Abstract
The SWEET (sugars will eventually be exported transporter) proteins, a family of sugar transporters, mediate sugar diffusion across cell membranes. Pathogenic fungi can acquire sugars from plant cells to satisfy their nutritional demands for growth and infection by exploiting plant SWEET sugar transporters. However, the mechanism underlying the sugar allocation in cotton plants infected by Verticillium dahliae, the causative agent of Verticillium wilt, remains unclear. In this study, observations of the colonization of cotton roots by V. dahliae revealed that a large number of conidia had germinated at 48-hour post-inoculation (hpi) and massive hyphae had appeared at 96 hpi. The glucose content in the infected roots was significantly increased at 48 hpi. On the basis of an evolutionary analysis, an association analysis, and qRT-PCR assays, GhSWEET42 was found to be closely associated with V. dahliae infection in cotton. Furthermore, GhSWEET42 was shown to encode a glucose transporter localized to the plasma membrane. The overexpression of GhSWEET42 in Arabidopsis thaliana plants led to increased glucose content, and compromised their resistance to V. dahliae. In contrast, knockdown of GhSWEET42 expression in cotton plants by virus-induced gene silencing (VIGS) led to a decrease in glucose content, and enhanced their resistance to V. dahliae. Together, these results suggest that GhSWEET42 plays a key role in V. dahliae infection in cotton through glucose translocation, and that manipulation of GhSWEET42 expression to control the glucose level at the infected site is a useful method for inhibiting V. dahliae infection.
Keywords: cotton, SWEET, Verticillium dahliae, glucose, sugar transporter
Introduction
Verticillium dahliae is a soil-borne fungal pathogen that can infect a broad range of plant species, including many economically important crops, and causes devastating diseases (Fradin and Thomma, 2006). During infection, V. dahliae hyphae pass through the epidermal cells and multiply in the vascular tissue (Zhao et al., 2014). The hyphae then produce toxins and block plant ducts, thereby disrupting water transport, and ultimately leading to plant death (Fradin and Thomma, 2006). All microorganisms that interact with plants must obtain metabolites from the host cells to meet the nutrient requirements for growth (Yamada et al., 2016). Since carbon is essential for growth and pathogenicity, obtaining sufficient sugars from plants is an important task for successful infection by plant pathogens (Chen et al., 2010; Klimes et al., 2015). However, little is known about the molecular mechanism of sugar uptake by V. dahliae from plants.
During long-term co-evolution, plants and microorganisms have developed a set of strategies enabling them to compete for nutrients (Chen et al., 2012). Plant hosts can prevent metabolite loss to pathogens by redistributing sugars away from the infection site (Berger et al., 2004). In Arabidopsis thaliana, the sugar transport protein AtSTP13 was activated by phosphorylation during bacterial challenge, which enhances its hexose uptake activity to compete with bacteria for extracellular sugars (Yamada et al., 2016). However, pathogens have evolved mechanisms that increase sugar concentrations at the infection site by modulating host sugar transporters, including SWEETs (sugars will eventually be exported transporters) (Cox et al., 2017). Both fungal and bacterial pathogens can induce the expression of different SWEET genes, suggesting that the sugar transport function of SWEETs may be targeted by pathogens and symbionts for nutrient acquisition (Chen et al., 2010). For example, in maize, Ustilago maydis infection was found to affect the local expression of the sugar transporter genes ZmSWEET4a and ZmSWEET4b, with expression levels correlated with fungal biomass (Sosso et al., 2019). In a previous study, Rhizoctonia solani upregulated OsSWEET11 expression 5- and 3-fold in rice leaves and sheaths, respectively, at 72 h after infection (Gao et al., 2018). Recent studies have shown that blocking pathogen access to host sugars resulting in its sugar starvation is a promising strategy for controlling plant diseases. For example, silencing the hexose transporter gene PsHXT1 restricted the normal growth and development of Puccinia striiformis f. sp. Tritici (Pst), leading to decreased fungal biomass and reduced symptoms of wheat stripe rust disease (Chang et al., 2020). In addition, the expression up-regulation of the wheat sugar transporter gene TaSTP6 contributes to the acquisition of host sugars by Pst, and knock-down of TaSTP6 reduced the susceptibility of wheat to Pst (Huai et al., 2019). The inhibition of OsSWEET11 function in leaf cells mitigated the symptoms of sheath blight disease in rice plants (Gao et al., 2018).
Sucrose is the major sugar translocated in most plants, and it is likely to be the type of sugar used by plant pathogenic fungi (Bezrutczyk et al., 2018). However, some studies have indicated that glucose, not sucrose, is the main carbon source obtained from the host by fungal mycelia (Sutton and Hall, 1999). A previous study found that, during infection of plants by U. maydis, the glucose content in the infected plant part was 20-times that in the uninfected part at 8 day post-infection (Doehlemann et al., 2008). Analyses of sugar acquisition and metabolism by U. maydis revealed a glucose concentration gradient in the hyphae, with a high concentration at the highly active hyphal tip (Sosso et al., 2019). Additionally, Hxt1 is a high-affinity glucose, fructose, and mannose transporter in U. maydis, and its deficient mutant strains showed significantly reduced growth in, and pathogenicity toward, their hosts (Schuler et al., 2015).
In this study, observations of the V. dahliae colonization process revealed that V. dahliae hyphae had penetrated into the roots of cotton (Gossypium hirsutum L.) plants at 48-hour post-inoculation (hpi). The glucose content in the infected roots had also significantly increased at 48 hpi. The transcript level of GhSWEET42, which encodes a plasma membrane-localized glucose transporter, was significantly induced at 48 hpi in the infected roots. Overexpression of GhSWEET42 in A. thaliana increased the glucose content in the transgenic plants, and made them more susceptible to V. dahlia infection. Conversely, knockdown of GhSWEET42 in cotton plants resulted in decreased glucose content, leading to improved resistance to V. dahliae. Our findings suggest that GhSWEET42 is involved in V. dahliae infection through glucose translocation.
Materials and Methods
Plant Materials and Growth Conditions
All A. thaliana lines used in this study were in the Columbia (Col-0) genetic background. Seeds were surface-sterilized with 0.1% mercuric chloride before being sown on the surface of the agar culture medium. Then seeds were vernalized at 4°C for 2 day in darkness and transferred to a growth chamber for germination. The growth chamber was set at 22°C and 60% relative humidity, with a 16-h light/8-h dark cycle.
The cotton cultivar Zhongzhimian 2, which is resistant to V. dahliae, was used in this study. Seeds were placed in sterile water and kept in an incubator at 30°C to germinate overnight. Seeds with undamaged radicles of the same length were selected and rinsed with water. Each seed was sown with the radicle pointing downwards in nutrient medium. Cotton seedlings were grown in a growth chamber set at 26°C and 60% relative humidity with a 16-h light/8-h dark cycle.
V. dahliae Growth Conditions and Plant Inoculations
The V. dahliae strain V592 was grown on potato dextrose agar medium for 4 day, after which it was transferred to Czapek’s medium [0.3% (w/v) NaNO3, 0.1% (w/v) MgSO4, 0.1% (w/v) KH2PO4, 0.0002% (w/v) FeSO4, 0.1% (w/v) KCl, and 3% (w/v) sucrose, pH 6.0] and incubated at 25°C for 5 day (Gao et al., 2013).
The A. thaliana inoculations were performed by root dipping, as follows: the roots of 4-week-old seedlings were rinsed with water and then immersed in a V. dahliae conidial suspension (1 × 107 conidia/mL) for 90 s, after which the seedlings were replanted in fresh soil. For cotton inoculations, cotton plants were grown in pots in a growth chamber for 1 month. Conidial suspensions were diluted with distilled water to a final concentration of about 1 × 107 conidia/mL. A 10-mL aliquot of the conidial suspension was added to each pot using a syringe to infect cotton seedlings (Gong et al., 2018).
Disease Assessment After V. dahliae Inoculation
To quantify the fungal biomass in plants, genomic DNA was extracted from various tissues collected after inoculating plants with V592 for quantitative real-time PCR (qRT-PCR) analysis. The internal transcribed spacer (ITS) region of ribosomal DNA was targeted using the fungal-specific ITS1-F primer and the V. dahliae-specific reverse primer STVe1-R (Fradin et al., 2011). The A. thaliana AtUBQ5 gene was used as an endogenous plant control (Gutierrez et al., 2008). The qRT-PCR analyses were completed using the extracted genomic DNA as previously described (Santhanam et al., 2013).
