:462–469. doi: 10.1038/s41591-019-0349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17:976–983. doi: 10.1016/S1470-2045(16)30053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng S, Coward J, McCaffrey E, Coucher J, Kalokerinos P, O'Byrne K. Pembrolizumab-Induced encephalopathy: a review of neurological toxicities with immune checkpoint inhibitors. J Thorac Oncol. 2017;12:1626–1635. doi: 10.1016/j.jtho.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 54.Kao JC, Liao B, Markovic SN, Klein CJ, Naddaf E, Staff NP, et al. Neurological complications associated with anti-programmed death 1 (PD-1) antibodies. JAMA Neurol. 2017;74:1216–1222. doi: 10.1001/jamaneurol.2017.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McGinnis GJ, Raber J. CNS side effects of immune checkpoint inhibitors: preclinical models, genetics and multimodality therapy. Immunotherapy. 2017;9:929–941. doi: 10.2217/imt-2017-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mirabile A, Brioschi E, Ducceschi M, Piva S, Lazzari C, Bulotta A, et al. PD-1 inhibitors-related neurological toxicities in patients with non-small-cell lung cancer: a literature review. Cancers (Basel) 2019, 11. [DOI] [PMC free article] [PubMed]
- 57.Fan DY, Wang YJ. Early intervention in Alzheimer’s disease: how early is early enough? Neurosci Bull. 2020;36:195–197. doi: 10.1007/s12264-019-00429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bharadwaj PR, Dubey AK, Masters CL, Martins RN, Macreadie IG. Abeta aggregation and possible implications in Alzheimer's disease pathogenesis. J Cell Mol Med. 2009;13:412–421. doi: 10.1111/j.1582-4934.2009.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saresella M, Calabrese E, Marventano I, Piancone F, Gatti A, Farina E, et al. A potential role for the PD1/PD-L1 pathway in the neuroinflammation of Alzheimer's disease. Neurobiol Aging. 2012;33:e611–622. doi: 10.1016/j.neurobiolaging.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Torres KC, Araujo Pereira P, Lima GS, Bozzi IC, Rezende VB, Bicalho MA, et al. Increased frequency of T cells expressing IL-10 in Alzheimer disease but not in late-onset depression patients. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:40–45. doi: 10.1016/j.pnpbp.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 61.Guillot-Sestier MV, Doty KR, Gate D, Rodriguez J, Jr, Leung BP, Rezai-Zadeh K, et al. Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron. 2015;85:534–548. doi: 10.1016/j.neuron.2014.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koronyo-Hamaoui M, Ko MHK, Koronyo Y, Azoulay D, Seksenyan A, Kunis G, et al. Attenuation of AD-like neuropathology by harnessing peripheral immune cells: local elevation of IL-10 and MMP-9. J Neurochem. 2009;111:1409–1424. doi: 10.1111/j.1471-4159.2009.06402.x. [DOI] [PubMed] [Google Scholar]
- 63.Kunis G, Baruch K, Rosenzweig N, Kertser A, Miller O, Berkutzki T, et al. IFN-gamma-dependent activation of the brain's choroid plexus for CNS immune surveillance and repair. Brain. 2013;136:3427–3440. doi: 10.1093/brain/awt259. [DOI] [PubMed] [Google Scholar]
- 64.Rosenzweig N, Dvir-Szternfeld R, Tsitsou-Kampeli A, Keren-Shaul H, Ben-Yehuda H, Weill-Raynal P, et al. PD-1/PD-L1 checkpoint blockade harnesses monocyte-derived macrophages to combat cognitive impairment in a tauopathy mouse model. Nat Commun. 2019;10:465. doi: 10.1038/s41467-019-08352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baruch K, Deczkowska A, Rosenzweig N, Tsitsou-Kampeli A, Sharif AM, Matcovitch-Natan O, et al. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer's disease. Nat Med. 2016;22:135–137. doi: 10.1038/nm.4022. [DOI] [PubMed] [Google Scholar]
- 66.Lin Y, Rajamohamedsait HB, Sandusky-Beltran LA, Gamallo-Lana B, Mar A, Sigurdsson EM. Chronic PD-1 checkpoint blockade does not affect cognition or promote tau clearance in a tauopathy mouse model. Front Aging Neurosci. 2019;11:377. doi: 10.3389/fnagi.2019.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Latta-Mahieu M, Elmer B, Bretteville A, Wang YM, Lopez-Grancha M, Goniot P, et al. Systemic immune-checkpoint blockade with anti-PD1 antibodies does not alter cerebral amyloid-beta burden in several amyloid transgenic mouse models. Glia. 2018;66:492–504. doi: 10.1002/glia.23260. [DOI] [PubMed] [Google Scholar]
