Abstract
Background
Cutaneous metastasis (CM) is the spread of cancer cells from a primary site to the skin and is rarely the first sign of silent cancer. We investigated the clinicopathological characteristics of CM from internal malignancies in Korean patients treated at our institution over 20 years.
Methods
The clinicopathological findings of 112 patients (62 females, 50 males) with CM diagnosed at Yeungnam University Hospital between 2000 and 2020 were retrospectively reviewed.
Results
Mean patient age was 58.6 years (range, 26 to 87 years), and the most common primary cancer site was breast (74.2%) in women and lung (36.0%) in men. Ninety-six patients (85.7%) presented with CM after primary tumor diagnosis. CM from the lung or biliary tract usually occurred within 2 years of primary tumor diagnosis, whereas metastases from the breast and kidney occurred several years later. The chest, abdomen, and scalp were common sites of CM. Breast cancer usually metastasized to chest skin, while gastrointestinal tract cancers commonly metastasized to the abdomen. The scalp was a common location for CM from various tumors. The most common dermatologic presentations were nodules and masses. Immunohistochemical studies helped identify underlying malignancies when primary tumors were unknown.
Conclusions
The relative frequency of CM parallels the overall incidence of primary malignant tumors, and CMs usually occur at anatomic sites close to the primary tumor. CM can be diagnosed based on clinical, radiological, and histological features; however, immunohistochemical study is required in some cases.
Keywords: Carcinoma; Metastasis; Skin, Immunohistochemistry
Cutaneous metastases (CMs) from internal malignancies are considered a sign of systemic cancer spread. However, CM can emerge at the same time as the internal malignancy and in rare cases can be the first clinical presentation of occult or unknown primary tumor. While single case reports of CM from various cancers have been reported in Korea [1-9], systematic reviews on the common clinical and morphological presentations and histological subtypes of CM are rare. A recent Korean study reviewed data from 401 patients with CM and reported that the two most common primary cancers were breast and lung cancer [10], and this finding was consistent with those of studies conducted in other ethnic groups [11-15].
This study was undertaken to document the clinicopathologic features of CM from internal malignancies and primary tumor types, anatomic locations, times between primary tumor diagnoses and development of CMs, and immunohistochemical results of metastatic carcinomas from an unknown origin at the time of CM diagnosis. In addition, we sought to determine whether the frequency of CM reflects the incidence of corresponding primary tumors in Korean patients.
MATERIALS AND METHODS
Case selection
Consecutive cases with cutaneous metastatic carcinoma biopsied at Yeungnam University Medical Center (YUMC) between January 2000 and July 2020 were selected for this study. CM presented as a tumor center in the dermis or subcutaneous tissue, and no features suggestive of primary neoplasm derived from skin appendages. When a patient had multiple synchronous CMs, the largest lesion was considered representative. Metastases from skin malignancies (melanomas or carcinomas), mammary Paget disease, sarcomas, hematolymphoid neoplasms, and/or carcinomas located in a biopsy scar or surgical wound site were excluded.
All histologic findings of hematoxylin and eosin– and immunohistochemically- stained slides of skin and corresponding primary tumors were reviewed. Medical records and pathology reports were reviewed for information about sites and dates of diagnoses of primary tumors and clinical presentations of CM. Anatomic locations of CM were classified into scalp, face, neck, chest, abdomen, back, perineum, upper extremity, or lower extremity. For cases with multiple CMs, anatomic location was categorized based on the largest lesion. Multifocality was defined as presence of multiple CMs in two or more anatomic locations.
Statistical analysis
Statistical analysis was performed using SPSS ver. 25.0 for Windows (IBM Corp., Armonk, NY, USA). The difference of time interval between primary tumor diagnosis and occurrence of CM according to tumor stage or histologic features was determined using Mann-Whitney or Kruskal-Wallis test followed by the Bonferroni method. Statistical significance was accepted for p-values < .05.
RESULTS
Incidence
A total of 112 patients (62 females, 50 males) with CM was included in this study. For diagnosis of CM, 79 (69.9%) patients underwent punch biopsy, and 34 (30.1%) underwent excision. Mean age at CM diagnosis was 58.6 years (median, 59 years; range, 26 to 87 years), and the mean age of women and men was 55.7 and 62.3 years, respectively. Primary tumor was confirmed histologically in 99 patients by surgical resection (64 patients at Yeungnam University Medical Center and 12 patients at other hospitals) or biopsy (23 patients). In 10 patients, presence of primary tumor was confirmed radiologically. Primary tumor was undetermined in two patients despite extensive workup, and one patient refused further study. Breast (42.0%) was the most common primary cancer site, followed by lung (18.8%), stomach (10.7%), colon and rectum (5.4%), biliary tract (4.5%), kidney (3.6%), and liver (2.7%) (Table 1). Breast cancer (74.2%) was the most common primary cancer in female patients, followed by stomach (6.5%), colorectal (4.8%), and lung (4.8%) cancer. In male patients, the most common primary cancer sites were lung (36.0%), stomach (16.0%), biliary tract (8.0%), and kidney (8.0%) (Table 1). Tumor stage, either pathologic or clinical, was available in 103 patients and distributed as follows: stage I in 7 (6.8%), stage II in 19 (18.4), stage III in 43 (41.7%), and stage IV in 34 (33.0%) patients. Six patients had no record of tumor stage (five patients underwent surgery at other hospitals and stage work-up was not performed in one patient), and primary tumors were unknown in three patients. Tumor stage according to primary site is listed in Table 1.