In the V. dahliae infection recovery assay, 1-cm sections cut from the base of the stem were surface-sterilized in 70% ethanol and rinsed with sterile water at 2 week after infection by V592. The stem segments were placed on potato dextrose agar medium supplemented with cephalosporin and incubated for 3 day at 25°C before being examined and photographed (Gong et al., 2018).
The disease symptom severity was recorded using an index ranging from 0 (healthy plants) to 4 (dead plants) (Zhang et al., 2017). The disease index was calculated using the formula described in the previous study (Gong et al., 2018).
Confocal Observation of V. dahliae Infection Process in Cotton Roots
Seeds of the cotton cultivar Zhongzhimian 2 were placed in a seed germination bag. After 5 day, cotton seedlings with uniform size were immersed in a V592-GFP conidial suspension (1 × 107 conidia/ML) for 90 s. The roots were cut lengthwise at 0, 6, 12, 24, 48, and 96 hpi and then examined under a confocal laser scanning microscope.
Phylogenetic and Association Analysis of SWEET Genes in Cotton
The protein sequences of SWEET genes in G. hirsutum, A. thaliana, and Vitis vinifera (Supplementary Table 1) were obtained from the literatures (Chen et al., 2010; Chong et al., 2014; Li et al., 2018). Multiple sequence alignments were performed using Clustal X (version 2.0) (Larkin et al., 2007). Phylogenetic trees were constructed using the neighbor-joining method with 1000 bootstrap replicates in MEGA (version 7.0) (Kumar et al., 2016).
Data related to the V. dahliae resistance of 258 modern G. hirsutum cultivars and enhanced germplasm lines were obtained from a previous study (Fang et al., 2017). The single nucleotide polymorphisms (SNPs) located in the clade II SWEET genes from G. hirsutum were identified and extracted. A general linear model (GLM), a GLM model with principle component analysis (GLM + PCA), and a mixed linear model (MLM) were used to perform association analyses between these SNPs and Verticillium wilt resistance using TASSEL (version 5.0) (Mao et al., 2020).
Ectopic Expression of GhSWEET42 in A. thaliana
The full-length GhSWEET42 coding sequence was cloned using gene-specific primers (Supplementary Table 2). The cloned sequence was inserted into a modified pCAMBIA2300 plant binary vector via homologous recombination. A freeze-thaw method was used to transform Agrobacterium tumefaciens strain GV3101 with the recombinant vector. The floral-dip method (Clough and Bent, 2010) was used to generate transgenic A. thaliana plants, which were selected on Murashige and Skoog medium containing kanamycin. Transgenic A. thaliana plants from the T3 generation were used for follow-up experiments.
Virus-Induced Gene Silencing (VIGS) of GhSWEET42 in Cotton
The tobacco rattle virus (TRV) system was used for a VIGS analysis in cotton as previously described (Wang et al., 2014). A 250-bp GhSWEET42 fragment was inserted into the pTRV2 vector via homologous recombination to generate the TRV:GhSWEET42 construct. Similarly, the TRV:GhCLA1 construct was produced as a positive control to monitor gene silencing efficiency. The empty vector (TRV:00) was used as a negative control. All vectors were inserted into A. tumefaciens strain GV3101 cells, which were then injected into the cotyledons of 7-day-old cotton seedlings (Long et al., 2019).
Total RNA Extraction, RT-PCR, and qRT-PCR
Total RNA was isolated using the RNAprep Pure Plant Kit (Tiangen, Beijing, China). First-strand cDNA was synthesized using the PrimeScriptTM II 1st Strand cDNA Synthesis Kit (Takara, Dalian, China). The RT-PCR assays were conducted as described in a previous study (Bustin, 2000). The qRT-PCR assays were performed using the Light Cycler 480 system and the SYBR Premix Ex Taq Kit (Takara). The G. hirsutum GhHIS3 and A. thaliana AtUBQ5 genes were used as internal controls. The primers used in this experiment are listed in Supplementary Table 2.
Subcellular Localization of GhSWEET42
Cells of A. tumefaciens GV3101 were transformed with the pCAMBIA2300 vector carrying GhSWEET42 under the control of the CaMV 35S promoter to produce GhSWEET42 with a C-terminal green fluorescent protein (GFP) tag. The transformed GV3101 cells were cultured and used to infiltrate leaves of tobacco (Nicotiana benthamiana) as previously described (Sosso et al., 2015). Bacterial cultures were grown for 16 h, after which the cells were collected by centrifugation and resuspended in infiltration buffer (10 mM MES, pH 5.6, 10 mM MgCl2, and 200 μM acetosyringone) to an optical density at 600 nm (OD600) of approximately 1.0–1.2. The abaxial side of N. benthamiana leaves was infiltrated with the cell solution using a needleless syringe. The infiltrated plants were incubated for 24 h, and then green fluorescence was visualized under a confocal laser scanning microscope.
Protoplasts were isolated from the young leaves of A. thaliana seedlings as previously described (Yoo et al., 2007). The protoplasts were transiently transformed by adding 10 μg vector to a 100-μL protoplast solution (105 protoplasts/mL), which was then gently mixed and incubated at room temperature for 12 h. Green fluorescence was detected by confocal laser scanning microscopy.
Yeast Complementation Assays
The GhSWEET42 coding sequence was cloned into the pDR196 expression vector. The sucrose transport-deficient S. cerevisiae mutant SUSY7/ura3 (Riesmeier et al., 1993) was transformed with the pDR196-GhSWEET42 recombinant vector and then grown on selective synthetic complete medium (without uracil) containing 2% glucose as the sole carbon source. Drop tests were used to assess the growth of the transformed yeast on agar-solidified SD medium supplemented with 2% sucrose (OD600 = 1; 1:10, 1:100, 1:1,000, and 1:10,000 dilutions). Yeast cells transformed with pDR196-AtSUT4 served as the positive control, and cells carrying the empty vector were used as the negative control. The transformed yeast cells were grown at 30°C for 4 day. Glucose-supplemented medium was used as the positive growth medium.
The hexose transport-deficient Saccharomyces cerevisiae mutant EBY.VW4000 (Wieczorke et al., 1999) was transformed with pDR196-GhSWEET42, pDR196-HXT7 (positive control), or pDR196 (negative control) for complementation assays. Drop tests were used to assess the growth of the transformed yeast on agar-solidified SD medium supplemented with 2% glucose or fructose (OD600 = 1; 1:10, 1:100, 1:1,000, and 1:10,000 dilutions). Yeast cells were grown at 30°C for 4 days. Maltose -supplemented medium was used as the positive growth medium. Specifically, the yeast sugar transport-defective strains SUSY7/ura3 and EBY.VW4000 have different growth states on media with glucose as the sole carbon source.
Determination of Glucose Contents
Each plant sample (0.2 g) was ground to a powder and then 500 μL of a mixture of methanol, isopropanol, and water (3:3:2, V/V/V) was added. The mixture was vortexed for 3 min, sonicated for 30 min, and then centrifuged at 14,000 rpm for 3 min at 4°C. The supernatant was collected and mixed with the internal standard and then evaporated under a stream of nitrogen. The evaporated samples were freeze-dried, and then each dried sample was derivatized with 100 μL pyridine solution of methoxylamine hydrochloride (15 mg/mL) at 37°C for 2 h. The mixture was added to 100 μL BSTFA (with 1% TMSC) and kept at 37°C for 30 min after vortexing. All mixtures were analyzed by as chromatography-mass spectrometry (GC-MS) as previously described (Gómez-González et al., 2010; Zheng et al., 2016).