- 68.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/S0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 69.Bodhankar S, Chen YX, Vandenbark AA, Murphy SJ, Offner H. IL-10-producing B-cells limit CNS inflammation and infarct volume in experimental stroke. Metab Brain Dis. 2013;28:375–386. doi: 10.1007/s11011-013-9413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ren XF, Akiyoshi K, Dziennis S, Vandenbark AA, Herson PS, Hurn PD, et al. Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J Neurosci. 2011;31:8556–8563. doi: 10.1523/JNEUROSCI.1623-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li PY, Mao LL, Liu XR, Gan Y, Zheng J, Thomson AW, et al. Essential role of program death 1-ligand 1 in regulatory T-cell-afforded protection against blood-brain barrier damage after stroke. Stroke. 2014;45:857–864. doi: 10.1161/STROKEAHA.113.004100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bodhankar S, Chen YX, Lapato A, Dotson AL, Wang JM, Vandenbark AA, et al. PD-L1 monoclonal antibody treats ischemic stroke by controlling central nervous system inflammation. Stroke. 2015;46:2926–2934. doi: 10.1161/STROKEAHA.115.010592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. PD-L1 enhances CNS inflammation and infarct volume following experimental stroke in mice in opposition to PD-1. J Neuroinflammation. 2013;10:111. doi: 10.1186/1742-2094-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han R, Luo J, Shi Y, Yao Y, Hao J. PD-L1 (programmed death ligand 1) protects against experimental intracerebral hemorrhage-induced brain injury. Stroke. 2017;48:2255–2262. doi: 10.1161/STROKEAHA.117.016705. [DOI] [PubMed] [Google Scholar]
- 75.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 76.Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14:331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li G, Fan ZK, Gu GF, Jia ZQ, Zhang QQ, Dai JY, et al. Epidural spinal cord stimulation promotes motor functional recovery by enhancing oligodendrocyte survival and differentiation and by protecting myelin after spinal cord injury in rats. Neurosci Bull. 2020;36:372–384. doi: 10.1007/s12264-019-00442-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang Q, Duan W, Sivanesan E, Liu S, Yang F, Chen Z, et al. Spinal cord stimulation for pain treatment after spinal cord injury. Neurosci Bull. 2019;35:527–539. doi: 10.1007/s12264-018-0320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009;60:202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zha J, Smith A, Andreansky S, Bracchi-Ricard V, Bethea JR. Chronic thoracic spinal cord injury impairs CD8+ T-cell function by up-regulating programmed cell death-1 expression. J Neuroinflammation. 2014;11:65. doi: 10.1186/1742-2094-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.He HF, Zhou YY, Zhou YL, Zhuang JY, He X, Wang SY, et al. Dexmedetomidine mitigates microglia-mediated neuroinflammation through upregulation of programmed cell death protein 1 in a rat spinal cord injury model. J Neurotrauma. 2018;35:2591–2603. doi: 10.1089/neu.2017.5625. [DOI] [PubMed] [Google Scholar]
- 83.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 84.Trabattoni D, Saresella M, Pacei M, Marventano I, Mendozzi L, Rovaris M, et al. Costimulatory pathways in multiple sclerosis: distinctive expression of PD-1 and PD-L1 in patients with different patterns of disease. J Immunol. 2009;183:4984–4993. doi: 10.4049/jimmunol.0901038. [DOI] [PubMed] [Google Scholar]
- 85.Kroner A, Mehling M, Hemmer B, Rieckmann P, Toyka KV, Maurer M, et al. A PD-1 polymorphism is associated with disease progression in multiple sclerosis. Ann Neurol. 2005;58:50–57. doi: 10.1002/ana.20514. [DOI] [PubMed] [Google Scholar]
- 86.Ortler S, Leder C, Mittelbronn M, Zozulya AL, Knolle PA, Chen L, et al. B7–H1 restricts neuroantigen-specific T cell responses and confines inflammatory CNS damage: implications for the lesion pathogenesis of multiple sclerosis. Eur J Immunol. 2008;38:1734–1744. doi: 10.1002/eji.200738071. [DOI] [PubMed] [Google Scholar]