Table 1.
Underlying primary tumors according to sex and stage
| Primary malignancy | Stage | Total | Female | Male |
|---|---|---|---|---|
| Breast | 47 (42.0) | 46 (74.2) | 1 (2.0) | |
| I | 5 | 5 | 0 | |
| II | 15 | 15 | 0 | |
| III | 22 | 22 | 0 | |
| IV | 4 | 4 | 0 | |
| Unknown | 1 | 0 | 1 | |
| Lung | 21 (18.8) | 3 (4.8) | 18 (36.0) | |
| I | 1 | 1 | 0 | |
| II | 1 | 0 | 1 | |
| III | 4 | 0 | 4 | |
| IV | 15 | 2 | 13 | |
| Stomach | 12 (10.7) | 4 (6.5) | 8 (16.0) | |
| II | 1 | 1 | 0 | |
| III | 8 | 2 | 6 | |
| IV | 3 | 1 | 2 | |
| Colorectum | 6 (5.4) | 3 (4.8) | 3 (6.0) | |
| III | 5 | 3 | 2 | |
| IV | 1 | 0 | 1 | |
| Biliary tract | 5 (4.5) | 1 (1.6) | 4 (8.0) | |
| III | 1 | 0 | 1 | |
| IV | 3 | 0 | 3 | |
| Unknown | 1 | 1 | 0 | |
| Kidney | 4 (3.6) | 0 | 4 (8.0) | |
| IV | 2 | 0 | 2 | |
| Unknown | 2 | 0 | 2 | |
| Liver | 3 (2.7) | 1 (1.6) | 2 (4.0) | |
| II | 1 | 1 | 0 | |
| III | 1 | 0 | 1 | |
| Unknown | 1 | 0 | 1 | |
| Salivary gland | 2 (1.8) | 0 | 2 (4.0) | |
| III | 1 | 0 | 1 | |
| IV | 1 | 0 | 1 | |
| Esophagus | Unknown | 1 (0.9) | 0 | 1 (2.0) |
| Lacrimal gland | II | 1 (0.9) | 0 | 1 (2.0) |
| Larynx | IV | 1 (0.9) | 0 | 1 (2.0) |
| Pancreas | IV | 1 (0.9) | 1 (1.6) | 0 |
| Paranasal sinus | IV | 1 (0.9) | 0 | 1 (2.0) |
| Prostate | IV | 1 (0.9) | 0 | 1 (2.0) |
| Thyroid | I | 1 (0.9) | 1 (1.6) | 0 |
| Tongue | III | 1 (0.9) | 1 (1.6) | 0 |
| Tonsil | IV | 1 (0.9) | 0 | 1 (2.0) |
| Unknown | Unknown | 3 (2.7) | 1 (1.6) | 2 (4.0) |
| Total | 112 (100) | 62 (100) | 50 (100) |
Values are presented as number (%).
Histologic features of primary tumors
The histologic types of common primary tumors with CM are listed in Table 2. Primary breast cancers were invasive carcinoma of no special type (invasive ductal carcinoma) in 42 (89.4%) patients, invasive micropapillary carcinoma in two (4.3%), invasive lobular carcinoma in one (2.1%), metaplastic carcinoma in one (2.1%), and unknown in one (2.1%). Breast molecular subtypes were luminal, triple-negative, human epidermal growth factor receptor 2-positive, and unknown in 21 (44.7%), 13 (27.7%), eight (17.0%), and five (10.6%) patients, respectively. Histologic grades were 1 in two (4.3%), 2 in 10 (21.3%), 3 in 30 (63.8%), and unknown in five (10.6%) patients. Presence or absence of lymphovascular invasion was available in 39 patients, 32 (82.1%) of whom showed lymphovascular invasion.
Table 2.
Histologic type of most frequent primary tumors
| Primary site | No. | Histologic type | No. |
|---|---|---|---|
| Breast | 47 | ||
| Invasive carcinoma, no special type | 42 | ||
| Invasive micropapillary carcinoma | 2 | ||
| Invasive lobular carcinoma | 1 | ||
| Metaplastic carcinoma | 1 | ||
| Unknown | 1 | ||
| Lung | 21 | ||
| Squamous cell carcinoma | 10 | ||
| Adenocarcinoma | 10 | ||
| Small cell carcinoma | 1 | ||
| Stomach | 12 | ||
| Adenocarcinoma | 10 | ||
| Mucinous carcinoma | 1 | ||
| Signet ring cell carcinoma | 1 | ||
| Colorectum | 6 | ||
| Adenocarcinoma | 4 | ||
| Invasive micropapillary carcinoma | 1 | ||
| Signet ring cell carcinoma | 1 |
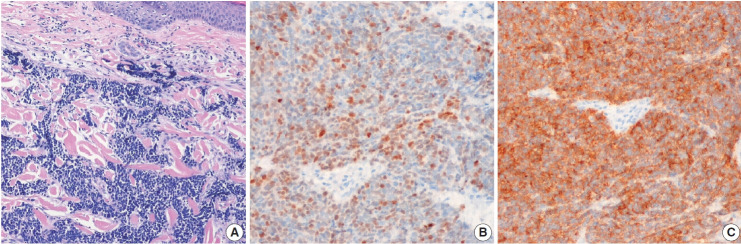
Of the 21 lung cancers with CM, 10 (47.6%) were squamous cell carcinomas, 10 (47.6%) were adenocarcinomas, and one (4.8%) was small cell carcinoma (Fig. 1). Of the 12 gastric adenocarcinomas with CM, eight (66.7%) were poorly differentiated, and the other four were either well differentiated, moderately differentiated, mucinous, or signet ring cell morphologies. For the six colorectal cancers, four were moderately differentiated adenocarcinomas, one was micropapillary carcinoma, and one was signet ring cell carcinoma.