Results
Colonization of Cotton Roots by V. dahliae and Subsequent Changes in Glucose Content
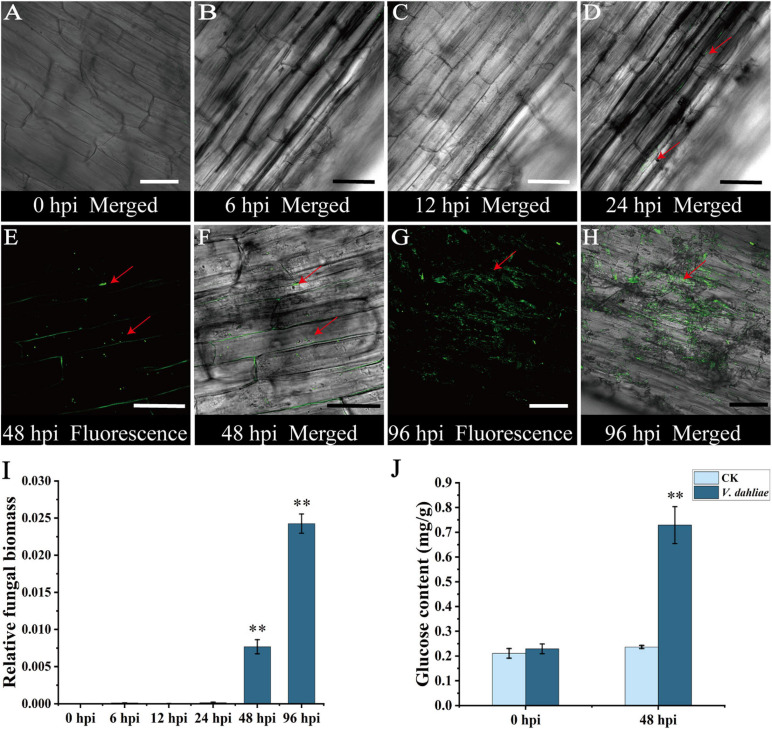
To clarify the process of colonization, cotton plants were inoculated with V. dahliae strain V592-GFP. The inoculated roots were cut longitudinally at various time-points and examined under a confocal laser scanning microscope. From 0 to 12 hpi, no obvious green fluorescence was detected in the cotton roots, implying that V. dahliae did not infect the roots before 12 hpi (Figures 1A–C and Supplementary Figure 1). Green fluorescence was first detected at 24 hpi, and the signal intensity increased significantly after 48 hpi (Figures 1D–F and Supplementary Figure 1). At 96 hpi, the green fluorescence from hyphae was observed in the longitudinal sections of cotton roots (Figures 1G,H and Supplementary Figure 2). To verify these results, the relative fungal biomass at each infection stage was determined by qRT-PCR. The V. dahliae fungal material was detectable at 24 hpi, and the biomass was significantly increased at 48 and 96 hpi, consistent with the fluorescence results (Figure 1I).
FIGURE 1.
Confocal micrographs of initial stage of cotton root colonization by Verticillium dahliae and post-infection changes in glucose content. (A) Longitudinal section of cotton roots with no detectable fluorescence at 0 hours post-inoculation (hpi). (B) Longitudinal section of cotton roots with no detectable fluorescence at 6 hpi. (C) Longitudinal section of cotton roots with no detectable fluorescence at 12 hpi. (D) Fluorescence at the surface of cotton roots at 24 hpi. (E) Longitudinal section of cotton roots at 48 hpi with hyphae multiplying in the intercellular space before penetrating the cell wall and invading root cells. Red arrow indicates the hyphal position. (F) Merged bright field transmission image and corresponding fluorescence image at 48 hpi. Red arrow indicates the hyphal position. (G) Longitudinal section of cotton roots at 96 hpi with hyphae covering the entire duct tissue and spreading within it. Red arrow indicates the hyphal position. (H) Merged bright field transmission image and corresponding fluorescence image at 96 hpi. Red arrow indicates the hyphal position. (I) qRT-PCR analysis of fungal biomass at various stages of infection. Asterisks indicate significant differences compared with 0 hpi. (J) Changes in the glucose content in cotton roots after V. dahliae infection. Asterisks indicate significant differences compared with CK. Error bars represent standard deviation of three biological replicates. Data were analyzed using Student’s t-test (∗∗P < 0.01). Scale bar = 50 μm.
Glucose is a nutrient as well as a signaling molecule that affects diverse processes (Sami et al., 2019; Yoon et al., 2021). Therefore, the glucose contents of cotton roots at 0 and 48 hpi were quantified using GC-MS technology. The data indicated the glucose content had increased significantly at 48 hpi in the infected cotton root (Figure 1J), suggesting that glucose might be involved in the infection of cotton roots by V. dahliae.
GhSWEET42 Is a Clade II SWEET Sugar Transporter Related to V. dahliae Resistance
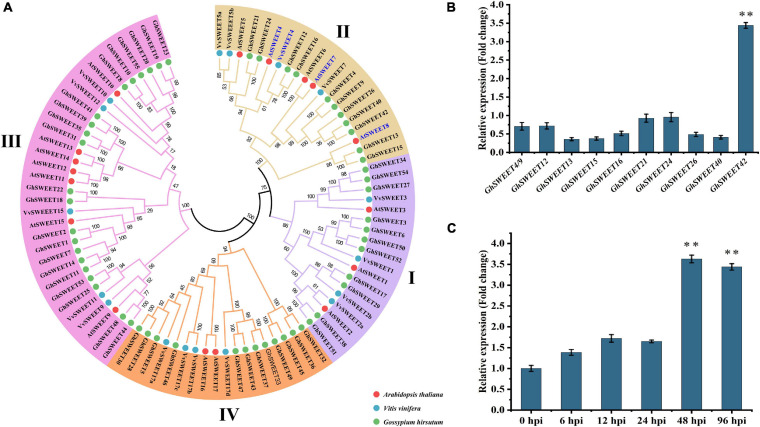
Plant pathogens usually hijack plant SWEET sugar transporters to obtain sufficient energy to successfully invade and grow in host plants (Cox et al., 2017; Phillips et al., 2017; Gupta, 2020). To explore why V. dahliae infection leads to increased glucose contents in cotton roots, a phylogenetic analysis was completed using the previously identified G. hirsutum SWEET genes as well as those from A. thaliana and V. vinifera (Figure 2A). Many SWEET genes related to glucose transport and disease resistance in A. thaliana and V. vinifera (Chong et al., 2014) clustered in clade II of the SWEET gene family. Therefore, we focused our research on the G. hirsutum SWEET genes in this clade.
FIGURE 2.
Phylogenetic and transcriptional analyses of Gossypium hirsutum SWEET genes. (A) Phylogenetic analysis of SWEET genes. Phylogenetic tree was constructed using protein sequences of SWEET genes from G. hirsutum (GhSWEETs), Arabidopsis thaliana (AtSWEETs), and Vitis vinifera (VvSWEETs). Blue mark indicates SWEET genes related to glucose transport and disease resistance in A. thaliana and V. vinifera. (B) Relative transcript levels of G. hirsutum clade II SWEET genes in roots at 48 h after infection by Verticillium dahliae. (C) Relative transcript levels of GhSWEET42 in roots infected with V. dahliae. For each gene, transcript level in control roots (wounded roots treated with 10 mL water) was set to 1 to determine relative transcript levels in inoculated roots [wounded roots treated with 10 mL V592 conidial suspension (1 × 107 conidia/mL)]. Asterisks represent significant difference in fold change. Error bars represent standard deviation of three biological replicates. Data were analyzed using Student’s t-test (∗∗P < 0.01).
To investigate the association between the G. hirsutum clade II SWEET genes and V. dahliae resistance, a candidate gene association analysis was conducted to determine whether the genetic variation in the G. hirsutum clade II SWEET genes was associated with phenotypic differences in responses to V. dahliae among cotton varieties. Earlier research indicated that the 200-kb regions upstream and downstream of significant SNPs can be defined as quantitative trait loci in G. hirsutum (Sun et al., 2017; Song et al., 2019). Therefore, SNP markers within a 400-kb interval centered on each SWEET gene were selected from 1,871,401 high-quality SNPs (major allele frequency > 0.05) identified in a previous study (Fang et al., 2017). The V. dahliae resistance phenotype data for the corresponding cotton varieties were also obtained from that study (Fang et al., 2017). Three statistical models, namely a GLM, a GLM + PCA, and an MLM were used to identify significant genotypic and phenotypic associations. The results of those analyses indicated that genetic variations within or near the G. hirsutum SWEET genes, including GhSWEET9, GhSWEET15/16, GhSWEET21, GhSWEET40, and GhSWEET42, are significantly associated with V. dahliae resistance (Supplementary Table 3).
To elucidate the potential role of individual G. hirsutum SWEET genes during plant responses to a V. dahliae infection, their transcript levels in cotton roots at 48 hpi were examined by qRT-PCR. Of the analyzed genes, only GhSWEET42 was strongly induced at 48 hpi. In contrast, the transcript levels of the other G. hirsutum clade II SWEET genes were unaffected by V. dahliae infection (Figure 2B). Additionally, GhSWEET42 more highly expressed in the susceptible cotton variety than in the resistant cotton varieties (Supplementary Figure 3). Monitoring of the dynamic changes in GhSWEET42 expression revealed high transcript levels of GhSWEET42 at 48 and 96 hpi (Figure 2C). This was consistent with the colonization process and changes in glucose content following inoculation with V. dahliae. Accordingly, GhSWEET42 may be important for mediating glucose translocation during V. dahliae infection of cotton.
