Abstract
Background: Depression is a leading cause of disability, burdened by high levels of non-response to conventional antidepressants. Novel therapeutic strategies targeting non-monoaminergic pathways are sorely needed. The widely available and safe statins have several putative mechanisms of action, especially anti-inflammatory, which make them ideal candidates for repurposing in the treatment of depression. A large number of articles has been published on this topic. The aim of this study is to assess this literature according to evidence-based medicine principles to inform clinical practise and research.
Methods: We performed a systematic review of the electronic databases MEDLINE, CENTRAL, Web of Science, CINAHL, and ClinicalTrials.gov, and an unstructured Google Scholar and manual search, until the 9th of April 2021, for all types of clinical studies assessing the effects of statins in depression.
Results: Seventy-two studies were retrieved that investigated the effects of statins on the risk of developing depression or on depressive symptoms in both depressed and non-depressed populations. Fifteen studies specifically addressed the effects of statins on inflammatory-related symptoms of anhedonia, psychomotor retardation, anxiety, and sleep disturbances in depression. Most studies suggested a positive effect of statins on the occurrence and severity of depression, with fewer studies showing no effect, while a minority indicated some negative effects.
Limitations: We provide a narrative report on all the included studies but did not perform any quantitative analysis, which limits the strength of our conclusions.
Conclusions: Robust evidence indicates that statins are unlikely to lead to depressive symptoms in the general population. Promising data suggest a potential role for statins in the treatment of depression. Further clinical studies are needed, especially in specific subgroups of patients identified by pre-treatment assessments of inflammatory and lipid profiles.
Keywords: statin, HMG 3-hydroxy-3-methylglutaryl, depression, antidepressant, mechanism, review
Introduction
1a Depression
Depression is a major contributor to the worldwide burden of disease (1). First-line antidepressant treatments are widely accessible but hampered by some critical issues: significant side effects, delayed therapeutic onset, and limited efficacy (2). Indeed the response rate of antidepressants ranges between 50 and 60% (3), and about one-third of depressed patients remain symptomatic after four treatment steps over one year (4). Most of the currently used antidepressants primarily affect monoaminergic (i.e., serotonin or 5-hydroxytryptamine, 5HT; noradrenaline, NA; dopamine, DA) neurotransmission (5), and scarce progress has been made over the last several years to develop novel antidepressant drugs. An innovative and promising approach favours instead the repurposing of existing medication with a well-defined safety profile and capable of targeting emerging physiopathological pathways implicated in depression (6).
Systemic and central nervous system (CNS) inflammatory processes appear causally involved in at least certain subtypes of depressive disorders (7). Inflammatory molecules such as C-reactive protein (CRP) and interleukin (IL)-6 are increased in depressed patients' peripheral blood (8, 9) and cerebrospinal fluid. Moreover, the prevalence of depression is higher among patients suffering from immune-mediated inflammatory disorders (10), and immunomodulation improves their depressive symptomatology irrespective of their effects on physical illness (11). Increased inflammatory markers have also been associated with specific subgroups of depressed patients, particularly those responding poorly to conventional antidepressants (12, 13), and those with high levels of anxiety (14), sleep disturbance (15), anhedonia (16), and psychomotor retardation (17, 18) — a cluster of symptoms that have been referred to as “depressive-inflammatory.” Therefore, targeting inflammation in depression may be a viable treatment strategy, and recent meta-analyses have described encouraging effects of anti-inflammatory agents as adjunctive treatments in depressed patients (19).
1b Statins in Depression
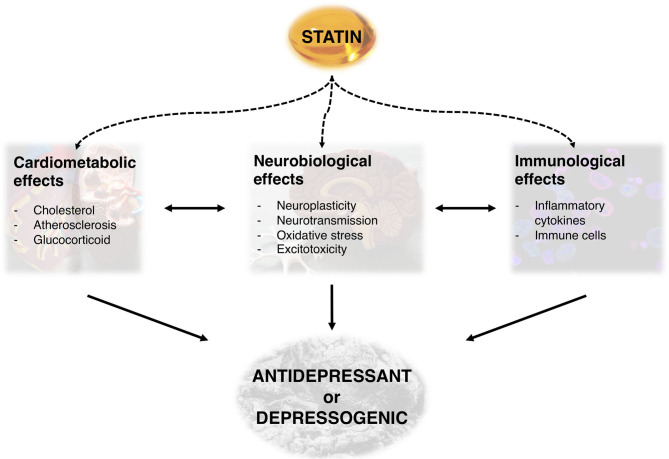
Statins or 3-hydroxy-3-methylglutaryl-Coenzyme A (HMG-CoA) reductase inhibitors are a class of medications capable of reducing blood cholesterol and a mainstay treatment for several cardiovascular, cerebrovascular, and metabolic disorders (20). Thanks to their well-established safety profile (21) and their ability to modulate inflammatory processes (22), they are considered excellent candidates for repurposing in the treatment of depression (23). Indeed, statins seem to possess numerous, and sometimes clashing neurobiological, cardiometabolic, and immunological effects (Figure 1) that might explain both their antidepressant and depressogenic properties (24), as we describe in the paragraphs below.
Figure 1.
Overview of potential mechanisms underlying the antidepressant or depressogenic effects of statins.
Firstly, the primary effect of statins on lipid metabolism, extensively interconnected with inflammatory processes (25), may have profound influences on the anatomy and physiology of the nervous system (26), and potentially interact with neurobiological pathways implicated in depression (27) and antidepressant mechanisms (28, 29). Moreover, in vitro studies show that statin-mediated cholesterol depletion alters 5HT1A receptor dynamics (30) and impairs the formation and release of synaptic vesicles (31). Some authors also suggest that, on the basis of the “vascular depression hypothesis” (32), the antidepressant effects of statins can be explained by their potential to prevent cerebrovascular accidents and therefore improve quality of life (33).
Statins however can affect neuronal homeostasis in several other ways that are not directly due to their action on cholesterol. There is extensive evidence that inflammation (34) and hypothalamic-pituitary-adrenal (HPA) (35) axis disturbances play a role in the pathophysiology of depression. The anti-inflammatory effects of statins are rapid (36) and independent of their lipid-lowering properties (37). Animal studies show that statins reduce depressive-like symptoms by reducing hippocampal neuroinflammation (38) and more broadly by inhibiting cytokine release in the central nervous system (CNS) and countering microglial and astrocyte activation (39–41). Likewise, human studies suggest that statins might affect mood by offsetting the peripheral pro-inflammatory effects of IL-6 and IL-18 (42). Statins also seem to reduce glucocorticoid levels in rats (43), although they increase serum cortisol in humans (44), which could be associated to the onset depressive symptoms. The depressive and anxiety behaviours caused in rats by chronic mild stress (45) or high-fat diet (46) were counteracted by statins, similarly to antidepressant medications. The depressogenic effect of reactive oxygen species (ROS) in the brain (47) could be reduced by statins both directly (48) and via peroxisome proliferator-activated receptor (PPAR)-γ activity and decreased nitrous oxide (NO) levels (49, 50) according to animal studies. Furthermore, glutamatergic N-methyl-D-aspartate (NMDA)-induced neuronal damage (51), whose modulation is associated to the mechanism of action of ketamine (52), is similarly affected by statins via direct (53) and PI3K/AKT/GSK3b/mTOR-mediated (54) antagonism.
Aside from these broadly neuroprotective effects, statins may also promote hippocampal neuroplasticity, especially implicated in the pathophysiology of depression and response to antidepressant treatment (55), through the increase of brain-derived neurotrophic factor (BDNF) via direct (53, 56) and tissue plasminogen activator (tPA) (57–59) and agmatine/imidazoline (60) pathways. However, higher hippocampal BDNF has also been associated to increased anxious behaviour in rats treated with statins (61).
Finally, the effects of statins on neurotransmitter turnover have also been reviewed. Conventional antidepressants act predominantly by modulating monoamines (dopamine, noradrenaline, serotonin) in the synaptic cleft (62). In this context, statins increase hippocampal serotonin levels (43), and induce serotonergic-dependent antidepressant-like effect that are counteracted by 5HT1A and 5HT2A/C receptor antagonists (63) and upregulate pre-frontal dopamine receptors expression (64) in animal models of depression. Moreover, statins seem to directly potentiate the serotonergic effects of some antidepressants in animals (65–67) and possibly in human trials (68). Non-monoaminergic pathways have also been explored in animal models of depression, showing that statins may elicit antidepressant action via opioid- (69) and endocannabinoid-mediated (70) neurotransmission.
Most of the evidence presented above comes from in vitro or animal studies, probably since many of the proposed antidepressant mechanisms of statins, especially in the CNS, are difficult to investigate in humans (24). Human clinical studies have been less consistent and reported both antidepressant and depressogenic effects.
1c Aim of the Study
Our review aims to systematically research and describe the literature regarding the role of statins in depression both in the general population and in depressed patients. Therefore, we searched for any studies that investigated the use of or exposure to statins in both depressed and non-depressed participants and their association with the risk of developing depression or their effect on depressive symptoms scores. We also specifically retrieved studies exploring the effect of statins on the symptom domains that appear associated with an inflammatory phenotype of depression, namely anhedonia, psychomotor retardation, anxiety, and sleep disturbance. This enabled us to discuss the available data and propose ways that it could be complemented by research.
Methods
We conducted an extensive literature search of the PubMed/MEDLINE, Cochrane CENTRAL, ISI Web of Science, CINAHL, and ClinicalTrials.gov databases from the date of inception until the 9th of April 2021, including non-English language articles. We used a well-validated search algorithm (PROSPERO international prospective register of systematic reviews reference CRD42020170938, available at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=170938) based on our previously conducted systematic review and meta-analysis of randomised controlled trials (71), which combined all the relevant terms for statins, depression, and antidepressants (Supplementary Material). References of the included papers were manually screened for further relevant material, and an unstructured search of Google Scholar was performed for additional grey literature. We contacted the corresponding authors to obtain information about unpublished or incomplete trials as reported on ClinicalTrials.gov.
All studies that reported on any statin and their effect on depression, mood scores, or depressive-inflammatory symptoms in depressed patients were included. We excluded in vitro and animal studies, though we summarised these in the introduction. We did not exclude on the basis of study design, comorbidity, concurrent medication use, outcome measures, or length of follow-up as not to compromise the inclusiveness of the review. We excluded previous narrative reviews, commentaries, protocols, and articles that did not report on the intervention/exposure (i.e., any statin) or outcome (i.e., depression, mood scores, depressive-inflammatory symptoms in depressed patients) of interest.
Three researchers (AQ, NRP, RDG) independently screened titles and abstracts for relevance and assessed the full texts for eligibility. Disagreements were discussed with a fourth researcher (FDC) and resolved by consensus. For the included studies, two researchers (AQ, NRP) extracted data about authors' names, year of publication, study design, sample size and characteristics, intervention/exposure, comparison, length of follow-up, primary outcome measures, and point estimates.
Finally, we identified four areas of study (i.e., the effects of statins on the risk of developing depression in non-depressed patients; on depressive symptoms scores in non-depressed patients; on the risk of developing depressive episodes or depressive symptoms scores in depressed patients; on depressive-inflammatory symptoms in depressed patients), and narratively described each article in its context.
Results
3a Studies Included
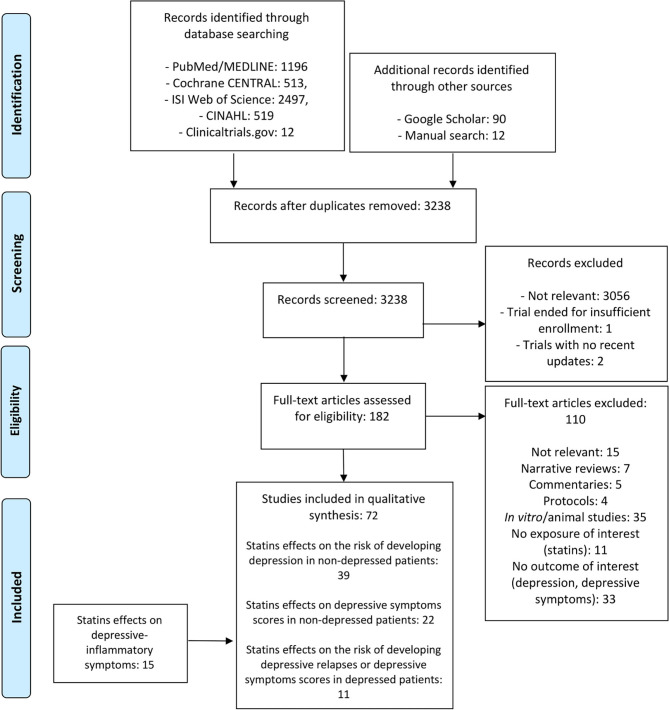
Our search was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (Figure 2) and retrieved 4,725 records from electronic databases (PubMed/MEDLINE = 1,196, Cochrane CENTRAL = 513, ISI Web of Science = 2,497, CINAHL = 519, Clinicaltrials.gov = 12) and further 102 papers from the Google Scholar and manual searches. After duplicates removal (= 1,589), 3,238 titles and abstracts were screened, of which 3,056 were excluded due to lack of relevance. The remaining 182 articles were assessed in full; a total of 110 were excluded as narrative reviews without meta-analysis (=7), commentaries (=5), protocols (=4), non-relevant (=15), in vitro/animal studies (=35), or lacking the intervention/exposure (= 11) or outcome (=33) of interest. The remaining 72 studies (Tables 1–3) were divided into three categories: effects of statins on the risk of developing depression in non-depressed patients (=39); effects of statins on depressive symptoms scores in non-depressed patients (=22); effects of statins on the risk of developing depressive episodes or depressive symptoms scores in depressed patients (=11); and a fourth category (Table 4) which included 15 studies that specifically investigated effects of statins on depressive-inflammatory symptoms in depressed patients. For all the included studies, we first attempted to report the effect sizes, either extracted from the study or calculated by the authors. Where this was not possible, we report the raw data available in the individual study.
Figure 2.
Flow chart of the included/excluded studies.
Table 1.
