ABSTRACT
Crocodilians are unique among vertebrates in that their hemoglobin (Hb) O2 binding is allosterically regulated by bicarbonate, which forms in red blood cells upon hydration of CO2. Although known for decades, this remarkable mode of allosteric control has not yet been experimentally verified with direct evidence of bicarbonate binding to crocodilian Hb, probably because of confounding CO2-mediated effects. Here, we provide the first quantitative analysis of the separate allosteric effects of CO2 and bicarbonate on purified Hb of the spectacled caiman (Caiman crocodilus). Using thin-layer gas diffusion chamber and Tucker chamber techniques, we demonstrate that both CO2 and bicarbonate bind to Hb with high affinity and strongly decrease O2 saturation of Hb. We propose that both effectors bind to an unidentified positively charged site containing a reactive amino group in the low-O2 affinity T conformation of Hb. These results provide the first experimental evidence that bicarbonate binds directly to crocodilian Hb and promotes O2 delivery independently of CO2. Using the gas diffusion chamber, we observed similar effects in Hbs of a phylogenetically diverse set of other caiman, alligator and crocodile species, suggesting that the unique mode of allosteric regulation by CO2 and bicarbonate evolved >80–100 million years ago in the common ancestor of crocodilians. Our results show a tight and unusual linkage between O2 and CO2 transport in the blood of crocodilians, where the build-up of erytrocytic CO2 and bicarbonate ions during breath-hold diving or digestion facilitates O2 delivery, while Hb desaturation facilitates CO2 transport as protein-bound CO2 and bicarbonate.
KEY WORDS: Adaptation, Oxygen, Carbon dioxide, Blood, Allosteric regulation
Summary: The first quantitative analysis of the separate effects of CO2 and bicarbonate on crocodilian hemoglobin, showing that both bind directly with high affinity at the same T-state site, promoting O2 unloading.
INTRODUCTION
The oxygenation properties of vertebrate hemoglobins (Hbs) are sensitive to a number of small-molecule effectors present in red blood cells (RBCs), including protons, CO2, chloride ions and organic phosphates. Allosteric regulation of Hb–O2 affinity by such effectors helps keep blood O2 supply in balance with tissue O2 consumption. These effectors bind preferentially to the low-affinity (deoxy) T-state of the Hb tetramer, thereby reducing Hb–O2 affinity by shifting the T-R equilibrium towards the T quaternary state (Perutz, 1976; Storz, 2019; Weber and Fago, 2004). Hence, increases in blood CO2 levels (as produced by increased metabolic activity) cause a reduction in Hb–O2 affinity, thereby facilitating O2 unloading to tissues. The underlying mechanism is the reaction of CO2 with N-terminal amino groups (-NH2) of the α- and β-chain subunits of the Hb tetramer (Kilmartin and Rossi-Bernardi, 1971), resulting in the formation of a carbamino product (-NH-COO−), which preferentially stabilizes the low-affinity T-state of Hb via interactions with positively charged amino acid residues. CO2 also exerts an indirect allosteric effect on Hb–O2 affinity by reducing RBC pH. Upon entry into the RBC, CO2 reacts with water to form bicarbonate ions and protons, a reaction catalyzed by the enzyme carbonic anhydrase. Protons bind to Hb and promote O2 unloading via the Bohr effect, while bicarbonate ions leave the RBC in exchange for chloride ions.
Among vertebrate Hbs, those of crocodilians are reportedly unique in that their O2 affinity is allosterically regulated by binding of bicarbonate ions to the Hb protein (Bauer et al., 1981; Perutz et al., 1981). This unusual bicarbonate sensitivity of crocodilian Hb is thought to support aerobic metabolism when blood bicarbonate levels are elevated. This occurs during breath-hold diving (Bautista et al., 2021; Hicks and White, 1992), and during the postprandial ‘alkaline tide’ where gastric acid secretion causes a pronounced metabolic alkalosis (Busk et al., 2000). In support of these interpretations, we recently found that the concentration of Hb-bound bicarbonate increased in the blood of diving caimans and correlated with the degree of O2 depletion (Bautista et al., 2021), providing strong in vivo evidence of the regulatory effect of bicarbonate ions on Hb oxygenation.
The bicarbonate sensitivity of crocodilian Hbs was first described in vitro in the spectacled caiman (Caiman crocodilus) (Bauer et al., 1981; Perutz et al., 1981), but the allosteric effect was inferred without direct experimental verification of bicarbonate binding to Hb. Subsequent studies on Hbs from other crocodilian species documented a direct allosteric effect of CO2 (Fago et al., 2020; Jensen et al., 1998; Weber et al., 2013), but the direct contribution of bicarbonate binding has been neither confirmed in these studies nor quantified. The reason is likely that it is difficult to experimentally distinguish the allosteric effects of CO2 from those of bicarbonate and protons because at equilibrium all these three compounds are present in solution. Moreover, previous studies (Bauer et al., 1981) used different experimental and buffer conditions and rarely accounted for the potential effect of other anions, such as chloride ions, which are potent allosteric modulators of crocodilian Hb–O2 affinity (Fago et al., 2020; Storz, 2019; Weber et al., 2017).
Here, we used kinetic and equilibrium approaches at a constant pH and in the absence of chloride ions or organic phosphates to separate the allosteric effects of CO2 and bicarbonate on crocodilian Hb oxygenation. We provide a detailed functional analysis on the allosteric regulation of the Hb of the spectacled caiman, the same species that was originally studied by Bauer and coworkers (Bauer et al., 1981; Bauer and Jelkmann, 1977; Jelkmann and Bauer, 1980). We also confirm the same mode of allosteric regulation in the Hbs of 13 other caiman, alligator and crocodile species, indicating that this unique property evolved in the common ancestor of extant crocodilians.
MATERIALS AND METHODS
Ethical statement
All protocols of animal handling and sampling were performed in accordance with the Danish Law for Animal Experimentation and were approved by the Danish Animal Experiments Inspectorate, under permit number 2018-15-0201-01507.
Animals and blood collection
Approximately 5 ml blood was drawn from the post-occipital sinus into heparinized syringes from three non-anesthetized adult spectacled caimans (Caiman crocodilus Linnaeus 1758; around 1.5 kg each) of undetermined sex. The procedure lasted less than 5 min. Blood was immediately centrifuged at 4°C (5000 g, 5 min) to remove plasma and RBCs were then washed 5 times in 0.9% NaCl, 0.5 mmol l−1 EDTA and stored at −80°C until further processing. As described previously (Fago et al., 2020), we sampled blood and prepared hemolysates from 13 other crocodilian species [American alligator, Alligator mississippiensis (Daudin 1802); Chinese alligator, Alligator sinensis (Fauvel 1879); broad-snouted caiman, Caiman latirostris Daudin 1802; yacaré caiman, Caiman yacare Daudin 1802; black caiman, Melanosuchus niger (Spix 1825); smooth-fronted caiman, Paleosuchus trigonatus (Schneider 1801); American crocodile, Crocodylus acutus Cuvier 1807; Philippine crocodile, Crocodylus mindorensis Schmidt 1935; Nile crocodile, Crocodylus niloticus Laurenti 1768; New Guinea crocodile, Crocodylus novaeguineae (Schmidt 1928); saltwater crocodile, Crocodylus porosus Schneider 1801; Cuban crocodile, Crocodylus rhombifer Cuvier 1807; and Siamese crocodile, Crocodylus siamensis Schneider 1801].
