Abstract
Chitosan scaffolds crosslinked by current methods insufficiently meet the demands of bone tissue engineering applications. We developed a novel effective crosslinking technique by using the natural and safe vanillin together with bioglass microparticles to generate an antibacterial, osteoconductive, and mechanically robust 3D porous chitosan-vanillin-bioglass (CVB) scaffold. In addition to the significantly improved mechanical properties, the CVB scaffolds had high porosity (>90%) and interconnected macroporous structures. Our data suggested that the crosslinking mainly resulted from the Schiff base reactions between the aldehydes of vanillin and amines of chitosan, together with the hydrogen and ionic bonds formed within them. Importantly, the CVB scaffolds not only showed good biocompatibility, bioactivity, and strong antibacterial ability but also significantly promoted osteoblastic differentiation, mineralization in vitro, and ectopic bone formation in vivo. Thus, the CVB scaffolds hold great promise for bone tissue engineering applications based on their robust mechanical properties, osteoconductivity, and antibacterial abilities.
Keywords: Chitosan, Schiff base, Bone tissue engineering, Bioglass, Vanillin, Antibacterial
Graphical Abstract
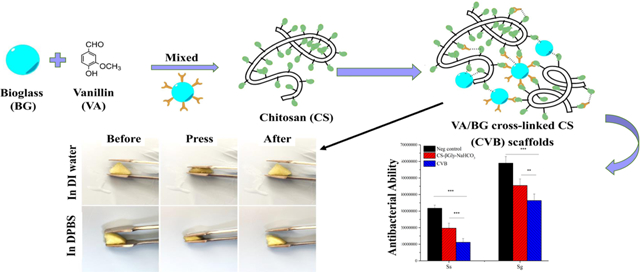
Introduction
Repair of the large bone defect remains a clinical challenge, due to the limited availability of autogenous bones (Guerado, & Caso, 2017; Verrier et al., 2016; Wang, Wu, Zhuo, & Cheng, 2014). One promising alternative strategy, scaffold-based bone tissue engineering, has seen increasing interest among researchers (Amini, Laurencin, & Nukavarapu, 2012). Therefore, it has been the central mission to develop an osteopromotive scaffold that determines the success of bone regeneration.
Chitosan (CS) has been widely used in different industries, e.g., water treatment (Wang, & Zhuang, 2017; Zhuang, Yin, & Wang, 2018). The excellent features of CS, including biocompatibility, biodegradability, and inherent anti-microbial ability have made it one of the most intriguing polysaccharides for biomedical applications, which has been successfully used in wound dressings (Liu et al., 2013; Murugan, & Ramakrishna, 2004; Song et al., 2018). Surprisingly, the clinical applications of chitosan in bone repair have been significantly impeded (very few are approved by the FDA), although numerous preclinical studies have been reported (Ahmed, Annu, Ali, & Sheikh, 2018; Bombaldi de Souza et al., 2020). In addition to poor osteoconductivity (Aguilar et al., 2019), the main limiting factor is the mechanical property/stability of chitosan, which requires strong crosslinking/blending with other materials to support tissue regeneration, even for non-loading bone repair (e.g., oral and craniofacial bone) (Aguilar et al., 2019). Glutaraldehyde is the most frequently used crosslinking agent for chitosan. However, glutaraldehyde is cytotoxic, in addition to many side effects of glutaraldehyde exposure, e.g., asthmatic symptoms, rhinitis, and skin irritation (Umashankar, Mohanan, & Kumari, 2012). Ionic crosslinkers (e.g., Cu2+ ions) (Lu et al., 2018) were also introduced for chitosan crosslinking, where the speed is difficult to control, and the mechanical properties are relatively low. Natural cross-linking agent genipin is an alternative crosslinker to glutaraldehyde with less cytotoxicity (Pinheiro, Cooley, Liao, Prabhu, & Elder, 2016). However, the reaction usually takes a few hours, and the characteristically dark blue color of the genipin cross-linked scaffolds may impede some practical applications (Sileika, Barrett, Zhang, Lau, & Messersmith, 2013). Therefore, it is critical to develop alternative methods to address current challenges for the application of chitosan in bone tissue engineering.
Vanillin (4-hydroxy-3-methoxy benzaldehyde), the primary component of vanilla bean extract, has been widely used as a flavoring agent in foods, beverages, and pharmaceuticals, and is generally recognized as safe (GRAS) (Ávila, Zougagh, Escarpa, & Ríos, 2007; Zou, Li, & Li, 2015). Recently, it has emerged as a promising natural non-toxic crosslinking agent for chitosan (Xu et al., 2018; Zou et al., 2015). The aldehyde group of vanillin and the amino groups of chitosan molecules can form a well-known Schiff base bond (Xu et al., 2018). However, a vanillin molecule has only one aldehyde group to form a Schiff base bond with chitosan, which deems it unable to form a stable network to achieve high mechanical properties. Therefore, most current applications of vanillin are limited to crosslinking microspheres and hydrogels with low mechanical properties (Xu et al., 2018; Zou et al., 2015), which are not suitable for bone tissue engineering applications. Bioactive glass (bioglass, BG) is a widely used biocompatible and osteoconductive inorganic material that can accelerate osteoblastic differentiation and bone formation (Brauer, 2015). Moreover, BG also can potentially prevent bacterial growth through the release of ions into its surroundings (Khvostenko, Hilton, Ferracane, Mitchell, & Kruzic, 2016), and induce alkalinization of the external medium (Laurent-Emmanuel Monfoulet et al., 2014; Zeng et al., 2016). It has been reported that higher pH favors imines formation for Schiff base reactions (Zeng et al., 2016). Thus, we hypothesize that biocompatible and biodegradable BG microparticles can promote vanillin-mediated Schiff reactions by increasing the pH of the microenvironment as well as promoting the hydrogen bonds and ionic bonds formed between vanillin and CS. In addition to the strong crosslinking-mediated high mechanical properties, we expected that BG would also further improve the osteoconductive and anti-microbial ability of the composite scaffold.
In this paper, we report for the first time, an innovative antibacterial 3D porous chitosanvanillin-BG (CVB) scaffolds based on vanillin and BG as the non-toxic and osteoconductive crosslinking agent. Our in vitro studies demonstrated that the CVB scaffolds have strong antibacterial ability, improved mechanical properties, and good osteoconductivity. Meanwhile, the in vivo data revealed that our CVB scaffolds strongly support BMP2-induced ectopic ossicle formation in mice.
2. Materials and Methods
2.1. Materials
Chitosan (Mw=100,000–300,000 Daltons, 85% deacetylated) was purchased from INDOFINE chemical company, Inc (Hillsborough, NJ, USA). Vanillin, ascorbic acid, β-Glycerophosphate salt, and ethanol were purchased from Sigma (St. Louis MO, USA). Bioglass (BG, 45%SiO2+24.5%Na2O+24.5%CaO+6%P2O5, >98%, 10μm) was bought from MO-SCI ONLINE (North Rolla, MO, USA). Bovine Serum Albumin (BSA) was purchased from Fisher Scientific (New Jersey, USA). Other chemical reagents were of analytical grade.
2.2. Chitosan-Vanillin-BG (CVB) scaffolds preparation
CVB hydrogels with a mass ratio (chitosan: vanillin: BG) of 32:16:2.5 were prepared as follows. Firstly, chitosan was dissolved in 1% (v/v) acetic acid to get a 4% (w/v) solution. Secondly, vanillin solution and BG solutions were mixed. Afterwards, 500 μl mixture was slowly added dropwise into 2 mL chitosan solution prepared above to induce gelation. The gelation time was around 5 to 7 min. Solutions with various mass ratios of chitosan: vanillin: BG were also prepared to screen the optimal gelling time and vanillin/BG concentration (Table. 1). The CVB scaffolds were prepared by freezing the CVB hydrogel at −80°C overnight followed by lyophilization for 48 hours. The fabrication of conventional ionic cross-linked CS-βGly-NaHCO3 hydrogel was also undertaken as a control (details in the Supporting Information).
Table 1.
Samples prepared with different concentrations of crosslink reagents
| Groups | CS (mg/mL) | CS/VA (w/w) | BG (mg/mL) | Gelation Time |
|---|---|---|---|---|
| A | 32 | 5:4 | 0 | After 24 hours |
| B | 32 | 5:0 | 5 | /** |
| C | 32 | 5:1 | 1 | /** |
| D | 32 | 5:1 | 2.5 | /** |
| E | 32 | 5:1 | 5 | At 20 min with obvious ↓*** |
| F | 32 | 5:2.5* | 1 | At 50 min |
| G | 32 | 5:2.5* | 2.5 | Within 7 min |
| H | 32 | 5:2.5* | 5 | Gelatinized during preparation process with ↓*** |
| I | 32 | 5:4 | 1 | Gelatinized during preparation process |
Vanillin (VA) was dissolved in 100% ETOH and the final concentration of vanillin in the gel was 16 mg/mL,
Unable to gelate,
BG precipitation.