Overview of studies regarding the effects of statins on depression diagnosis in non-depressed participants.
| Publication | Study design | Population | Exposure | Comparison | Follow-up | Primary outcomes | Major Findings* | Association |
|---|---|---|---|---|---|---|---|---|
| Meta-analysis | ||||||||
| Lee et al. (72) | Meta-analysis | 13 observational studies, 5,035,070 participants | Statin use | No use | NR | Diagnosis of depression | OR = 0.87 95% CI = 0.74 to 1.02 P = NR | = |
| Parsaik et al. (73) | Meta-analysis | 7 observational studies, 9,187 participants | Statin use | No use | 5 years | Diagnosis of depression | OR = 0.68 95% CI = 0.52–0.89 P = 0.01 | + |
| Cohort studies | ||||||||
| Asplund and Eriksson (74) | Cohort | 70,706? ischemic stroke patients | Discharge on statin | No prescription | 3 months | Diagnosis of post-stroke depression or antidepressant prescription (self-reported) | OR = 0.99 95% CI = 0.95–1.03 P = NR | = |
| Chuang et al. (75) | Historical cohort | 26,852 hyperlipidaemic patients | Statin use | No use | 4 years | Diagnosis of depression | HR = 0.81 95% CI = 0.69–0.96 P < 0.05 | + |
| Dave et al. (76) | Historical cohort | 299,298 statin users | Lipophilic statin use (atorvastatin, lovastatin, simvastatin) | Hydrophilic statin use (pravastatin, rosuvastatin) | 3 years | Diagnosis of depression | Lipophilic vs. hydrophilic statins: HR = 1.05 95% CI = 1.00–1.10 P = 0.078 Simvastatin vs. hydrophilic statins: HR = 1.09 95% CI = 1.02–1.16 P = 0.003 | = / - |
| Glaus et al. (77) | Cohort | 1,631 adults aged 35–66 years | Statin use (self-reported) | No use | 5.2 years | Diagnosis of depression | HR = 1.25 95% CI = 0.73–2.14 P > 0.05 | = |
| Huang et al. (78) | Cohort | 408 HNC hyperlipidaemic patients | Statin use | No use | 1 year | Diagnosis of depression | HR = 0.85 95% CI = 0.46–1.57 P = 0.4252 | = |
| Kang et al (79) | Cohort | 286 ischemic stroke patients | Statin use | No use | 1 year | Diagnosis of post-stroke depression | Wald = 8.477 95% CI = NR P = 0.004 | + |
| Kang et al. (80) | Cohort | 11,218 ischemic stroke patients | Statin use | No use | 1 year | Diagnosis of post-stroke depression | HR = 1.59 95% CI = 1.30–1.95 P < 0.001 | - |
| Kessing et al. (81) | Historical cohort | 497,080 statin users | Statin use | No use | Up to 21 years | Diagnosis of depression or antidepressant prescription | Trend test for statin prescription = 0.92 95% CI = 0.92–0.95 P < 0.001 | + |
| Kim et al. (42) | Cohort | 711 ACS patients | Statin use | No use | 1 year | Diagnosis of depression | Prevalence difference = −9.5% (statin users) 95% CI = NR P = 0.037 | + |
| Kim et al. (82) | Cohort | 288 ischemic stroke patients | Statin use | No use | 1 year | Diagnosis of post-stroke depression | OR = 0.54 95% CI = 0.49–0.87 P = NR | + |
| Khokhar et al. (83) | Historical cohort | 100,515 TBI patients aged >/=65 years | Statin use | No use | 6 months | Diagnosis of depression | RR = 0.85 95% CI = 0.79–0.90 P = NR | + |
| Köhler-Forsberg et al. (84) | Historical cohort | 387,954 adults | Statin prescription redemption | No prescription | 6.8 years | Diagnosis of depression | HR = 1.33 95% CI = 1.31–1.35 P = NR | - |
| Mansi et al. (85) | Historical cohort | 13,944 adults aged 30–85 years | Statin use | No use | 4.5 years | Diagnosis of mood disorder | OR = 1.02 95% CI = 0.94–1.11 P = 0.6 | = |
| Medici et al. (86) | Cohort | 12,176 ICU patients | Statin use | No use | 3 years | Diagnosis of depression or antidepressant prescription | RR = 1.04 95% CI = 0.96–1.13 P = NR | = |
| Molero et al. (87) | Historical cohort | 1,149,384 statin-users aged >/=15 years | Statin use | No use | 8 years | Diagnosis of depressive episode (unplanned hospital visit or specialised outpatient care) | HR = 0.91 95% CI = 0.88–0.94 P = NR | + |
| Otte et al. (88) | Cohort | 776 CAD patients | Statin use | No use | 6 years | Diagnosis of depression (PHQ>/=10) | OR = 0.62 95% CI = 0.41–0.95 P = 0.026 | + |
| Cross sectional | 965 CAD patients | Statin use | No use | – | Diagnosis of depression (PHQ>/=10) | OR = 0.66 95% CI = 0.45–0.98 P = 0.04 | + | |
| Pasco et al. (89) | Historical cohort | 345 women aged >/=50 years | Statin or aspirin use (self-reported) | No use | 10 years | Diagnosis of depression (first episode) | HR = 0.20 95% CI = 0.04–0.85 P = 0.03 | + |
| Case-control | 345 women aged >/=50 years | Statin use prior to depression onset (self-reported) | No use | 10 years | Diagnosis of depression (first episode) | OR = 0.13 95% CI = 0.02–1.02 P > 0.05 | = | |
| Redlich et al. (90) | Cohort | 4,607,990 adults aged >/=40 years | Statin prescription | No prescription | 3 years | Diagnosis of depression | OR = 0.92 95% CI = 0.89–0.96 P = 0.016 | + |
| Smeeth et al. (91) | Cohort | 729,529 adults aged >/=40 years | Statin prescription | No prescription | 4.3 years | New antidepressant prescription | HR = 1.01 99% CI = 0.96–1.06 P = NR | = |
| Stafford and Berk (92) | Cohort | 193 MI, PTCA, or CABG hospitalised patients | Statin prescription | No prescription | 9 months | Diagnosis of depression | OR = 0.21 95% CI = 0.052–0.876 P = 0.032 | + |
| Wee et al. (93) | Cohort | 3,792 TBI patients | Statin use in hyperlipidaemia (SHL) | Untreated hyperlipidaemia (UHL) or normolipidaemia (NL) | 3 years | Diagnosis of depression | SHL vs. NL HR= 1.02 95% CI = 0.55–1.89 P = 0.9611 SHL vs. UHL HR = 0.63 95% CI = 0.34–1.17 P = 0.1433 | = |
| Williams et al. (94) | Historical cohort | 836 adult men | Statin or aspirin use | No use | 6 years | Diagnosis of mood disorder | HR = 0.55 95% CI = 0.23–1.32 P = 0.18 | = |
| Case-control | 937 adult men | Statin or aspirin use | No use | – | Diagnosis of mood disorder | OR = 0.1 95% CI = 0.1–0.4 P < 0.001 | + | |
| Wium-Andersen et al. (95) | Historical cohort | 91,842 ACS patients, 91,860 matched individuals | Statin use | No use | 12 years | Diagnosis of early (<1 year) or late (1–12 years) depression or antidepressant prescription | Early depression ACS patients: HR = 0.94 95% CI = 0.86–0.94 P = NR Non ACS: HR = 1.04 95% CI = 0.96–1.12 P = NR Late depression ACS patients: HR = 0.96 95% CI = 0.82–0.90 P = NR Non ACS: HR = 1.00 95% CI = 0.95–1.06 P = NR | + |
| Wium-Andersen et al. (96) | Historical cohort | 147,487 ischemic stroke patients, 160,235 matched individuals | Statin use | No use | 1 year | Diagnosis of early (<1 year) or late (1–12 years) depression or antidepressant prescription | Early depression stroke patients: HR = 0.71 95% CI = 0.70–0.73 P = NR Non stroke: HR = 1.00 95% CI = 0.94–1.05 P = NR Late depression stroke patients: HR = 0.90 95% CI = 0.87–0.93 P = NR Non stroke: HR = 0.90 95% CI = 0.86–0.94 P = NR | + |
| Yeh et al. (97) | Historical cohort | 9,139 Asthma-COPD overlap syndrome patients | Statin use | No use | Up to 11 years | Diagnosis of depression | HR = 0.36 95% CI = 0.25–0.53 P < 0.001 | + |
| Young-Xu et al. (98) | Cohort | 371 CAD patients | Statin use | No use | 4 years | Diagnosis of depression (Kellner Symptom questionnaire >/=7) | OR = 0.63 95% CI = 0.43–0.93 P = NR | + |
| Case-control studies | ||||||||
| Yang (33) | Case-control | 366 hyperlipidaemic patients aged 40–79 years | Statin use | No use | – | Diagnosis of depression | OR = 0.4 95% CI = 0.2–0.9 P < 0.05 | + |
| Cross-sectional studies | ||||||||
| Agustini et al. (99) | Cross sectional | 19,114 community-dwelling participants aged >/= 70 years | Statin use (self-reported) | No use | – | Diagnosis of depression (CES-D >/= 8) | OR = 1.09 95% CI = 0.98–1.20 P = 0.11 | = |
| Boumendil and Tubert-Bitter (100) | Cross sectional | 17,244 adults | Simvastatin use (self-reported) | No use | – | Absenteeism due to depression | PR = 2.18 95% CI = 1.18–4.03 P = NR | – |
| Feng et al. (101) | Cross sectional | 2,804 adults aged >/= 55 years | Statin use (self-reported) | No use | – | Diagnosis of depression (GDS >/=5) | OR = 0.71 95% CI = 0.52–0.97 P = NR | + |
| Lindberg and Hallas (102) | Cross sectional | 166 users of antidepressant and statins | Statin use | No prescription | – | Antidepressant prescription redemption before vs after redemption of statin | RR= 1.06 95% CI = 0.79 to 1.45 P= NR | = |
| Williams et al. (103) | Cross sectional | 638 White Europeans, 695 South Asians and African-Caribbean | Statin prescription | No prescription | – | Diagnosis of depression (GDS >/=4) | White Europeans: OR = 0.54 95% CI = 0.26 to 1.13 P = NR South Asian and African-Caribbean: OR = 1.67 95% CI = 0.97–2.88 P = NR | = |
| Case series | ||||||||
| Cham et al. (104) | Case series | 11 male and 1 female patients | Statin treatment (simvastatin, atorvastatin, rosuvastatin, lovastatin, pravastatin) for 1 day to several months | – | – | – | Episodes of violent ideation, irritability, depression and suicide were reported. All 12 cases resolved upon discontinuation and recurred with re-challenge when attempted. Four cases met Naranjo criteria for definite causality, 4 for probable causality, 4 for possible causality. | – |
| Duits and Bos (105) | Case series | 4 female patients aged 32–59 | Simvastatin | – | – | – | Two patients developed psychotic, obsessive, depressive symptoms and suicidal/homicidal thoughts, and required treatment with clomipramine and cognitive therapy. One patient developed paranoid thoughts, suicidality, agitation and depressive symptoms after 4 days of simvastatin; symptoms resolved upon discontinuation. One patient suffered a depressive syndrome with psychotic features after 3 months of simvastatin; management with discontinuation, antipsychotic and antihypertensive medications was necessary. | – |
| Lechleitner et al. (106) | Case series | 4 female patients with primary hypercholesterolaemia, aged 44–66 | Pravastatin 10 mg for 12 weeks | - | - | - | Three patients developed mild-moderate depressive symptoms reversed by discontinuation. One patient developed severe psychiatric symptoms and suicidality, improved on discontinuation and didn't reoccurred with lovastatin treatment. | - |
| Rosenson Goranson (107) | Case series | 2 male hyperlipidaemic patients, aged 51–53 | Lovastatin 20–60 mg/die | – | – | – | Two patients developed sleep disturbances, anxious mood and irritability after several weeks of lovastatin treatment (20 mg/die and 60 mg/die); symptoms reversed 48 h after discontinuation, and reoccurred upon re-challenge, but not upon starting of pravastatin treatment. | – |
| Tatley and Savage (108) | Case series | Adverse reaction reports to New Zealand Centre for Adverse Reaction Monitoring | Statin treatment (simvastatin, atorvastatin, Fluvastatin, pravastatin) | – | – | – | 203 reports of psychiatric adverse events associated with statins (67 reports of mood disorders, 30 of cognitive disorders, 51 of sleep disorders, 14 of perception disorders, 107 other reactions such as asthenia, fatigue, lethargy). 57 reactions were severe. 34 had documented recurrence upon re-challenge | – |
The effect size of the main findings, either extracted from the study or calculated by the authors. Where this was not possible, we report the raw data.
ACS, acute coronary syndrome; CABG, coronary artery bypass graft; CAD, coronary artery disease; CES-D, centre for epidemiological studies – depression scale; CI, confidence interval; COPD, chronic obstructive pulmonary disease; GDS, geriatric depression scale; HNC, head and neck cancer; HR, hazard ratio; ICU, intensive care unit; MD, mean difference; MI, myocardial infarction; PHQ, patient health questionnaire; PR, prevalence ratio; PTCA, percutaneous transluminal coronary angioplasty; OR, odds ratio; RR, relative risk; SD, standard deviation; TBI, traumatic brain injury.
3b Effects of Statins on the Risk of Developing Depression in Non-depressed Patients
We identified 39 records (2 meta-analyses, 23 cohort studies, 2 cohort and case-control studies, 1 cohort and cross-sectional study, 1 case-control study, 5 cross-sectional studies, and 5 case series – see Table 1) investigating the effect of statins on the risk of depression diagnosis in non-depressed participants.
3b-Z Meta-Analyses
One meta-analysis, by Parsaik and colleagues (73), included 4 cohort, 2 nested case-control, and 1 cross-sectional studies on a total of 9,187 non-depressed participants with median follow-up of 5 years. The pooled adjusted odds ratio (OR) of depression for statin users compared to non-users was 0.68 (95% CI = 0.52–0.89), showing statin users were 32% less likely to develop depression. One of the included studies that reported lack of association (102) accounted for most of the meta-analysis' heterogeneity (Cochran's Q-test P = 0.014, I2 = 55%). When it was removed from the analysis, the antidepressant effect of statins was stronger (OR = 0.63, 95% CI = 0.43–0.93) and heterogeneity decreased (Cochran's Q-test P = 0.40, I2 = 2%) (73). A more recent and larger meta-analysis of 13 observational studies and 5,035,070 participants reported comparable results (OR = 0.85; 95% CI = 0.72–0.99); however, no association between statin use and depression risk when the trim-and-fill analysis (i.e., a method to correct for publication bias) was used (OR = 0.87, 95% CI = 0.74–1.02), which suggests that some smaller studies with negative results may have not been published (72).
3b-II Cohort Studies
Of the 26 cohort studies, 14 used a prospective cohort and 12 a historical cohort design.
Nine studies investigated the association between use vs. non-use of statins in non-psychiatric populations with non-specific physical comorbidities; of these studies, half found no association between statins and depression. A small study on 1,631 adults followed for 5.2 years found no evidence of association between self-reported statins use and a formal diagnosis of depression (HR = 1.25, 95% CI = 0.73–2.14) (77). A strong protective effect (HR = 0.20, 95% CI 0.04–0.85), of statins or aspirin use on the risk of developing depression was confirmed in 345 women aged 50+ years (89). In contrast, a study by Williams and colleagues did not find any significant association (HR = 0.55, 95% CI = 0.23–1.32) between the use of either statins or aspirin and further diagnosis of mood disorder in a sample of 836 men (94). Lack of association (OR = 1.02, 95% CI = 0.94–1.11) between statins use and diagnosis of depression or bipolar disorder was also reported in a study of 13,944 adults followed for 4.5 years (85). A large study on 129,288 statin users and 600,241 matched non-users followed for 4.3 years did not identify any association (HR= 1.01, 99% CI= 0.96–1.06) between statin use and initiation of antidepressant treatment (91). A more recent study of 193,977 statin users and 193,977 matched non-users showed an increased risk of depression diagnosis (HR= 1.33, 95% CI = 1.31–1.35) in statin users, though this association became non-significant when adjusting for antidepressant use (84). Conversely, a study on prescription data from 497,080 statin users found that use of statins decreased the rate of incident depression (Trend test for statin prescription = 0.92, 95% CI = 0.92–0.95) (81), confirming the results of a previous nationwide cohort study (N = 4,607,990) (OR = 0.92, 95% CI = 0.89–0.96) (90). The latter article also indicated a protective effect for simvastatin (OR = 0.93, 95% CI = 0.89–0.97) and a harmful effect for atorvastatin (OR = 1.11, 95% CI = 1.01–1.22) on the risk of developing depression (despite both being lipophilic molecules) (90), whereas another study on 299,298 participants comparing lipophilic vs. hydrophilic statins highlighted an increased risk of diagnosing depression for simvastatin only (HR = 1.09, 95% CI = 1.02–1.16), but not when all lipophilic statins were compared to hydrophilic ones (HR = 1.05 95% CI = 1.00–1.10) (76). A recent within-subject epidemiological study conducted on a large nationwide register of 1,149,384 statin users followed for 8 years found that presentation for depressive disorders was less frequent during periods on statins, compared to periods off statins (HR = 0.91, 95% CI = 0.88–0.94) (87).