- 87.Salama AD, Chitnis T, Imitola J, Ansari MJ, Akiba H, Tushima F, et al. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med. 2003;198:71–78. doi: 10.1084/jem.20022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harari D, Kuhn N, Abramovich R, Sasson K, Zozulya AL, Smith P, et al. Enhanced in vivo efficacy of a type I interferon superagonist with extended plasma half-life in a mouse model of multiple sclerosis. J Biol Chem. 2014;289:29014–29029. doi: 10.1074/jbc.M114.602474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carter LL, Leach MW, Azoitei ML, Cui J, Pelker JW, Jussif J, et al. PD-1/PD-L1, but not PD-1/PD-L2, interactions regulate the severity of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2007;182:124–134. doi: 10.1016/j.jneuroim.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 90.Bodhankar S, Wang C, Vandenbark AA, Offner H. Estrogen-induced protection against experimental autoimmune encephalomyelitis is abrogated in the absence of B cells. Eur J Immunol. 2011;41:1165–1175. doi: 10.1002/eji.201040992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng X, Zhao Z, Ventura E, Gran B, Shindler KS, Rostami A. The PD-1/PD-L pathway is up-regulated during IL-12-induced suppression of EAE mediated by IFN-gamma. J Neuroimmunol. 2007;185:75–86. doi: 10.1016/j.jneuroim.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao J, Ji RR. Anti-PD-1 treatment as a neurotherapy to enhance neuronal excitability, synaptic plasticity and memory. BioRxiv. 2019 doi: 10.1101/870600. [DOI] [Google Scholar]
- 93.Xiao X, Zhao XT, Xu LC, Yue LP, Liu FY, Cai J, et al. Shp-1 dephosphorylates TRPV1 in dorsal root ganglion neurons and alleviates CFA-induced inflammatory pain in rats. Pain. 2015;156:597–608. doi: 10.1097/01.j.pain.0000460351.30707.c4. [DOI] [PubMed] [Google Scholar]
- 94.Liu BL, Cao QL, Zhao X, Liu HZ, Zhang YQ. Inhibition of TRPV1 by SHP-1 in nociceptive primary sensory neurons is critical in PD-L1 analgesia. JCI Insight 2020, 5. [DOI] [PMC free article] [PubMed]
- 95.Wang Z, Jiang C, He Q, Matsuda M, Han Q, Wang K, et al. Anti-PD-1 treatment impairs opioid antinociception in rodents and nonhuman primates. Sci Transl Med 2020, 12. [DOI] [PMC free article] [PubMed]
- 96.Sheng HY, Zhang YQ. Emerging molecular targets for the management of cancer pain. Neurosci Bull. 2020;36:1225–1228. doi: 10.1007/s12264-020-00526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Acosta C, Djouhri L, Watkins R, Berry C, Bromage K, Lawson SN. TREK2 expressed selectively in IB4-binding C-fiber nociceptors hyperpolarizes their membrane potentials and limits spontaneous pain. J Neurosci. 2014;34:1494–1509. doi: 10.1523/JNEUROSCI.4528-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang K, Gu Y, Liao Y, Bang S, Donnelly CR, Chen O, et al. PD-1 blockade inhibits osteoclast formation and murine bone cancer pain. J Clin Invest. 2020;130:3603–3620. doi: 10.1172/JCI133334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci. 2002;22:4860–4868. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thibult ML, Mamessier E, Gertner-Dardenne J, Pastor S, Just-Landi S, Xerri L, et al. PD-1 is a novel regulator of human B-cell activation. Int Immunol. 2013;25:129–137. doi: 10.1093/intimm/dxs098. [DOI] [PubMed] [Google Scholar]
- 101.Goodman A, Patel SP, Kurzrock R. PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev Clin Oncol. 2017;14:203–220. doi: 10.1038/nrclinonc.2016.168. [DOI] [PubMed] [Google Scholar]
- 102.Wang X, Wang G, Wang Z, Liu B, Han N, Li J, et al. PD-1-expressing B cells suppress CD4+ and CD8+ T cells via PD-1/PD-L1-dependent pathway. Mol Immunol. 2019;109:20–26. doi: 10.1016/j.molimm.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 103.Lim TS, Chew V, Sieow JL, Goh S, Yeong JP, Soon AL, et al. PD-1 expression on dendritic cells suppresses CD8+ T cell function and antitumor immunity. Oncoimmunology. 2016;5:e1085146. doi: 10.1080/2162402X.2015.1085146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yao S, Wang S, Zhu Y, Luo L, Zhu G, Flies S, et al. PD-1 on dendritic cells impedes innate immunity against bacterial infection. Blood. 2009;113:5811–5818. doi: 10.1182/blood-2009-02-203141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qin W, Hu L, Zhang X, Jiang S, Li J, Zhang Z, et al. The diverse function of PD-1/PD-L pathway beyond cancer. Front Immunol. 2019;10:2298. doi: 10.3389/fimmu.2019.02298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bally AP, Lu P, Tang Y, Austin JW, Scharer CD, Ahmed R, et al. NF-κB regulates PD-1 expression in macrophages. J Immunol. 2015;194:4545–4554. doi: 10.4049/jimmunol.1402550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Norris S, Coleman A, Kuri-Cervantes L, Bower M, Nelson M, Goodier MR. PD-1 expression on natural killer cells and CD8+ T cells during chronic HIV-1 infection. Viral Immunol. 2012;25:329–332. doi: 10.1089/vim.2011.0096. [DOI] [PubMed] [Google Scholar]