2.3. Characterization of CVB composite scaffolds
2.3.1. Scanning electron microscopy (SEM) and Transmission electron microscopy (TEM)
CVB scaffolds were characterized by a Hitachi S-4800 Scanning Electron Microscopes with an installed IXRF EDS system for EDS imaging. Samples were sputter-coated with gold, imaged and analyzed according to a previous study (Hu, Miszuk, Stein, & Sun, 2020). The BG distribution in CVB scaffolds were detected by both SEM and TEM (JEOL JEM 1230, Japan).
2.3.2. Fourier transform infrared spectroscopy (FTIR)
Fourier transform infrared (FTIR) spectra of CVB composite scaffolds were recorded by a Nicolet™ iS50 FTIR spectrometer from 500 to 4000cm−1, using 32 scans at a resolution of 4cm−1. All the samples were prepared by potassium bromide (KBr)-disk method (details described in supplementary data).
2.3.3. Porosity
The porosity of CVB scaffolds was calculated by the liquid displacement method using a 100% ethanol bath of known volume according to the literature (Ho, & Hutmacher, 2006). Scaffold porosity was calculated from the following Equation (1):
| (1) |
where V1 is the initial known volume of 100% ethanol, V2 the volume sum of ethanol and submerged scaffolds, and V 3 the remaining volume of ethanol in the bath after removing scaffolds.
2.3.4. Mechanical Properties
The mechanical properties of the wet/dry CVB scaffolds were studied by atomic force microscopy (AFM) and compression tests, respectively, and the elastic modulus and compressive modulus were recorded.
AFM tests were carried out in a liquid cell at room temperature using a molecular force probe 3D AFM (Asylum Research, Santa Barbara, CA) according to a previous report (Lansakara, Tong, Bardeen, & Tivanski, 2020). The approach data of force-indentation curves were fit to the Hertzian elastic contact model to calculate Young’s modulus of both hydrated substrates.
The dry mechanical properties of both scaffolds were studied in compression using a Zwick/Roell 1445 RetroLine Material testing system (Zwick USA, Kennesaw, GA). Scaffolds size were measured carefully with an Electronic Digital Caliper. All the scaffolds were compressed to 50% of deformation at a 1 mm/min rate, with load cell of 10kN. The upper-force limit of the instrument was set as 25~30 N. The Young’s modulus of each sample was calculated from the slope/elastic part of the stress-strain curve.
2.3.5. Swelling test
Swelling studies were performed to determine the water absorption ability. All the scaffolds (with the size of 5×5×5mm) were immersed in DI water (Deionized water) at 37 °C. The initial dry weight of the scaffold was determined before immersion (Wi). After 24 h immersion, the scaffolds were taken out and the wet weight was recorded (Ws). The swelling ratio was evaluated using Equation (2):
| (2) |
2.3.6. In vitro Biodegradability
The in vitro bio-degradation of the scaffolds was studied in PBS at 37 °C. Scaffolds were immersed in PBS for 3, 7, 14, and 21 days. Initial weights of the scaffolds were obtained as Wi. After immersion, the scaffolds were washed in DI water and lyophilized. The dry weights were noted as Wd. The degradation ratio was calculated using Equation (3):
| (3) |
Degradation of BG was also studied using the PBS collected above (supplementary data, Fig. S2).
2.4. In vitro cell study and anti-microbial test
2.4.1. Cell culture and cell seeding
Mouse calvaria-derived pre-osteoblasts (MC3T3-E1, from ATCC) were cultured in Corning® T-75 flasks under a humidified atmosphere with 5% CO2 at 37 °C according to our previous report (Hu et al., 2020). Minimum Essential Medium Alpha (1X) (α-MEM, Gibco, Waltham, MA), supplemented with 10% fetal bovine serum (FBS), 100 μg/mL streptomycin sulfate, and 100 U/mL penicillin was used as growth medium (GM). To induce MC3T3-E1 cells osteoblastic differentiation, cells were cultured in an osteoconductive medium (OC, GM containing 50 μg/mL L-ascorbic acid and 2.5 mM β-glycerophosphate salt).
CVB scaffolds were first to cut into rectangles (5mm ×5mm ×1mm thickness), and the samples were then immersed in 70% ethanol for 45min followed by being washed with PBS 3 times. Subsequently, the scaffold was dried with sterile gauze before cells were seeded into the scaffold (1.5×105 cells per scaffold in a 24-well plate).
2.4.2. Cell viability and morphology
Cytotoxicity of the CVB scaffolds was quantitatively measured using a CellTiter 96® Aqueous One Solution Cell Proliferation Assay (MTS) according to the product instructions (Promega, Madison, WI, USA) on day 0, day 1, and day 3. Reacted medium of each sample was transferred into a 96-well plate and read at 490 nm using a SpectraMax M2e Microplate Reader (Molecular Devices, San Jose, CA, USA).
Cell morphology of MC3T3-E1 on day 1 was visualized by staining with fluorescent Phalloidin-FITC (Life Technologies, OR, USA) and DAPI (Sigma, St. Louis MO, USA) according to our previous report (Hu et al., 2020). Samples were then observed under confocal microscopy (Zeiss LSM 710, Germany).
2.4.3. Antibacterial test
Two bacterial species common in dental plaque [Streptococcus gordonii– (American Type Culture Collection (ATCC) 33399) and Streptococcus sanguinis– (ATCC 10556)] were chosen for testing the scaffold’s antibacterial properties. The bacteria were cultured on a blood agar plate at 37°C, 5% CO2, for 24h. Each species was then suspended in PBS to a final concentration of 1 × 105 CFU/ml. For culture plate preparation, the Brain Heart Infusion (BHI) agar while still liquid was mixed with 2% test materials, or mixed with 2% PBS as a control, and then poured into Petri dishes. The plates were inoculated with 10 μl aliquots from a 10-fold serial dilution of each bacterial test strain. Plates were incubated at 37°C, 5% CO2, for 48h. Colonies were counted and the colony forming units (CFU)/ml was calculated for each strain and condition.
2.5. In vitro osteoconductive capability of CVB scaffolds
2.5.1. In vitro bio-mineralization
CVB scaffolds were immersed in 10 times simulated body fluid (10×SBF) solution and then incubated at 37 °C in a closed Falcon tube for predetermined periods. The SBF solution was prepared according to the previously reported method (Chahal, Fathima, & Yusoff, 2014). After 3, 7, 14 and 21 days, the scaffolds were removed and washed three times with DI water, lyophilized and characterized using the SEM and EDS. X-ray diffraction (XRD, Rigaku SmartLab, USA) was used to determine crystallinity of the mineral on CVB scaffolds. The scaffolds were scanned from 2θ = 10° to 2θ = 90° using Cu-Kα radiation.
2.5.2. Alkaline phosphatase (ALP) activity
After 7 days of cell culture, the ALP activity was quantified with an EnzoLyte pNPP Alkaline Phosphatase Assay Kit (AnaSpec, San Jose, CA, USA), following our former protocol (Miszuk et al., 2018). After 30min incubation, the absorbance of samples was read at 405 nm and normalized against total protein content measured by a BCA kit (Thermo Scientific™, Waltham, MA, USA) according to the user guide.
2.5.3. Calcium content
Calcium deposition on the cell-scaffold constructs was evaluated by a total calcium LiquiColor® kit (Stanbio Laboratory, TX) according to our previous research work (Yao, Fuglsby, Zheng, & Sun, 2020) after 3 weeks of cell culture. The calcium content was calculated from the following Equation (4):
| (4) |
where Au and As are the absorbance values of sample and standard at 550 nm, respectively. 2.5.4. RNA extraction and quantitative real-time PCR.
The osteogenic biomarkers including Runt-related transcription factor 2 (RUNX2), osteocalcin (OCN), and bone sialoprotein (BSP) were analyzed on varied periods using real-time PCR according to our previous report (Hu et al., 2020). Gene primers of RUNX2 (qMmuCED0049270) and OCN (qMmuCED0041364) were purchased from Bio-Rad, while the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and BSP were synthesized at the University of Iowa (Table S1).