The remaining cohort studies focussed on groups of patients with specific physical illness. Five studies included patients with heart conditions (42, 88, 92, 95, 98). One large historical cohort study on 91,842 patients with acute coronary syndrome (ACS) and 91,860 non-ACS controls found that statins use was associated with decreased risk of both early (within 1 year) (HR = 0.94, 95% CI = 0.86–0.94) and late (within 12 years) (HR = 0.96, 95% CI = 0.82–0.90) depression, but only in the ACS patients (95). The other studies on cardiological patients were prospective and included smaller samples between 193 and 711 participants. Stafford and Berk (92) reported decreased risk of post-discharge depression at 9 months (OR = 0.21, 95% CI = 0.052–0.876). Another study found lower incidence of depression at 1 year follow-up in ACS patients taking statins (23.3%) compared to statin non-users (32.8%) (42). Two studies on patients with coronary artery disease reported a reduced risk of depressive illness as measured with mood questionnaires at 6 years (OR = 0.62, 95% CI = 0.41–0.95) (88) and 4 years (OR = 0.63, 95% CI = 0.43–0.93) (98).
Five studies were conducted on stroke patients (74, 79, 80, 82, 96). One found increased risk of post-stroke depression among statin users compared to non-users (N = 11,218, HR = 1.59, 95% CI = 1.30–1.95) (80). A larger study on 70,706 stroke patients found no effect (OR = 0.99, 95% CI = 0.95–1.03) of statin prescription on self-reported low mood or antidepressant use at 3 months follow-up (74). The remaining reports indicated a beneficial effect of statins on depression at 1 year follow-up: two were on small samples of 288 (OR = 0.54, 95% CI = 0.49–0.87) (82) and 286 participants (Wald = 8.477, P = 0.004) (79), whereas the third was conducted on a large historical cohort of 147,487 stroke patients and 160,235 matched individuals, and showed a risk reduction among both stroke patients (HR = 0.90, 95% CI = 0.87–0.93) and non-stroke patients (HR = 0.90, 95% CI = 0.86–0.94) (96). Chuang and colleagues investigated the relationship between hyperlipidaemia, statins use, and depression: 26,852 hyperlipidaemic and 107,408 non-hyperlipidaemic patients were compared, showing a lower risk of depression among hyperlipidaemic participants who received statins (HR = 0.81, 95% CI = 0.69–0.96), but in the non- hyperlipidaemic (75).
Two studies were conducted on survivors of traumatic brain injury. One found that statin use was associated with fewer depression diagnoses at 6 months (N = 100,515, RR = 0.85, 95% CI = 0.79–0.90) (83). The other (N = 3,792) reported higher risk of depression in patients that were also hyperlipidaemic vs. normolipidaemic (HR = 1.61, 95% CI = 1.03–2.53), but no significant difference between patients treated with statins or not, regardless of their hyperlipidaemic (HR = 0.63, 95% CI = 0.34–1.17) or normolipidaemic (HR = 1.02, 95% CI = 0.55–1.89) status (93).
One study conducted on 9,139 patients affected by asthma-COPD overlap syndrome and found that statin users were at decreased risk of depression for up to 11 years compared to non-users (HR = 0.36, 95% CI = 0.25–0.53) (97). Finally, two studies on 408 hyperlipidaemic head and neck cancer patients (HR = 0.85, 95% CI = 0.46–1.57) (78) and 12,176 ICU patients (RR = 1.04, 95% CI = 0.96–1.13) (86) showed no effect of statins on the risk of depression.
3b-III Case-Control Studies
Two cohort studies on non-depressed patients also included a case-control analysis: one reported non-significant results (N = 345 females, OR = 0.13, 95% CI = 0.02–1.02) (89), whilst the other showed a strong protective effect of statins (N = 937 males, OR = 0.1, 95% CI = 0.1–0.4) (94). Another case-control study on 366 hyperlipidaemic participants reported decreased risk of new onset depression in statin users vs. non-users (OR = 0.4, 95% CI = 0.2–0.9) (33).
3b-IV Cross-Sectional Studies
Six cross-sectional studies were retrieved. One showed increased absenteeism from work due to depression among employees reporting statin use (N = 17,244, Prevalence Ratio = 2.18, 95% CI = 1.18–4.03) (100). Another within-subjects study found no significant change (RR = 1.06, 95% CI = 0.79–1.45) in the redemption of antidepressants prescription before vs. after the initiation of statins (102). One article on patients with heart conditions found a reduction (N = 965, OR = 0.66, 95% CI = 0.45–0.98) (88) in risk of developing depression in statin users. The remaining studies involved elderly participants. Outcomes based on a Geriatric Depression Scale (GDS) or a Centre for Epidemiologic Studies – Depression (CES-D) score above threshold for diagnosis of depression were reported in three studies with conflicting results: one showed a decreased prevalence of depression in statin users (N = 2,804, OR = 0.71, 95% CI = 0.52–0.97) (101), another reported no difference in prevalence among South Asian and African-Caribbean statin users (N = 695, OR = 1.67, 95% CI = 0.97–2.88) nor White Europeans (N = 638, OR = 0.54, 95% CI = 0.26–1.13) (103) though a significant ethnicity-statin interaction (P = 0.041), while a further study in 19,114 elderly community-dwelling participants found no association (OR = 1.09, 95% CI = 0.98–1.20) (99).
3b-V Case Series
We retrieved 5 case series that we report for completeness. Tatley and colleagues investigated reports from the New Zealand Centre for Adverse Reaction Monitoring and found that, of the 203 reports of psychiatric adverse events associated with statins, 67 concerned mood disorders (108). Another record reported 12 cases of onset of violent ideation, irritability, depression, and suicide that resolved upon statins discontinuation and recurred when re-challenging (104). Two older case series included 8 reports of female patients suffering depressive and psychotic symptoms after initiation of pravastatin and simvastatin that resolved after discontinuation (105, 106); a similar clinical course was described in another case series concerning 2 males treated with lovastatin (107).
3c Effects of Statins on Depressive Symptoms Scores in Non-depressed Patients
We identified 22 records (3 meta-analyses, 13 clinical trials, 3 cohort studies, 3 cross-sectional studies – see Table 2) investigating the effect of statins on depressive symptoms scores in non-depressed participants.
Table 2.
Overview of studies regarding the effects of statins on depressive symptoms scores in non-depressed participants.
| Publication | Study design | Population |
Intervention/
exposure |
Comparison | Follow-up | Primary outcomes | Major Findings | Association |
|---|---|---|---|---|---|---|---|---|
| Meta-analysis | ||||||||
| Köhler-Forsberg et al. (84) | Meta-analysis | 7 RCTs, 1,576 depressed and non-depressed participants | Statin add-on or monotherapy | Placebo | 6 weeks to 4 years | HDRS, CES-D, GHQ | SMD = −0.26 95% CI = −0.48 to −0.04 P = 0.02 | + |
| O'Neil et al. (109) | Meta-analysis | 7 RCTs, 2,105 participants | Simvastatin, lovastatin, pravastatin | Placebo | 4 weeks to 4 years | HDRS, MSQ, BSI, HADS, BDI, GHQ, CES-D | SMD= −0.43 95% CI = −0.61 to −0.24 P = NR | = |
| Yatham et al. (110) | Meta-analysis | 10 RCTs, 2,517 depressed and non-depressed participants | Atorvastatin, simvastatin, lovastatin, pravastatin | Placebo | 6 weeks to 4 years | HDRS, HADS, CES-D, GHQ, POMS | SMD= −0.309 95% CI = −0.525 to −0.094 P = 0.005 | + |
| Randomised controlled trials | ||||||||
| Carlsson et al. (111) | RCT crossover | 41 hyperlipidaemic adults aged >/=70 years | Pravastatin 20 mg pravastatin 20 mg + tocopherol 400 IU | Placebo + tocopherol pravastatin + tocopherol | 1 year | GDS | [From KKöhler-Forsberg (84)] SMD = 0.09 95% CI = −0.57 to 0.76 P = 0.622 | = |
| Chan et al. (112) | RCT | 140 secondary progressive multiple sclerosis patients | Simvastatin 80 mg | Placebo | 2 years | HDRS | SMD = −1.0 95% CI = −3.2 to 1.2 P = 0.37 | = |
| Gengo et al. (113) | RCT crossover | 36 hyperlipidaemic patients | Lovastatin 40 mg, pravastatin 40 mg | Placebo | 4 weeks | POMS | SMD= −0.633 95% CI = −1.213 to −0.053 P = 0.032 | + |
| Harrison and Ashton (114) | RCT crossover | 25 healthy volunteers | Simvastatin 40 mg, pravastatin 40 mg | Placebo | 4 weeks | HADS | SMD= 0.048 95% CI = −0.507 to 0.602 P = 0.866 | = |
| Hyyppä et al. (115) | RCT crossover | 120 hyperlipidaemic men aged 35–64 years | Simvastatin 20 mg | Placebo | 24 weeks | BDI | MD = 0.06 95% CI = 0.01–0.12 P = 0.01590 | – |
| Krysiak et al. (116) | Non-randomised non-controlled trial | 14 hyperlipidaemic women, 14 normolipidaemic women | Atorvastatin 20−40 mg in hyperlipidaemia | No treatment in normolipidaemia | 24 weeks | BDI | Baseline: 11.6 (3.7) vs. 7.6 (3.9) SMD = NR 95% CI = NR P < 0.05 End of study: 9.4 (3.0) vs. 8.0 (4.3) SMD = NR 95% CI = NR P = NR | + |
| Morales et al. (117) | RCT | 80 older adults aged >/=65 years | Simvastatin | Placebo | 15 weeks | CES-D | SMD = 0.00 95% CI = −0.46 to 0.46 P = NR | = |
| Muldoon et al. (118) | RCT | 209 hyperlipidaemic adults | Lovastatin 20 mg | Placebo | 6 months | HDRS | SMD = 0.21 95% CI = −0.07 to 0.49 P > 0.2 | = |
| Ormiston et al. (119) | Non-controlled trial | 12 healthy volunteers | Atorvastatin 10–20 mg, lovastatin 20–40 mg | – | 1 year | BDI | T = 2.27, df = 11, P < 0.05 | + |
| Robertson et al. (120) | RCT | 52 mild TBI patients aged 18–50 years | Atorvastatin 1mg/kg/die (up to 80 mg/die) | Placebo | 3 months | CES-D | SMD = 0.05 95% CI = −0.495 to 0.595 P = 0.857 | = |
| Santanello et al. (121) | RCT | 431 adults aged >/= 65 years | Lovastatin 20–40 mg | Placebo | 6 months | CES-D | SMD = −0.08 95% CI = −0.29 to 0.14 P = 0.53 | = |
| Stewart et al. (122) | RCT | 1,130 adults with CAD and hyperlipidaemia | Pravastatin 40 mg | Placebo | 4 years | GHQ | MD = 0.49 95% CI = −0.30 to 1.28 P = 0.23 | = |
| Wardle et al. (123) | RCT | 621 adults with CAD aged 40–75 years | Simvastatin 20–40 mg | Placebo | 152 weeks | POMS | SMD = −0.405 95% CI = −0.596 to −0.213 P = 0.000 | = |
| Cohort studies | ||||||||
| Al Badarin et al. (124) | Cohort | 1,691 ACS patients | Statin prescription | No prescription | 1 year | PHQ-8 | MD = −0.05 95% CI = −0.67 to 0.58 P = 0.88 | = |
| Feng et al. (125) | Cohort | 1,803 adults aged >/=55 years | Statin use | No use | 1.5 years | GDS | Regression coefficient = −0.12 95% CI = NR F = 1.44 P = 0.23 | = |
| Hoogwegt et al. (126) | Cohort | 409 ICD-implanted patients | Statin use | No use | 1 year | HADS | MD = −0.97 95% CI = −1.99 to 0.05 P = 0.6 | = |
| Cross sectional studies | ||||||||
| Agostini et al. (127) | Cross sectional | 756 community-dwelling veterans aged >/= 65 years | Statin use | No use | – | CES-D | SMD = −0.18 95% CI = −0.69 to 0.33 P = 0.49 | = |
| Mandas et al. (128) | Cross sectional | 329 adults with dyslipidaemia aged >/= 65 years | Statin use | No use | – | GDS | SMD = 0.4573 95% CI = NR P = 0.01828 | – |
| Olson et al. (129) | Cross sectional | 525 women undergoing coronary angiography | Cholesterol-lowering drug use (self-reported) | No use | – | BDI | BDI: SMD = NR 95% CI = NR P = 0.94 | = |
*The effect size of the main findings, either extracted from the study or calculated by the authors. Where this was not possible, we report the raw data.
ACS, acute coronary syndrome; BDI, beck depression inventory; BSI, brief symptom inventory; CAD, coronary artery disease; CES-D, centre for epidemiological studies – depression scale; CI, confidence interval; GDS, geriatric depression scale; GHQ, general health questionnaire; HADS, hospital anxiety and depression scale; HDRS, hamilton depression rating scale; ICD, implantable cardioverter-defibrillator; MD, mean difference; MSQ, mood states questionnaire; PHQ, patient health questionnaire; POMS, profile of mood scores; PR, prevalence ratio; OR, odds ratio; RCT, randomised controlled trial; SD, standard deviation; SMD, standardised mean difference; TBI, traumatic brain injury.
3c-I Meta-Analyses
Of the three meta-analyses we identified, two reported the antidepressant effect of statins and one found no association. A meta-analysis of 7 RCTs (N = 2105) with follow-up between 4 weeks and 4 years reported no overall difference in depressive scores between participants receiving statins or placebo (SMD = −0.08, 95% CI = −0.29 to 0.12) (109). A more recent meta-analysis assessed the effects of all anti-inflammatory drugs in a mixed sample of non-depressed patients with baseline depressive symptoms and patients with a diagnosis of depression; with regards to the statins' trials (either in add-on to antidepressants, or in monotherapy), 7 RCTs (N = 1,576) were retrieved by the author and indicated an antidepressant effect of statins (SMD = −0.26, 95% CI = −0.48 to −0.04) (138). Another meta-analysis on statins only, again on a mixed sample of non-depressed and depressed patients, included 10 RCTs (N = 2,517) and confirmed that statins reduced depressive scores (SMD = −0.309, 95% CI = −0.525 to −0.094), though with high heterogeneity and poorly determined risk of bias (110). Interestingly, despite the essentially overlapping inclusion/exclusion criteria of these meta-analyses, only one study was included in all three of them (122). Two studies (115, 117) were only included in the older one (109). Two studies were only included by Köhler-Forsberg and colleagues (111, 121), and one only by Yatham and colleagues (120). Four studies were included by Yatham and colleagues and O'Neil and colleagues, but not by Köhler-Forsberg and colleagues (113, 114, 118, 123).