All protocols of animal handling and sampling were performed in accordance with the Danish Law for Animal Experimentation and were approved by the Danish Animal Experiments Inspectorate, under permit number 2018-15-0201-01507.
Preparation of the hemolysate
For each individual spectacled caiman (N=3), the hemolysate was prepared by dilution (1:5) of washed RBCs in chloride-free 10 mmol l−1 Hepes pH 7.6, 0.5 mmol l−1 EDTA, and incubated on ice for 30 min. The hemolysate was then centrifuged at 4°C (12000 g, 20 min) to remove cell debris and then passed (‘stripped’) on a PD-10 desalting column (GE Healthcare) equilibrated with 10 mmol l−1 Hepes pH 7.6, 0.5 mmol l−1 EDTA to remove small molecular weight molecules, including allosteric effectors of Hb. Chloride-free Hepes buffers were used throughout. The absence of chloride in solutions was verified by a model 926S Mark II chloride analyzer (Sherwood Scientific Ltd, Cambridge, UK).
Discriminating the effects of CO2 and bicarbonate
To discriminate between the effects of CO2 and bicarbonate on Hb oxygenation, we measured the time course of changes in O2 saturation of stripped hemolysate and gel-filtration purified Hb (see ‘Hb purification and mass spectrometry analysis’, below) after addition and removal of 1% CO2 gas at a fixed PO2. These experiments were performed in the presence and absence of 1 mmol l−1 acetazolamide, a specific inhibitor of carbonic anhydrase contained in the hemolysate, in order to manipulate the rate of bicarbonate formation from CO2 and distinguish between effects due to bicarbonate or CO2 on Hb oxygenation (Fago et al., 1999). Experiments were performed at 25°C using a thin-layer modified diffusion chamber technique (Cadiz et al., 2019; Natarajan et al., 2018; Storz et al., 2020; Weber et al., 2017). In brief, 4 µl samples were placed in the temperature-controlled gas diffusion chamber connected to a Cary 60 UV-Vis spectrophotometer equipped with fiber optic probes (Agilent Technologies), and O2 saturation was determined from the absorption trace at 415 nm. Discrete values of PO2 and PCO2 within the chamber were obtained by mixing O2 and CO2 with ultrapure N2 gas using a programmable Gas Mixing System (GMS, Loligo Systems, Viborg, Denmark), with the gas mixture constantly flowing above the sample surface. The experiments were performed at a fixed PO2 corresponding approximately to 50% O2 saturation, under the same conditions as in our previous study on crocodilian Hbs (0.3 mmol l−1 heme, 0.1 mol l−1 Hepes pH 7.2, 0.5 mmol l−1 EDTA) (Fago et al., 2020). Heme concentration of samples was measured spectrophotometrically using published extinction coefficients at 577 and 542 nm (Van Assendelft, 1970).
Throughout this study, the pH of Hb samples equilibrated with the same CO2 of the experiments was routinely measured using a pH electrode (InLab® Micro, Mettler Toledo) and it did not change appreciably (range 7.16–7.21).
Measuring O2 equilibrium curves
We measured O2 equilibrium curves of stripped hemolysate and purified Hb (see ‘Hb purification and mass spectrometry analysis’, below) using the diffusion chamber technique described above at 25°C under identical buffer conditions (0.3 mmol l−1 heme, 0.1 mol l−1 Hepes pH 7.2, 0.5 mmol l−1 EDTA). O2 affinity (P50, the PO2 at 50% O2 saturation) and Hill's cooperativity coefficient (n) were calculated by non-linear regression fitting the Hill equation (Eqn 1) to the saturation data (Janecka et al., 2015; Jendroszek et al., 2018):
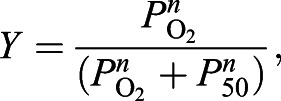 |
(1) |
where Y is the O2 saturation and PO2 is the O2 tension. The accuracy of the thin layer diffusion chamber technique was investigated in technical triplicates, yielding standard error of mean P50 below 2.5% (Table S1).
Hb purification and mass spectrometry analysis
Hb was purified from endogenous carbonic anhydrase by gel filtration using fast protein liquid chromatography (FPLC) on an ÄKTA Pure Protein Purification System equipped with a Superdex 75 10/300 GL gel-filtration column and a 100 µl loop (GE Healthcare Life Sciences). Buffer was 10 mmol l−1 Hepes pH 7.6, 0.5 mmol l−1 EDTA, 0.15 mol l−1 NaCl, and flow rate was 0.5 ml min−1. Absorbance was measured at 280 nm. The column was calibrated with standard proteins (Sigma Aldrich) of known molecular mass: horse myoglobin (17.6 kDa), bovine carbonic anhydrase II (30 kDa) and human adult Hb (64.6 kDa). The Hb fractions purified by gel filtration were concentrated by ultracentrifugation and dialyzed against 10 mmol l−1 Hepes pH 7.5, 0.5 mmol l−1 EDTA to remove NaCl. After dialysis, the absence of Cl− in the samples was confirmed by using a MK II Chloride Analyzer 926S (Sherwood Scientific Ltd). Hb fractions were analyzed for the presence or absence of intermolecular disulfide bonds by SDS polyacrylamide gel electrophoresis (range 4–12%) using NuPAGE® Novex® Bis-Tris Pre-Cast Gels and stained with Coomassie Blue, in the presence and absence of the reducing agent DTT.
To establish whether carbonic anhydrase (∼30 kDa) co-eluted with Hb by FPLC, we ran the purified Hb fractions on a mini-protean precast 4–20% SDS PAGE gel (Bio-Rad, Hercules, CA, USA), which we then stained with Coomassie Brilliant Blue-G. We conducted tandem mass spectrometry (MS/MS) analyses on the bands ranging from ∼25 to 50 kDa in size (Fig. S3). The stained bands were excised and processed for in-gel tryptic digestion (Shevchenko et al., 2006) and the eluted peptides were then analyzed using a Thermo Orbitrap Fusion Lumos Tribrid (Thermo Scientific™) mass spectrometer in data-dependent acquisition mode. Peptides were identified by searching MS/MS data against the UniprotKB/Swiss-Prot database. The search was set up for full tryptic peptides with a maximum of two missed cleavage sites. The precursor mass tolerance threshold was set as 10 ppm and maximum fragment mass error was set at 0.02 Da. Qualitative analysis was performed using PEAKS X software. The significance threshold of the ion score was calculated based on a false discovery rate of ≤1%.