2.6. In vivo bone regeneration
Laboratory animals’ care and use were followed by the protocols approved by the office of the Institutional Animal Care and Use Committee (IACUC) of the University of Iowa. Inbred C57BL/6NHsd female mice (6 weeks, ENVIGO) were adopted for the study. Mice were shaved and an antiseptic (70% ethanol) was applied to the surgical area. As per our previous protocol, each mouse received a total of 4 scaffolds on their dorsal sides (Yao et al., 2020). CVB and gelfoam scaffolds were prepared with or without 2μg BMP2. Mice were euthanized 4 weeks after surgery, and retrieved ossicles were fixed in 10% neutral buffered formalin for 2 days and then transferred into cold 70% ethanol for further analysis.
4 weeks post-implantation, the radiographic analysis was performed on the fixed constructs using an In-Vivo Xtreme small animal imaging μCT system (Bruker, Billerica, MA, USA). Total Ossicle Volume (mm3) of each specimen was analyzed quantitively. After μCT analysis, the fixed ossicles were decalcified using 15% EDTA (Fisher Scientific, New Jersey, USA, pH adjusted to 7.2) solution for 2 days and then stored in 70% ethanol. After that, the samples were cut and stained with Hematoxylin and Eosin (H&E) for microscopic observation.
2.7. Statistical analysis.
Three independent experiments at least, unless otherwise stated, were performed and data were presented as mean ± standard deviation. The means for three or more parallel groups were analyzed by one-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001 were identified as statistically significant between individual groups.
3. Results
3.1. Preparation and characterization of CVB scaffolds
The optimal dose of vanillin and BG was screened, and the experiment details were summarized in Table 1. Homogeneous CVB hydrogel was successfully formed within 7 min when the final concentration of vanillin and BG was 16mg/mL and 2.5mg/mL, respectively, which was then chosen as the optimal crosslinking reagent dose for our further experiments. The main potential mechanism of CVB hydrogel formation process is schematically illustrated here (Fig.1), and more detailed explanation was presented in the discussion part.
Figure.1.
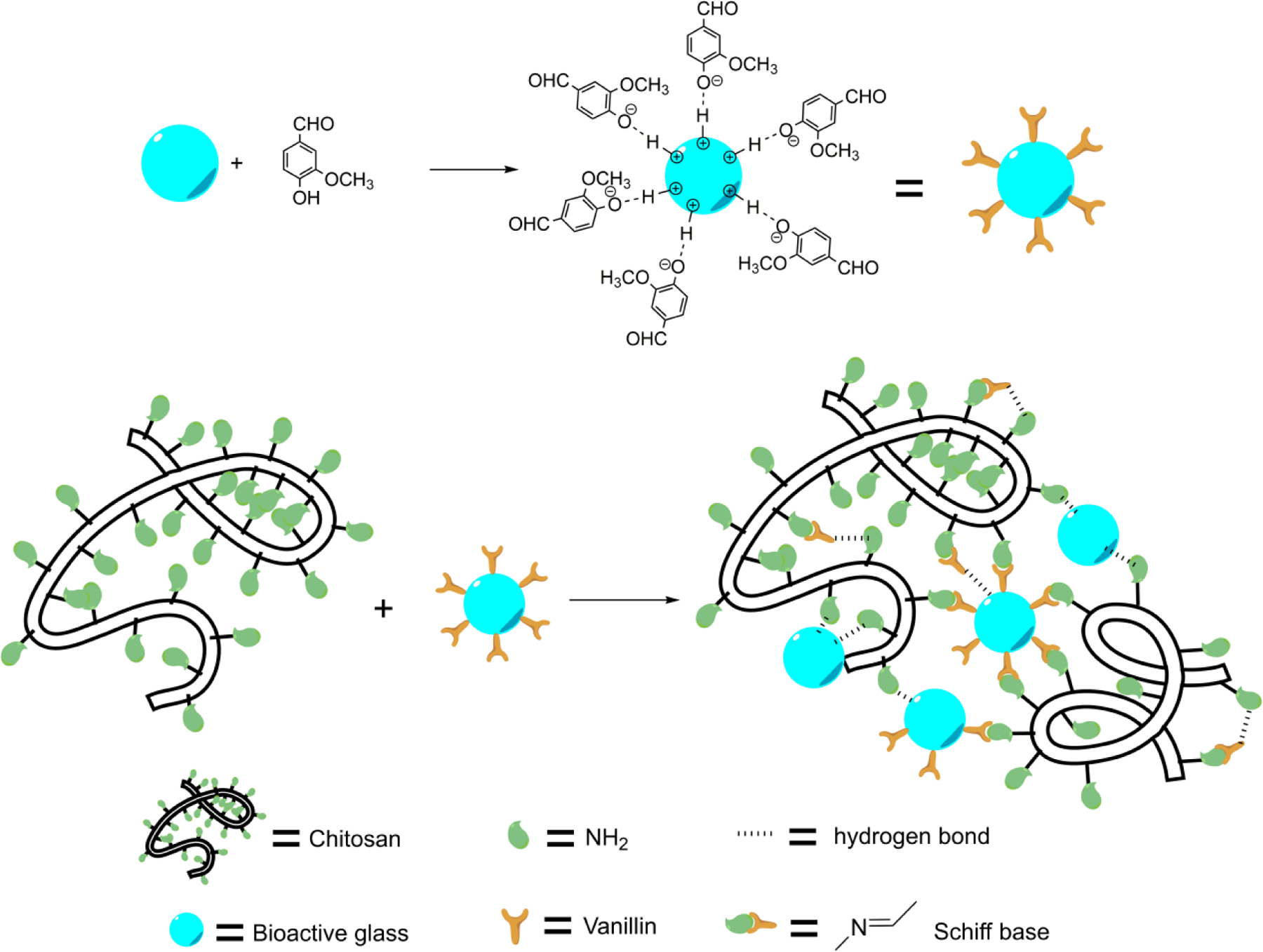
schematic illustration of the CVB hydrogel formation process
The SEM images indicated that our innovative CVB scaffolds had highly interconnected macroporous structures (Fig. 2A). Although the average pore sizes were similar, CVB scaffolds had significantly higher porosity compared to the CS-βGly-NaHCO3 group (Table 2). The presence of BG in CVB scaffolds was confirmed by SEM under high magnification and TEM (Fig. S4). The FTIR spectrum of vanillin showed the feature peaks of the aldehyde group (C=O) at 1663 cm−1 and the phenolic hydroxyl group at 1261 cm−1 as per previous reports (Xu et al., 2018). For CVB scaffolds, a new peak appeared at 1640 cm−1 which corresponds to the C=N stretching vibration according to the previous report (Peng, Wang, Tan, & Cheng, 1998). The results of the FTIR spectrum confirmed the formation of Schiff base between chitosan and vanillin in CVB scaffolds (Fig.2 B).
Figure.2.