3c-II Clinical Trials
We identified 11 RCTs evaluating the effect of statins on mood scores in non-depressed participants. Two RCTs demonstrated opposing significant effects of statin use on depressive scores. A crossover RCT including 36 hypercholesteraemic patients followed-up for 4 weeks showed a reduction in depressive scores associated with statins' use (SMD = −0.633, 95% CI = −1.213 to −0.053) (113), whereas another crossover RCT assessing the separate and combined effects of simvastatin and Mediterranean-type diet in 120 hyperlipidaemic men followed-up for 24 weeks highlighted an increase in depressive scores in the statin group [Mean Difference (MD) = 0.06, 95% CI = 0.01–0.12] (115).
None of the remaining 9 RCTs reported significant changes in depressive scores associated with statins. One study on 25 healthy volunteers found no effect of simvastatin or pravastatin compared to placebo on depressive symptoms at 4 weeks (MD = 0.05, 95% CI = −0.51 to 0.60) (114). Similar results were reported in a sample of 209 hyperlipidaemic adults treated with lovastatin vs. placebo for 6 months (SMD = 0.21, 95% CI = −0.07 to 0.49) (118). Two further RCTs were conducted on larger samples of individuals with heart conditions (N = 1,130 and N = 621) and had longer follow-up (4 years and 152 weeks). Both Stewart and colleagues (122) and Wardle and colleagues (123), respectively, showed no differences in depressive scores depending on intervention (MD = 0.49, 95% CI = −0.30 to 1.28; χ2 heterogeneity = 1.66, linear trend = 0.08). Three trials on 431 (121), 80 (117), and 41 (111) older adults, respectively, found no differences (SMD = −0.08, 95% CI = −0.29 to 0.14; SMD = 0.00, 95% CI = −0.46 to 0.46; SMD = 0.09, 95% CI = −0.57 to 0.76) at 15 weeks, 6 months, and 1 year in depressive scores. Chan and colleagues conducted a trial on 140 patients with multiple sclerosis and found no significative difference in depressive scores (SMD = −1.0, 95% CI = −3.2 to 1.2) between simvastatin treatment and placebo at 2 years (112). Similar results (SMD = 0.05, 95% CI = −0.495 to 0.595) were reported in 52 mild traumatic brain injury patients using atorvastatin for 3 months (120).
Two further trials were non-controlled and/or non-randomised. One small (n = 12) double blind pilot study did not include a placebo arm, and reported an improvement in depressive scores (t = 2.27, df = 11) following treatment with statins (119). Another small non-randomised non-placebo controlled study on 14 hypercholesteraemic women receiving 24 weeks treatment with atorvastatin and 14 normolipidaemic patients left untreated found that the depressive scores at baseline were significantly higher among the hypercholesteraemic (p < 0.05) and treatment with statins normalised depressive scores (116).
3c-III Cohort Studies
All 3 cohort studies identified showed no effect of statins on depressive scores. A cohort study on 1,691 patients with acute myocardial infarction showed that statin prescription did not affect the natural decrease in depressive scores (MD = −0.05, 95% CI = −0.67 to 0.58) at 1 year follow-up (124). Similar results (MD = −0.97, 95% CI = −1.99 to 0.05) were reported by Hoogwegt and colleagues in 409 ICD-implanted patients (126). Another study on 1,803 elderly participants concluded that statin use did not correlate with increased scores of the Geriatric Depression Scale (p = 0.23) at 1.5 years follow-up, though post hoc analyses suggested a protective effect of statins in female participants and an opposite effect in men (125).
3c-IV Cross-Sectional Studies
Of the three cross-sectional studies, two did not show any association between statins use and depressive scores: one was conducted on 756 elderly veterans (beta = −0.18, 95% CI = −0.69 to 0.33) (127) and the other on 525 female patients with heart conditions (P = 0.94) (129). The remaining study was again in elderly dyslipidaemic patients but this one reported an increase in depressive scores in statin-users (N = 329, P = 0.018) (128).
3d Effects of Statins on the Risk of Developing Depressive Episodes or on Depressive Symptoms Scores in Depressed Patients
We identified 11 records (3 meta-analysis, 6 clinical trials, 2 cohort studies – see Table 3) investigating the effect of statins on depression in depressed patients.
Table 3.
Overview of studies regarding the effects of statins on the risk of developing depressive episodes or on depressive symptoms scores in depressed patients.
| References | Study design | Population |
Intervention/
exposure |
Comparison | Follow-up | Primary outcomes | Major Findings | Association |
|---|---|---|---|---|---|---|---|---|
| Meta-analysis | ||||||||
| Bai et al. (19) | Meta-analysis | 3 RCTs, 166 MDD patients | TAU + Statin | TAU + placebo | 6–12 weeks | HDRS | SMD = −0.65 95% CI = −0.96 to −0.33 P < 0.0001 | + |
| De Giorgi et al. (71) | Meta-analysis | 4 RCTs, 255 MDD patients | TAU + Statin | TAU + placebo | 8 weeks | HDRS/MADRS | SMD = −0.48 95% CI = −0.74 to −0. 22 P = NR | + |
| Salagre et al. (130) | Meta-analysis | 3 RCTs, 165 MDD patients | TAU + Statin | TAU + placebo | 6–12 weeks | HDRS | SMD = −0.73 95% CI = −1.04 to −0.42 P < 0.001 | + |
| Randomised controlled trials | ||||||||
| Abbasi et al. (131) | RCT | 46 post CABG patients with mild to moderate depression | Simvastatin 20 mg | Atorvastatin 20 mg | 6 weeks | HDRS | SMD = 3.63 95% CI = 0.44–6.51 P = 0.03 | + |
| Berk et al. (132) | RCT | 130 MDD patients aged 15–25 years | TAU + rosuvastatin 10 mg | TAU + placebo | 12 weeks | MADRS | SMD = −4.2 95% CI = −9.1 to 0.6 P = 0.089 | = |
| Ghanizadeh and Hedayati (133) | RCT | 68 MDD patients | Fluoxetine 40 mg + lovastatin 30 mg | Fluoxetine 40 mg + placebo | 6 weeks | HDRS | SMD = −0.77 95% CI = −1.30 to −0.24 P < 0.001 | + |
| Gougol et al. (134) | RCT | 48 MDD patients | Fluoxetine 20 mg + simvastatin 20 mg | Fluoxetine 20 mg + placebo | 6 weeks | HDRS | SMD = −0.73 95% CI = −1.34 to −0.11 P = NR | + |
| Haghighi et al. (135) | RCT | 60 MDD patients | Citalopram 40 mg + atorvastatin 20 mg | Citalopram 40 mg + placebo | 12 weeks | HDRS | SMD= −0.70 95% CI = −1.22 to −0.18 P = NR | + |
| Soh et al. (136) | RCT | 60 patients with mood disorder | Lithium + atorvastatin 20 mg | Lithium + placebo | 12 weeks | Relapse (MADRS≥10) | χ2 (1) = 0.148 95% CI = NR P = 0.70 | = |
| Cohort studies | ||||||||
| Kim et al. (68) | Cohort | 300 patients with comorbid ACS and depression | Escitalopram + statin, statin-only use | Escitalopram-only, placebo-only | 1 year | Response (HDRS, BDI) | HDRS response: OR = 2.23 95% CI = 1.11–4.51 P = 0.025 BDI response: OR = 2.82 95% CI = 1.35–5.90 P = 0.006 | + |
| 146 patients with comorbid ACS and depression | Statin use | TAU | 1 year | Response (HDRS, BDI) | HDRS response: OR = 1.19 95% CI = 0.45–3.18 P = 0.726 BDI response: OR = 0.89 95% CI = 0.36–2.22 P = 0.798 | = | ||
| Köhler et al. (137) | Historical cohort | 872,216 SSRI users | SSRI + statin use | SSRI-only use | 3 years | Depressive episode (hospital contact) | HR = 0.64 95% CI = 0.55–0.75 P = NR | + |
*The effect size of the main findings, either extracted from the study or calculated by the authors. Where this was not possible, we report the raw data.
ACS, acute coronary syndrome; BDI, beck depression inventory; CABG, coronary artery bypass graft; CI, confidence interval; HDRS, hamilton depression rating scale; HR, hazard ratio; MADRS, montgomery-åsberg depression rating scale; MD, mean difference; MDD, major depressive disorder; PR, prevalence ratio; OR, odds ratio; RCT, randomised controlled trial; SD, standard deviation; SMD, standardised mean difference; SSRI, selective serotonin reuptake inhibitors; TAU, treatment as usual.
3d-I Meta-Analyses
Two meta-analyses reported results on the effect of statins as add-on treatment in depressed patients, with similar results. They both included 3 RCTs (133–135) and found a significant improvement in depressive symptoms [SMD = −0.73, 95% CI = −1.04 to −0.42 (130); SMD = −0.65, 95% CI = −0.96 to −0.33 (19)] associated with statins add-on. A more recent meta-analysis added a further RCT (132) and confirmed the above results (SMD = −0.48, 95% CI = −0.74 to −0.22], whilst also supporting the acceptability, tolerability, and safety of statins in the treatment of depression (71).
3d-II Clinical Trials
We found 6 clinical trials investigating the effect of statins in depressed patients (3 of which were included in the meta-analyses commented on above). In the oldest of these RCTs, 68 depressed patients were randomised to 6 weeks of either fluoxetine plus lovastatin or fluoxetine plus placebo: depressive scores decreased significantly in both groups, but more noticeably in the treatment group [mean change = 12.8 (SD = 6.3) vs. 8.2 (SD = 4.0, t = 3.4, df = 60)] (133). A similar 6 week trial on simvastatin randomised 48 depressed patients and found comparable results (SMD = 4.81, P = 0.02), though remission rates were not significantly different (59 vs. 45%, P = 0.36) (134). In a 12-week trial, 60 depressed patients received citalopram for 1 week and were then randomised to either atorvastatin or placebo adjunction. Results showed significantly lower depressive scores [Time × Group interaction: F(3,174) = 8.93] and increased partial remission (OR = 8.83, 95% CI = 1.02–76.96) for the statin group (135). A recent RCT included 130 MDD patients aged 15 to 25 years old, who were randomised to receive either treatment as usual (TAU) plus placebo, or TAU plus aspirin, or TAU plus rosuvastatin. Differences in changes in Montgomery-Asberg Depression Rating Scale (MADRS) scores were not significant when comparing rosuvastatin and placebo group (SMD = −4.2, 95% CI = −9.1 to 0.6, P = 0.089) nor when comparing rosuvastatin vs. aspirin groups (SMD = −6.4, 95% CI = −11.7 to 1.2) (132). The most recent RCT compared the relapse risk in a sample of 60 BD or MDD patients randomised to either lithium plus atorvastatin or lithium plus placebo, and reported non-significant effect (χ2(1) = 0.148, P = 0.70) (136).
We also retrieved a RCT comparing the antidepressant effects of simvastatin vs. atorvastatin, without a placebo control, in 46 post-CABG patients with comorbid mild to moderate depression. Depressive scores at 6 weeks decreased more prominently in the simvastatin group (SMD = 3.63, 95% CI = 0.44–6.51, P = 0.03) (131).
3d-III Cohort Studies
Two cohort studies assessed the effect of statins in depressed patients. Kim and colleagues analysed 1-year follow-up data of a previous 24-week RCT of escitalopram in 300 patients with comorbid ACS and depression. Incidental statin-users showed higher response rates on both the Hamilton Depression Rating Scale (HAM-D) (OR = 2.23, 95% CI = 1.11–4.51) and the Beck's Depressive Inventory (BDI) (OR = 2.82, 95% CI = 1.35–5.90) at 1 year (68). The same paper however found no differences in response (HAM-D: OR = 1.19, 95% CI = 0.45–3.18; BDI: OR = 0.89, 95% CI = 0.36–2.22) when considering the effect of statins' monotherapy in patients who were not on antidepressants (N = 146). A higher response rate, though only on the HAM-D, was observed in users of lipophilic statin vs. all others statins users (OR = 2.91, 95% CI = 1.21–6.99) (68). Finally, a large (N = 872,216) historical cohort study compared several outcomes associated with depression in a group of SSRIs-plus-statins users against a group of SSRIs-only users and found a significantly lower risk for psychiatric hospital contacts (HR = 0.75, 95% CI = 0.69–0.82) and psychiatric hospital contacts specifically due to depression (HR = 0.64, 95% CI = 0.55–0.75) among statin users. No significant differences were reported in all-cause mortality (HR = 1.04, 95% CI = 0.96–1.12) and suicidality (HR = 0.85, 95% CI = 0.61–1.18) (137).
3e Effects of Statins on Depressive-Inflammatory Symptoms in Depressed Patients
Fifteen of the studies described above also investigated the effect of statins on specific depressive-inflammatory symptoms in patients with depression (see Table 4).
Table 4.