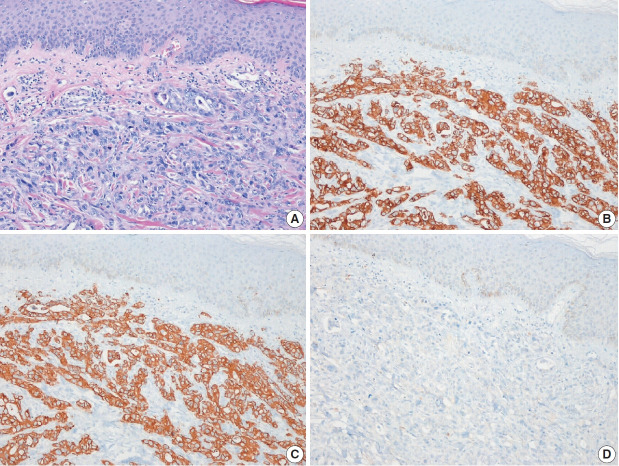
Fig. 1.
Metastatic small cell carcinoma of the lung. (A) Tumor cells infiltrated dermal collagen bundles and were diffusely positive for thyroid transcription factor 1 (B) and synaptophysin (C).
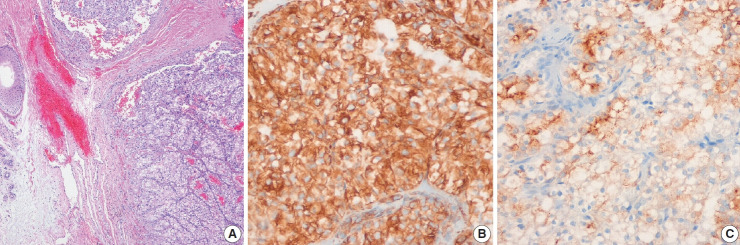
All metastatic renal cell carcinomas were of the clear cell type (Fig. 2). The unusual CMs were from invasive ductal carcinoma from the lacrimal gland, adenoid cystic carcinoma from the submandibular gland, and papillary carcinoma from the thyroid gland. Four cases of squamous cell carcinoma of the esophagus, paranasal sinus, tongue, or tonsil developed CM.
Fig. 2.
Metastatic renal cell carcinoma. (A) The tumor was clear cell type, and tumor cells were positive for vimentin (B) and renal cell carcinoma (C).
Clinical presentation of CMs
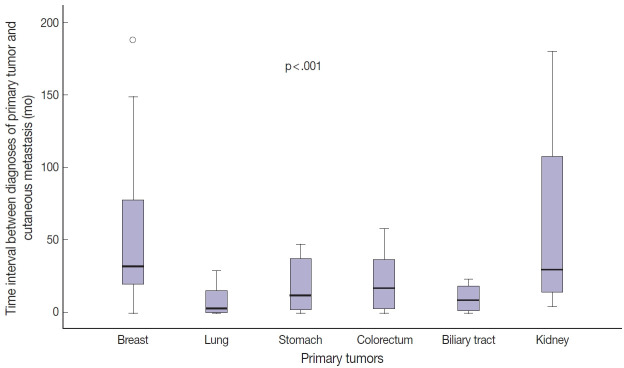
Among the 112 patients, 96 (85.7%) presented with CM after primary tumor diagnosis (mean, 41.8 months; range, 1 to 325 months). In 12 patients (10.7%), CM was found at the same time as internal malignancies, and in four patients (3.6%), CM was the first sign of internal malignancy. Time between diagnosis of primary tumors and CM was greater for patients with breast or kidney cancer than for those with lung or biliary tract cancer (Table 3, Fig. 3). In breast cancer patients, tumor stage was significantly associated with time interval between primary tumor diagnosis and CM (p < .001). Stage II or III tumors showed a longer time interval between primary tumor diagnosis and CM than did stage IV tumors (Table 3). However, there was no statistical difference in time interval between diagnosis of breast cancer and CM according to presence or absence of lymphovascular invasion or histologic grade of the primary tumor (Supplementary Table S1). Due to the small number of patients, the same statistical analysis could not be performed for other primary tumors.
Table 3.