GhSWEET42 Encodes a Plasma Membrane-Localized Glucose Transporter
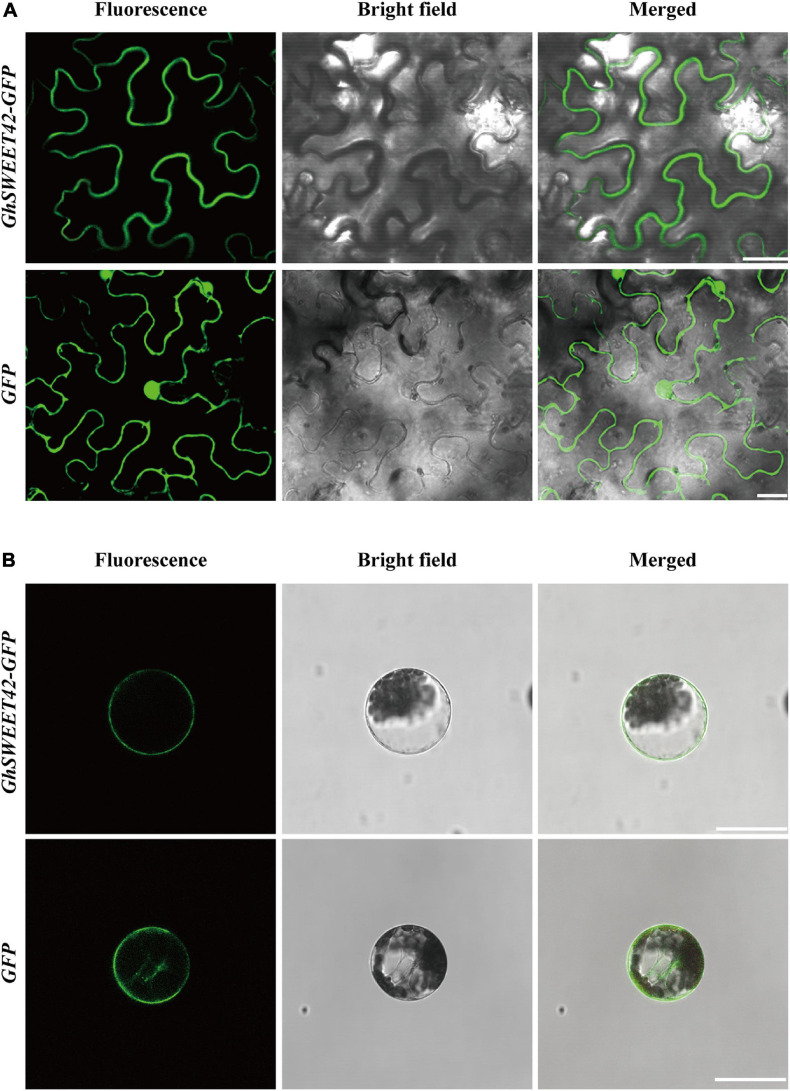
On the basis of a multiple sequence alignment and transmembrane structural analysis (Supplementary Figure 4), the GhSWEET42 amino acid sequence was predicted to include seven transmembrane domains, which are conserved among SWEET proteins (Chen et al., 2010). To confirm the subcellular localization of GhSWEET42, the gene encoding the GhSWEET42-GFP fusion protein was expressed under the control of the CaMV 35S promoter in transiently transformed N. benthamiana epidermal cells and A. thaliana protoplasts. Confocal microscopy images of the GFP signal confirmed that GhSWEET42-GFP is localized in the plasma membrane (Figures 3A,B).
FIGURE 3.
Subcellular localization of GhSWEET42. (A) Subcellular localization of GhSWEET42 in tobacco epidermal cells. (B) Subcellular localization of GhSWEET42 in Arabidopsis thaliana protoplasts. Scale bar = 25 μm. Plasmid containing 35S-GhSWEET42-GFP fusion (GhSWEET42-GFP) and 35S-GFP (GFP) was introduced into tobacco epidermal cells and A. thaliana protoplasts. GhSWEET42-GFP fusion protein localized in the plasma membrane.
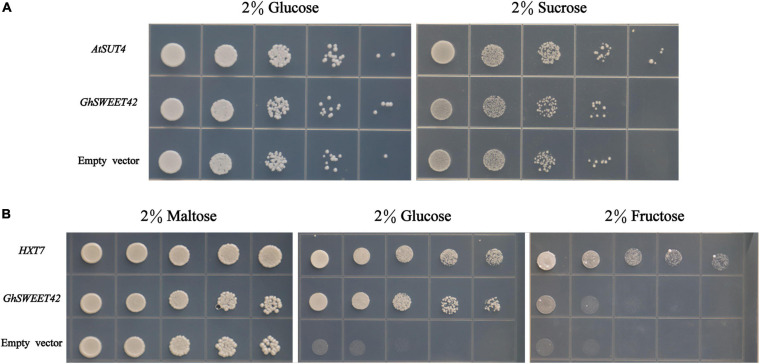
Previous research confirmed that SWEET proteins mediate the transport of sucrose, glucose, and fructose (Sosso et al., 2015; Sun et al., 2019). To identify which sugar is transported by GhSWEET42, the sucrose transport-deficient yeast mutant SUSY7/ura3 (Riesmeier et al., 1993) was transformed with GhSWEET42. The growth of the GhSWEET42-expressing yeast cells on medium supplemented with sucrose was similar to that of yeast cells carrying the empty vector (Figure 4A), indicating that GhSWEET42 does not transport sucrose. The expression of GhSWEET42 in the hexose transport-deficient yeast mutant EBY.VW4000 (Wieczorke et al., 1999) was unable to restore the growth of the yeast mutant on medium supplemented with fructose. However, GhSWEET42 expression enabled the yeast mutant to grow on medium supplemented with glucose, similar to the positive control expressing the yeast carbohydrate transporter gene HXT7 (Figure 4B), implying that GhSWEET42 is a glucose transporter.
FIGURE 4.
Functional characterization of GhSWEET42 using yeast sugar transport-defective strains SUSY7/ura3 and EBY.VW4000. (A) Complementation of sucrose transport deficiency in the SUSY7/ura3 yeast mutant by GhSWEET42 (positive control: AtSUT4; negative control: empty vector). (B) Complementation of hexose transport deficiency in the EBY.VW4000 yeast mutant by GhSWEET42 (positive control: yeast HXT7; negative control: empty vector).
Overexpression of GhSWEET42 in A. thaliana Increases Glucose Content and Weakens Resistance to V. dahliae
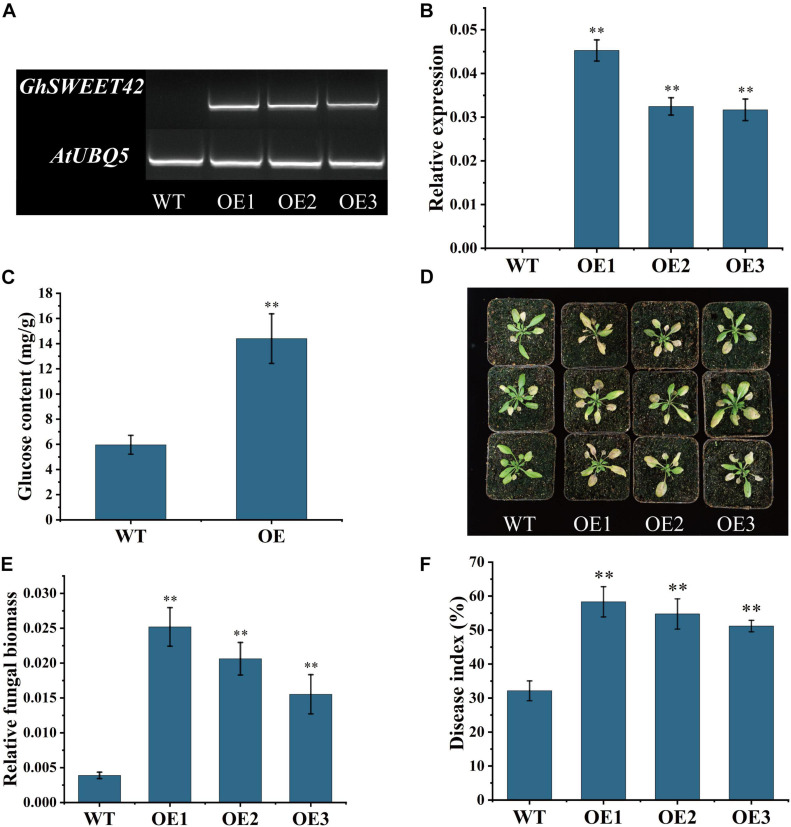
Transgenic A. thaliana lines expressing GhSWEET42 were generated to characterize its function. The expression of GhSWEET42 in the T3 homozygous transgenic lines was evaluated by RT-PCR and qRT-PCR. Three independent transgenic lines with high GhSWEET42 expression levels (Figures 5A,B) were chosen for further analyses. When grown under the same conditions, the transgenic plants accumulated more glucose than did wild-type plants (Figure 5C), indicating that the overexpression of GhSWEET42 affects glucose metabolism in A. thaliana. The transgenic and wild-type plants were also compared in terms of their responses to infection by V. dahliae strain V592. The inoculated transgenic plants showed more severe disease symptoms (e.g., wilting, chlorosis, early senescence, and necrosis) than did the wild-type plants (Figure 5D). Moreover, the qRT-PCR data confirmed that the fungal biomass was higher in transgenic plants than in wild-type plants (Figure 5E). The disease index calculated from at least 20 plants per treatment was approximately 32 for wild-type plants, but significantly higher for the three transgenic lines overexpressing GhSWEET42 (i.e., 58, 55, and 51) (Figure 5F). These results indicate that overexpression of GhSWEET42 increases the glucose content in A. thaliana and its sensitivity to V. dahliae.