CO2 effect on O2 affinity and CO2 saturation curves
To establish whether the purified Hb fractions had different sensitivity to CO2, we determined O2 equilibrium curves for each gel-filtration purified Hb fraction at 25°C (0.3 mmol l−1 heme, 0.1 mol l−1 Hepes pH 7.2, 0.5 mmol l−1 EDTA) at discrete CO2 tensions in the gas mixture, and calculated P50 and n at each PCO2 as described above (see ‘Measuring O2 equilibrium curves’).
For each purified Hb fraction, we determined apparent CO2 saturation curves by measuring the decrease in O2 saturation upon addition of CO2 to the gas mixture, while keeping a fixed PO2 (close to the P50 of that Hb fraction in the absence of CO2). A new Hb sample (4 µl) was used for measurements at each given PCO2. Experiments were performed using the modified diffusion chamber technique at 25°C, 0.3 mmol l−1 heme, 0.1 mol l−1 Hepes pH 7.2, 0.5 mmol l−1 EDTA. Apparent CO2 saturation curves for each purified Hb were obtained by non-linear regression fitting of a single hyperbola (Eqn 2) to the data:
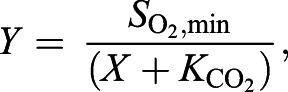 |
(2) |
where Y is O2 saturation, SO2,min is the O2 saturation at saturating PCO2 values and X is PCO2. This analysis provided the apparent affinity constant for the allosteric binding of CO2 to Hb (KCO2, the PCO2 at 50% CO2 saturation). We also examined the possibility of two binding sites by fitting a double hyperbola equation to the data (see Table S1).
Bicarbonate effect on O2 release and bicarbonate saturation curves
To determine the effect of bicarbonate on Hb oxygenation, we equilibrated samples of each gel-filtration purified Hb fraction (0.05 mmol l−1 heme, 0.1 mol l−1 Hepes buffer pH 7.2, 0.5 mmol l−1 EDTA) in Eschweiler tonometers for at least 40 min at 25°C with a gas mixture of 2% air and 98% N2 generated by a gas-mixing pump (Wösthoff, Bochum, Germany). The same gas mixture (corresponding to a PO2 close to the P50 of the Hb) was also used to equilibrate a freshly prepared stock solution of sodium bicarbonate (NaHCO3) dissolved in MilliQ H2O in a separate tonometer at the same temperature. After equilibration, the Hb sample was transferred with a gastight syringe into a 2.5 ml Tucker chamber (Tucker, 1967), previously flushed with 2% air and 98% N2. The chamber was equipped with a fiber optic O2 probe connected to a FireSting-O2 (FSO2-1) oxygen and temperature meter and controlled by FireSting logger V.2.365 software for PO2 signal recording (PyroScience, Germany). Following stabilization of the PO2 signal of the Hb sample in the chamber, a small volume (<60 µl) of the NaHCO3 solution was injected into the Tucker chamber to achieve discrete NaHCO3 concentrations (2, 4, 7.7, 15.4, 23.1 and 30 mmol l−1), and the subsequent increase in PO2 in the chamber (due to bicarbonate-induced O2 unloading by the Hb) was recorded. The bicarbonate concentration of the stock solution was 0.5, 1, 1.2 or 1.3 mol l−1, chosen based on the desired final concentration within the chamber, to maintain a similar injection volume (<60 µl) in all samples. A new Hb sample was used for each bicarbonate addition and separate experiments were performed for the Hb fractions of each of the three individuals. Two-point calibration of the oxygen probe was performed immediately before recording each trace by flushing the chamber with water-saturated air or N2 and taking into account the barometric pressure on the day. The pH of the bicarbonate-containing Hb solution was measured at the end of each experiment using a pH electrode (InLab® Micro, Mettler Toledo) and no significant change was observed (range 7.19–7.23). The accuracy of the Tucker chamber technique was investigated in technical triplicates, yielding standard error of final mean PO2 of 0.5% (Table S2).
Apparent bicarbonate saturation curves for each purified Hb were obtained by non-linear regression fitting of a single hyperbola (Eqn 3) to the data:
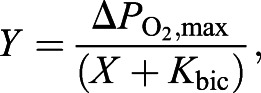 |
(3) |
where Y is ΔPO2, ΔPO2,max is the ΔPO2 at saturating bicarbonate concentrations (plateau) and X is [NaHCO3] in mmol l−1. This analysis provided the apparent affinity constant for the allosteric binding of bicarbonate to Hb (Kbic, the bicarbonate concentration at half-bicarbonate saturation).
Statistical analysis
Statistical analysis and curve fitting were performed using SigmaPlot version 12 (SigmaStat) and Prism V.8 (GraphPad). The obtained parameters SO2,min and KCO2 were statistically compared by two-way ANOVA considering CO2 and Hb fraction as factors. The KCO2 values obtained for the Hb fractions were statistically compared by t-test. Similarly, the ΔPO2,max and Kbic values obtained for the Hb fractions were statistically compared by t-test. Statistical significance was considered at α<0.05. Data are given as means±1 s.e.m. (corresponding 95% confidence intervals, CI, are provided in Tables S1 and S2).
RESULTS
Reversible effects of CO2 on hemolysate oxygenation
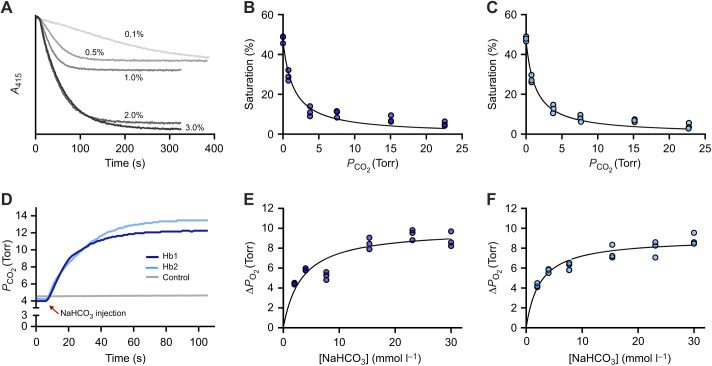
To investigate the mode of allosteric regulation of crocodilian Hb, we first tested whether the CO2 effect on O2 saturation of Hb could be attributed exclusively to bicarbonate, as originally proposed by Bauer and co-workers (1981). In these experiments, we used stripped hemolysates of spectacled caiman (N=3 individuals) and 13 other crocodilian species representing the two most speciose families, Alligatoridae and Crocodylidae, which together capture the deepest evolutionary split among all living crocodilians. We used a kinetic approach originally developed by us to demonstrate that hagfish Hb is sensitive to bicarbonate, but insensitive to CO2 (Fago et al., 1999). This method takes advantage of the presence of active endogenous carbonic anhydrase in the hemolysate that rapidly converts added CO2 to bicarbonate, and of the carbonic anhydrase inhibitor acetazolamide that specifically inhibit this fast enzymatic reaction. Thus, with active carbonic anhydrase, a fast decrease of Hb O2 saturation upon addition of CO2 means that either CO2 or bicarbonate can act as allosteric effectors, whereas with carbonic anhydrase inhibited by acetazolamide, a slower decrease of O2 saturation upon addition of CO2 indicates that CO2 must first be converted to bicarbonate (via the slow non-catalyzed reaction) to be able to affect oxygenation and that bicarbonate – not CO2 – is the true allosteric effector (Fago et al., 1999). As shown in Fig. 1, at a fixed PO2 (yielding ∼50% O2 saturation), addition of 1% CO2 in the gas mixture caused a rapid and strong decrease in the Hb O2 saturation down to ∼10% in the stripped hemolysate of spectacled caiman (Fig. 1A). This CO2-mediated decrease in Hb O2 saturation was fully reversible, and 50% saturation was restored when CO2 was removed from the gas mixture (Fig. 1A). Remarkably, the decrease in Hb-O2 saturation upon addition of CO2 was equally fast when endogenous carbonic anhydrase was inhibited by acetazolamide (Fig. 1B). We also observed the same response in the hemolysates of other crocodilian species (Fig. S1).