Time interval between diagnosis of primary malignancy and cutaneous metastasis in patients who developed cutaneous metastasis after diagnosis of primary malignancy
| Primary site | Stage | No. | Time interval between diagnosis of primary tumor and occurrence of cutaneous metastasisa (mo) |
|---|---|---|---|
| Breast | 46 | 1–325 (58.9 ± 60.5) | |
| I | 5 | 25–149 (84.0 ± 58.4) | |
| II | 15 | 11–188 (71.9 ± 51.4) | |
| III | 22 | 1–325 (48.1 ± 70.1) | |
| IV | 3 | 24–53 (36.0 ± 15.1) | |
| Unknown | 1 | 42 | |
| Lung | 14 | 1–29 (12.5 ± 9.5) | |
| I | 1 | 3 | |
| II | 1 | 13 | |
| III | 4 | 2–26 (15.8 ± 10.4) | |
| IV | 8 | 1–29 (12.0 ± 10.2) | |
| Stomach | 11 | 1–47 (20.8 ± 17.9) | |
| II | 1 | 31 | |
| III | 8 | 1–47 (19.9 ± 19.1) | |
| IV | 2 | 3-36 | |
| Colorectum | III | 5 | 3–58 (26.4 ± 21.5) |
| Kidney | 4 | 4–180 (61.0 ± 80.4) | |
| IV | 2 | 4–35 | |
| Unknown | 2 | 25–180 | |
| Biliary tract | 3 | 3–23 (13.3 ± 10.0) | |
| III | 1 | 23 | |
| IV | 1 | 3 | |
| Unknown | 1 | 14 | |
| Liver | 3 | ||
| II | 1 | 64 | |
| III | 1 | 87 | |
| Unknown | 1 | - | |
| Salivary gland | 2 | ||
| III | 1 | 30 | |
| IV | 1 | 25 | |
| Esophagus | Unknown | 1 | 7 |
| Lacrimal gland | II | 1 | 39 |
| Larynx | IV | 1 | 30 |
| Pancreas | IV | 1 | 9 |
| Paranasal sinus | IV | 1 | 2 |
| Thyroid | I | 1 | 120 |
| Tongue | III | 1 | 17 |
| Tonsil | IV | 1 | 11 |
| Total | 96 | 1–325 (41.8 ± 50.6) |
Values are presented as range (mean ± standard deviation).
Fig. 3.
Comparison of time interval between diagnosis of primary tumor and occurrence of cutaneous metastasis according to primary tumor site.
Forty-nine patients (43.4%) had multiple CM lesions, and 12 (10.7%) presented with multiple CMs at two or more anatomic locations (Table 4). About 70% of the 112 patients were asymptomatic, but others experienced tenderness (16.1%), oozing (6.3%), and pain (5.4%). The most common dermatologic presentations of CM were nodules (53.6%) or masses (19.6%), followed by papules (12.5%), plaques (9.8%), or ulcers (2.7%) (Table 4).
Table 4.
Clinical presentation of cutaneous metastasis
| Clinical presentation | No. of cases (%) |
|---|---|
| No. of lesions | |
| Single | 63 (56.3) |
| Multiple | 49 (43.8) |
| Focality | |
| Unifocal | 100 (89.3) |
| Multifocal | 12 (10.7) |
| Symptom | |
| Asymptomatic | 78 (69.6) |
| Tenderness | 18 (16.1) |
| Oozing | 7 (6.3) |
| Pain/Tenderness | 4 (3.6) |
| Pain | 2 (1.8) |
| Itching | 2 (1.8) |
| Bleeding | 1 (0.9) |
| Dermatologic presentation | |
| Nodule | 60 (53.6) |
| Mass | 22 (19.6) |
| Papule | 14 (12.5) |
| Plaque | 11 (9.8) |
| Ulcer | 3 (2.7) |
| Patch | 1 (0.9) |
| Vesicle | 1 (0.9) |
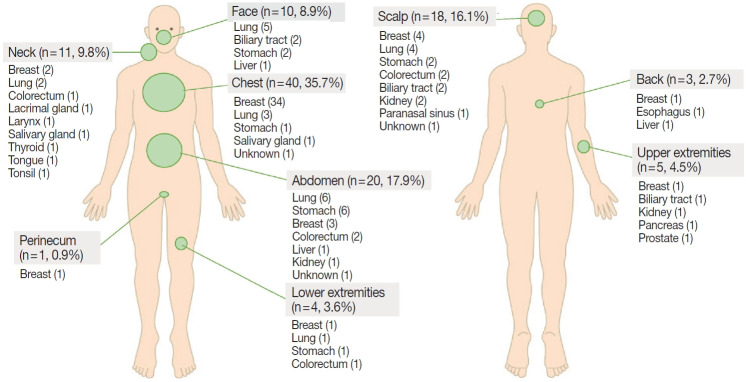
The common sites of CM were chest (35.7%), head and neck (34.8%; scalp, 16.1%; neck, 9.8%; face, 8.9%), and abdomen (17.9%) (Fig. 4). Breast cancer usually metastasized to the chest, lung cancer to the head and neck or chest, and gastrointestinal tract cancer to the abdomen or scalp.
Fig. 4.
Incidence of cutaneous metastasis by anatomic location and primary tumor site.