FIGURE 5.
Decreased Verticillium dahliae resistance of Arabidopsis thaliana plants overexpressing GhSWEET42. (A) Transcript levels of GhSWEET42 in wild-type and GhSWEET42-overexpressing plants as determined by RT-PCR. AtUBQ5 was used as an internal control. (B) Transcript levels of GhSWEET42 in wild-type and GhSWEET42-overexpressing plants as determined by qRT-PCR. AtUBQ5 was used as an internal control. Error bars represent standard deviation of three biological replicates. Asterisks indicate significant differences compared with WT. (C) Glucose contents in wild-type and GhSWEET42-overexpressing plants. Error bars represent standard deviation of three biological replicates. Asterisks indicate significant differences compared with WT. (D) Symptoms of wild-type and GhSWEET42-overexpressing plants inoculated with V. dahliae. Two-week-old A. thaliana plants were inoculated with V. dahliae and replanted in soil. Plants were photographed at 7 day after inoculation. Analysis was completed using at least 20 plants per line. (E) Fungal biomass in wild-type and GhSWEET42-overexpressing plants as determined by qRT-PCR. Error bars represent standard deviation of three biological replicates. Asterisks indicate significant differences compared with WT. (F) Disease indices of wild-type and GhSWEET42-overexpressing plants. Asterisks indicate significant differences compared with WT. Data were generated for three replicates, each comprising 20 A. thaliana plants. Data were analyzed using Student’s t-test (∗∗P < 0.01). WT: wild-type plants; OE: GhSWEET42-overexpressing plants.
Down-Regulation of GhSWEET42 in Cotton Decreases the Glucose Content and Enhances Resistance to V. dahliae
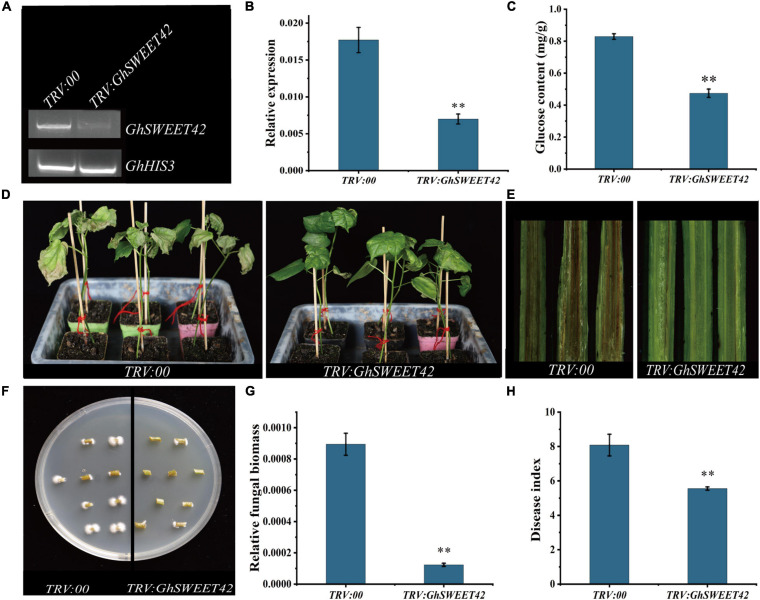
To elucidate the role of GhSWEET42 during the infection of cotton by V. dahliae, GhSWEET42 expression was knocked-down using a VIGS method. After the plants injected with TRV:GhCLA1 exhibited the strong albino phenotype (Supplementary Figure 5), the roots from cotton seedlings injected with TRV:00 and TRV:GhSWEET42 were sampled for RT-PCR and qRT-PCR analyses. The results confirmed that the transcript level of GhSWEET42 was lower in the TRV:GhSWEET42 plants than in the TRV:00 plants (Figures 6A,B). And the transcript level of GhSWEET40, which have a close phylogenetic relationship to GhSWEET42 (Figure 2A), did not differ significantly between TRV:GhSWEET42 and TRV:00 plants (Supplementary Figure 6), showing that GhSWEET42 was efficiently and specifically silenced in the TRV:GhSWEET42 plants. Compared with the TRV:00 plants, the TRV:GhSWEET42 plants showed significantly decreased glucose content in the roots (Figure 6C). Moreover, the TRV:GhSWEET42 plants were more resistant to V. dahliae than were the TRV:00 plants, with less wilting and fewer etiolated and abscised leaves (Figure 6D). A stereo microscope was used to examine the accumulation of V. dahliae in vascular tissues. And the results showed that the TRV:GhSWEET42 plants were less affected by V. dahliae than were the TRV:00 plants (Figure 6E). We conducted a recovery assay to assess the extent of the colonization of stems by V. dahliae, and found that there was substantially less fungal growth from the stems of TRV:GhSWEET42 plants than from the stems of TRV:00 plants (Figure 6F). This result was consistent with the results of the qRT-PCR analysis of fungal biomass (Figure 6G). Finally, we calculated the disease index from at least 30 plants per treatment. The disease index of TRV:00 plants (83.8) was higher than that of the TRV:GhSWEET42 plants (55.5) (Figure 6H). These observations suggest that silencing GhSWEET42 can decrease the glucose level in cotton plants, thereby increasing their resistance to V. dahliae.
FIGURE 6.
Increased Verticillium dahliae resistance of GhSWEET42-silenced cotton plants. (A) RT-PCR analysis confirming gene-silencing efficiency in roots of TRV:00 and TRV:GhSWEET42 plants. GhHIS3 served as the internal reference control. (B) qRT-PCR analysis confirming gene-silencing efficiency in roots of TRV:00 and TRV:GhSWEET42 plants. GhHIS3 served as the internal reference control. Error bars represent standard deviation of three biological replicates. Asterisks indicate significant differences compared with TRV:00. (C) Glucose contents in roots of TRV:00 and TRV:GhSWEET42 plants. Error bars represent standard deviation of three biological replicates. Asterisks indicate significant differences compared with TRV:00. (D) Representative images of TRV:00 and TRV:GhSWEET42 plants infected with V. dahliae. After the positive seedlings were completely albino, both plant lines were inoculated with V. dahliae. Each biological repeat consisted of at least 30 seedlings. (E) Vascular tissues of TRV:00 and TRV:GhSWEET42 plants infected with Verticillium dahliae. Samples were examined using a stereo microscope. (F) Fungal recovery experiments. Stem sections of TRV:00 and TRV:GhSWEET42 plants were placed on potato dextrose agar medium and incubated at 25°C. Samples were photographed 3 day later. (G) Fungal biomass in TRV:00 and TRV:GhSWEET42 plants as determined by qRT-PCR. Error bars represent standard deviation of three biological replicates. Asterisks indicate significant differences compared with TRV:00. (H) Disease indices of TRV:00 and TRV:GhSWEET42 plants. Data were generated for three replicates, each comprising 30 cotton plants. Asterisks indicate significant differences compared with TRV:00.Data were analyzed using Student’s t-test (∗∗P < 0.01).
Discussion
Verticillium dahliae is a soil-borne fungus that attacks plants through the roots (Fradin and Thomma, 2006). V. dahliae needs to penetrate the root epidermal cells to complete the colonization process, and this stage is critical for successful infection by V. dahliae (Bowers et al., 1996). Since marker-expressing fungal strains can be used to analyze the fungal colonization process, GFP transformants have become a common tool for analyzing various colonization processes of fungi in plants (Oren et al., 2003; Pantelides et al., 2009). In this study, cotton roots were infected with V592-GFP to observe the colonization process of V. dahliae. The results identified 48 hpi as a critical period for the colonization of cotton roots by V. dahliae. Another study also found that 48 h is a critical period for V. dahliae to penetrate epidermal cells, and the hyphae swell slightly to form penetrating structures when penetrating the epidermal cells of A. thaliana (Zhao et al., 2014). After successful penetration into the epidermal cells, hyphae grow and branch within the root cortex and begin to form networks (Zhang et al., 2012). In the study, the observation showed that many hyphae appeared and colonized the root cells at 96 hpi.