Fig. 1.
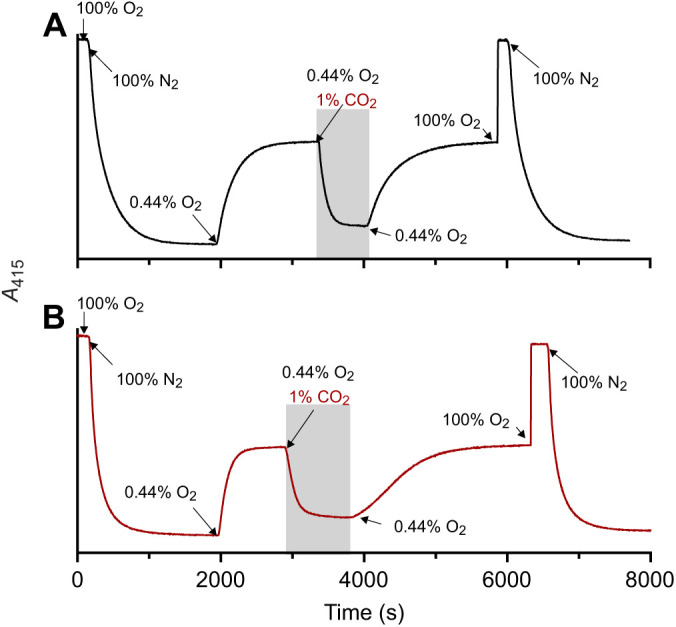
Reversibility of CO2 effect on O2 saturation in the stripped hemolysate of the spectacled caiman. Representative absorbance traces (absorbance at 415 nm; range ∼0.3–0.8) in the absence (A) and presence (B) of acetazolamide monitored by the diffusion chamber technique. After equilibration with 100% O2 (100% O2 saturation) and 100% N2 (0% O2 saturation), the hemolysate was equilibrated with 0.44% O2 to achieve approximately 50% O2 saturation before equilibration with 1% CO2, 0.44% O2, as indicated by the shaded area. Changes in the percentage gas in the mixture are indicated by arrows. Traces are representative of one sample out of N=3 biological replicates.
The fact that the recovery to ∼50% O2 saturation upon removal of CO2 was slower in the presence of acetazolamide (Fig. 1; Fig. S1) was puzzling and could indicate (1) that carbonic anhydrase was involved in the dissociation of Hb-bound CO2, perhaps by direct interaction with Hb, or (2) that acetazolamide could somehow interfere with the dissociation of CO2 from Hb. The next set of experiments was aimed to test these possibilities. The addition of bovine carbonic anhydrase II to spectacled caiman hemolysate produced similar traces to those of Fig. 1 (Fig. S2), indicating that endogenous carbonic anhydrase was indeed present in the hemolysate.
Functionally identical Hb fractions differing in molecular mass
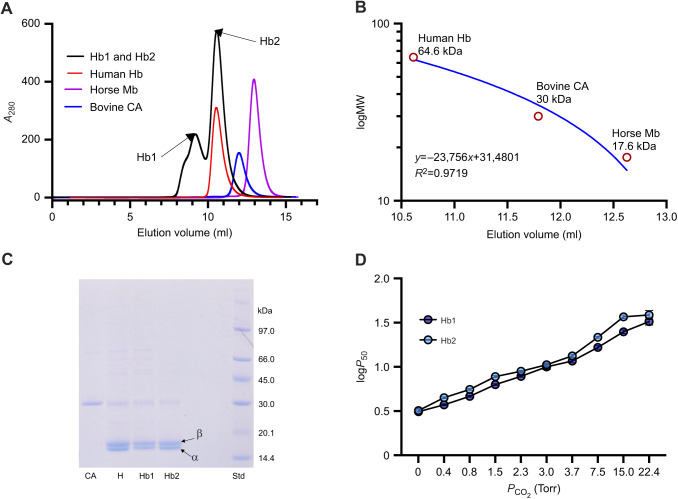
In order to determine whether endogenous carbonic anhydrase was somehow involved in the reversibility of the CO2-mediated effect on Hb oxygenation, we passed spectacled caiman hemolysate through a gel-filtration column to remove carbonic anhydrase. Spectacled caiman expresses a single adult Hb isoform (Jelkmann and Bauer, 1980), similar to all other crocodilians examined (Fago et al., 2020; Hoffmann et al., 2018). Results from gel filtration showed differences in the apparent molecular mass of Hb, with some Hb assembled into a higher-order aggregate – presumably an octamer – with an apparent molecular mass of 95.7 kDa, while most Hb was present as a regular tetramer with an apparent molecular mass of 62.7 kDa (Fig. 2A,B), as reported earlier in the same species (Bauer and Jelkmann, 1977). We named these Hb fractions Hb1 and Hb2, respectively. Analysis of Hb1 and Hb2 by SDS-PAGE under reducing and non-reducing conditions (Fig. 2C and Fig. S3, respectively) showed identical patterns and intensity of bands, indicating that high-order aggregates are not formed via disulfide bonds as in the Hb aggregates of other crocodilians (Weber et al., 2013) and non-avian reptiles (Petersen et al., 2017; Storz et al., 2015), but via some other interaction.
Fig. 2.
FPLC purification, SDS-PAGE and functional analysis of hemoglobin (Hb) fractions of spectacled caiman. (A) Elution profile of caiman hemolysate (absorbance at 280 nm; representative of one sample out of N=3 biological replicates), resolving in two peaks (Hb1 and Hb2) indicated by arrows, is shown superimposed on that of standard proteins with known molecular mass: human Hb (64.6 kDa), horse myoglobin (Mb, 17.6 kDa) and bovine carbonic anhydrase II (CA, 30 kDa). (B) Calibration curve by regression analysis for molecular weight (MW, kDa) against elution volume. (C) SDS PAGE under reducing conditions (10 mmol l−1 DTT) of (left to right) bovine CA II, spectacled caiman hemolysate (H), purified Hb1, purified Hb2 and standard protein (Std) as indicated. The bands corresponding to α- and β-type chains in the hemolysate and in the two purified Hb fractions are indicated. (D) logP50 measured at various PCO2 values, showing an identical effect of CO2 on the O2 affinity of purified Hb1 and Hb2 fractions (N=3).