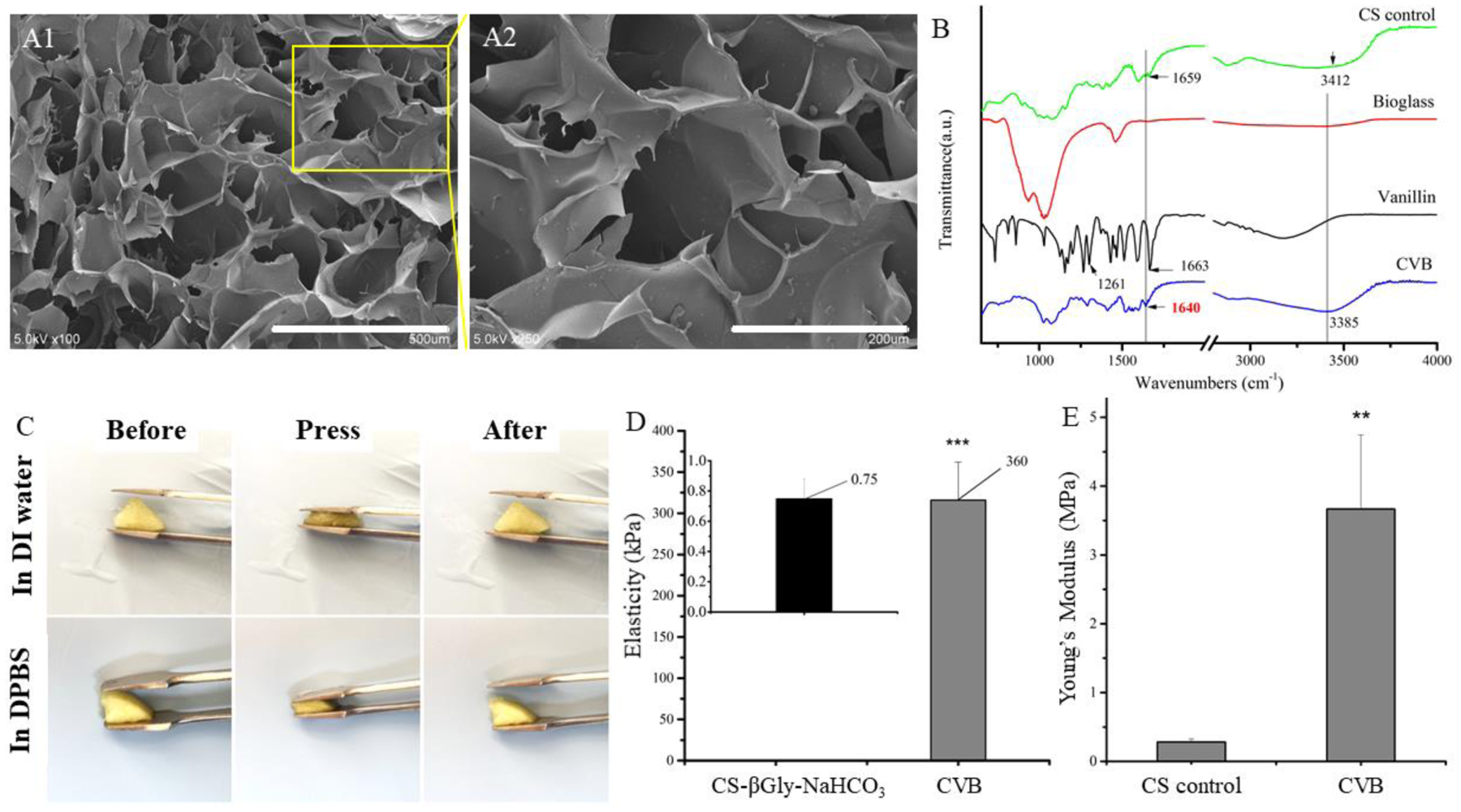
Characterization of CVB and control scaffolds. (A1–A2) SEM images of CVB scaffolds at low (scale bars=500 μm, left panel) and high (scale bars=200 μm, middle panel) magnifications, (B) FTIR spectrum of CVB scaffolds, (C) Highly elastic CVB scaffolds in aqueous buffer, (D) Young’s modulus examination of CVB hydrogel and CS-βGly-NaHCO3 hydrogel has been measured using AFM analysis (wet), (E) compression Young’s modulus testing of CVB and CS scaffolds (dry).
Table 2.
Characteristics of scaffold pore size and porosity
| Morphological aspects | CS-βGly-NaHCO3 | CVB |
|---|---|---|
| Average pore size (μm) | 100.05 ± 102.81 | 115.38 ± 58.52 |
| Porosity (%) | 82.02 ± 0.52 | 94.64 ± 0.71 |
Additionally, the CVB scaffolds were highly elastic after becoming fully hydrated in either DI water or DPBS (Fig. 2C). AFM test revealed that the CVB hydrogel had an extremely high average elasticity (360 kPa) compared to the ionic cross-linked CS-βGly-NaHCO3 hydrogel (0.75 kPa) (Fig. 2D). After lyophilization, the CVB scaffolds showed significantly higher compressive Young’s modulus compared to neat chitosan scaffolds that served as the control for dry CVB scaffold because CS-βGly-NaHCO3 scaffolds became collapsed after freeze-dry process. (Fig. 2E, P<0.01).
After 24 h of incubation in DI water (room temperature, Fig. 3A), a significant increase of the swelling ratio from 2.72 ± 0.32 % to 27.3 ± 2.4% was observed for CS-βGly-NaHCO3 scaffolds and CVB scaffolds, respectively. Additionally, the degradation rate of CVB scaffolds was significantly slower than that of CS-βGly-NaHCO3 scaffolds. These data suggested that CVB scaffolds had much better elasticity, structural stability, and mechanical properties compared to the CS-βGly-NaHCO3 scaffolds, especially in an aqueous environment.
Figure. 3.
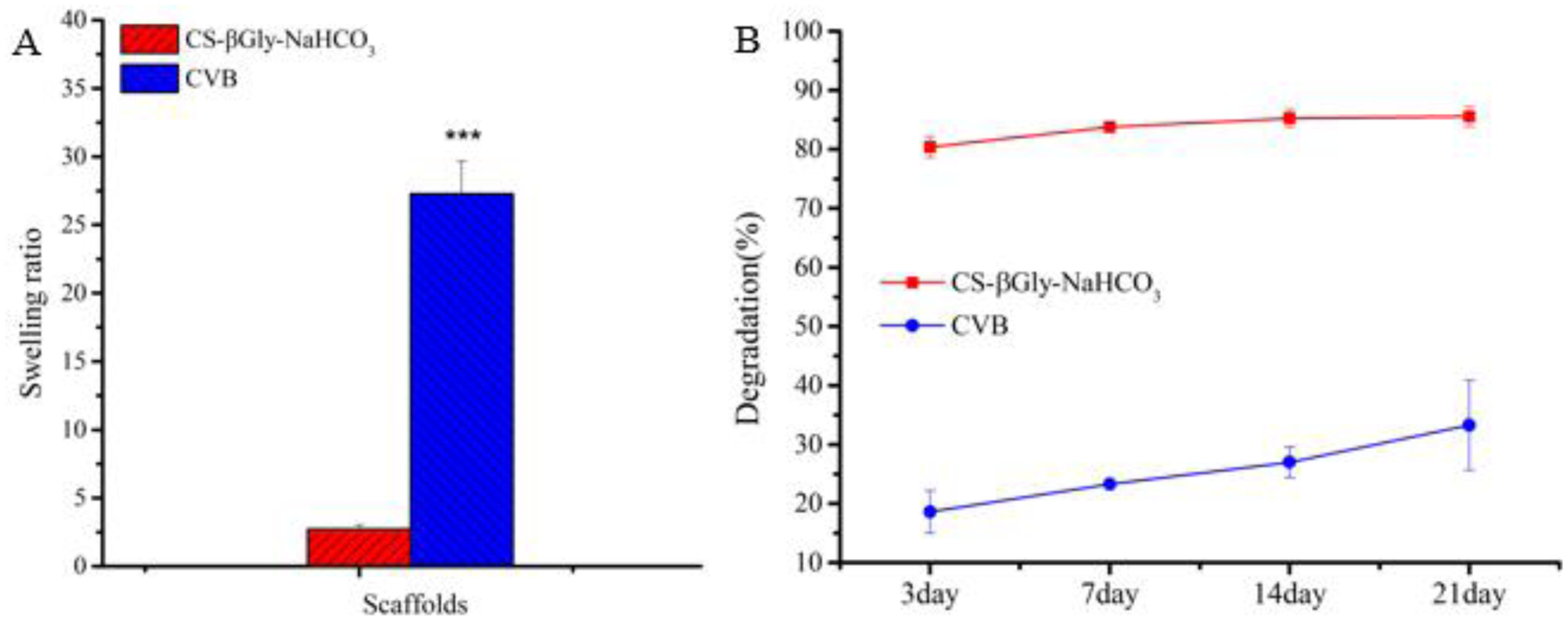
Swelling and degradation properties of CVB and CS-βGly-NaHCO3 scaffolds.
3.2. Biocompatibility and antibacterial ability
As the data indicated, the relative MC3T3-E1 cell number (absorbance at OD=490 nm) significantly increased with the culture time (Fig. 4A). Additionally, confocal imaging showcased that the MC3T3-E1 cells attached and spread throughout the entirety of CVB scaffolds after 24 hours of seeding (Fig.4B). Overall, the data indicated that the 3D CVB scaffolds had good biocompatibility and well-supported mouse osteoblasts attachment and proliferation in vitro.
Figure.4.