Immunohistochemical markers that define primary sites
The primary tumor was unable to be confirmed histologically in 12 patients with CM. In these cases, primary tumor sites were identified based on immunohistochemical staining results of skin lesions and radiologic findings (Table 5).
Table 5.
Clinicopathologic features of cases with cutaneous metastasis in which the primary tumor was not confirmed histologically
| No. | Age (yr) | Sex | Cutaneous metastasis |
Radiologic findings | Suggested primary site | ||
|---|---|---|---|---|---|---|---|
| Location | Histology | Immunohistochemical results | |||||
| 1 | 62 | M | Axilla | Adenocarcinoma | TTF-1+, CK7+, CK20–, CDX2– | Multiple masses in lungs, liver, bone, and LNs on PET-CT | Lung |
| 2 | 61 | M | Abdomen | Adenocarcinoma | TTF-1–, CK7+, CK20–, Napsin A– | Lung mass on CT | Lung |
| 3 | 58 | M | Scalp, Abdomen | Adenocarcinoma | TTF-1–, CK7+, CK20– | Lung mass on CT, suspicious metastases to hilar, subaortic, subcarninal LNs, and both adrenals | Lung |
| 4 | 55 | M | Scalp | Adenocarcinoma | TTF-1+, CK7+, CK20– | Lung mass on PET-CT, metastases to multiple LNs and bones | Lung |
| 5 | 76 | M | Chest | Squamous cell carcinoma | CK7 focal+, p63+, p40+ | Lung mass on CT | Lung |
| 6 | 68 | M | Face | Adenocarcinoma | CK7+, CK19+, CK20+, TTF-1–, CDX2– | Liver masses on PET-CT without histologic confirmation | Biliary tract |
| 7 | 58 | F | Chest | Carcinoma | ER–, PR+, Mammaglobin+, GCDFP-15– | Left breast mass on PET-CT, metastases to bone and axillary LNs | Breast |
| 8 | 72 | M | Upper extremity | Adenocarcinoma | PSA–, P504S– | Prostatic mass on PET-CT, multiple lung masses, metastases to multiple bones | Prostate |
| Lung mass: TTF-1–, c-Met+, CK7+, P504S+, ERG– | Kidney | ||||||
| 9 | 74 | M | Abdomen | Renal cell carcinoma | PAX8+, RCC– | Right kidney mass on PET-CT, metastases to multiple bones | Unknown |
| 10 | 36 | F | Scalp | Adenocarcinoma | CK7+, CK19+, PAX8+, CK20–, CDX2–, TTF-1–, WT-1–, ER–, PR– | Multiple masses in liver, adrenals, kidneys, ovaries, and omentum on PET-CT | Unknown |
| 11 | 67 | M | Axilla | Carcinoma | GATA3+, CK7–, CK20–, PSA–, TTF-1–, CDX2– | No suggested primary tumor on PET-CT | Unknown |
| 12 | 57 | M | Trunk | Adenocarcinoma | CK7+, CK19+, CK20–, CDX2 focal+, TTF-1– | No further study | |
M, male; TTF-1, thyroid transcription factor 1; CK, cytokeratin; LN, lymph node; PET-CT, positron emission tomography-computed tomography; ER, estrogen receptor; PR, progesterone receptor; GCDFP-15, gross cystic disease fluid protein 15; PSA, prostate specific antigen; RCC, renal cell carcinoma.
Five patients had lung masses revealed by chest computed tomography (CT) or positron emission tomography (PET)–CT. In four of these cases, the CM was adenocarcinomas; the other was squamous cell carcinoma. In all five patients, CMs were positive for cytokeratin 7 (CK7), four adenocarcinomas were negative for CK20, and two patients were positive for thyroid transcription factor 1 (TTF-1). Metastatic squamous cell carcinoma was positive for p63 and p40.
One patient had metastatic adenocarcinoma masses of the face and liver by PET-CT. Tumor cells in this patient were positive for CK7, CK19, and CK20 and negative for TTF-1 and caudaltype homeobox 2 (CDX2), which suggested biliary tract origin (Fig. 5). Interestingly, one female patient with metastatic carcinoma of the left chest skin was suspected of having breast cancer because tumor cells were positive for mammaglobin and progesterone receptor (PR) but negative for estrogen receptor (ER) and gross cystic disease fluid protein 15 (GCDFP-15). PET-CT of this patient depicted hypermetabolic areas in the left breast, axillary lymph nodes, and multiple bones.
Fig. 5.
Representative histologic features and immunohistochemical staining results for metastatic adenocarcinoma of the face. (A) Poorly differentiated adenocarcinoma was mainly located in the dermis. Tumor cells were positive for cytokeratin (CK) 7 (B) and CK19 (C) but negative for thyroid transcription factor 1 (D). Although no primary tumor was confirmed histologically, the metastatic adenocarcinoma was considered of biliary tract origin.