Numerous studies have found that sugars play a central role in the host-pathogen interaction (Chen et al., 2010; Gupta, 2020). Sugar transport is crucial for the pathogenicity of the fungus (Doidy et al., 2012). Pathogen infection triggers changes in sugar transport in host plants to improve nutrient supply to the pathogen (Lemoine et al., 2013). For example, previous studies have shown that sucrose and hexose significantly accumulate in Pst-challenged wheat leaves (Chang et al., 2013), and the glucose content increases in adult leaves of Zea mays infected with U. maydis (Sosso et al., 2019). A study on nutrient transfer from the host to the fungus found that glucose is the main carbon and energy source transferred to the fungal hyphae (Sutton and Hall, 1999). The increased sugar contents in infected tissues may be due to increased import of materials from adjacent uninfected tissues and healthy leaves (Chou et al., 2000; Hayes et al., 2010). We found that the glucose content was significantly increased at 48 hpi in the cotton roots infected by V. dahliae, suggesting that V. dahliae infection might result in glucose transport from uninfected cells to infected cells in cotton.
Disease resistance in plants is generally controlled by dominant resistance (R) genes. In a few cases, the gene that provides resistance is recessive, so plants carrying the corresponding dominant allele are sensitive (Navathe et al., 2020). In these cases, the dominant allele is a susceptibility (S) gene. The SWEET family, which is involved in sucrose or glucose transport, is among the most intensively studied class of S genes (Chen et al., 2010; Yuan and Wang, 2013). These sugar transporters are often hijacked by pathogens to supply sugars as a source of nutrition for themselves, leading to successful infection (Chen et al., 2010). Infection by pathogens leads to up-regulation of clade II SWEET genes in A. thaliana and V. vinifera (Chen et al., 2010; Chong et al., 2014). We found that the infection by V. dahliae up-regulated the clade II SWEET gene GhSWEET42. And overexpression of GhSWEET42 in A. thaliana increased the glucose accumulation, and enhanced susceptibility to V. dahlia. Previous studies showed that pathogens generally attack the host by secreting transcription activator-like effectors (TALEs), which enter the nuclei of host cells and mimic transcription factors to increase the expression of SWEET genes (Cox et al., 2017; Deng et al., 2017). For example, PthXo1 secreted by Xanthomonas oryzae pv. Oryzae (Xoo) strain PXO99A is a TALE, which directly interacts with the OsSWEET11 promoter and specifically activates the transcription of OsSWEET11 in rice (Yang et al., 2006). Another study found that Avrb6, a TALE determining Xanthomonas citri subsp. malvacearum pathogenicity, upregulates GhSWEET10 expression in cotton (Cox et al., 2017). The increased expression of sugar transporters stimulated by pathogenic bacteria would divert host nutrition toward infection sites (Walerowski et al., 2018). Therefore, GhSWEET42 sugar transporter might be the target of V. dahliae, and may facilitate glucose accumulation at the site of infection to enhance V. dahliae growth.
In many cases, the pathogenicity of pathogens depends on the sugar supply by the sugar transporters encoded by SWEET genes of the hosts. Therefore, modulating the host SWEET genes to block pathogen access to nutrients has emerged as an attractive strategy to achieve disease resistance (Oliva and Quibod, 2017). In our study, silencing GhSWEET42 expression in cotton decreased the glucose content and improved resistance to V. dahliae. Previous studies demonstrated that the A. thaliana sweet4 mutant shows enhanced resistance to gray mold (Chong et al., 2014), and inhibiting OsSWEET11 function in mesophyll cells increases the resistance of rice to sheath blight disease (Gao et al., 2018). Additionally, altering the TALE binding elements in SWEET gene promoters by CRISPR/Cas9-mediated genome editing endows rice lines with robust, broad-spectrum resistance to bacterial blight (Eom et al., 2019; Oliva et al., 2019). Thus, the identification of GhSWEET42 provides a candidate gene for using the sugar starvation strategy to inhibit infection by V. dahliae in cotton.
In conclusion, the results of this study revealed 48 hpi as a key period during the colonization of cotton roots by V. dahliae. Additionally, we proved that V. dahliae infection increased the transport of glucose to the infection site. We observed that the ectopic expression of GhSWEET42 in A. thaliana increased the glucose content in the transformed plants. During infection of these transgenic A. thaliana plants by V. dahliae, the pathogen grew quickly, leading to accelerated development of disease symptoms. Conversely, silencing of GhSWEET42 decreased the glucose content in roots and reduced disease symptoms and restricted hyphal growth when infected by V. dahliae. Therefore, GhSWEET42 was involved in infection of V. dahliae in cotton through glucose translocation and could be as a target gene to strengthen cotton resistance to V. dahliae.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
WL, XM, and DY conceived and designed the research. MS, ZZ, ZR, XW, and JZ performed the experiments. WS, HF, and FZ provided the materials. MS, ZZ, WL, WS, and HF analyzed the data. MS and WL prepared the figures and wrote the manuscript. XM and DY revised the manuscript. All authors have read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Dr. Eckhard Boles and Dr. Kaijing Zuo for the yeast deficient mutant strain EBY.VW4000, Dr. Wolf B. Frommer and Dr. Kaijing Zuo for the yeast deficient mutant strain SUSY7/ura3, and Xiaoyu Pei and Yangai Liu for their technical assistance.
Footnotes
Funding. This work was supported by the National Natural Science Foundation of China (32072114 and 31901580) and the National Key R&D Program of China for Crop Breeding (2016YFD0100306).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.690754/full#supplementary-material
Fluorescence images at 0, 6, 12, and 24 h after infection by Verticillium dahliae.
Bright field images at 48 and 96 h after infection by Verticillium dahliae.
Relative transcript levels of GhSWEET42 in roots of resistant and susceptible cotton varieties at 48 h after infection by Verticillium dahliae. The relative transcript levels of GhSWEET42 were detected by qRT-PCR, and GhHIS3 served as the internal reference control. Resistant variety: Zhongzhimian 2; susceptible variety: TM-1. Asterisks indicate significant differences compared with Zhongzhimian 2. Error bars represent standard deviation of three biological replicates. Data were analyzed using Student’s t-test (**P < 0.01).
Multiple sequence alignment and transmembrane structural analysis of GhWEET42.
Albino phenotype of TRV:GhCLA1 plants.
The expression analysis of GhSWEET40 in roots of TRV:00 and TRV:GhSWEET42 plants by qRT-PCR. GhHIS3 served as the internal reference control. Error bars represent standard deviation of three biological replicates. n.s. indicates no significant difference compared with TRV:00. Data were analyzed using Student’s t-test (**P < 0.01).
Protein sequences of SWEET genes from Gossypium hirsutum, Arabidopsis thaliana, and Vitis vinifera.
Primers used in this study.
Association analysis of natural variations around SWEET genes with Verticillium dahliae resistance in cotton lines and cultivars.