SDS-PAGE of Hb1 and Hb2 revealed not only the α- and β-type Hb chains but also less intense bands of higher molecular mass that could correspond to carbonic anhydrase, having a molecular mass of 30 kDa (Fig. 2C). The identity of these bands, corresponding to a size range of ∼25–50 kDa, was established by mass spectrometry. The ∼30 kDa band corresponded to stable intact heterodimers (Hb α- and β-chains), possibly associated with aggregate formation of Hb, and the ∼42 kDa band corresponded to actin protein. Thus, carbonic anhydrase had been effectively removed from both Hb1 and Hb2 by gel filtration.
As a next step, we investigated the reversibility of CO2 binding on O2 saturation for the purified Hb1 and Hb2 fractions in the absence and presence of acetazolamide (Fig. S4). As with the stripped hemolysate (Fig. 1), return to the original O2 saturation upon removal of CO2 was slower in the presence of acetazolamide. This result suggests that acetazolamide somehow interferes with Hb–CO2 dissociation, but not with Hb–CO2 binding.
To establish whether purified Hb1 and Hb2 had different O2 binding affinities and CO2 sensitivities, we measured O2 equilibrium curves under identical buffer conditions at different PCO2 values. These experiments yielded similar P50 at a range of PCO2 values for the two Hb fractions (Fig. 2D) and similar cooperativity coefficients (n ∼1.0–1.8), indicating that the assembly of Hb into higher-order aggregates did not alter the oxygenation properties and CO2 sensitivity.
CO2 apparent binding curves and affinity of Hb fractions
We used the diffusion chamber technique to determine the apparent CO2 affinity of the purified Hb1 and Hb2 fractions (Fig. 3A–C). These experiments measured the decrease in O2 saturation at different PCO2 values, while maintaining a fixed PO2 roughly corresponding to the P50 of each Hb measured in the absence of CO2 (Fig. 3A). The Hb1 and Hb2 fractions exhibited changes in O2 saturation upon addition of CO2 that were not significantly different from each other (two-way ANOVA, P=0.543), so the data were pooled. These data showed a single hyperbolic relationship (Fig. 3B,C), indicating the presence of a single type of binding site for CO2 in each Hb, with apparent affinities (expressed as PCO2) corresponding to 1.48±0.22 Torr (95% CI, 1.10–1.96 Torr; Table S1; where 1 Torr=0.133 kPa) and 1.32±0.15 Torr (95% CI, 1.06–1.66 Torr; Table S1) for Hb1 and Hb2, respectively, that were not significantly different from each other (t-test, P=0.570). The use of a double hyperbola equation did not narrow the confidence interval of the fitted parameters (Table S1), so the data support the existence of a single type of binding site for CO2 on caiman Hb.
Fig. 3.
Effect of CO2 and bicarbonate on the O2 saturation of purified Hb1 and Hb2 from spectacled caiman and apparent CO2 and bicarbonate binding curves. (A) Representative traces measured using the thin-layer diffusion chamber of the decrease in Hb saturation induced by changes in PCO2 at a fixed PO2 close to P50 (0.44% O2, PO2 ∼3.3 Torr). (B,C) Apparent CO2 saturation curves of (B) Hb1 and (C) Hb2 purified from N=3 individuals. Non-linear regression fitting of a single hyperbola equation is shown (details and fitted parameters are in Table S1). (D) Representative traces (one sample out of N=3) showing the increase in O2 released from Hb1 and Hb2 upon injection of bicarbonate at a fixed PO2 of ∼4.2 Torr. The gray line represents a control trace in which bicarbonate was added to buffer alone. (E,F) Apparent bicarbonate saturation curves of (E) Hb1 and (F) Hb2 obtained as shown in D. Non-linear regression fitting of a single hyperbola is shown (details and fitted parameters are in Table S2).
Bicarbonate apparent binding curves and affinity of Hb fractions
To verify experimentally the direct allosteric effect of bicarbonate, we used purified Hb1 and Hb2 fractions of spectacled caiman in a Tucker chamber, equipped with an O2 probe. In these experiments, we kept the Hb solution at a fixed PO2 (close to the P50) and measured the increase in PO2 in the chamber following the injection of a measured small volume of a bicarbonate stock solution that was maintained at the same PO2 (Fig. 3D–F). The results of these experiments show that bicarbonate had a dose-dependent effect on O2 release from Hb following a simple hyperbolic function, indicating the existence of a single type of allosteric binding site. Injection of bicarbonate solutions in buffer alone did not increase PO2 in the chamber (Fig. 3D). From the bicarbonate binding curves, the apparent bicarbonate affinities were 3.31±0.35 mmol l−1 (95% CI, 1.89–5.49 mmol l−1; Table S2) and 2.48±0.16 mmol l−1 (95% CI, 1.72–3.45 mmol l−1; Table S2) for Hb1 and Hb2, respectively, which are not significantly different from each other (P=0.094). Interestingly, the apparent affinity for bicarbonate of spectacled caiman Hb in the deoxy T-state reported by Bauer and co-workers (1981) was ∼0.7 mmol l−1, close to our estimated values at the same pH and temperature.
DISCUSSION
This study provides the first experimental evidence that bicarbonate binds directly to crocodilian Hb and promotes O2 delivery (Fig. 3), independently of CO2. In addition, this study demonstrates that CO2 binds to crocodilian Hb directly without being converted to bicarbonate, as shown by the fast and acetazolamide-independent decrease in Hb O2 saturation upon addition of CO2 (Fig. 1; Fig. S1). This indicates that CO2 is a potent allosteric effector of crocodilian Hbs in its own right, in contrast with the original study by Bauer and coworkers (1981) performed under different experimental conditions, including the use of chloride-containing solutions. Also, the fact that the CO2-induced decrease in Hb O2 saturation was of the same magnitude in the presence and absence of acetazolamide (Fig. 1; Fig. S1) indicates that there is no additional binding of bicarbonate (which would form rapidly without acetazolamide) elsewhere on the protein. Thus, the allosteric effect of bicarbonate occurs via the binding of bicarbonate to the same protein binding site for CO2.