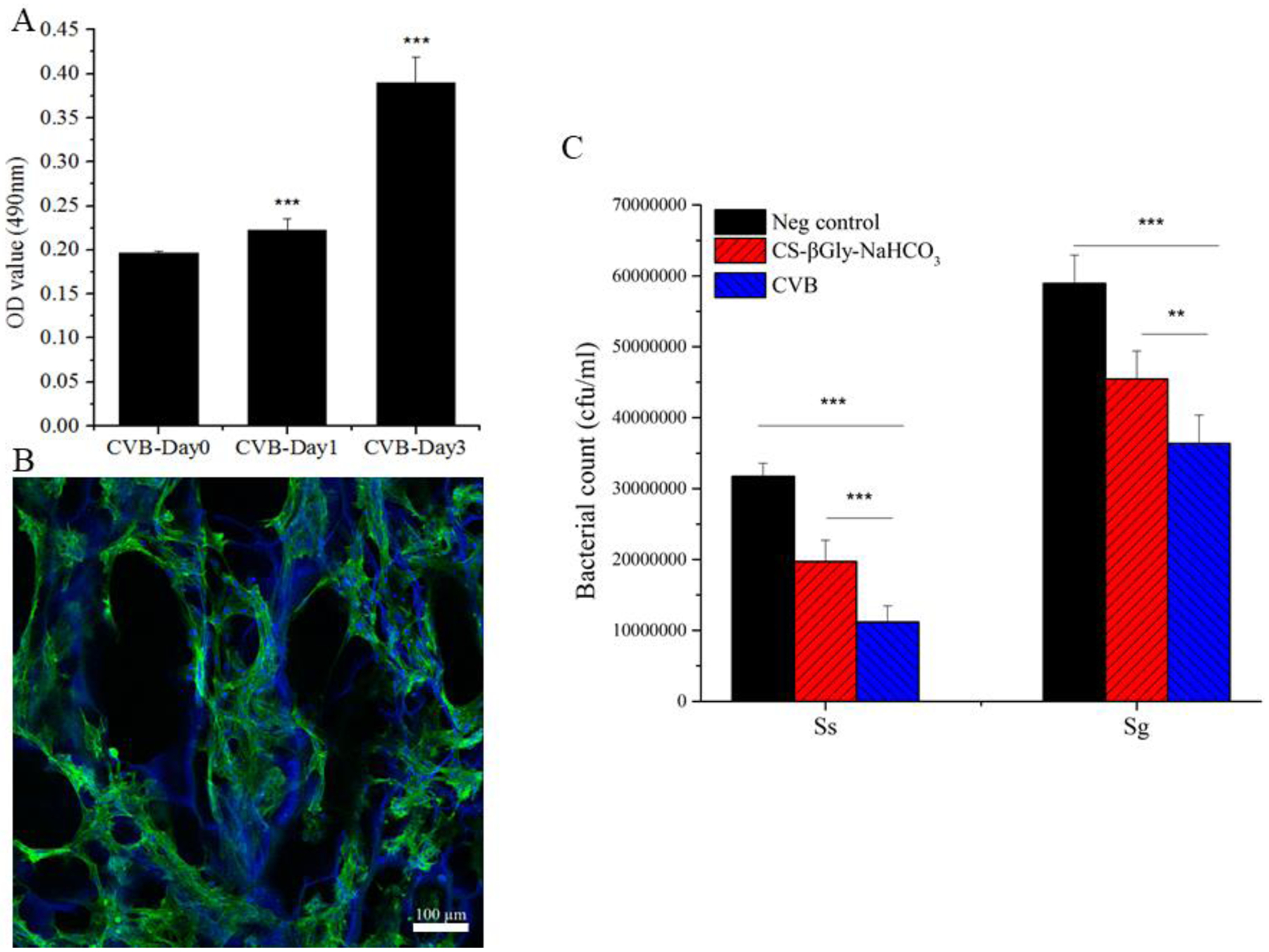
Scaffold biocompatibility and anti-microbial test. (A) Cell viability; (B) Confocal images; Cytoskeletons were shown in green (Fluorescein Phalloidin), while the cell nuclei were blue (DAPI), the CVB scaffolds also appeared as blue due to the great dye adsorption capacity of chitosan. Scale bar = 100 μm. (C) The bacterial inhibition test was carried out for S. gordonii (Sg) and S. sanguinis (Ss).
The antibacterial test revealed that the numbers of viable bacteria colonies of both S. gordonii and S. sanguinis on CVB containing culture plates were significantly less than those on the negative control (PBS group, P < 0.001) and the CS-βGly-NaHCO3 containing plates (Fig. 4C, P < 0.01), suggesting that the main components of CVB scaffolds could significantly inhibit bacterial growth.
3.3. CVB scaffolds promote in vitro osteoblastic differentiation
3.3.1. Bioactivity of CVB scaffolds
The bioactivity of CS-βGly-NaHCO3 (Fig.5A) and CVB (Fig.5B) scaffolds was studied by incubation in the biomimetic SFB solution (10×SBF). SEM imaging revealed the appearance of the largely different mineral on CS-βGly-NaHCO3 and CVB scaffolds. The HA deposition on CS-βGly-NaHCO3 scaffolds was a non-crystalline shape (Fig. 5C), while the minerals on CVB scaffolds exhibited a crystalline, sphere-shaped morphology (Fig. 5D).
Figure.5.
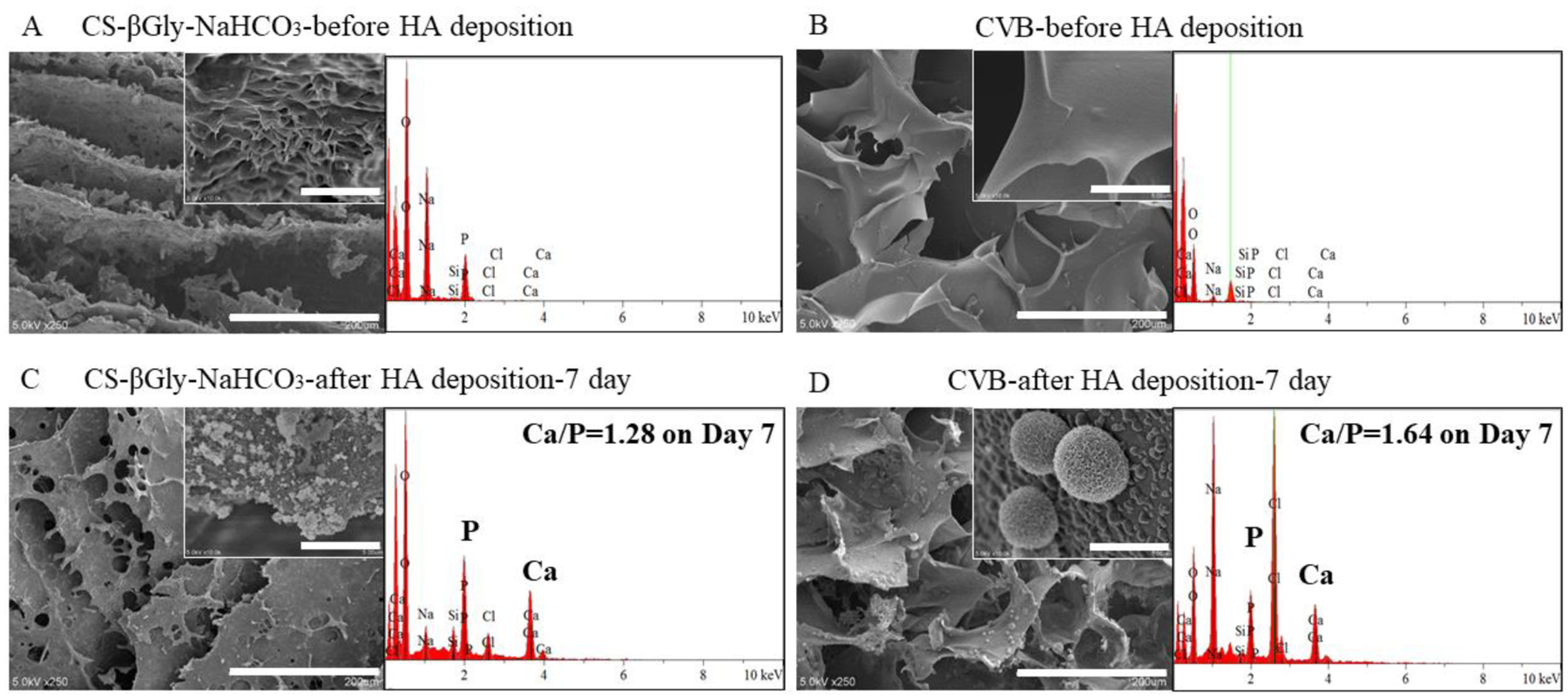
Bio-mineralized scaffold characterization. (A, B) SEM images and EDS analysis of non-mineralized scaffolds. (C, D) SEM and EDS data showing the presence of calcium element within mineralized scaffolds. Scale bars: 200μm (large images) and 5μm (inserted images).
The elemental analysis (EDS) further confirmed the presence of CaP minerals within the mineralized scaffolds, and the Ca/P ratio of the minerals on CS-βGly-NaHCO3 and CVB scaffold was estimated to be 1.28 and 1.64 (Fig. 5C, D), respectively. The Ca/P ratio of the minerals on CVB scaffolds is much closer to natural hydroxyapatite (HA), which has a Ca/P ratio of 1.67 (Liu, Yazici, Ergun, Webster, & Bermek, 2008). Moreover, SEM of mineralized scaffolds on days 1 and 3 revealed that the growth of the minerals on CVB scaffolds was faster than that on CS-βGly-NaHCO3 scaffolds (Support information, Fig. S1). To further confirm the HA deposition, the scaffolds with different incubation time were detected by XRD (Support information, Fig.S2). Therefore, CVB demonstrated better bioactivity compared to CS-βGly-NaHCO3 scaffolds based on the in vitro mineralization data.