References
- Berger S., Papadopoulos M., Schreiber U., Kaiser W., Roitsch T. (2004). Complex regulation of gene expression, photosynthesis and sugar levels by pathogen infection in tomato. Physiol. Plant. 122 419–428. 10.1111/j.1399-3054.2004.00433.x [DOI] [Google Scholar]
- Bezrutczyk M., Yang J., Eom J. S., Prior M., Sosso D., Hartwig T., et al. (2018). Sugar flux and signaling in plant-microbe interactions. Plant J. 93 675–685. 10.1111/tpj.13775 [DOI] [PubMed] [Google Scholar]
- Bowers J. H., Nameth S. T., Riedel R. M., Rowe R. C. (1996). Infection and colonization of potato roots by Verticillium dahliae as affected by Pratylenchus penetrans and P. crenatus. Phytopathology 86 614–621. 10.1094/phyto-86-614 [DOI] [Google Scholar]
- Bustin S. A. (2000). Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 25 169–193. 10.1677/jme.0.0250169 [DOI] [PubMed] [Google Scholar]
- Chang Q., Lin X., Yao M., Liu P., Guo J., Huang L., et al. (2020). Hexose transporter PsHXT1-mediated sugar uptake is required for pathogenicity of wheat stripe rust. Plant Biotechnol. J. 18 2367–2369. 10.1111/pbi.13398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q., Liu J., Wang Q., Han L., Liu J., Li M., et al. (2013). The effect of Puccinia striiformis f. sp. tritici on the levels of water-soluble carbohydrates and the photosynthetic rate in wheat leaves. Physiol. Mol. Plant Pathol. 84 131–137. 10.1016/j.pmpp.2013.09.001 [DOI] [Google Scholar]
- Chen L.-Q., Hou B.-H., Frommer W. B. (2012). Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 6065 207–211. 10.1126/science.1213351 [DOI] [PubMed] [Google Scholar]
- Chen L.-Q., Hou B.-H., Lalonde S., Takanaga H., Hartung M. L., Qu X.-Q., et al. (2010). Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468 527–532. 10.1038/nature09606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J., Piron M. C., Meyer S., Merdinoglu D., Bertsch C., Mestre P. (2014). The SWEET family of sugar transporters in grapevine: VvSWEET4 is involved in the interaction with Botrytis cinerea. J. Exp. Bot. 65 6589–6601. 10.1093/jxb/eru375 [DOI] [PubMed] [Google Scholar]
- Chou H. M., Bundock N., Rolfe S. A., Scholes J. D. (2000). Infection of Arabidopsis thaliana leaves with Albugo candida (white blister rust) causes a reprogramming of host metabolism. Mol. Plant Pathol. 1 99–113. 10.1046/j.1364-3703.2000.00013.x [DOI] [PubMed] [Google Scholar]
- Clough S. J., Bent A. F. (2010). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. 10.1046/j.1365-313x.1998.00343.x [DOI] [PubMed] [Google Scholar]
- Cox K. L., Meng F., Wilkins K. E., Li F., Wang P., Booher N. J., et al. (2017). TAL effector driven induction of a SWEET gene confers susceptibility to bacterial blight of cotton. Nat. Commun. 8:15588. 10.1038/ncomms15588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Marshall N. C., Rowland J. L., McCoy J. M., Worrall L. J., Santos A. S., et al. (2017). Assembly, structure, function and regulation of type III secretion systems. Nat. Rev. Microbiol. 15 323–337. 10.1038/nrmicro.2017.20 [DOI] [PubMed] [Google Scholar]
- Doehlemann G., Wahl R., Horst R. J., Voll L. M., Usadel B., Poree F., et al. (2008). Reprogramming a maize plant: transcriptional and metabolic changes induced by the fungal biotroph Ustilago maydis. Plant J. 56 181–195. 10.1111/j.1365-313X.2008.03590.x [DOI] [PubMed] [Google Scholar]
- Doidy J., Grace E., Kühn C., Simon-Plas F., Casieri L., Wipf D. (2012). Sugar transporters in plants and in their interactions with fungi. Trends Plant Sci. 17 413–422. 10.1016/j.tplants.2012.03.009 [DOI] [PubMed] [Google Scholar]
- Eom J. S., Luo D., Atienza-Grande G., Yang J., Ji C., Thi Luu V., et al. (2019). Diagnostic kit for rice blight resistance. Nat. Biotechnol. 37 1372–1379. 10.1038/s41587-019-0268-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L., Wang Q., Hu Y., Jia Y., Chen J., Liu B., et al. (2017). Genomic analyses in cotton identify signatures of selection and loci associated with fiber quality and yield traits. Nat. Genet. 49 1089–1098. 10.1038/ng.3887 [DOI] [PubMed] [Google Scholar]
- Fradin E. F., Abd-El-Haliem A., Masini L., van den Berg G. C., Joosten M. H., Thomma B. P. (2011). Interfamily transfer of tomato Ve1 mediates Verticillium resistance in Arabidopsis. Plant Physiol. 156 2255–2265. 10.1104/pp.111.180067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin E. F., Thomma B. P. (2006). Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 7 71–86. 10.1111/j.1364-3703.2006.00323.x [DOI] [PubMed] [Google Scholar]
- Gao W., Long L., Zhu L. F., Xu L., Gao W. H., Sun L. Q., et al. (2013). Proteomic and virus-induced gene silencing (VIGS) analyses reveal that gossypol, brassinosteroids, and jasmonic acid contribute to the resistance of cotton to Verticillium dahliae. Mol. Cell Proteomics 12 3690–3703. 10.1074/mcp.M113.031013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Zhang C., Han X., Wang Z. Y., Ma L., Yuan P., et al. (2018). Inhibition of OsSWEET11 function in mesophyll cells improves resistance of rice to sheath blight disease. Mol. Plant Pathol. 19 2149–2161. 10.1111/mpp.12689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-González S., Ruiz-Jiménez J., Priego-Capote F., Luque de Castro M. D. (2010). Qualitative and quantitative sugar profiling in olive fruits, leaves, and stems by gas chromatography-tandem mass spectrometry (GC-MS/MS) after ultrasound-assisted leaching. J. Agric. Food Chem. 58 12292–12299. 10.1021/jf102350s [DOI] [PubMed] [Google Scholar]
- Gong Q., Yang Z., Chen E., Sun G., He S., Butt H. I., et al. (2018). A phi-class glutathione S-transferase gene for verticillium wilt resistance in gossypium arboreum identified in a genome-wide association study. Plant Cell Physiol. 59 275–289. 10.1093/pcp/pcx180 [DOI] [PubMed] [Google Scholar]
- Gupta P. K. (2020). SWEET genes for disease resistance in plants. Trends Genet. 36 901–904. 10.1016/j.tig.2020.08.007 [DOI] [PubMed] [Google Scholar]
- Gutierrez L., Mauriat M., Guenin S., Pelloux J., Lefebvre J. F., Louvet R., et al. (2008). The lack of a systematic validation of reference genes: a serious pitfall undervalued in reverse transcription-polymerase chain reaction (RT-PCR) analysis in plants. Plant Biotechnol. J. 6 609–618. 10.1111/j.1467-7652.2008.00346.x [DOI] [PubMed] [Google Scholar]
- Hayes M. A., Feechan A., Dry I. B. (2010). Involvement of abscisic acid in the coordinated regulation of a stress-inducible hexose transporter (VvHT5) and a cell wall invertase in grapevine in response to biotrophic fungal infection. Plant Physiol. 153 211–221. 10.1104/pp.110.154765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huai B., Yang Q., Qian Y., Qian W., Kang Z., Liu J. (2019). ABA-induced sugar transporter TaSTP6 promotes wheat susceptibility to stripe rust. Plant Physiol. 181 1328–1343. 10.1104/pp.19.00632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimes A., Dobinson K. F., Thomma B. P., Klosterman S. J. (2015). Genomics spurs rapid advances in our understanding of the biology of vascular wilt pathogens in the genus Verticillium. Annu. Rev. Phytopathol. 53 181–198. 10.1146/annurev-phyto-080614-120224 [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K. (2016). MEGA7: molecular evolutionary genetics analysis Version 7.0 for bigger datasets. Mol. Biol. Evol. 33 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., et al. (2007). Clustal W and Clustal X version 2.0. Bioinformatics 23 2947–2948. 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- Lemoine R., La Camera S., Atanassova R., Dédaldéchamp F., Allario T., Pourtau N., et al. (2013). Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 4:272. 10.3389/fpls.2013.00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Ren Z., Wang Z., Sun K., Pei X., Liu Y., et al. (2018). Evolution and stress responses of Gossypium hirsutum SWEET genes. Int. J. Mol. Sci. 19:769. 10.3390/ijms19030769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L., Yang W.-W., Liao P., Guo Y.-W., Kumar A., Gao W. (2019). Transcriptome analysis reveals differentially expressed ERF transcription factors associated with salt response in cotton. Plant Sci. 281 72–81. 10.1016/j.plantsci.2019.01.012 [DOI] [PubMed] [Google Scholar]