A structural model for CO2 and bicarbonate allosteric binding
In analogy with other Hbs, we propose that CO2 chemically binds to the crocodilian Hb protein to form a carbamino compound (-NH-COO−) at a non-protonated amino group (-NH2) (see reaction scheme in Fig. 4A). The negatively charged carbamino group would then be stabilized by nearby positively charged amino acid residues on a specific T-state binding site (Fig. 4B), thereby causing a decrease in the O2 affinity of Hb and promoting O2 unloading. As pH does not change upon addition of CO2, as reported here and earlier (Jensen et al., 1998), the proton generated in carbamino formation (Fig. 4A) is picked up by the protein molecule, likely contributing to further electrostatic stabilization of the negative carbamino derivative. We note that there is very little structural difference between covalent CO2 binding via carbamino formation and non-covalent bicarbonate binding to the Hb protein (Fig. 4B), consistent with the inference from our results that the two allosteric effectors bind with high affinity (Fig. 3) to the same site (Fig. 1). Thus, the degree of site occupancy by either CO2 or bicarbonate depends on their relative concentrations. In addition, protein binding of CO2 but not of bicarbonate requires an unprotonated amino group (Fig. 4B), and should be favored by an increase in pH (which, however, increases bicarbonate concentration). These aspects should be investigated further once the binding site is known. It is possible that the evolution of a particularly strong interaction with CO2 in crocodilian Hbs could have caused the strong interaction with bicarbonate ions, which characterize these Hbs. Hagfish Hb, whose function is not regulated by T–R allosteric transitions (Fago et al., 2001), is thus the only one among chordates to be sensitive to bicarbonate but not CO2 (Fago et al., 1999).
Fig. 4.
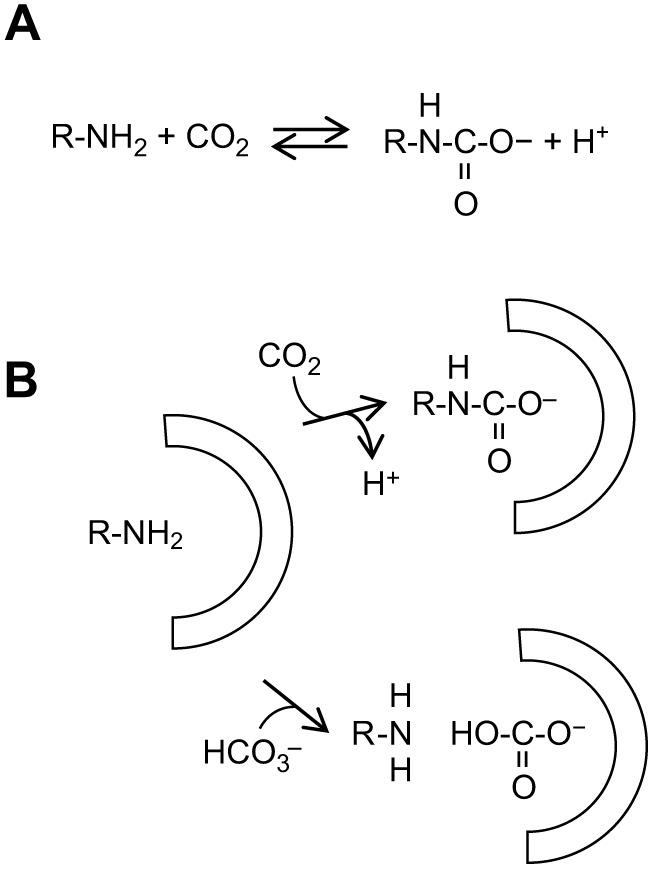
Proposed mechanistic model of the observed CO2- and bicarbonate-dependent changes in Hb oxygenation in crocodilians. (A) General scheme of carbamino protein derivative formation (R-NH-COO−) at a non-protonated amino group (R-NH2) from CO2. (B) Identical putative binding site for CO2 (top) and bicarbonate (bottom) on the T-state of crocodilian Hb, including a reactive uncharged amino group surrounded by positively charged residues (indicated by the half-circle). The negatively charged carbamino formed at the amino group (top) is stabilized by the positively charged amino acid residues, stabilizing the T-state and causing a decrease in O2 saturation. The proton generated in carbamino formation is picked up by the protein molecule, likely contributing to further electrostatic stabilization of the carbamino. Note the very small structural difference between covalent CO2 binding via carbamino formation (top) and non-covalent bicarbonate binding (bottom) to the protein.
Consistent with previous studies (Bauer et al., 1981; Perutz et al., 1981), our results (Fig. 3) suggest the existence of a single physical binding site for CO2 and bicarbonate in the T-state of crocodilian Hb or a pair of identical binding sites due to the two-fold symmetry of the Hb tetramer. We propose that this binding site (or pair of identical sites) comprises a reactive non-protonated amino group close to positively charged residues able to chemically bind CO2. Carbamino formation in human Hb occurs at the α- and β-subunit N-termini (Bauer and Schröder, 1972; Kilmartin and Rossi-Bernardi, 1971; Perrella et al., 1975), but in crocodilian Hbs, these groups are acetylated to a variable extent without affecting the strong sensitivity to CO2 (Fago et al., 2020; Natarajan et al., 2020). In the original structural model proposed by Perutz and co-workers (1981), two bicarbonate ions bind two identical sites lining the central cavity of T-state crocodilian Hb, where organic phosphates normally bind, comprising Lys82β, Glu144β and the N-terminus of the α subunit. However, the smooth-fronted caiman (Paleosuchus trigonatus) possesses Gln at 82β rather than positively charged Lys (Fago et al., 2020), and yet the Hb of this species exhibits the same CO2/bicarbonate effect as that of other crocodilians (Fig. S1). Moreover, acetylation of the α-chain N-termini of crocodilian Hbs does not appear to affect CO2 sensitivity (Natarajan et al., 2020). Based on site-directed mutagenesis of recombinant human Hb, other studies (Komiyama et al., 1996, 1995) have proposed a more complex mechanism, involving numerous residues at the α1β2 interface of the Hb tetramer, in particular, Lys38β, which is present in all crocodilian Hbs. Molecular modeling has revealed numerous positively charged residues in the central cavity of alligator Hb, including Arg135β involved in several interactions (Fago et al., 2020). In the vicinity of other positively charged residues, the amino group of Lys or Arg side chains, which normally have high pKa values and are protonated, would be largely in the non-protonated form at physiological pH values and would therefore be available for carbamino formation. Mutagenesis studies using recombinant crocodile Hb, rather than human Hb, are underway in our lab to resolve these possibilities. Although the residues involved remain to be identified, it appears from our study that there is great diversity among chordate Hbs in the allosteric control of Hb function by CO2 and bicarbonate, with Hbs sensitive to only CO2 (e.g. human Hb), only bicarbonate (hagfish) or a combination of the two (crocodile).