3.3.2. Osteoblastic differentiation of MC3T3-E1 cells on the CVB scaffolds
The osteoblastic differentiation of MC3T3-E1 cells on the CVB scaffolds was investigated by measuring both ALP activity and calcium content. MC3T3-E1 cells were cultured in either growth medium (GM) or osteoconductive (OC) medium. After 7 days of culture, the cells on CVB scaffolds cultured in OC (OC+CVB) group showed the highest ALP activity compared to the cells on tissue culture plate (TCP) cultured in GM (GM-con), and in OC (OC-con), and on CVB scaffolds cultured in GM (GM+CVB) group (Fig. 6A, P<0.001). Please note, significantly higher ALP activity was observed from the GM+CVB group compared to the OC control group (P<0.001), indicating that even cultured in a non-osteoconductive GM, the CVB scaffolds could still promote osteoblastic differentiation and have stronger osteopromotive ability than the chemicals.
Figure.6.
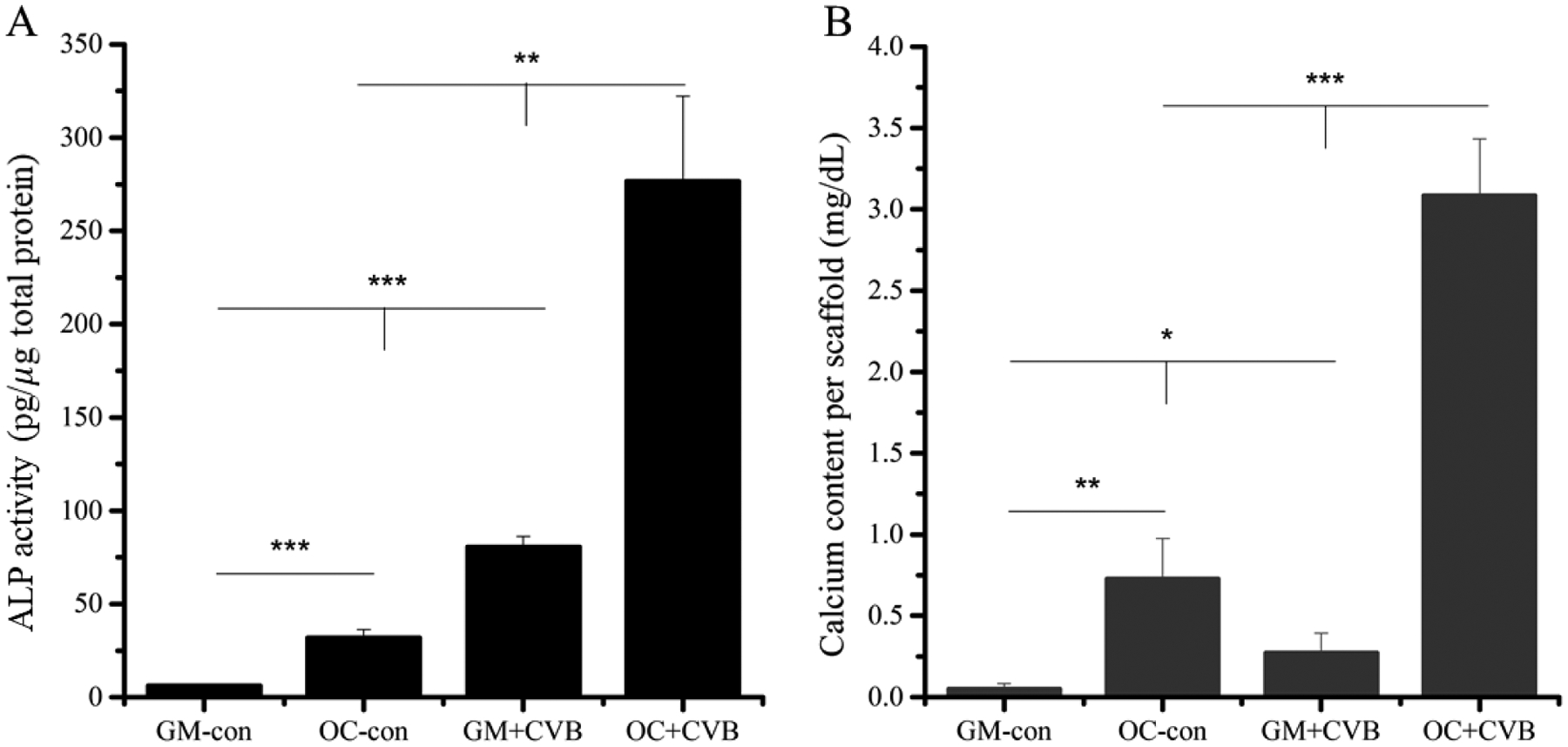
Effects of CVB on MC3T3-E1 cells differentiation. (A) 7 days ALP quantification, (B) calcium content test of MC3T3-E1 cells after 3 weeks incubation in growth medium (GM) and osteoconductive (OC) medium.
To further study the effects of the CVB scaffolds on MC3T3-E1 cells osteoblastic differentiation, we measured the mineralization of cells cultured on CVB scaffolds after 3 weeks of culture. Although not much calcium was detected on cell/CVB constructs cultured in GM (GM+CVB), it was still significantly higher than the GM-con (P<0.05). Compared with OC-con group, a significantly higher amount of calcium was produced by cells on CVB constructs cultured in OC (OC+CVB), (Fig.6B, P< 0.001), which was consistent with our ALP activity results.
3.3.3. Gene expression analysis
After cells were cultured in either GM or OC for 3, 7, 10 days, the quantitative gene expression of osteogenic markers, including RUNX2, OCN, and BSP, were evaluated to further study the effects of CVB scaffolds on the osteoblastic differentiation of MC3T3-E1 cells. RUNX2 expression was significantly improved by CVB scaffolds in OC on day 7 and a similar trend was observed on other time points (Fig. 7, left column). In addition to the early marker, the mature osteogenic marker genes, OCN (Fig. 7, middle column) and BSP (Fig. 7, right column) were significantly improved by CVB scaffolds in almost all three-time points, especially when they were cultured in OC. These quantitative gene expression data were consistent with both ALP activity and mineralization data (Fig.6).
Figure. 7.
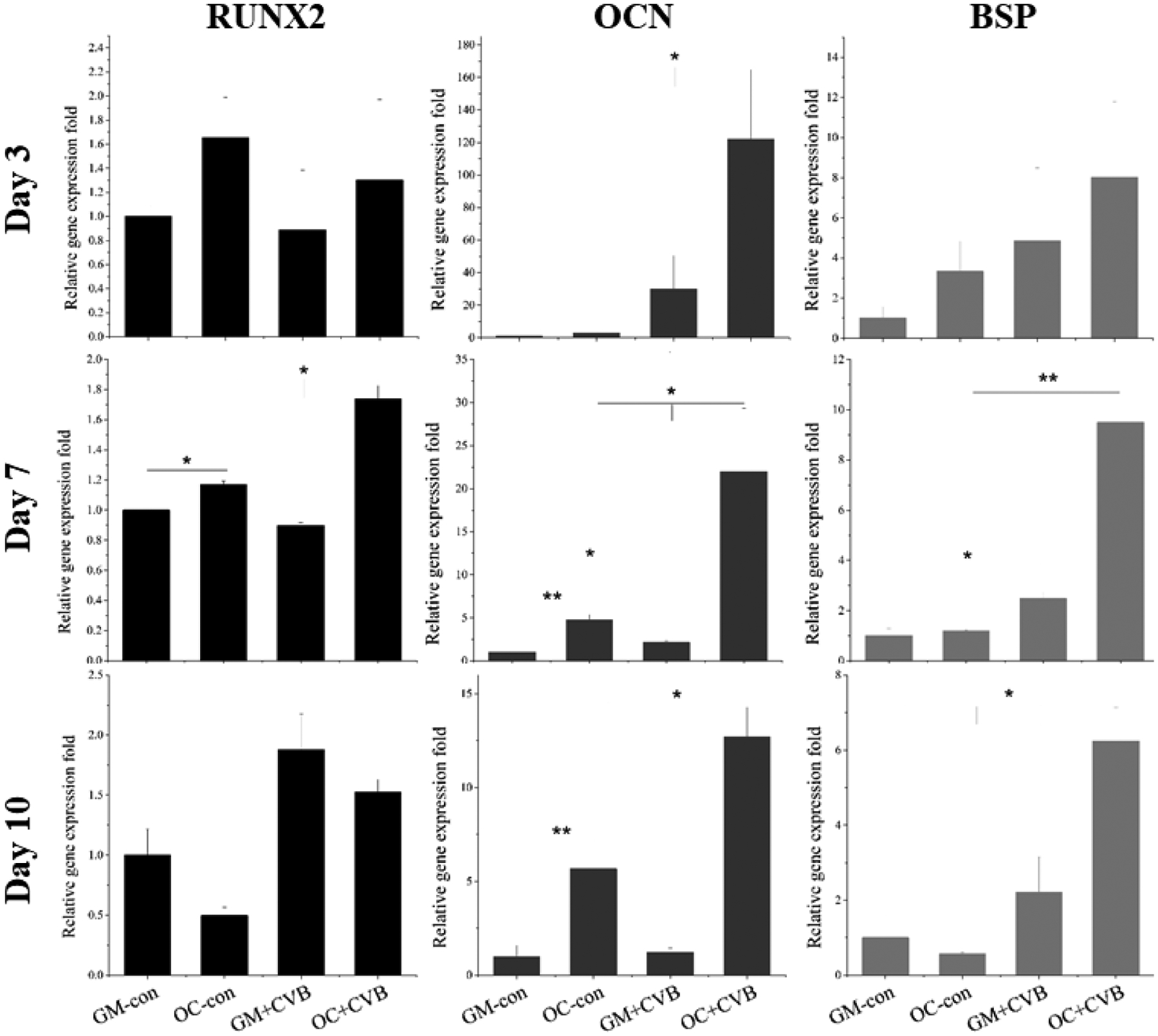
Osteogenic marker gene expressions (RUNX2, OCN, and BSP) were quantified by real-time PCR assay on varied days. (*P < 0.05; **P < 0.01 vs. control group)
Therefore, all of these in vitro data indicated that our innovative 3D porous CVB scaffolds highly promoted osteoblastic differentiation, mineralization, and demonstrated excellent osteoconductivity.