Overview of studies regarding the effects of statins on depressive-inflammatory symptoms.
| Publication | Study design | Population |
Intervention/
exposure |
Comparison | Follow-up | Primary outcomes | Major Findings | Association |
|---|---|---|---|---|---|---|---|---|
| ANHEDONIA | ||||||||
| Psychomotor retardation | ||||||||
| Randomised Controlled Trials | ||||||||
| Harrison and Ashton (114) | RCT crossover | 25 healthy volunteers | Simvastatin 40 mg, pravastatin 40 mg | Placebo | 4 weeks | DSST | Pravastatin: mean = 74.3 95% CI = 70.3–78.3 Simvastatin: mean = 74.6 95% CI = 70.3–78.9 Placebo: mean = 74.6 95% CI = 70.9–78.3 SMD = NR 95% CI = NR P = NR | = |
| Muldoon et al. (118) | RCT | 209 hyperlipidaemic adults | Lovastatin 20 mg | Placebo | 6 months | DSST | MD = 0.06 95% CI = 0.01–0.12 P = 0.016 | + |
| Santanello et al. (121) | RCT | 431 adults aged >/= 65 years or older | Lovastatin 20, lovastatin 40 mg | Placebo | 6 months | DSST | Mean (SD) = Placebo 0.33 (13.6) Lovastatin 20 mg −0.80 (13.28) Lovastatin 40 mg 1.66 (8.98) SMD = NR 95% CI = NR P = 0.66 | = |
| ANXIETY | ||||||||
| Randomised controlled trials | ||||||||
| Berk et al. (132) | RCT | 130 MDD patients aged 15–25 years | TAU + rosuvastatin 10 mg | TAU + placebo | 12 weeks | GAD | SMD = −0.6 95% CI = −1.7 to 3.0 P = 0.684 | = |
| Harrison and Ashton (114) | RCT crossover | 25 healthy volunteers | Simvastatin 40 mg, pravastatin 40 mg | Placebo | 4 weeks | HADS (anxiety subscale) | Pravastatin: mean score = 3.2 95% CI = 2.0–4.4 Simvastatin: mean score = 2.5 95% CI = 1.7–3.3 Placebo mean score = 3.1 95% CI = 2.2–4.0 SMD = NR 95% CI = NR P = NR | = |
| Hyyppä et al. (115) | RCT crossover | 120 hyperlipidaemic men aged 35–64 years | Simvastatin 20 mg | Placebo | 24 weeks | BDI (anxiety items) | Mean change= 0.00 95%CI = −0.05 to 0.05 P= 0.9474 | = |
| Stewart et al. (122) | RCT | 1,130 adults with CAD and hyperlipidaemia | Pravastatin 40 mg | Placebo | 4 years | GHQ (anxiety items) | MD= NR 95% CI = NR P= “non-significant” | = |
| Wardle et al. (123) | RCT | 621 adults with CAD aged 40–75 years | Simvastatin 20–40 mg | Placebo | 152 weeks | POMS (tension/anxiety items) | X2 = 3.57 95% CI = NR P = “non-significant” | = |
| Cohort studies | ||||||||
| Hoogwegt et al. (126) | Cohort | 409 ICD-implanted patients | Statin use | No use | 1 year | HADS (anxiety subscale) | MD = −0.81 95% CI = −1.80 – 0.18 P = 0.11 | = |
| Molero et al. (87) | Historical cohort | 1,149,384 statin users aged >/= 15 years | Statin use | No use | 8 years | Diagnosis of anxiety disorder (unplanned hospital visit or specialised outpatient care) | HR = 0.99 95% CI = 0.95–1.02 P = NR | = |
| Young-xu et al. (98) | Cohort | 371 CAD patients | Statin use | No use | 4 years | Diagnosis of anxiety (Kellner Symptom questionnaire >/=8) | OR = 0.69 95% CI = 0.47 – 0.99 P = NR | + |
| SLEEP | ||||||||
| Randomised controlled trials | ||||||||
| Carlsson et al. (111) | RCT crossover | 41 hyperlipidaemic adults aged >/= 70 years | Pravastatin 20 mg pravastatin 20 mg + tocopherol 400 IU | Placebo + tocopherol pravastatin + tocopherol | 1 year | SDS | SMD = NR 95% CI = NR P = 0.761 | = |
| Harrison and Ashton (114) | RCT crossover | 25 healthy volunteers | Simvastatin 40 mg, pravastatin 40 mg | Placebo | 4 weeks | LSQ | Pravastatin: mean = 51.4 95% CI = 48.4–54.6 Simvastatin: mean = 47.0 95% CI = 44.9–49.1 Placebo: mean = 50.1 95% CI = 46.4–53.8 SMD = NR 95% CI = NR P = NR | = |
| Santanello et al. (121) | RCT | 431 adults aged >/= 65 years | Lovastatin 20–40 mg | Placebo | 6 months | SDS | Mean change (SD) = Placebo −0.07 (2.39) Lovastatin 20 mg −0.05 (2.53) Lovastatin 40 mg 0.46 (3.07) SMD = NR 95% CI = NR P = 0.93 | = |
| Wardle et al. (123) | RCT | 621 adults with CAD aged 40–75 years | Simvastatin 20–40 mg | Placebo | 152 weeks | Sleep symptoms report | Prevalence difference= −5.3% (statin users) MD = NR 95% CI = NR P = NR | + |
*The effect size of the main findings, either extracted from the study or calculated by the authors. Where this was not possible, we report the raw data.
BDI, beck depression inventory; CAD, coronary artery disease; CI, confidence interval; DSST, digit symbol substitution test; GHQ, general health questionnaire; HADS, hospital anxiety and depression scale; HR, hazard ratio; ICD, implantable cardioverter-defibrillator; LSQ, leeds sleep questionnaire; MD, mean difference; MDD, major depressive disorder; POMS, profile of mood scores; PR, prevalence ratio; OR, odds ratio; RCT, randomised controlled trial; SD, standard deviation; SDS, sleep dysfunction scale; SMD, standardised mean difference; TAU, treatment as usual.
3e-I Anhedonia
We could not identify any paper specifically addressing anhedonia.
3e-II Psychomotor Retardation
Three of the mentioned RCTs investigated the effects of statins on measures of psychomotor retardation. A study conducted on 209 hyperlipidaemic adults reported a statistically better psychomotor speed for the placebo group vs. lovastatin-treated subjects (Z score = 0.17, 95% CI = 0.05–0.28) (118). The two remaining studies reported no significant difference in Digit Symbol Substitution Test (DSST) scores between statin and placebo groups (114, 121).
3e-III Anxiety
Eight studies measured anxiety as well as depressive scores. The most recent RCT found no significant difference in Generalised Anxiety Disorder 7-items scale score reduction between a rosuvastatin and placebo group (MD = −0.6, 95% CI = −1.7 to 3.0, P = 0.684) (132). A crossover RCT on 25 healthy volunteers did not show any difference on the anxiety items of a hospital anxiety and depression scale between pravastatin (mean = 3.2, 95% CI = 2.0–4.4), simvastatin (mean = 2.5, 95% CI = 1.7–3.3), and placebo (mean = 3.1, 95% CI = 2.2–4.0) groups (114). Another RCT on 621 adults with increased risk of coronary artery disease (CAD) found no difference in anxiety scores (χ2 = 3.57, linear trend = 0.07) between simvastatin and placebo after a 152-week follow-up (123). Two RCTs were conducted on patients with previous ACS (N = 1,130) (122) and hyperlipidaemia (N = 120) (115), and could not find any significant effect of statins on anxiety scores (MD = 0.49, 95% CI = −0.30–1.28; MD = 0.00, 95% CI = −0.05 to 0.05, respectively). Conversely, a cohort study on 371 CAD patients showed an improvement in anxiety among statin users (OR = 0.69, 95% CI = 0.47–0.99) (98). Another cohort study conducted on patients with ICD (N = 409) reported non-significant differences in anxiety scores when statins were used (MD = −0.81, 95% CI = −1.80 to 0.18) (126). Last, a recent cohort study on Swedish nationwide register of statin users (N = 1,149,384) reported no difference in the risk anxiety disorders presentation when on statins compared to periods off statins (HR = 0.99, 95% CI = 0.95–1.02) (87).
3e-IV Sleep
Four papers also investigated the effect of statins on sleep symptoms in depression and found no association. Harrison and colleagues did not find significant differences on Leeds Sleep Questionnaire (LSQ) scores between simvastatin (mean = 47.0, 95% CI = 44.9–49.1), pravastatin (mean = 51.4, 95% CI = 48.4–54.6), or placebo groups (mean = 50.1, 95% CI = 46.4–53.8) (114). A positive effect of simvastatin, though of unclear statistical value, was seen in a sample of patients at increased CAD risk, with sleep disturbances reported by 48.8% of patients taking simvastatin and 54% of patients taking placebo (123). Two further RCTs on, respectively, 431 (121) and 41 (111) older adults randomised to statins or placebo did not identify any changes on a sleep dysfunction scale (P = 0.93; P = 0.76, respectively).
Discussion
In this paper, we illustrated the mechanisms whereby statins may play a role in ameliorating the pathophysiological changes associated with depression and reported on 72 clinical studies on the effects of statins in both non-depressed and depressed patients. To our knowledge, this is the largest review to date that retrieved and discussed this extensive literature following a systematic, evidence-based methodology. Although our aim was chiefly to provide the reader with a comprehensive, descriptive overview of the available literature, the collected data allow to draw some important conclusions.
The high number of articles retrieved as well as the presence of a definite trend for larger and more robust studies emphasise the interest of the scientific community to this research area, which may have significant implications for routine clinical practise. Such awareness likely stems from two important observations: firstly, statins are among the most commonly prescribed medications (139), hence the discovery that their use is associated with either antidepressant or depressogenic effects would have a very substantial impact on public health; and secondly, the relative lack of any breakthrough development of new antidepressant drugs capable of targeting alternative biological pathways (140), or that are free from concerns about adverse events and misuse [e.g., ketamine (141)], immediately make the potential use of statins especially appealing.
The issue of whether statins are to be considered a public health concern because of a depressogenic potential in non-depressed people appears less likely, from a purely numerical perspective, by looking at the large majority of studies reporting either no effect (=29) or at best a positive effect (=25) on depression as compared to studies reporting a negative effect (=10). From this perspective, data from large epidemiological studies can be particularly informative: the latest meta-analysis of observational studies included over 5 million non-depressed participants and did not observe any negative effect of statins on the risk of receiving a diagnosis of depression (72). This result appears in contrast with a previous smaller meta-analysis that had highlighted a risk reduction for depression in statins' users (73), with such difference mainly driven by the addition of a large Danish cohort study showing an increased risk of developing depression associated with the prescription of statins (84). However, the latter authors reported that this negative effect of statins became non-significant after adjusting for clinical-demographic variables and seemed mainly driven by confounders (84); moreover, the meta-analysis by Lee and colleagues (72) did not include the more recently published results on more than 1 million statins' users showing instead a significant reduction in clinical presentations for depressive episodes when participants were taking statins compared to when they were not (87). Data on the effects of statins on depressive scores in non-depressed or mixed populations, which could be more sensitive to smaller changes as compared to new diagnosis of or presentations for depression, are only available on smaller samples mainly from RCTs (109, 110, 138) rather than larger observational studies. This is likely due to national registers not routinely recording measures on scales of depression — surely an important avenue for further research. Still, taken together, these results provide reassurance that statins are unlikely to provoke the onset of depressive syndrome in otherwise healthy populations.
When considering studies specifically directed at groups of depressed patients, the numbers are even more favourable (positive effect =8 studies, no effect =2 studies, negative effect = no studies). Perhaps unsurprisingly, this crude dichotomy suggests that statins might indeed have an antidepressant action in people suffering from depression, but they are unable to improve mood in non-depressed subjects; similar to traditional antidepressants (142). In line with this, the large meta-analysis of RCTs on a mixed population by Yatham and colleagues (110) revealed via a subgroup analysis that only the depressed sample showed significantly improved mood on statins, whereas such effect was not apparent in the non-depressed subgroup. Translating this concept into neurobiological terms, statins would be able to express an antidepressant activity only in those people who present an underlying condition, such as increased inflammation, which directly contributes to their depressive symptomatology, whereas they would have no effect or even a negative effect if they act to perturb physiological processes, such as the regulatory functions of inflammatory cytokines, that are necessary for neuronal integrity (143). To paraphrase, both “too much” and “too little” inflammation are problematic when it comes to mood homeostasis, so an anti-inflammatory drug can be beneficial only if it hits the “sweet spot” in between these two conditions (34).
It follows that, if the antidepressant effect of statins was mainly explained by their anti-inflammatory properties, only a subset of patients whose depression was related to increased inflammation (12) would benefit from their use. For example, a previous study in depressed participants showed that, whilst patients who had raised inflammatory markers appeared to improve when on the anti-cytokine medication infliximab, those who did not have features of increased baseline inflammation indeed displayed a worsening of their depressive symptoms (144). Matters are complicated further when considering that there is no real consensus about the mechanistic processes contributing to statins' activity on mood, which likely involves complex interactions between several biological systems (24); therefore, for instance, it may be important to also consider lipid profiles when exploring the effect of stains on depressive symptoms. It is therefore conceivable that the use of statins could indeed lead to insubstantial or even harmful effects on mood because of the confounding heterogeneity of non-depressed, non-inflamed, or non-dyslipidaemic participants. In this respect, it will be intriguing to learn the results of two concurrent clinical trials investigating the antidepressant potential of simvastatin in patients with depression and comorbid obesity [i.e., a condition associated with abnormal lipid metabolism, inflammation, and antidepressant treatment resistance (145)] (146), and in patients with treatment-resistant depression whilst accounting for the mediating effects of blood lipids and CRP (147).
Other demographic and clinical variables that have been linked with baseline inflammatory status could likewise play a significant role. For example, elderly people are more likely to present with higher levels of inflammation (148), and indeed the older the patient, the higher the apparent benefit of statins on depression (90), and perhaps vice versa in younger populations (132). Similarly, sex differences in immune functions might explain why women, who are generally more liable to increased activity of the immune system (149), responded more than men to the antidepressant effect of statins (125). Also, a large cohort of non-depressed participants on statin treatment following an acute coronary event, a condition associated with increased systemic inflammation, showed a reduced likelihood of developing depression, but such effect was not seen in the group without underlying coronary syndrome (95). Results in patients with post-stroke depression were more heterogeneous (74, 80, 82, 96), but when the mediating effect of the pro-inflammatory cytokine IL-6 was taken into account, an antidepressant effect of statins once again emerged (79).
Despite significant evidence connecting inflammation with the beneficial effects of statins in depression, we could not identify any study explicitly addressing whether statins affect the cardinal depressive-inflammatory symptom cluster of anhedonia (150). Although the design of a study targeting such a specific symptom may seem impractical or restrictive, its importance has been highlighted by a recent trial of the anti-inflammatory sirukumab, which failed to show any effect on total depressive scores but was associated with a significant improvement in a rating scale measuring aspects of anhedonia (151). Anhedonia is reportedly one of the most impairing depressive symptoms and responds relatively poorly to treatment with conventional antidepressants (152); therefore a beneficial activity of statins on this symptom cluster would have an important clinical impact. Evidence about the effect of statins on psychomotor retardation, a symptom closely related to anhedonia (153) probably due to a shared common pathway involving dopaminergic dysfunction, was likewise sparse and inconsistent. Instead, more data were available for the depressive-inflammatory symptoms of anxiety and sleep disturbances. With regards to the former, despite animal studies suggesting that statins could lead to increased anxiety in rats (61), several clinical studies have shown no negative effects (87, 132), or indeed an improvement (98) of anxiety scores in statin-users. Then, contrary to a wide literature describing a potential association between statins' use and sleep problems in non-depressed populations (154), we could not identify any study that reported similar outcomes in the context of depressive symptoms, including in subgroups of elderly patients who are generally more likely to suffer from sleep difficulties (121).
Another factor potentially contributing to the heterogeneity of the published findings might be related to the notion that all statins are equally capable of expressing an antidepressant effect or indeed any neurobiological effect at all. Although most statins share similar pharmacodynamic properties, their pharmacokinetics and especially their lipophilicity (and thus arguably their potential to penetrate the blood-brain barrier) vary dramatically (155). For example, all the retrieved RCTs (133–135) that employed a lipophilic statin (respectively, lovastatin, atorvastatin, and simvastatin) in depressed patients observed an improvement of depressive symptoms, whereas the only trial that could not replicate this effect used the hydrophilic molecule, rosuvastatin (132). Interestingly, simvastatin, the most lipophilic statin, showed a more pronounced antidepressant effect compared to the less lipophilic atorvastatin in another RCT (131) and fared better than any other statin in a recent exploratory network meta-analysis (71). Likewise, evidence from most observational studies in non-depressed populations reported that simvastatin had the most beneficial effects (87, 90), though another study reported a conflicting finding (76). Again, data from the previously mentioned ongoing trials (146, 147), which are both using the highly lipophilic simvastatin, possibly corroborated by further observational studies comparing the effects of individual lipophilic and hydrophilic statins, could provide further useful evidence.