Physiological implications
This study aimed to unravel CO2 and bicarbonate binding to caiman Hb under in vitro controlled buffer conditions without the confounding effect of organic phosphates and chloride ions, which are normally present in crocodile RBCs. As an intended consequence, the experimental conditions in our study did not mimic the in vivo conditions within intact RBCs. In a recent study on caiman blood (Bautista et al., 2021), we found progressive accumulation of CO2 and bicarbonate in RBCs during diving, and that most of the RBC bicarbonate was bound to Hb rather than exported to the plasma. Most importantly, Hb-bound bicarbonate was inversely correlated with the degree of Hb O2 saturation in the blood (Bautista et al., 2021), which is fully consistent with the binding of bicarbonate to the T-state of the Hb described in the present study. Although the direct CO2 binding to Hb was not measured (Bautista et al., 2021), the progressive increase of CO2 in RBCs during diving is strongly consistent with our present demonstration that Hb acts as a sink for CO2 as Hb O2 saturation decreases. Physiologically, the strong effect of blood CO2 and bicarbonate on Hb oxygenation during diving is to facilitate O2 delivery to sustain aerobic metabolism of tissues. At the same time, Hb desaturation facilitates CO2 transport in the venous blood as protein-bound CO2 and bicarbonate. A study on blood properties from digesting alligators (Busk et al., 2000) proposed that the increase in plasma bicarbonate (the ‘alkaline tide’) should safeguard tissue O2 delivery by allosterically reducing Hb–O2 affinity, thereby helping to sustain the large increase in metabolic rate during digestion. However, whether CO2 and bicarbonate content within RBCs also increases during digestion remains to be investigated. In addition, the possibility of right-to-left shunts in the unique crocodilian heart adds further complexity in these situations through their influence O2 and CO2/bicarbonate concentrations in the blood (Conner et al., 2019; Malte et al., 2016).
Conclusions
This is the first study of crocodilian Hb to quantify the direct allosteric binding of both CO2 and bicarbonate. Both effectors bind to T-state Hb with high affinity at the same positively charged site involving a reactive amino group. Physiologically, the strong Hb sensitivity to CO2 and bicarbonate provides the basis for a unique linkage between O2 and CO2 transport in the blood of crocodilians, where the build-up of CO2 and bicarbonate ions during breath-hold diving or digestion facilitates O2 delivery to tissues to sustain aerobic metabolism. These distinct effects of CO2 and bicarbonate on Hb–O2 affinity are shared by a phylogenetically diverse set of caiman, alligator and crocodile species, indicating that these unique regulatory properties evolved prior to the diversification of extant crocodilians >80–100 million years ago in the late Cretaceous, but after the stem lineage of crocodilians diverged from the ancestors of dinosaurs and modern birds.
Supplementary Material
Acknowledgements
We thank Elin E. Petersen and Marie Skou Pedersen for their technical assistance. We also would like to thank Rene Hedegaard and the staff from the Krokodille Zoo (Eskilstrup, Denmark) for assistance in blood sampling. We also thank Vikas Kumar (Mass Spectrometry and Proteomics Core Facility, University of Nebraska Medical Center) for assistance with the MS/MS data analysis.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: J.F.S., A.F.; Methodology: H.M., A.F.; Validation: H.M., A.F.; Formal analysis: N.M.B., H.M., C.N.; Investigation: N.M.B., C.N.; Resources: H.M., T.W., J.F.S., A.F.; Writing - original draft: N.M.B., A.F.; Writing - review & editing: N.M.B., H.M., C.N., T.W., J.F.S., A.F.; Visualization: N.M.B.; Supervision: A.F.; Project administration: A.F.; Funding acquisition: H.M., T.W., J.F.S., A.F.
Funding
This research was supported by funding from the National Institutes of Health (HL087216) (J.F.S. and A.F.), the National Science Foundation (OIA-1736249) (J.F.S.), and Danmarks Frie Forskningsfond (T.W. and H.M.). Deposited in PMC for release after 12 months.
References
- Bauer, C. and Jelkmann, W. (1977). Carbon dioxide governs the oxygen affinity of crocodile blood. Nature 269, 825-827. 10.1038/269825a0 [DOI] [PubMed] [Google Scholar]
- Bauer, C. and Schröder, E. (1972). Carbamino compounds of haemoglobin in human adult and foetal blood. J. Physiol. 227, 457-471. 10.1113/jphysiol.1972.sp010042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, C., Forster, M., Gros, G., Mosca, A., Perrella, M., Rollema, H. S. and Vogel, D. (1981). Analysis of bicarbonate binding to crocodilian hemoglobin. J. Biol. Chem. 256, 8429-8435. 10.1016/S0021-9258(19)68861-7 [DOI] [PubMed] [Google Scholar]
- Bautista, N. M., Damsgaard, C., Fago, A. and Wang, T. (2021). Carbon dioxide and bicarbonate accumulation in caiman erythrocytes during diving. J. Exp. Biol. 224, 1-6. 10.1242/jeb.242435 [DOI] [PubMed] [Google Scholar]
- Busk, M., Overgaard, J., Hicks, J. W., Bennett, A. F. and Wang, T. (2000). Effects of feeding on arterial blood gases in the American alligator Alligator mississippiensis. J. Exp. Biol. 203, 3117-3124. 10.1242/jeb.203.20.3117 [DOI] [PubMed] [Google Scholar]
- Cadiz, L., Bundgaard, A., Malte, H. and Fago, A. (2019). Hypoxia enhances blood O2 affinity and depresses skeletal muscle O2 consumption in zebrafish (Danio rerio). Comp. Biochem. Physiol. B 234, 18-25. 10.1016/j.cbpb.2019.05.003 [DOI] [PubMed] [Google Scholar]
- Conner, J. L., Crossley, J. L., Elsey, R., Nelson, D., Wang, T. and Crossley, D. A. (2019). Does the left aorta provide proton-rich blood to the gut when crocodilians digest a meal? J. Exp.Biol. 222, jeb201079. 10.1242/jeb.201079 [DOI] [PubMed] [Google Scholar]
- Fago, A., Malte, H. and Dohn, N. (1999). Bicarbonate binding to hemoglobin links oxygen and carbon dioxide transport in hagfish. Resp. Physiol. 115, 309-315. 10.1016/S0034-5687(98)00102-9 [DOI] [PubMed] [Google Scholar]
- Fago, A., Giangiacomo, L., D'Avino, R., Carratore, V., Romano, M., Boffi, A. and Chiancone, E. (2001). Hagfish hemoglobins: Structure, function, and oxygen-linked association. J. Biol. Chem. 276, 27415-27423. 10.1074/jbc.M100759200 [DOI] [PubMed] [Google Scholar]