3.4. Ectopic bone regeneration CVB scaffolds
To study the in vivo bone formation ability of the CVB scaffolds, subcutaneous implantation of gelfoam (used as a control) and CVB scaffolds was studied on dorsal sides of mice for up to 4 weeks. Gelfoam or CVB scaffolds were supplemented with or without exogenous BMP2 protein. The macro-views, radiographic examination and H&E staining indicated that BMP2 was essential for bone formation since ossicles (bone + marrow) were only observed in BMP2+CVB and BMP2+ Gelfoam groups, whereas not in either CVB or gelfoam alone groups (Fig.8 A–B). Moreover, μCT analysis indicated that a significantly higher total ossicle volume was detected in the BMP2+CVB group compared to in the BMP2+Gelfoam group (Fig. 8C).
Figure. 8.
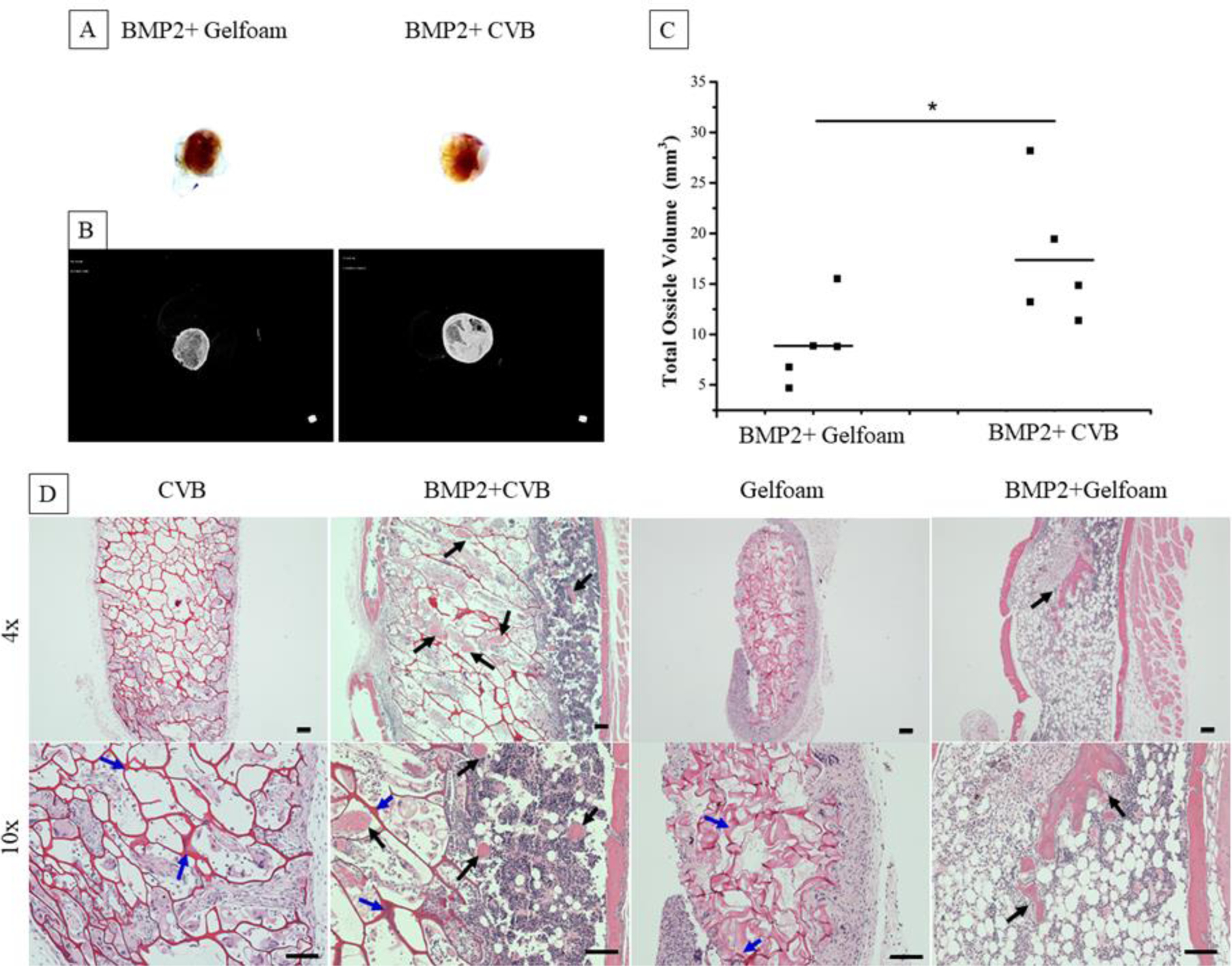
Characterization of ossicles harvesting from mice after 4 weeks post-implantation. (A) Digital images of BMP2+scaffolds (BMP2=2μg/scaffold), (B) Radiographic examination of ossicles, (C) Quantification of total ossicle volume, (n = 5, *P < 0.05 vs. BMP2+Gelfoam group). (D) Microscope images of H&E staining ossicles at low magnification (4×, upper panel), and high magnification (10×, lower panel). Scale bars = 100μm. (Black and blue arrows indicate new bone formation and the residual scaffolds inside of the ossicles, respectively).
Although no ossicles formed in the CVB scaffolds without BMP2 addition, the CVB scaffold maintained the interconnected macroporous structure, and the host cells penetrated throughout the scaffolds, indicating their good stability/mechanical properties as well as biocompatibility. In contrast, gelfoams shrunk significantly after 4-week of transplantation, and very limited number of cells were found inside of the scaffold without the addition of BMP2 (Fig.8 D). Typical ossicles were formed in both gelfoam and CVB scaffolds with most bone on the edges and marrow tissue inside of the structures. It was noted that both gelfoam and CVB degraded much faster with the presence of BMP2, although significantly more CVB scaffolds were still maintained and gelfoams were completely replaced by host tissues. Interestingly, many more adipocytes were observed in the ossicles from gelfoam compared to the ones from CVB scaffolds.