One male patient with metastatic adenocarcinoma of the upper extremity skin had a prostatic mass and multiple lung and bone lesions. Tumor cells in a lung mass biopsy were positive for P504S but negative for TTF-1, although the metastatic adenocarcinoma was negative for P504S and prostatic specific antigen. Another patient with metastatic carcinoma in the abdominal skin that was morphologically consistent with clear renal cell carcinoma had a hypermetabolic mass on PET-CT in the right kidney. Tumor cells from the skin lesion were positive for paired box gene 8 (PAX8). In one female patient with metastatic adenocarcinoma of the scalp, PET-CT depicted multiple masses in the liver, adrenals, kidneys, ovaries, and omentum. Tumor cells from the scalp were positive for CK7, CK19, and PAX8 but negative for CK20, CDX2, TTF-1, WT-1, ER, and PR. In this patient, a primary tumor originating from the ovary or biliary tract was suspected but not confirmed. Despite extensive work-up, primary cancer was not determined in one patient. Another patient refused further work-up aimed at identifying the primary site.
DISCUSSION
In this study, we retrospectively reviewed reviewed 112 cases with CM from internal malignancies in patients treated over 20 years at our institution. Common primary tumor sites differed in female and male patients. Breast and stomach cancer were the most common primary tumors in female patients, while lung and stomach cancers were most common in male patients. Considering that the numbers of breast, gastric, lung, and colorectal cancer patients newly registered at our institution over the past 20 years (January 2000 to December 2020) were 10,890, 9,968, 8,409, and 8,188 patients, respectively, the incidence of CM might reflect the overall incidence of cancers. According to Korean cancer statistics for 2017 [16], the most common cancer sites were the stomach (12.8%), colorectum (12.1%), lung (11.6%), thyroid (11.3%), breast (9.6%), liver (6.6%), prostate (5.5%), pancreas (3%), biliary tract and gallbladder (2.9%), and kidney (2.3%). The most common sites by sex were breast (20.3%), thyroid (18.3%), colorectum (10.4%), and stomach (8.9%) in women and stomach (16.3%), lung (15.3%), colorectum (13.6%), and prostate (10.5%) in men. These results indicate that the incidence of CM is proportional to the overall incidence of cancer, with exceptions that lung cancer tended to metastasize more easily than stomach cancer, and that thyroid and prostate cancers rarely gave rise to skin metastases. In addition, the frequency of CM from breast cancer corresponded to the frequency of histologic and molecular subtypes of primary breast cancer. Previous studies on different ethnic groups, including a Korean study, reported similar results regarding correspondence between CM frequency and the overall incidence of primary malignant tumors [10,12-15,17,18].
However, time between primary diagnosis and development of CM varied considerably and depended on primary tumor type. For example, CM from lung and biliary tract cancers usually occurred within two years of primary diagnosis, whereas CM from breast cancer and hepatocellular and renal cell carcinoma occurred several years after primary diagnosis. Choi et al. [10] also reported that CM occurred several years (mean, 48.2 months) after diagnosis of hepatocellular carcinoma, while lung and pancreatic cancers metastasized to the skin within one year of primary diagnosis. Furthermore, it was reported in a Taiwanese study that CM occurred early in lung cancer (mean, 15.7 months; range, 1 month to 5 years) but several years after breast cancer excision (mean, 47.2 months; range, 1 month to 10 years) [13].
With regard to metastatic sites, any skin region might be involved, but the chest, head and neck, and abdomen were most affected by CM. In particular, breast cancer usually metastasized to the chest, whereas gastrointestinal tract cancers commonly metastasized to abdominal skin. These results support the notion that CM is usually found in an anatomic location close to the primary tumor, presumably because epithelial cancer cells predominantly disseminate via lymphatic channels. However, some CMs occurred in regions distant from the primary tumors; for example, the scalp was a common CM location for various tumors. The possible mechanism as to why the scalp is a common destination for CM is thought to be due to its high vascularity [19].
It has been reported that 2.9% to 21% of CMs are found prior to diagnosis of primary cancers [11,12]. In the present study, 11.5% of patients developed CM as the first manifestation of internal malignancy. In this clinical setting, pathologists can use morphologic examinations and immunohistochemical studies to identify underlying primary tumors and provide valuable information for subsequent clinical workup. In metastatic adenocarcinoma, positivity for CK7, TTF-1, and napsin A and negativity for CK20 indicate a pulmonary origin. Squamous cell carcinoma of the lung typically exhibits CK5/6 and p40 positivity, and small cell lung carcinoma is positive for TTF-1 but negative for CK7 and CK20 [20]. Metastatic small cell carcinoma of the lung and primary cutaneous Merkel cell carcinoma must be precisely differentiated as they share a neuroendocrine origin and have similar histologic features. CK20 and TTF-1 have been routinely used in this context because small cell lung carcinoma is usually CK20 negative but TTF-1 positive, while primary cutaneous Merkel cell carcinoma is CK20 positive (characteristically paranuclear dotlike) and TTF-1 negative in most cases [21,22]. However, about 10% of Merkel cell carcinomas are positive for TTF-1 and negative for CK20 [23].