- Mao H., Li S., Wang Z., Cheng X., Li F., Mei F., et al. (2020). Regulatory changes in TaSNAC8-6A are associated with drought tolerance in wheat seedlings. Plant Biotechnol. J. 18 1078–1092. 10.1111/pbi.13277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navathe S., Yadav P. S., Chand R., Mishra V. K., Vasistha N. K., Meher P. K., et al. (2020). ToxA–Tsn1 interaction for spot blotch susceptibility in indian wheat: an example of inverse gene-for-gene relationship. Plant Dis. 104 71–81. 10.1094/pdis-05-19-1066-re [DOI] [PubMed] [Google Scholar]
- Oliva R., Ji C., Atienza-Grande G., Huguet-Tapia J. C., Perez-Quintero A., Li T., et al. (2019). Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat. Biotechnol. 37 1344–1350. 10.1038/s41587-019-0267-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva R., Quibod I. L. (2017). Immunity and starvation: new opportunities to elevate disease resistance in crops. Curr. Opin. Plant. Biol. 38 84–91. 10.1016/j.pbi.2017.04.020 [DOI] [PubMed] [Google Scholar]
- Oren L., Ezrati S., Cohen D., Sharon A. (2003). Early events in the Fusarium verticillioides-maize interaction characterized by using a green fluorescent protein-expressing transgenic isolate. Appl. Environ. Microbiol. 69 1695–1701. 10.1128/aem.69.3.1695-1701.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelides I. S., Tjamos S. E., Striglis I. A., Chatzipavlidis I., Paplomatas E. J. (2009). Mode of action of a non-pathogenic Fusarium oxysporum strain against Verticillium dahliae using real time QPCR analysis and biomarker transformation. Biol. Control 50 30–36. 10.1016/j.biocontrol.2009.01.010 [DOI] [Google Scholar]
- Phillips A. Z., Berry J. C., Wilson M. C., Vijayaraghavan A., Burke J., Bunn J. I., et al. (2017). Genomics-enabled analysis of the emergent disease cotton bacterial blight. PLoS Genet. 13:e1007003. 10.1371/journal.pgen.1007003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier J. W., Willmitzer L., Frommer W. B. (1993). Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J. 11 4705–4713. 10.1002/j.1460-2075.1992.tb05575.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sami F., Siddiqui H., Hayat S. (2019). Interaction of glucose and phytohormone signaling in plants. Plant Physiol. Biochem. 135 119–126. 10.1016/j.plaphy.2018.11.005 [DOI] [PubMed] [Google Scholar]
- Santhanam P., van Esse H. P., Albert I., Faino L., Nurnberger T., Thomma B. P. (2013). Evidence for functional diversification within a fungal NEP1-like protein family. Mol. Plant Microbe Interact. 26 278–286. 10.1094/MPMI-09-12-0222-R [DOI] [PubMed] [Google Scholar]
- Schuler D., Wahl R., Wippel K., Vranes M., Munsterkotter M., Sauer N., et al. (2015). Hxt1, a monosaccharide transporter and sensor required for virulence of the maize pathogen Ustilago maydis. New Phytol. 206 1086–1100. 10.1111/nph.13314 [DOI] [PubMed] [Google Scholar]
- Song C., Li W., Pei X., Liu Y., Ren Z., He K., et al. (2019). Dissection of the genetic variation and candidate genes of lint percentage by a genome-wide association study in upland cotton. Theor. Appl. Genet. 132 1991–2002. 10.1007/s00122-019-03333-0 [DOI] [PubMed] [Google Scholar]
- Sosso D., Luo D., Li Q. B., Sasse J., Yang J., Gendrot G., et al. (2015). Seed filling in domesticated maize and rice depends on SWEET-mediated hexose transport. Nat. Genet. 47 1489–1493. 10.1038/ng.3422 [DOI] [PubMed] [Google Scholar]
- Sosso D., van der Linde K., Bezrutczyk M., Schuler D., Schneider K., Kamper J., et al. (2019). Sugar partitioning between Ustilago maydis and its host zea mays L during infection. Plant Physiol. 179 1373–1385. 10.1104/pp.18.01435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Gao Z., Wang J., Huang Y., Chen Y., Li J., et al. (2019). Cotton fiber elongation requires the transcription factor GhMYB212 to regulate sucrose transportation into expanding fibers. New Phytol. 222 864–881. 10.1111/nph.15620 [DOI] [PubMed] [Google Scholar]
- Sun Z., Wang X., Liu Z., Gu Q., Zhang Y., Li Z., et al. (2017). Genome-wide association study discovered genetic variation and candidate genes of fibre quality traits in Gossypium hirsutum L. Plant Biotechnol. J. 15 982–996. 10.1111/pbi.12693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton P. N., Hall M. (1999). Glucose, and not sucrose, is transported from wheat to wheat powdery mildew. Planta 208 426–430. 10.1007/s004250050578 [DOI] [Google Scholar]
- Walerowski P., Gundel A., Yahaya N., Truman W., Sobczak M., Olszak M., et al. (2018). Clubroot disease stimulates early steps of phloem differentiation and recruits SWEET sucrose transporters within developing galls. Plant Cell 30 3058–3073. 10.1105/tpc.18.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.-Y., Lü K., Cai C.-P., Xu J., Guo W.-Z. (2014). Establishment and application of TRV-mediated virus-induced gene silencing in cotton. Acta Agronomica Sinica 40:1356. 10.3724/SP.J.1006.2014.01356 [DOI] [Google Scholar]
- Wieczorke R., Krampe S., Weierstall T., Freidel K., Hollenberg C. P., Boles E. (1999). Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett. 464 123–128. 10.1016/S0014-5793(99)01698-1 [DOI] [PubMed] [Google Scholar]
- Yamada K., Saijo Y., Nakagami H., Takano Y. (2016). Regulation of sugar transporter activity for antibacterial defense in Arabidopsis. Science 354 1427–1430. 10.1126/science.aah5692 [DOI] [PubMed] [Google Scholar]
- Yang B., Sugio A., White F. (2006). Os8N3 is a host disease-susceptibility gene for bacterial blight of rice. Proc. Natl. Acad. Sci. U. S. A. 103 10503–10508. 10.1073/pnas.0604088103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S. D., Cho Y. H., Sheen J. (2007). Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2 1565–1572. 10.1038/nprot.2007.199 [DOI] [PubMed] [Google Scholar]
- Yoon J., Cho L. H., Tun W., Jeon J. S., An G. (2021). Sucrose signaling in higher plants. Plant Sci. 302:110703. 10.1016/j.plantsci.2020.110703 [DOI] [PubMed] [Google Scholar]
- Yuan M., Wang S. (2013). Rice MtN3/saliva/SWEET family genes and their homologs in cellular organisms. Mol. Plant 6 665–674. 10.1093/mp/sst035 [DOI] [PubMed] [Google Scholar]
- Zhang T., Zhang B., Hua C., Meng P., Wang S., Chen Z., et al. (2017). VdPKS1 is required for melanin formation and virulence in a cotton wilt pathogen Verticillium dahliae. Sci. China Life Sci. 60 868–879. 10.1007/s11427-017-9075-3 [DOI] [PubMed] [Google Scholar]
- Zhang W.-W., Jiang T.-F., Cui X., Qi F.-J., Jian G.-L. (2012). Colonization in cotton plants by a green fluorescent protein labelled strain of Verticillium dahliae. Eur. J. Plant Pathol. 135 867–876. 10.1007/s10658-012-0131-1 [DOI] [Google Scholar]
- Zhao P., Zhao Y. L., Jin Y., Zhang T., Guo H. S. (2014). Colonization process of Arabidopsis thaliana roots by a green fluorescent protein-tagged isolate of Verticillium dahliae. Protein Cell 5 94–98. 10.1007/s13238-013-0009-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Zhang Q., Quan J., Zheng Q., Xi W. (2016). Determination of sugars, organic acids, aroma components, and carotenoids in grapefruit pulps. Food Chem. 205 112–121. 10.1016/j.foodchem.2016.03.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fluorescence images at 0, 6, 12, and 24 h after infection by Verticillium dahliae.
Bright field images at 48 and 96 h after infection by Verticillium dahliae.
Relative transcript levels of GhSWEET42 in roots of resistant and susceptible cotton varieties at 48 h after infection by Verticillium dahliae. The relative transcript levels of GhSWEET42 were detected by qRT-PCR, and GhHIS3 served as the internal reference control. Resistant variety: Zhongzhimian 2; susceptible variety: TM-1. Asterisks indicate significant differences compared with Zhongzhimian 2. Error bars represent standard deviation of three biological replicates. Data were analyzed using Student’s t-test (**P < 0.01).
Multiple sequence alignment and transmembrane structural analysis of GhWEET42.
Albino phenotype of TRV:GhCLA1 plants.
The expression analysis of GhSWEET40 in roots of TRV:00 and TRV:GhSWEET42 plants by qRT-PCR. GhHIS3 served as the internal reference control. Error bars represent standard deviation of three biological replicates. n.s. indicates no significant difference compared with TRV:00. Data were analyzed using Student’s t-test (**P < 0.01).
Protein sequences of SWEET genes from Gossypium hirsutum, Arabidopsis thaliana, and Vitis vinifera.
Primers used in this study.
Association analysis of natural variations around SWEET genes with Verticillium dahliae resistance in cotton lines and cultivars.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.