- 109.Concha-Benavente F, Kansy B, Moskovitz J, Moy J, Chandran U, Ferris RL. PD-L1 mediates dysfunction in activated PD-1+ NK cells in head and neck cancer patients. Cancer Immunol Res. 2018;6:1548–1560. doi: 10.1158/2326-6066.CIR-18-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen L, Sham CW, Chan AM, Francisco LM, Wu Y, Mareninov S, et al. Role of the immune modulator programmed cell death-1 during development and apoptosis of mouse retinal ganglion cells. Invest Ophthalmol Vis Sci. 2009;50:4941–4948. doi: 10.1167/iovs.09-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang W, Chan A, Qin Y, Kwong JMK, Caprioli J, Levinson R, et al. Programmed cell death-1 is expressed in large retinal ganglion cells and is upregulated after optic nerve crush. Exp Eye Res. 2015;140:1–9. doi: 10.1016/j.exer.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fourcade J, Sun ZJ, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–138. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 114.Mizuno R, Sugiura D, Shimizu K, Maruhashi T, Watada M, Okazaki IM, et al. PD-1 primarily targets TCR signal in the inhibition of functional T cell activation. Front Immunol. 2019;10:630. doi: 10.3389/fimmu.2019.00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schwartz M, Arad M, Ben-Yehuda H. Potential immunotherapy for Alzheimer disease and age-related dementia. Dialogues Clin Neurosci. 2019;21:21–25. doi: 10.31887/DCNS.2019.21.1/mschwartz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25:477–486. doi: 10.1038/s41591-018-0337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Caccese M, Indraccolo S, Zagonel V, Lombardi G. PD-1/PD-L1 immune-checkpoint inhibitors in glioblastoma: A concise review. Crit Rev Oncol Hematol. 2019;135:128–134. doi: 10.1016/j.critrevonc.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 118.Litak J, Mazurek M, Grochowski C, Kamieniak P, Rolinski J. PD-L1/PD-1 axis in glioblastoma multiforme. Int J Mol Sci 2019, 20. [DOI] [PMC free article] [PubMed]
- 119.Hardcastle J, Mills L, Malo CS, Jin F, Kurokawa C, Geekiyanage H, et al. Immunovirotherapy with measles virus strains in combination with anti-PD-1 antibody blockade enhances antitumor activity in glioblastoma treatment. Neuro-Oncol. 2017;19:493–502. doi: 10.1093/neuonc/now179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hugo W, Zaretsky JM, Sun L, Song CY, Moreno BH, Hu-Lieskovan S, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165:35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kleffel S, Posch C, Barthel SR, Mueller H, Schlapbach C, Guenova E, et al. Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell. 2015;162:1242–1256. doi: 10.1016/j.cell.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Matson V, Fessler J, Bao R, Chongsuwat T, Zha YY, Alegre ML, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pittet CL, Newcombe J, Prat A, Arbour N. Human brain endothelial cells endeavor to immunoregulate CD8 T cells via PD-1 ligand expression in multiple sclerosis. J Neuroinflammation. 2011;8:155. doi: 10.1186/1742-2094-8-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Javan MR, Aslani S, Zamani MR, Rostamnejad J, Asadi M, Farhoodi M, et al. Downregulation of immunosuppressive molecules, PD-1 and PD-L1 but not PD-L2, in the patients with multiple sclerosis. Iran J Allergy Asthma Immunol. 2016;15:296–302. [PubMed] [Google Scholar]
- 126.Shi S, Han Y, Wang D, Guo P, Wang J, Ren T, et al. PD-L1 and PD-1 expressed in trigeminal ganglia may inhibit pain in an acute migraine model. Cephalalgia. 2020;40:288–298. doi: 10.1177/0333102419883374. [DOI] [PubMed] [Google Scholar]
- 127.Hirth M, Gandla J, Kuner R. A checkpoint to pain. Nat Neurosci. 2017;20:897–899. doi: 10.1038/nn.4586. [DOI] [PubMed] [Google Scholar]
- 128.Zhang J, Zhang H, Luo Y. Association between activation of the programmed cell death-1 (PD-1)/programmed death-ligand 1 (PD-L1) pathway and pain in patients with cancer. Med Sci Monit. 2019;25:1275–1282. doi: 10.12659/MSM.912632. [DOI] [PMC free article] [PubMed] [Google Scholar]