Our study has several limitations. Firstly, although we collated the largest number of studies on the effects of statins in depression to date, we did not perform any quantitative pooled analysis. Here we defer to the numerous meta-analyses published thus far (19, 72, 73, 109, 110, 130, 138); however, our aim was not to replicate the findings from such an extensive amount of previous secondary research. but rather to use a sensitive and systematic search strategy to ensure a very high level of inclusiveness. In this way we hoped to provide readers with a comprehensive, unbiased narrative review on the topic of statins and depression, complemented by the involvement of several authors in the process of literature search and data extraction. Nevertheless, it is possible that some significant records have been missed. Finally, many of the included studies, especially the older literature, did not report or were indeed lacking methodological detail, which may have affected our attempted interpretation and contextualisation of the findings. However, the identification of such methodological issues is likewise important as it will inform further research studies.
In summary, our broad evidence-based overview of the mechanisms and clinical studies on statins and their effects in depression adds to the wide literature investigating this important research and clinical subject. In view of the substantial amount of evidence suggesting an effect of statins on depressive symptoms, and the potential implications for clinical practise and public health should these effects be confirmed, we advocate for further mechanistic, observational, and interventional studies to definitively shed light on this matter.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
RD, NR, PC, and CH contributed to conception of the study. RD and NR designed the study. RD, NR, AQ, and FD contributed to the organisation of the database. RD and FD performed the literature search. NR and AQ extracted the data. RD wrote the first draught of the paper. NR devised the figures and tables. PC and CH supervised the study. All authors contributed to the article and approved the submitted version.
Conflict of Interest
CH has received consultancy fees from P1vital, Janssen, Sage Pharmaceuticals, Zogenix, Pfizer, and Lundbeck outside of the current work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
Funding. This project was funded by the Wellcome Trust, award: 102176/Z/13/Z, grant: 216452/Z/19/Z, and title: The effects of anti-inflammatory drugs on emotional and reward processing. CH is supported by the Oxford Health NIHR Biomedical Research Centre. FD is supported by the National Institute for Health Research (NIHR) Research Professorship to Professor Andrea Cipriani (grant RP-2017-08-ST2-006) and by the NIHR Oxford Health Biomedical Research Centre (grant BRC-1215-20005). The views expressed are those of the authors and not necessarily those of the Wellcome Trust, the NIHR, or the NHS.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.702617/full#supplementary-material
References
- 1.James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1789–858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy SH. A review of antidepressant therapy in primary care: current practices and future directions. Prim Care Comp J Clin Psychiatry. (2013) 15:PCC.12r01420. 10.4088/PCC.12r01420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. (2018) 391:1357–66. 10.1016/S0140-6736(17)32802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. (2006) 163:1905–17. 10.1176/ajp.2006.163.11.1905 [DOI] [PubMed] [Google Scholar]
- 5.Harmer CJ, Duman RS, Cowen PJ. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry. (2017) 4:409–18. 10.1016/S2215-0366(17)30015-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebada ME. Drug repurposing may generate novel approaches to treating depression. J Pharm Pharmacol. (2017) 69:1428–36. 10.1111/jphp.12815 [DOI] [PubMed] [Google Scholar]
- 7.Lamers F, Milaneschi Y, Vinkers CH, Schoevers RA, Giltay EJ, Penninx BWJH. Depression profilers and immuno-metabolic dysregulation: longitudinal results from the NESDA study. Brain Behav Immun. (2020) 88:174–83. 10.1016/j.bbi.2020.04.002 [DOI] [PubMed] [Google Scholar]
- 8.Çakici N, Sutterland AL, Penninx BWJH, Dalm VA, de Haan L, van Beveren NJM. Altered peripheral blood compounds in drug-naïve first-episode patients with either schizophrenia or major depressive disorder: a meta-analysis. Brain Behav Immun. (2020) 88:547–58. 10.1016/j.bbi.2020.04.039 [DOI] [PubMed] [Google Scholar]
- 9.Yuan N, Chen Y, Xia Y, Dai J, Liu C. Inflammation-related biomarkers in major psychiatric disorders: a cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl Psychiatry. (2019) 9:233. 10.1038/s41398-019-0570-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marrie RA, Walld R, Bolton JM, Sareen J, Walker JR, Patten SB, et al. Rising incidence of psychiatric disorders before diagnosis of immune-mediated inflammatory disease. Epidemiol Psychiatr Sci. (2019) 28:333–42. 10.1017/S2045796017000579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wittenberg GM, Stylianou A, Zhang Y, Sun Y, Gupta A, Jagannatha PS, et al. Effects of immunomodulatory drugs on depressive symptoms: a mega-analysis of randomized, placebo-controlled clinical trials in inflammatory disorders. Mol Psychiatry. (2019) 25:1–11. 10.1038/s41380-019-0471-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamberlain SR, Cavanagh J, De Boer P, Mondelli V, Jones DNC, Drevets WC, et al. Treatment-resistant depression and peripheral C-reactive protein. Br J Psychiatry. (2019) 214:11–9. 10.1192/bjp.2018.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benedetti F, Poletti S, Vai B, Mazza MG, Lorenzi C, Brioschi S, et al. Higher baseline interleukin-1β and TNF-α hamper antidepressant response in major depressive disorder. Eur Neuropsychopharmacol. (2021) 42:35–44. 10.1016/j.euroneuro.2020.11.009 [DOI] [PubMed] [Google Scholar]
- 14.Costello H, Gould RL, Abrol E, Howard R. Systematic review and meta-analysis of the association between peripheral inflammatory cytokines and generalised anxiety disorder. BMJ Open. (2019) 9:e027925. 10.1136/bmjopen-2018-027925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang M, Wei J, Yang X, Ni P, Wang Y, Zhao L, et al. The level of IL-6 was associated with sleep disturbances in patients with major depressive disorder. Neuropsychiatr Dis Treat. (2019) 15:1695–700. 10.2147/NDT.S202329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Eeden WA, van Hemert AM, Carlier IVE, Penninx BWJH, Lamers F, Fried EI, et al. Basal and LPS-stimulated inflammatory markers and the course of individual symptoms of depression. Transl Psychiatry. (2020) 10:235. 10.1038/s41398-020-00920-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou FC, Lee JWY, Zhang QH, Sun ZL, Bo Q, He XX, et al. Higher serum c-reactive protein levels in catatonic patients: a comparison to non-catatonic patients and healthy controls. Schizophr Bull. (2020) 46:1155–64. 10.1093/schbul/sbaa041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Primo de Carvalho Alves L, Sica da Rocha N. Diffe18.pagerent cytokine patterns associate with melancholia severity among inpatients with major depressive disorder. Ther Adv Psychopharmacol. (2020) 10. 10.1177/2045125320937921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai S, Guo W, Feng Y, Deng H, Li G, Nie H, et al. Efficacy and safety of anti-inflammatory agents for the treatment of major depressive disorder: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry. (2020) 91:21–32. 10.1136/jnnp-2019-320912 [DOI] [PubMed] [Google Scholar]
- 20.Sizar O, Khare S, Jamil RT, Talati R. Statin Medications. StatPearls Publishing; (2021). Available online at: http://www.ncbi.nlm.nih.gov/pubmed/28613690 (accessed April 10, 2021). [PubMed] [Google Scholar]
- 21.Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. (2016) 388:2532–61. 10.1016/S0140-6736(16)31357-5 [DOI] [PubMed] [Google Scholar]
- 22.Devaraj S, Rogers J, Jialal I. Statins and biomarkers of inflammation. Curr Atheroscler Rep. (2007) 9:33–41. 10.1007/BF02693938 [DOI] [PubMed] [Google Scholar]
- 23.Köhler-Forsberg O, Gasse C, Berk M, Østergaard SD. Do statins have antidepressant effects? CNS Drugs. (2017) 31:335–43. 10.1007/s40263-017-0422-3 [DOI] [PubMed] [Google Scholar]
- 24.Köhler-Forsberg O, Otte C, Gold SM, Østergaard SD. Statins in the treatment of depression: hype or hope? Pharmacol Ther. (2020) 215:107625. 10.1016/j.pharmthera.2020.107625 [DOI] [PubMed] [Google Scholar]
- 25.van Diepen JA, Berbée JFP, Havekes LM, Rensen PCN. Interactions between inflammation and lipid metabolism: relevance for efficacy of anti-inflammatory drugs in the treatment of atherosclerosis. Atherosclerosis. (2013) 228:306–15. 10.1016/j.atherosclerosis.2013.02.028 [DOI] [PubMed] [Google Scholar]
- 26.Schneider M, Levant B, Reichel M, Gulbins E, Kornhuber J, Müller CP. Lipids in psychiatric disorders and preventive medicine. Neurosci Biobehav Rev. (2017) 76:336–62. 10.1016/j.neubiorev.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 27.Walther A, Cannistraci CV, Simons K, Durán C, Gerl MJ, Wehrli S, et al. Lipidomics in major depressive disorder. Front Psychiatry. (2018) 9:459. 10.3389/fpsyt.2018.00459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee LHW, Shui G, Farooqui AA, Wenk MR, Tan CH, Ong WY. Lipidomic analyses of the mouse brain after antidepressant treatment: Evidence for endogenous release of long-chain fatty acids? Int J Neuropsychopharmacol. (2009) 12:953–64. 10.1017/S146114570900995X [DOI] [PubMed] [Google Scholar]
- 29.Gulbins E, Palmada M, Reichel M, Lüth A, Böhmer C, Amato D, et al. Acid sphingomyelinase-ceramide system mediates effects of antidepressant drugs. Nat Med. (2013) 19:934–8. 10.1038/nm.3214 [DOI] [PubMed] [Google Scholar]
- 30.Shrivastava S, Pucadyil TJ, Paila YD, Ganguly S, Chattopadhyay A. Chronic cholesterol depletion using statin impairs the function and dynamics of human serotonin 1a receptors †. Biochemistry. (2010) 49:5426–35. 10.1021/bi100276b [DOI] [PubMed] [Google Scholar]
- 31.Mailman T, Hariharan M, Karten B. Inhibition of neuronal cholesterol biosynthesis with lovastatin leads to impaired synaptic vesicle release even in the presence of lipoproteins or geranylgeraniol. J Neurochem. (2011) 119:1002–15. 10.1111/j.1471-4159.2011.07474.x [DOI] [PubMed] [Google Scholar]
- 32.Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. (2013) 18:963–74. 10.1038/mp.2013.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang C-C, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. (2003) 163:1926. 10.1001/archinte.163.16.1926 [DOI] [PubMed] [Google Scholar]
- 34.Miller AH, Haroon E, Felger JC. Therapeutic implications of brain–immune interactions: treatment in translation. Neuropsychopharmacol Rev. (2017) 42:334–59. 10.1038/npp.2016.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. (2008) 31:464–8. 10.1016/j.tins.2008.06.006 [DOI] [PubMed] [Google Scholar]
- 36.Macin SM, Perna ER, Farías EF, Franciosi V, Cialzeta JR, Brizuela M, et al. Atorvastatin has an important acute anti-inflammatory effect in patients with acute coronary syndrome: results of a randomized, double-blind, placebo-controlled study. Am Heart J. (2005) 149:451–7. 10.1016/j.ahj.2004.07.041 [DOI] [PubMed] [Google Scholar]
- 37.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. (2005) 4:977–87. 10.1038/nrd1901 [DOI] [PubMed] [Google Scholar]
- 38.Wu H, Lv W, Pan Q, Kalavagunta PK, Liu Q, Qin G, et al. Simvastatin therapy in adolescent mice attenuates HFD-induced depression-like behavior by reducing hippocampal neuroinflammation. J Affect Disord. (2019) 243:83–95. 10.1016/j.jad.2018.09.022 [DOI] [PubMed] [Google Scholar]
- 39.Lim S-W, Shiue Y-L, Liao J-C, Wee H-Y, Wang C-C, Chio C-C, et al. Simvastatin therapy in the acute stage of traumatic brain injury attenuates brain trauma-induced depression-like behavior in rats by reducing neuroinflammation in the hippocampus. Neurocrit Care. (2017) 26:122–32. 10.1007/s12028-016-0290-6 [DOI] [PubMed] [Google Scholar]
- 40.Yu X-B, Zhang H-N, Dai Y, Zhou Z-Y, Xu R, Hu L-F, et al. Simvastatin prevents and ameliorates depressive behaviors via neuroinflammatory regulation in mice. J Affect Disord. (2019) 245:939–49. 10.1016/j.jad.2018.11.086 [DOI] [PubMed] [Google Scholar]
- 41.Hai-Na Z, Xu-Ben Y, Cong-Rong T, Yan-Cheng C, Fan Y, Lei-Mei X, et al. Atorvastatin ameliorates depressive behaviors and neuroinflammatory in streptozotocin-induced diabetic mice. Psychopharmacology. (2020) 237:695–705. 10.1007/s00213-019-05406-w [DOI] [PubMed] [Google Scholar]
- 42.Kim S-W, Kang H-J, Bae K-Y, Shin I-S, Hong YJ, Ahn Y-K, et al. Interactions between pro-inflammatory cytokines and statins on depression in patients with acute coronary syndrome. Prog Neuro Psychopharmacol Biol Psychiatry. (2018) 80:250–4. 10.1016/j.pnpbp.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 43.ElBatsh MM. Antidepressant-like effect of simvastatin in diabetic rats. Can J Physiol Pharmacol. (2015) 93:649–56. 10.1139/cjpp-2014-0560 [DOI] [PubMed] [Google Scholar]
- 44.Sahebkar A, Rathouska J, Simental-Mendía LE, Nachtigal P. Statin therapy and plasma cortisol concentrations: a systematic review and meta-analysis of randomized placebo-controlled trials. Pharmacol Res. (2016) 103:17–25. 10.1016/j.phrs.2015.10.013 [DOI] [PubMed] [Google Scholar]
- 45.Lin P-Y, Chang AYW, Lin T-K. Simvastatin treatment exerts antidepressant-like effect in rats exposed to chronic mild stress. Pharmacol Biochem Behav. (2014) 124:174–9. 10.1016/j.pbb.2014.06.006 [DOI] [PubMed] [Google Scholar]
- 46.Can ÖD, Ulupinar E, Özkay ÜD, Yegin B, Öztürk Y. The effect of simvastatin treatment on behavioral parameters, cognitive performance, and hippocampal morphology in rats fed a standard or a high-fat diet. Behav Pharmacol. (2012) 23:582–92. 10.1097/FBP.0b013e328356c3f2 [DOI] [PubMed] [Google Scholar]
- 47.Bhatt S, Nagappa AN, Patil CR. Role of oxidative stress in depression. Drug Discov Today. (2020) 25:1270–6. 10.1016/j.drudis.2020.05.001 [DOI] [PubMed] [Google Scholar]