The histologic features of metastatic breast cancer can be similar to those of primary cutaneous adnexal malignancies, and both are positive for CK7 and negative for CK20. ER, PR, mammaglobin, and GCDFP-15 are useful markers of breast origin [21,24,25], but only 50% of metastatic breast carcinomas are ER positive and 65%–70% are mammaglobin positive. Gynecological malignancies occasionally exhibit ER and/or PR positivity [26-28]. These findings indicate that immunohistochemical results should be considered alongside clinical and radiological findings. For gastrointestinal tract malignancies, CK7, CK20, and CDX2 can help determine tumor origin. Gastric cancer is generally positive for CK7 and CK20, whereas colorectal cancer is negative for CK7 and positive for CK20 and CDX2. In hepatocellular carcinoma, hepatocyte paraffin 1 (HepPar-1), arginase 1, and α-fetoprotein can help confirm diagnosis [20,29]. Renal cell carcinoma shows negativity for CK7 and CK20 and positivity for renal cell carcinoma marker and PAX8 [20]. Gynecological cancers exhibit a variety of immunohistochemical profiles and are generally positive for cancer antigens 125, CK7, and CK20 [21], though ovarian cancer and endometrial cancer are also PAX8 positive [30].
In conclusion, although CMs are rarely observed after diagnosis of primary internal malignancies, they occur at rates that are approximately proportional to the frequency of the primary tumor. The most common primary cancer sites in this retrospective study was breast in women and lung in men. Lung and biliary tract cancers usually metastasized to the skin within 2 years of primary cancer diagnosis, whereas metastases from breast cancer, hepatocellular carcinoma, and renal cell carcinoma occurred several years later. Although metastasis to the skin can occur at any location, the chest, head and neck, and abdomen were most commonly affected. CM can be diagnosed by clinical, radiological, and histological examinations, although in some cases immunohistochemical studies are required.
Footnotes
Ethics Statement
This study was approved by the Institutional Review Board (IRB) of Yeungnam University Medical Center (YUMC2020-06-092), with a waiver of the requirement for informed consent.
Availability of Data and Material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: YKB. Data curation: HMK, GYK. Formal analysis: GYK, YKB. Funding acquisition: YKB. Investigation: YKB, DHS. Methodology: HMK, GYK, DHS, YKB. Project administration: YKB. Validation: YKB. Writing—original draft: HMK, GYK, YKB. Writing—review & editing: HMK, GYK, DHS, YKB. Approval of final manuscript: HMK, GYK, DHS, YKB. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
This work was supported by the 2020 Yeungnam University Research Grant (220A480010).
Supplementary Materials
The Data Supplement is available with this article at https://doi.org/10.4132/jptm.2021.05.24.
Time interval between diagnosis of primary malignancy and cutaneous metastasis in breast cancer patients according to lymphovascular invasion and histologic grade
References
- 1.Moon JI, Park JY, Jeon TJ, et al. Non-umbilical cutaneous metastasis of pancreatic adenocarcinoma as the first clinical manifestation: a case report. Korean J Gastroenterol. 2016;68:221–4. doi: 10.4166/kjg.2016.68.4.221. [DOI] [PubMed] [Google Scholar]
- 2.Kwon MJ, Ryu SH, Jo SY, et al. A case of hepatocellular carcinoma presenting as a gingival mass. Korean J Gastroenterol. 2016;68:321–5. doi: 10.4166/kjg.2016.68.6.321. [DOI] [PubMed] [Google Scholar]
- 3.Song YW, Kim WS, Yun GY, et al. A case of early gastric cancer with nodular tumor-like scalp metastasis. Korean J Gastroenterol. 2016;68:36–9. doi: 10.4166/kjg.2016.68.1.36. [DOI] [PubMed] [Google Scholar]
- 4.Yang HJ, Kang SY. Cutaneous metastatic renal cell carcinoma to the scalp. Arch Craniofac Surg. 2019;20:392–6. doi: 10.7181/acfs.2019.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JH, Kim MJ, Sim WY, Lew BL. Alopecia neoplastica due to gastric adenocarcinoma metastasis to the scalp, presenting as alopecia: a case report and literature review. Ann Dermatol. 2014;26:624–7. doi: 10.5021/ad.2014.26.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim MK, Kim SH, Lee YY, et al. Metastatic skin lesions on lower extremities in a patient with recurrent serous papillary ovarian carcinoma: a case report and literature review. Cancer Res Treat. 2012;44:142–5. doi: 10.4143/crt.2012.44.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.An MK, Park BW, Cho EB, Park EJ, Kim KH, Kim KJ. A case of cutaneous metastasis of nasopharyngeal carcinoma on the face. Korean J Dermatol. 2019;57:80–3. [Google Scholar]
- 8.Hong SG, Jo SY, Jeong HH, et al. Cutaneous metastasis originating from esophageal carcinoma: a case report. Korean J Dermatol. 2020;58:269–72. [Google Scholar]
- 9.Lee JY, Shin SJ, Yoo CS, Kil MS, Kim CW, Kim SS. A case of zosteriform cutaneous metastasis from lung cancer. Korean J Dermatol. 2013;51:272–5. [Google Scholar]