- Fago, A., Natarajan, C., Pettinati, M., Hoffmann, F. G., Wang, T., Weber, R. E., Drusin, S. I., Issoglio, F., Martí, M. A., Estrin, D.et al. (2020). Structure and function of crocodilian hemoglobins and allosteric regulation by chloride, ATP, and CO2. Am. J. Physiol. Regul. Integr. Comp. Physiol. 318, R657-R667. 10.1152/ajpregu.00342.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks, J. W., White, F. N. (1992). Pulmonary gas exchange during intermittent ventilation in the American alligator. Resp. Physiol. 88, 23-46. 10.1016/0034-5687(92)90026-S [DOI] [PubMed] [Google Scholar]
- Hoffmann, F. G., Vandewege, M. W., Storz, J. F. and Opazo, J. C. (2018). Gene turnover and diversification of the α- and β-globin gene families in sauropsid vertebrates. Genome Biol. Evol. 10, 344-358. 10.1093/gbe/evy001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janecka, J. E., Nielsen, S. S. E., Andersen, S. D., Hoffmann, F. G., Weber, R. E., Anderson, T., Storz, J. F. and Fago, A. (2015). Genetically based low oxygen affinities of felid hemoglobins: lack of biochemical adaptation to high-altitude hypoxia in the snow leopard. J. Exp. Biol. 218, 2402-2409. 10.1242/jeb.125369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelkmann, W. and Bauer, C. (1980). Oxygen binding properties of caiman blood in the absence and presence of carbon dioxide. Comp. Biochem. Physiol. A 65, 331-336. 10.1016/0300-9629(80)90037-7 [DOI] [Google Scholar]
- Jendroszek, A., Malte, H., Overgaard, C. B., Beedholm, K., Natarajan, C., Weber, R. E., Storz, J. F. and Fago, A. (2018). Allosteric mechanisms underlying the adaptive increase in hemoglobin–oxygen affinity of the bar-headed goose. J. Exp. Biol. 221, jeb185470. 10.1242/jeb.185470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, F. B., Wang, T., Jones, D. R. and Brahm, J. (1998). Carbon dioxide transport in alligator blood and its erythrocyte permeability to anions and water. Am. J. Physiol. Regul. Integr. Comp. Physiol. 274, R661-R671. 10.1152/ajpregu.1998.274.3.R661 [DOI] [PubMed] [Google Scholar]
- Kilmartin, J. V. and Rossi-Bernardi, L. (1971). The binding of carbon dioxide by horse haemoglobin. Biochem. J. 124, 31-45. 10.1042/bj1240031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama, N. H., Miyazaki, G., Tame, J. and Nagai, K. (1995). Transplanting a unique allosteric effect from crocodile into human haemoglobin. Nature 373, 244-246. 10.1038/373244a0 [DOI] [PubMed] [Google Scholar]
- Komiyama, N., Tame, J. and Nagai, K. (1996). A hemoglobin-based blood substitute: transplanting a novel allosteric effect of crocodile Hb. Biol. Chem. 377, 543-548. 10.1515/bchm3.1996.377.9.543 [DOI] [PubMed] [Google Scholar]
- Malte, C. L., Malte, H., Reinholdt, L. R., Findsen, A., Hicks, J. W. and Wang, T. (2016). Right-to-left shunt has modest effects on CO2 delivery to the gut during digestion, but compromises oxygen delivery. J. Exp. Biol. 220, 531-536. 10.1242/jeb.149625 [DOI] [PubMed] [Google Scholar]
- Natarajan, C., Jendroszek, A., Kumar, A., Weber, R. E., Tame, J. R. H., Fago, A. and Storz, J. F. (2018). Molecular basis of hemoglobin adaptation in the high-flying bar-headed goose. PLoS Genet. 14, 1-19. 10.1371/journal.pgen.1007331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan, C., Signore, A. V., Kumar, V., Weber, R. E., Fago, A. and Storz, J. F. (2020). Effect of NH2-terminal acetylation on the oxygenation properties of vertebrate haemoglobin. Biochem. J. 477, 3839-3850. 10.1042/BCJ20200623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrella, M., Bresciani, D. and Rossi-Bernardi, L. (1975). The binding of CO2 to human hemoglobin. J. Biol. Chem. 250, 5413-5418. 10.1016/S0021-9258(19)41197-6 [DOI] [PubMed] [Google Scholar]
- Perutz, M. F. (1976). Structure and mechanisms of haemoglobin. Br. Med. Bull. 32, 195-208. 10.1093/oxfordjournals.bmb.a071363 [DOI] [PubMed] [Google Scholar]
- Perutz, M. F., Bauer, C., Gros, G., Leclercq, F., Vandecasserie, C., Schnek, A. G., Braunitzer, G., Friday, A. E. and Joysey, K. A. (1981). Allosteric regulation of crocodilian haemoglobin. Nature 291, 682-684. 10.1038/291682a0 [DOI] [PubMed] [Google Scholar]
- Petersen, A. G., Petersen, S. V., Frische, S., Drakulic, S., Golas, M. M., Sander, B. and Fago, A. (2017). Hemoglobin polymerization via disulfide bond formation in the hypoxia-tolerant turtle Trachemys scripta: implications for antioxidant defense and O2 transport. Am. J. Physiol. Regul. Integr. Comp. Physiol. 314, R84-R93. 10.1152/ajpregu.00024.2017 [DOI] [PubMed] [Google Scholar]
- Shevchenko, A., Tomas, H., Havli, J., Olsen, J. V. and Mann, M. (2006). In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 1, 2856-2860. 10.1038/nprot.2006.468 [DOI] [PubMed] [Google Scholar]
- Storz, J. F. (2019). Hemoglobin: Insights into Protein Structure, Function, and Evolution, pp. 1-233. Oxford University Press. [Google Scholar]
- Storz, J. F., Natarajan, C., Moriyama, H., Hoffmann, F. G., Wang, T., Fago, A., Malte, H., Overgaard, J. and Weber, R. E. (2015). Oxygenation properties and isoform diversity of snake hemoglobins. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R1178-R1191. 10.1152/ajpregu.00327.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz, J. F., Natarajan, C., Grouleff, M. K., Vandewege, M., Hoffmann, F. G., You, X., Venkatesh, B. and Fago, A. (2020). Oxygenation properties of hemoglobin and the evolutionary origins of isoform multiplicity in an amphibious air-breathing fish, the blue-spotted mudskipper (Boleophthalmus pectinirostris). J. Exp. Biol. 223, jeb217307. 10.1242/jeb.217307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker, V. A. (1967). Method for oxygen content and dissociation curves on microliter blood samples. J. Appl. Physiol. 23, 410-414. 10.1152/jappl.1967.23.3.410 [DOI] [PubMed] [Google Scholar]
- Van Assendelft, O. W. (1970). Spectrophotometry of Haemoglobin Derivatives. Assen, The Netherlands: Royal Vangorcum Ltd. [Google Scholar]
- Weber, R. E. and Fago, A. (2004). Functional adaptation and its molecular basis in vertebrate hemoglobins, neuroglobins and cytoglobins. Resp. Physiol. Neurobiol. 144, 141-159. 10.1016/j.resp.2004.04.018 [DOI] [PubMed] [Google Scholar]
- Weber, R. E., Fago, A., Malte, H., Storz, J. F. and Gorr, T. A. (2013). Lack of conventional oxygen-linked proton and anion binding sites does not impair allosteric regulation of oxygen binding in dwarf caiman hemoglobin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305, R300-R312. 10.1152/ajpregu.00014.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, R. E., Jarvis, J. U. M., Fago, A. and Bennett, N. C. (2017). O2 binding and CO2 sensitivity in haemoglobins of subterranean African mole rats. J. Exp. Biol. 220, 3939-3948. 10.1242/jeb.160457 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.