- 48.Ludka FK, Dal-Cim T, Binder LB, Constantino LC, Massari C, Tasca CI. Atorvastatin and fluoxetine prevent oxidative stress and mitochondrial dysfunction evoked by glutamate toxicity in hippocampal slices. Mol Neurobiol. (2017) 54:3149–61. 10.1007/s12035-016-9882-6 [DOI] [PubMed] [Google Scholar]
- 49.Shahsavarian A, Javadi S, Jahanabadi S, Khoshnoodi M, Shamsaee J, Shafaroodi H, et al. Antidepressant-like effect of atorvastatin in the forced swimming test in mice: the role of PPAR-gamma receptor and nitric oxide pathway. Eur J Pharmacol. (2014) 745:52–8. 10.1016/j.ejphar.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 50.Naserzadeh R, Abad N, Ghorbanzadeh B, Dolatshahi M, Mansouri MT. Simvastatin exerts antidepressant-like activity in mouse forced swimming test: role of NO-cGMP-KATP channels pathway and PPAR-gamma receptors. Pharmacol Biochem Behav. (2019) 180:92–100. 10.1016/j.pbb.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 51.Amidfar M, Woelfer M, Réus GZ, Quevedo J, Walter M, Kim YK. The role of NMDA receptor in neurobiology and treatment of major depressive disorder: evidence from translational research. Prog Neuro Psychopharmacol Biol Psychiatry. (2019) 94:109668. 10.1016/j.pnpbp.2019.109668 [DOI] [PubMed] [Google Scholar]
- 52.Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. (2018) 23:801–11. 10.1038/mp.2017.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ludka FK, Zomkowski ADE, Cunha MP, Dal-Cim T, Zeni ALB, Rodrigues ALS, et al. Acute atorvastatin treatment exerts antidepressant-like effect in mice via the l-arginine–nitric oxide–cyclic guanosine monophosphate pathway and increases BDNF levels. Eur Neuropsychopharmacol. (2013) 23:400–12. 10.1016/j.euroneuro.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 54.Ludka FK, Constantino LC, Dal-Cim T, Binder LB, Zomkowski A, Rodrigues ALS, et al. Involvement of PI3K/Akt/GSK-3β and mTOR in the antidepressant-like effect of atorvastatin in mice. J Psychiatr Res. (2016) 82:50–7. 10.1016/j.jpsychires.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 55.Arosio B, Guerini FR, Voshaar RCO, Aprahamian I. Blood brain-derived neurotrophic factor (BDNF) and major depression: do we have a translational perspective? Front Behav Neurosci. (2021) 15:626906. 10.3389/fnbeh.2021.626906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang C-R, Yu X-B, Zhang H-N, Cao Y-C, Yang F, Xu L-M, et al. Lovastatin Prevents Depressive Behaviors and Increased Hippocampal Neurogenesis in Streptozotocin-Induced Diabetic Mice. Pharmacology. (2020) 105:339–48. 10.1159/000503865 [DOI] [PubMed] [Google Scholar]
- 57.Tsai S-J. Statins may enhance the proteolytic cleavage of proBDNF: implications for the treatment of depression. Med Hypoth. (2007) 68:1296–9. 10.1016/j.mehy.2006.09.043 [DOI] [PubMed] [Google Scholar]
- 58.Ludka FK, Cunha MP, Dal-Cim T, Binder LB, Constantino LC, Massari CM, et al. Atorvastatin protects from Aβ1–40-induced cell damage and depressive-like behavior via ProBDNF cleavage. Mol Neurobiol. (2017) 54:6163–73. 10.1007/s12035-016-0134-6 [DOI] [PubMed] [Google Scholar]
- 59.Taniguti EH, Ferreira YS, Stupp IJV, Fraga-Junior EB, Doneda DL, Lopes L, et al. Atorvastatin prevents lipopolysaccharide-induced depressive-like behaviour in mice. Brain Res Bull. (2019) 146:279–86. 10.1016/j.brainresbull.2019.01.018 [DOI] [PubMed] [Google Scholar]
- 60.Rahangdale S, Fating R, Gajbhiye M, Kapse M, Inamdar N, Kotagale N, et al. Involvement of agmatine in antidepressant-like effect of HMG-CoA reductase inhibitors in mice. Eur J Pharmacol. (2021) 892:173739. 10.1016/j.ejphar.2020.173739 [DOI] [PubMed] [Google Scholar]
- 61.Okudan N, Belviranli M. High dose simvastatin and rosuvastatin impair cognitive abilities of healthy rats via decreasing hippocampal neurotrophins and irisin. Brain Res Bull. (2020) 165:81–9. 10.1016/j.brainresbull.2020.09.019 [DOI] [PubMed] [Google Scholar]
- 62.Morilak DA, Frazer A. Antidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int J Neuropsychopharmacol. (2004) 7:193–218. 10.1017/S1461145704004080 [DOI] [PubMed] [Google Scholar]
- 63.Ludka FK, Constantino LC, Kuminek G, Binder LB, Zomkowski ADE, Cunha MP, et al. Atorvastatin evokes a serotonergic system-dependent antidepressant-like effect in mice. Pharmacol Biochem Behav. (2014) 122:253–60. 10.1016/j.pbb.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 64.Wang Q, Ting WL, Yang H, Wong PTH. High doses of simvastatin upregulate dopamine D 1 and D 2 receptor expression in the rat prefrontal cortex: possible involvement of endothelial nitric oxide synthase. Br J Pharmacol. (2005) 144:933–9. 10.1038/sj.bjp.0706106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Renshaw PF, Parsegian A, Yang CK, Novero A, Yoon SJ, Lyoo IK, et al. Lovastatin potentiates the antidepressant efficacy of fluoxetine in rats. Pharmacol Biochem Behav. (2009) 92:88–92. 10.1016/j.pbb.2008.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Santos T, da Cruz JGP, Baungratz MM, da Cruz JN, Dal Magro DD, da Cruz JGP. Behavioral interactions of simvastatin and fluoxetine in tests of anxiety and depression. Neuropsychiatr Dis Treat. (2012) 8:413–22. 10.2147/NDT.S31714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Al-Asmari AK, Ullah Z, Al Masoudi AS, Ahmad I. Simultaneous administration of fluoxetine and simvastatin ameliorates lipid profile, improves brain level of neurotransmitters, and increases bioavailability of simvastatin. J Exp Pharmacol. (2017) 9:47–57. 10.2147/JEP.S128696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim SW, Bae KY, Kim JM, Shin IS, Hong YJ, Ahn Y, et al. The use of statins for the treatment of depression in patients with acute coronary syndrome. Transl Psychiatry. (2015) 5:e620. 10.1038/tp.2015.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dolatshahi M, Davoudi S, Paridar Y, Naserzadeh R, Ghorbanzadeh B. Pharmacological evidence for the involvement of the opioid system in the antidepressant-like effect of simvastatin in mice: without tolerance and withdrawal syndrome. Neurosci Lett. (2020) 714:134578. 10.1016/j.neulet.2019.134578 [DOI] [PubMed] [Google Scholar]
- 70.Wang H, Zhou J, Liu QZ, Wang LL, Shang J. Simvastatin and bezafibrate ameliorate emotional disorder induced by high fat diet in C57BL/6 mice. Sci Rep. (2017) 7:2335. 10.1038/s41598-017-02576-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Giorgi R, Crescenzo F De, Pesci NR, Martens M, Howard W, Cowen PJ, et al. Statins for major depressive disorder: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE. (2021) 71:e0249409. 10.1371/journal.pone.0249409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee MC, Peng TR, Lee CH, Wang JY, Lee JA, Chen SM, et al. Statin use and depression risk: a systematic review and meta-analysis. J Affect Disord. (2021) 282:308–15. 10.1016/j.jad.2020.12.164 [DOI] [PubMed] [Google Scholar]
- 73.Parsaik AK, Singh BM, Hassan M, Williams MD, Singh K, Rummans TA, et al. Statins use and risk of depression: A systematic review and meta-analysis. J Affect Disord. (2014) 160:62–7. 10.1016/j.jad.2013.11.026 [DOI] [PubMed] [Google Scholar]
- 74.Asplund K, Eriksson M. Inflammation, poststroke depression and statins. Int J Stroke. (2011) 6:567–8. 10.1111/j.1747-4949.2011.00691.x [DOI] [PubMed] [Google Scholar]
- 75.Chuang C-S, Yang T-Y, Muo C-H, Su H-L, Sung F-C, Kao C-H. Hyperlipidemia, statin use and the risk of developing depression: a nationwide retrospective cohort study. Gen Hosp Psychiatry. (2014) 36:497–501. 10.1016/j.genhosppsych.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 76.Dave CV, Winterstein AG, Park H, Cook RL, Hartzema AG. Comparative risk of lipophilic and hydrophilic statins on incident depression: a retrospective cohort study. J Affect Disord. (2018) 238:542–6. 10.1016/j.jad.2018.06.021 [DOI] [PubMed] [Google Scholar]
- 77.Glaus J, Vandeleur CL, Lasserre AM, Strippoli M-PPF, Castelao E, Gholam-Rezaee M, et al. Aspirin and statin use and the subsequent development of depression in men and women: results from a longitudinal population-based study. J Affect Disord. (2015) 182:126–31. 10.1016/j.jad.2015.03.044 [DOI] [PubMed] [Google Scholar]
- 78.Huang CI, Lin LC, Tien HC, Que J, Ting WC, Chen PC, et al. Hyperlipidemia and statins use for the risk of new-onset anxiety/depression in patients with head and neck cancer: a population-based study. PLoS ONE. (2017) 12:e0174574. 10.1371/journal.pone.0174574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kang H-J, Bae K-Y, Kim S-W, Kim J-T, Park M-S, Cho K-H, et al. Effects of interleukin-6, interleukin-18, and statin use, evaluated at acute stroke, on post-stroke depression during 1-year follow-up. Psychoneuroendocrinology. (2016) 72:156–60. 10.1016/j.psyneuen.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 80.Kang J-H, Kao L-T, Lin H-C, Tsai M-C, Chung S-D. Statin use increases the risk of depressive disorder in stroke patients: a population-based study. J Neurol Sci. (2015) 348:89–93. 10.1016/j.jns.2014.11.013 [DOI] [PubMed] [Google Scholar]
- 81.Kessing LV, Rytgaard HC, Gerds TA, Berk M, Ekstrøm CT, Andersen PK. New drug candidates for depression - a nationwide population-based study. Acta Psychiatr Scand. (2019) 139:68–77. 10.1111/acps.12957 [DOI] [PubMed] [Google Scholar]
- 82.Kim J-M, Stewart R, Kang H-J, Bae K-Y, Kim S-W, Shin I-S, et al. A prospective study of statin use and poststroke depression. J Clin Psychopharmacol. (2014) 34:72–9. 10.1097/JCP.0000000000000051 [DOI] [PubMed] [Google Scholar]
- 83.Khokhar B, Simoni-Wastila L, Slejko JF, Perfetto E, Zhan M, Smith GS. Mortality and associated morbidities following traumatic brain injury in older medicare statin users. J Head Trauma Rehabil. (2018) 1:E68–76. 10.1097/HTR.0000000000000369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Köhler-Forsberg O, Gasse C, Petersen L, Nierenberg AA, Mors O, Østergaard SD. Statin treatment and the risk of depression. J Affect Disord. (2019) 246:706–15. 10.1016/j.jad.2018.12.110 [DOI] [PubMed] [Google Scholar]
- 85.Mansi I, Frei CR, Pugh MJ, Mortensen EM. Psychologic disorders and statin use: a propensity score-matched analysis. Pharmacotherapy. (2013) 33:615–26. 10.1002/phar.1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Medici CR, Gradus JL, Pedersen L, Sørensen HT, Østergaard SD, Christiansen CF. No impact of preadmission anti-inflammatory drug use on risk of depression and anxiety after critical illness*. Crit Care Med. (2017) 45:1635–41. 10.1097/CCM.0000000000002571 [DOI] [PubMed] [Google Scholar]
- 87.Molero Y, Cipriani A, Larsson H, Lichtenstein P, D'Onofrio BM, Fazel S. Associations between statin use and suicidality, depression, anxiety, and seizures: a Swedish total-population cohort study. Lancet Psychiatry. (2020) 7:982–90. 10.1016/S2215-0366(20)30311-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Otte C, Zhao S, Whooley MA. Statin use and risk of depression in patients with coronary heart disease. J Clin Psychiatry. (2012) 73:610–5. 10.4088/JCP.11m07038 [DOI] [PubMed] [Google Scholar]
- 89.Pasco JA, Jacka FN, Williams LJ, Henry MJ, Nicholson GC, Kotowicz MA, et al. Clinical implications of the cytokine hypothesis of depression: the association between use of statins and aspirin and the risk of major depression. Psychother Psychosom. (2010) 79:323–5. 10.1159/000319530 [DOI] [PubMed] [Google Scholar]
- 90.Redlich C, Berk M, Williams LJ, Sundquist J, Sundquist K, Li X. Statin use and risk of depression: a Swedish national cohort study. BMC Psychiatry. (2014) 14:348. 10.1186/s12888-014-0348-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol. (2009) 67:99–109. 10.1111/j.1365-2125.2008.03308.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stafford L, Berk M. The use of statins after a cardiac intervention is associated with reduced risk of subsequent depression. J Clin Psychiatry. (2011) 72:1229–35. 10.4088/JCP.09m05825blu [DOI] [PubMed] [Google Scholar]
- 93.Wee H-Y, Ho C-H, Fu Liang W, Hsieh K-Y, Wang C-C, et al. Increased risk of new-onset depression in patients with traumatic brain injury and hyperlipidemia: the important role of statin medications. J Clin Psychiatry. (2016) 77:505–11. 10.4088/JCP.14m09749 [DOI] [PubMed] [Google Scholar]
- 94.Williams LJ, Pasco JA, Mohebbi M, Jacka FN, Stuart AL, Venugopal K, et al. Statin and aspirin use and the risk of mood disorders among men. Int J Neuropsychopharmacol. (2016) 19:pyw008. 10.1093/ijnp/pyw008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wium-Andersen IK, Wium-Andersen MK, Jørgensen MB, Osler M. Anti-inflammatory treatment and risk of depression in 91,842 patients with acute coronary syndrome and 91,860 individuals without acute coronary syndrome in Denmark. Int J Cardiol. (2017) 246:1–6. 10.1016/j.ijcard.2017.05.105 [DOI] [PubMed] [Google Scholar]
- 96.Wium-Andersen IK, Wium-Andersen MK, Jørgensen MB, Osler M. Anti-inflammatory treatment and risk for depression after first-time stroke in a cohort of 147 487 Danish patients. J Psychiatry Neurosci. (2017) 42:320–30. 10.1503/jpn160244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yeh J-J, Syue S-H, Lin C-L, Hsu CY, Shae Z, Kao C-H. Effects of statins on anxiety and depression in patients with asthma-chronic obstructive pulmonary disease overlap syndrome. J Affect Disord. (2019) 253:277–84. 10.1016/j.jad.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 98.Young-Xu Y, Chan KA, Liao JK, Ravid S, Blatt CM. Long-term statin use and psychological well-being. J Am Coll Cardiol. (2003) 42:690–7. 10.1016/S0735-1097(03)00785-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Agustini B, Mohebbi M, Woods RL, McNeil JJ, Nelson MR, Shah RC, et al. Association between statin use and depressive symptoms in a large community-dwelling older population living in Australia and the USA: a cross-sectional study. CNS Drugs. (2019) 33:685–94. 10.1007/s40263-019-00633-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boumendil E, Tubert-Bitter P. Depression-induced absenteeism in relation to antihyperlipidemic treatment: a study using GAZEL cohort data. Epidemiology. (1995) 6:322–5. 10.1097/00001648-199505000-00023 [DOI] [PubMed] [Google Scholar]