- 10.Choi ME, Jung CJ, Lee WJ, et al. Clinicopathological study of Korean patients with cutaneous metastasis from internal malignancies. Australas J Dermatol. 2020;61:e139–42. doi: 10.1111/ajd.13135. [DOI] [PubMed] [Google Scholar]
- 11.Brownstein MH, Helwig EB. Spread of tumors to the skin. Arch Dermatol. 1973;107:80–6. [PubMed] [Google Scholar]
- 12.Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma: a retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19–26. doi: 10.1016/0190-9622(90)70002-y. [DOI] [PubMed] [Google Scholar]
- 13.Hu SC, Chen GS, Lu YW, Wu CS, Lan CC. Cutaneous metastases from different internal malignancies: a clinical and prognostic appraisal. J Eur Acad Dermatol Venereol. 2008;22:735–40. doi: 10.1111/j.1468-3083.2008.02590.x. [DOI] [PubMed] [Google Scholar]
- 14.Sittart JA, Senise M. Cutaneous metastasis from internal carcinomas: a review of 45 years. An Bras Dermatol. 2013;88:541–4. doi: 10.1590/abd1806-4841.20131165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handa U, Kundu R, Dimri K. Cutaneous metastasis: a study of 138 cases diagnosed by fine-needle aspiration cytology. Acta Cytol. 2017;61:47–54. doi: 10.1159/000453252. [DOI] [PubMed] [Google Scholar]
- 16.Hong S, Won YJ, Park YR, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2017. Cancer Res Treat. 2020;52:335–50. doi: 10.4143/crt.2020.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gul U, Kilic A, Gonul M, Kulcu Cakmak S, Erinckan C. Spectrum of cutaneous metastases in 1287 cases of internal malignancies: a study from Turkey. Acta Derm Venereol. 2007;87:160–2. doi: 10.2340/00015555-0199. [DOI] [PubMed] [Google Scholar]
- 18.Mok ZR, Yong AM, Leung AJ, Tan KB, Aw DC. Cutaneous metastasis: experience from a tertiary healthcare institution in Singapore. Int J Dermatol. 2017;56:1497–8. doi: 10.1111/ijd.13650. [DOI] [PubMed] [Google Scholar]
- 19.Shah SR, Applebaum DS, Potenziani S, Huttenbach YT, Wolf J, Orengo IF. Cutaneous metastasis to the scalp as the primary presentation of colorectal adenocarcinoma. Dermatol Online J. 2017 Nov 15; doi: 10.5070/D32311037274. [Epub]. [DOI] [PubMed]
- 20.Habermehl G, Ko J. Cutaneous metastases: a review and diagnostic approach to tumors of unknown origin. Arch Pathol Lab Med. 2019;143:943–57. doi: 10.5858/arpa.2018-0051-RA. [DOI] [PubMed] [Google Scholar]
- 21.Hussein MR. Skin metastasis: a pathologist's perspective. J Cutan Pathol. 2010;37:e1–20. doi: 10.1111/j.1600-0560.2009.01469.x. [DOI] [PubMed] [Google Scholar]
- 22.Yang DT, Holden JA, Florell SR. CD117, CK20, TTF-1, and DNA topoisomerase II-alpha antigen expression in small cell tumors. J Cutan Pathol. 2004;31:254–61. doi: 10.1111/j.0303-6987.2003.00175.x. [DOI] [PubMed] [Google Scholar]
- 23.Kervarrec T, Tallet A, Miquelestorena-Standley E, et al. Diagnostic accuracy of a panel of immunohistochemical and molecular markers to distinguish Merkel cell carcinoma from other neuroendocrine carcinomas. Mod Pathol. 2019;32:499–510. doi: 10.1038/s41379-018-0155-y. [DOI] [PubMed] [Google Scholar]
- 24.Green AR, Young P, Krivinskas S, et al. The expression of ERalpha, ERbeta and PR in lobular carcinoma in situ of the breast determined using laser microdissection and real-time PCR. Histopathology. 2009;54:419–27. doi: 10.1111/j.1365-2559.2009.03233.x. [DOI] [PubMed] [Google Scholar]
- 25.Matsuoka K, Ohsumi S, Takashima S, Saeki T, Aogi K, Mandai K. Occult breast carcinoma presenting with axillary lymph node metastases: follow-up of eleven patients. Breast Cancer. 2003;10:330–4. doi: 10.1007/BF02967653. [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Fernandez C, Daneshbod Y, Nassiri M, Milikowski C, Alvarez C, Nadji M. Immunohistochemically determined estrogen receptor phenotype remains stable in recurrent and metastatic breast cancer. Am J Clin Pathol. 2008;130:879–82. doi: 10.1309/AJCPD1AO3YSYQYNW. [DOI] [PubMed] [Google Scholar]
- 27.Liu H. Application of immunohistochemistry in breast pathology: a review and update. Arch Pathol Lab Med. 2014;138:1629–42. doi: 10.5858/arpa.2014-0094-RA. [DOI] [PubMed] [Google Scholar]
- 28.Monaco SE, Wu Y, Teot LA, Cai G. Assessment of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status in the fine needle aspirates of metastatic breast carcinomas. Diagn Cytopathol. 2013;41:308–15. doi: 10.1002/dc.21841. [DOI] [PubMed] [Google Scholar]
- 29.Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303–10. doi: 10.1097/00000478-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Kandalaft PL, Gown AM. Practical applications in immunohistochemistry: carcinomas of unknown primary site. Arch Pathol Lab Med. 2016;140:508–23. doi: 10.5858/arpa.2015-0173-CP. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time interval between diagnosis of primary malignancy and cutaneous metastasis in breast cancer patients according to lymphovascular invasion and histologic grade