- 101.Feng L, Tan C-H, Merchant RA, Ng T-P. Association between depressive symptoms and use of HMG-CoA reductase inhibitors (Statins), corticosteroids and histamine H2 receptor antagonists in community-dwelling older persons. Drugs Aging. (2008) 25:795–805. 10.2165/00002512-200825090-00005 [DOI] [PubMed] [Google Scholar]
- 102.Lindberg G, Hallas J. Cholesterol-lowering drugs and antidepressants—A study of prescription symmetry. Pharmacoepidemiol Drug Saf. (1998) 7:399–402. [DOI] [PubMed] [Google Scholar]
- 103.Williams ED, Eastwood SV, Tillin T, Stewart R, Chaturvedi N, Hughes AD. Statin use is associated with reduced depressive symptoms in Europeans, but increased symptoms in ethnic minorities in the UK: an observational study. Br J Clin Pharmacol. (2015) 80:172–3. 10.1111/bcp.12599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cham S, Koslik HJ, Golomb BA. Mood, personality, and behavior changes during treatment with statins: a case series. Drug Saf Case Reports. (2016) 3:1. 10.1007/s40800-015-0024-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Duits N, Bos FM. Depressive symptoms and cholesterol-lowering drugs. Lancet. (1993) 341:114. 10.1016/0140-6736(93)92591-G [DOI] [PubMed] [Google Scholar]
- 106.Lechleitner M, Hoppichler F, Konwalinka G, Patsch J, Braunsteiner H. Depressive symptoms in hypercholesterolaemic patients treated with pravastatin. Lancet. (1992) 340:910. 10.1016/0140-6736(92)93318-H [DOI] [PubMed] [Google Scholar]
- 107.Rosenson RS, Goranson NL. Lovastatin-associated sleep and mood disturbances. Am J Med. (1993) 95:548–9. 10.1016/0002-9343(93)90343-N [DOI] [PubMed] [Google Scholar]
- 108.Tatley M, Savage R. Psychiatric adverse reactions with statins, fibrates and ezetimibe. Drug Saf. (2007) 30:195–201. 10.2165/00002018-200730030-00003 [DOI] [PubMed] [Google Scholar]
- 109.O'Neil A, Sanna L, Redlich C, Sanderson K, Jacka F, Williams LJ, et al. The impact of statins on psychological wellbeing: a systematic review and meta-analysis. BMC Med. (2012) 10:154. 10.1186/1741-7015-10-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yatham MS, Yatham KS, Ravindran A V., Sullivan F.Do statins have an effect on depressive symptoms? A systematic review and meta-analysis. J Affect Disord. (2019) 257:55–63. 10.1016/j.jad.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 111.Carlsson CM, Papcke-Benson K, Carnes M, McBride PE, Stein JH. Health-related quality of life and long-term therapy with pravastatin and tocopherol (Vitamin E) in older adults. Drugs Aging. (2002) 19:793–805. 10.2165/00002512-200219100-00008 [DOI] [PubMed] [Google Scholar]
- 112.Chan D, Binks S, Nicholas JM, Frost C, Cardoso MJ, Ourselin S, et al. Effect of high-dose simvastatin on cognitive, neuropsychiatric, and health-related quality-of-life measures in secondary progressive multiple sclerosis: secondary analyses from the MS-STAT randomised, placebo-controlled trial. Lancet Neurol. (2017) 16:591–600. 10.1016/S1474-4422(17)30113-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gengo F, Cwudzinski D, Kinkel P, Block G, Stauffer L, Lines C. Effects of treatment with lovastatin and pravastatin on daytime cognitive performance. Clin Cardiol. (1995) 18:209–14. 10.1002/clc.4960180406 [DOI] [PubMed] [Google Scholar]
- 114.Harrison R, Ashton C. Do cholesterol-lowering agents affect brain activity? A comparison of simvastatin, pravastatin, and placebo in healthy volunteers. Br J Clin Pharmacol. (1994) 37:231–6. 10.1111/j.1365-2125.1994.tb04268.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hyyppä MT, Kronholm E, Virtanen A, Leino A, Jula A. Does simvastatin affect mood and steroid hormone levels in hypercholesterolemic men? A randomized double-blind trial. Psychoneuroendocrinology. (2003) 28:181–94. 10.1016/S0306-4530(02)00014-8 [DOI] [PubMed] [Google Scholar]
- 116.Krysiak R, Drosdzol-Cop A, Skrzypulec-Plinta V, Okopień B. The effect of atorvastatin on sexual function and depressive symptoms in young women with elevated cholesterol levels — a pilot study. Endokrynol Pol. (2018) 69:688–94. 10.5603/EP.a2018.0062 [DOI] [PubMed] [Google Scholar]
- 117.Morales K, Wittink M, Datto C, DiFilippo S, Cary M, TenHave T, et al. Simvastatin causes changes in affective processes in elderly volunteers. J Am Geriatr Soc. (2006) 54:70–6. 10.1111/j.1532-5415.2005.00542.x [DOI] [PubMed] [Google Scholar]
- 118.Muldoon MF, Barger SD, Ryan CM, Flory JD, Lehoczky JP, Matthews KA, et al. Effects of lovastatin on cognitive function and psychological well-being. Am J Med. (2000) 108:538–46. 10.1016/S0002-9343(00)00353-3 [DOI] [PubMed] [Google Scholar]
- 119.Ormiston T, Wolkowitz OM, Reus VI, Manfredi F. Behavioral implications of lowering cholesterol levels: a double-blind pilot study. Psychosomatics. (2003) 44:412–4. 10.1176/appi.psy.44.5.412 [DOI] [PubMed] [Google Scholar]
- 120.Robertson CS, McCarthy JJ, Miller ER, Levin H, McCauley SR, Swank PR, et al. Phase II clinical trial of atorvastatin in mild traumatic brain injury. J Neurotrauma. (2017) 34:1394–401. 10.1089/neu.2016.4717 [DOI] [PubMed] [Google Scholar]
- 121.Santanello NC, Barber BL, Applegate WB, Elam J, Curtis C, Hunninghake DB, et al. Effect of pharmacologic lipid lowering on health-related quality of life in older persons: results from the cholesterol reduction in seniors program (CRISP) pilot study. J Am Geriatr Soc. (1997) 45:8–14. 10.1111/j.1532-5415.1997.tb00971.x [DOI] [PubMed] [Google Scholar]
- 122.Stewart RA, Sharples KJ, North FM, Menkes DB, Baker J, Simes J. Long-term assessment of psychological well-being in a randomized placebo-controlled trial of cholesterol reduction with pravastatin. Arch Intern Med. (2000) 160:3144. 10.1001/archinte.160.20.3144 [DOI] [PubMed] [Google Scholar]
- 123.Wardle J, Armitage J, Collins R, Wallendszus K, Keech A, Lawson A. Randomised placebo controlled trial of effect on mood oflowering cholesterol concentration. BMJ. (1996) 313:75–8. 10.1136/bmj.313.7049.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Al Badarin FJ, Spertus JA, Gosch KL, Buchanan DM, Chan PS. Initiation of statin therapy after acute myocardial infarction is not associated with worsening depressive symptoms: insights from the prospective registry evaluating outcomes after myocardial infarctions: events and recovery (PREMIER) and translational R. Am Heart J. (2013) 166:879–86. 10.1016/j.ahj.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Feng L, Yap KB, Kua EH, Ng TP. Statin use and depressive symptoms in a prospective study of community-living older persons. Pharmacoepidemiol Drug Saf. (2010) 19:942–8. 10.1002/pds.1993 [DOI] [PubMed] [Google Scholar]
- 126.Hoogwegt MT, Theuns DAMJ, Kupper N, Jordaens L, Pedersen SS. Relation of statin therapy to psychological functioning in patients with an implantable cardioverter defibrillator. Am J Cardiol. (2013) 111:1169–74. 10.1016/j.amjcard.2012.12.047 [DOI] [PubMed] [Google Scholar]
- 127.Agostini JV, Tinetti ME, Han L, McAvay G, Foody JM, Concato J. Effects of statin use on muscle strength, cognition, and depressive symptoms in older adults. J Am Geriatr Soc. (2007) 55:420–5. 10.1111/j.1532-5415.2007.01071.x [DOI] [PubMed] [Google Scholar]
- 128.Mandas A, Congiu MG, Abete C, Dessì S, Manconi PE, Musio M, et al. Cognitive decline and depressive symptoms in late-life are associated with statin use: evidence from a population-based study of Sardinian old people living in their own home. Neurol Res. (2014) 36:247–54. 10.1179/1743132813Y.0000000287 [DOI] [PubMed] [Google Scholar]
- 129.Olson MB, Kelsey SF, Matthews KA, Bairey Merz CN, Eteiba W, McGorray SP, et al. Lipid-lowering medication use and aggression scores in women: a report from the NHLBI-sponsored WISE study. J Womens Health. (2008) 17:187–94. 10.1089/jwh.2007.0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Salagre E, Fernandes BS, Dodd S, Brownstein DJ, Berk M. Statins for the treatment of depression: a meta-analysis of randomized, double-blind, placebo-controlled trials. J Affect Disord. (2016) 200:235–42. 10.1016/j.jad.2016.04.047 [DOI] [PubMed] [Google Scholar]
- 131.Abbasi SH, Mohammadinejad P, Shahmansouri N, Salehiomran A, Beglar AA, Zeinoddini A, et al. Simvastatin versus atorvastatin for improving mild to moderate depression in post-coronary artery bypass graft patients: a double-blind, placebo-controlled, randomized trial. J Affect Disord. (2015) 183:149–55. 10.1016/j.jad.2015.04.049 [DOI] [PubMed] [Google Scholar]
- 132.Berk M, Mohebbi M, Dean OM, Cotton SM, Chanen AM, Dodd S, et al. Youth depression alleviation with anti-inflammatory agents (YoDA-A): a randomised clinical trial of rosuvastatin and aspirin. BMC Med. (2020) 18:16. 10.1186/s12916-019-1475-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ghanizadeh A, Hedayati A. Augmentation of fluoxetine with lovastatin for treating major depressive disorder, a randomized double-blind placebo controlled-clinical trial. Dep Anxiety. (2013) 30:1084–8. 10.1002/da.22195 [DOI] [PubMed] [Google Scholar]
- 134.Gougol A, Farokhnia M, Salimi S, Iranpour N, Zareh-Mohammadi N, Yekehtaz H, et al. Simvastatin as an adjuvant therapy to fluoxetine in patients with moderate to severe major depression: a double-blind placebo-controlled trial. J Psychopharmacol. (2015) 29:575–81. 10.1177/0269881115578160 [DOI] [PubMed] [Google Scholar]
- 135.Haghighi M, Holsboer-Trachsler E, Jahangard L, Brand S, Bajoghli H, Ahmadpanah M, et al. In a randomized, double-blind clinical trial, adjuvant atorvastatin improved symptoms of depression and blood lipid values in patients suffering from severe major depressive disorder. J Psychiatr Res. (2014) 58:109–14. 10.1016/j.jpsychires.2014.07.018 [DOI] [PubMed] [Google Scholar]
- 136.Fotso Soh J, Almadani A, Beaulieu S, Rajji T, Mulsant BH, Su CL, et al. The effect of atorvastatin on cognition and mood in bipolar disorder and unipolar depression patients: a secondary analysis of a randomized controlled trial. J Affect Disord. (2020) 262:149–54. 10.1016/j.jad.2019.11.013 [DOI] [PubMed] [Google Scholar]
- 137.Köhler O, Gasse C, Petersen L, Ingstrup KG, Nierenberg AA, Mors O, et al. The effect of concomitant treatment with SSRIs and statins: a population-based study. Am J Psychiatry. (2016) 173:807–15. 10.1176/appi.ajp.2016.15040463 [DOI] [PubMed] [Google Scholar]
- 138.Köhler-Forsberg ON, Lydholm C, Hjorthøj C, Nordentoft M, Mors O, Benros ME. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. (2019) 139:404–19. 10.1111/acps.13016 [DOI] [PubMed] [Google Scholar]
- 139.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA J Am Med Assoc. (2015) 314:1818–31. 10.1001/jama.2015.13766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dubovsky SL. What is new about new antidepressants? Psychother Psychosom. (2018) 87:129–39. 10.1159/000488945 [DOI] [PubMed] [Google Scholar]
- 141.Gautam C, Mahajan S, Sharma J, Singh H, Singh J. Repurposing potential of ketamine: opportunities and challenges. Indian J Psychol Med. (2020) 42:22–9. 10.4103/IJPSYM.IJPSYM_228_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Depression Medications: Antidepressants. Available online at: https://psychcentral.com/lib/depression-medications-antidepressants#1 (accessed April 11, 2021).
- 143.Golia MT, Poggini S, Alboni S, Garofalo S, Ciano Albanese N, Viglione A, et al. Interplay between inflammation and neural plasticity: both immune activation and suppression impair LTP and BDNF expression. Brain Behav Immun. (2019) 81:484–94. 10.1016/j.bbi.2019.07.003 [DOI] [PubMed] [Google Scholar]
- 144.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. Arch Gen Psychiatry. (2013) 70:31–41. 10.1001/2013.jamapsychiatry.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Capuron L, Lasselin J, Castanon N. Role of adiposity-driven inflammation in depressive morbidity. Neuropsychopharmacology. (2017) 42:115–28. 10.1038/npp.2016.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Otte C, Chae WR, Nowacki J, Kaczmarczyk M, Piber D, Roepke S, et al. Simvastatin add-on to escitalopram in patients with comorbid obesity and major depression (SIMCODE): study protocol of a multicentre, randomised, double-blind, placebo-controlled trial. BMJ Open. (2020) 10:e040119. 10.1136/bmjopen-2020-040119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Husain MI, Carvalho AF, Husain MO, Rahman RR, Hodsoll J, Chaudhry IB, et al. Adjunctive simvastatin for treatment-resistant depression: study protocol of a 12-week randomised controlled trial. BJPsych Open. (2019) 5:1–7. 10.1192/bjo.2018.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. (2018) 14:576–90. 10.1038/s41574-018-0059-4 [DOI] [PubMed] [Google Scholar]
- 149.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. (2016) 16:626–38. 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- 150.Miller AH. Beyond depression: the expanding role of inflammation in psychiatric disorders. World Psychiatry. (2020) 19:108–9. 10.1002/wps.20723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Salvadore G, Nash A, Bleys C, Hsu B, Drevets WC. A double-blind, placebo-controlled, multicenter study of sirukumab as adjunctive treatment to a monoaminergic antidepressant in adults with major depressive disorder (2019). [Google Scholar]
- 152.Buckner JD, Joiner TE, Pettit JW, Lewinsohn PM, Schmidt NB. Implications of the DSM's emphasis on sadness and anhedonia in major depressive disorder. Psychiatry Res. (2008) 159:25–30. 10.1016/j.psychres.2007.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lemke MR, Puhl P, Koethe N, Winkler T. Psychomotor retardation and anhedonia in depression. Acta Psychiatr Scand. (1999) 99:252–6. 10.1111/j.1600-0447.1999.tb07221.x [DOI] [PubMed] [Google Scholar]
- 154.Broncel M, Gorzelak-Pabiś P, Sahebkar A, Serejko K, Ursoniu S, Rysz J, et al. Sleep changes following statin therapy: a systematic review and meta-analysis of randomized placebo-controlled polysomnographic trials. Arch Med Sci. (2015) 11:915–26. 10.5114/aoms.2015.54841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.McFarland A, Davey A, McDermott C, Anoopkumar-Dukie S, Grant G, Arora D, et al. Molecular mechanisms underlying the effects of statins in the central nervous system. Int J Mol Sci. (2014) 15:20607–37. 10.3390/ijms151120607 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.