4. Discussion
To develop a sustainable and strong cross-linking technique for chitosan scaffolds fabrication, we investigated the possibility of Schiff base formation between the primary amines from chitosan and the aldehydes from vanillin. However, a vanillin molecule has only one aldehyde group to form a Schiff base bond with chitosan (Xu et al., 2018), which is not able to form a strong chitosan scaffold for bone regeneration. Although the remaining −NH2 could form hydrogen bonding with the amino or the hydroxyl groups in another chitosan molecule, the hydrogen bond is too weak, thus leading to the formation of hydrogel with a low mechanical property (Xu et al., 2018). Moreover, a high dose of vanillin (>30mg/mL) was used for the crosslinking of chitosan hydrogel (Xu et al., 2018), which may cause a safety concern in cell culture and future in vivo study (Bezerra, Soares, & de Sousa, 2016). Since the Schiff base formation is favored in a slightly higher pH environment (under neutral conditions) (Zeng et al., 2016; Zhang, Tao, Li, & Wei, 2011), this can be achieved by BG without using a toxic organic reagent (Fiume, Barberi, Verné, & Baino, 2018). In this work, we developed an innovative CVB scaffold to combine the functions of vanillin with BG that is used as a non-toxic pH adjustor. However, the same pH of chitosan solution adjusted by NaOH failed to form a hydrogel, suggesting that the role of BG played in chitosan-vanillin crosslinking was more significant than simply changing the pH.
In our preliminary experiments, we added vanillin solution into chitosan solution firstly then followed by the BG suspension and failed to form the homogenous hydrogel. Only by mixing BG microparticles and vanillin solution first before adding it into the chitosan solution can form a CVB hydrogel successfully. Based on these experiments, we believed that the role of BG could be both the pH adjustor and a co-crosslinker. During the gelation process, BG microparticles not only raised the surrounding pH that favors Schiff base formation but also increased the number of binding sites between chitosan and vanillin (as illustrated in Fig.1). Hydrogen and ionic bonds were formed among BG microparticles and vanillin, which was confirmed by UV absorbance from 200–400 nm (supplementary data, Fig. S5). Therefore, the BG microparticles were covered with numerous reactive aldehyde groups from vanillin, which ensured multiple crosslinks and formation of a strong chitosan hydrogel network in a few minutes. Moreover, we expect that BG will be a promising co-crosslinker with vanillin for bone tissue engineering because it has been known for its strong osteoconductivity (Brauer, 2015).
One important feature of our innovative CVB scaffolds is the higher elasticity, structural stability, and significantly improved mechanical properties compared to the ionic cross-linked CS-βGly-NaHCO3 scaffolds. The higher elasticity of CVB scaffolds should be strongly related to the matrix structure difference (Table 2, Fig.5). The larger porosity and interconnected pores make water penetration more easily into the CVB scaffolds, thus increasing the swelling degree. The slower degradation rate of CVB scaffolds could be due to the higher crosslinking degree. The improved mechanical stiffness of CVB scaffolds could be attributed to the higher crosslinking degree promoted by BG as we discussed above and the interactions between BG and chitosan polymers (Wers, Oudadesse, Lefeuvre, Merdrignac-Conanec, & Barroug, 2015). It is well known that the mechanical properties of biomaterials play important role in stem cell osteogenic differentiation in vitro and bone formation in vivo (Sun, Zhu, Hu, & Krebsbach, 2014; Yao et al., 2020). Therefore, we assumed that the improved mechanical properties of CVB were one of the main reasons that CVB scaffolds promoted strong osteoblastic differentiation. Besides, our in vivo histological data also confirmed that CVB remained stable after 4 weeks of transplantation while gelfoam was completely absorbed in both BMP2-added groups. The stronger mechanical support of CVB also could be the main reason for the improved BMP2-induced ossicle formation.
Our CVB scaffolds revealed good biocompatibility for MC3T3-E1 cells together with the strong anti-microbial ability, which may be mainly due to the intrinsic antibacterial activity of chitosan (Shariatinia, & Jalali, 2018) and vanillin (Oliveira et al., 2014). BG could have a potential contribution to the improved anti-microbial activity because it has been reported to have good antibacterial ability via the high pH value and debris caused by BG dissolution (Hu, Chang, Liu, & Ning, 2009). Additionally, CVB scaffolds displayed significantly improved bio-mineralization ability compared to ionic cross-linked chitosan scaffolds. Notably, CVB scaffolds could improve ALP activity even without the addition of osteoconductive chemicals. This may be attributed to the strong osteoconductivity of BG (Brauer, 2015) and chitosan itself because chitosan has been found to promote osteoblast differentiation in vitro in some previous reports (El-Meliegy, Abu-Elsaad, El-Kady, & Ibrahim, 2018).
5. Conclusions
We have developed a novel 3D porous chitosan-vanillin-BG (CVB) scaffold with improved mechanical properties, anti-microbial ability, and osteoconductivity by using the natural crosslinking agent vanillin in combination with BG microparticles. The innovative CVB scaffolds significantly promoted osteoblastic differentiation, mineralization, and inhibited bacterial growth in vitro. The improved mechanical properties and osteopromotive ability of the CVB scaffolds were largely because that the osteoconductive BG microparticles significantly improved the vanillin-mediated Schiff base reaction through servicing as a co-crosslinker and a pH adjustor. Consistently, our in vivo data also indicated the CVB 3D scaffolds could strongly support BMP2-induced ectopic bone/ossicle formation.
Supplementary Material
Highlights.
A novel vanillin-bioglass microparticles-based crosslinking method for chitosan scaffold.
Innovative 3D porous chitosan-vanillin-BG (CVB) scaffold was developed.
CVB showed significantly improved mechanical properties, stability, and bioactivity.
MC3T3-E1 cells showed significantly enhanced osteoblastic differentiation on CVB.
CVB showed strong antibacterial activity and osteoconductivity in vivo.
Acknowledgments
This work was supported by the startup funds from the Department of Oral and Maxillofacial Surgery at the University of Iowa, the National Institute of Dental & Craniofacial Research of the National Institutes of Health under Award Numbers R01DE029159 and T90DE023520. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors also would like to thank Drs. Brad A. Amendt, Cristina De Mattos Pimenta Vidal, Scott K. Shaw, and the members of their lab for their technical support in equipment usage.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Aguilar A, et al. (2019). Application of Chitosan in Bone and Dental Engineering. Molecules, 24(16), 3009–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Annu, Ali A, & Sheikh J (2018). A review on chitosan centred scaffolds and their applications in tissue engineering. International Journal of Biological Macromolecules, 116, 849–862. [DOI] [PubMed] [Google Scholar]
- Amini AR, Laurencin CT, & Nukavarapu SP (2012). Bone tissue engineering: recent advances and challenges. Critical Reviews in Biomedical Engineering, 40(5), 363–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ávila M, Zougagh M, Escarpa A, & Ríos Á (2007). Supported liquid membrane-modified piezoelectric flow sensor with molecularly imprinted polymer for the determination of vanillin in food samples. Talanta, 72(4), 1362–1369. [DOI] [PubMed] [Google Scholar]
- Bezerra DP, Soares AKN, & de Sousa DP (2016). Overview of the Role of Vanillin on Redox Status and Cancer Development. Oxidative Medicine and Cellular Longevity, 2016, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombaldi de Souza RF, et al. (2020). Phosphorylation of chitosan to improve osteoinduction of chitosan/xanthan-based scaffolds for periosteal tissue engineering. International Journal of Biological Macromolecules, 143, 619–632. [DOI] [PubMed] [Google Scholar]
- Brauer DS (2015). Bioactive Glasses—Structure and Properties. Angewandte Chemie, 54(14), 4160–4181. [DOI] [PubMed] [Google Scholar]
- Chahal S, Fathima SJ, & Yusoff MB (2014). Biomimetic growth of bone-like apatite via simulated body fluid on hydroxyethyl cellulose/polyvinyl alcohol electrospun nanofibers. Bio-Medical Materials and Engineering, 24(1), 799–806. [DOI] [PubMed] [Google Scholar]
- El-Meliegy E, Abu-Elsaad NI, El-Kady AM, & Ibrahim MA (2018). Improvement of physico-chemical properties of dextran-chitosan composite scaffolds by addition of nano-hydroxyapatite. Scientific Reports, 8(1), 12180–12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiume E, Barberi J, Verné E, & Baino F (2018). Bioactive Glasses: From Parent 45S5 Composition to Scaffold-Assisted Tissue-Healing Therapies. Journal of Functional Biomaterials, 9(1), 24–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerado E, & Caso E (2017). Challenges of bone tissue engineering in orthopaedic patients. World journal of orthopedics, 8(2), 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho ST, & Hutmacher DW (2006). A comparison of micro CT with other techniques used in the characterization of scaffolds. Biomaterials, 27(8), 1362–1376. [DOI] [PubMed] [Google Scholar]
- Hu J, Miszuk JM, Stein KM, & Sun H (2020). Nanoclay promotes mouse cranial bone regeneration mainly through modulating drug binding and sustained release. Applied Materials Today, 21, 100860–100870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Chang J, Liu M, & Ning C (2009). Study on antibacterial effect of 45S5 Bioglass®. Journal of Materials Science: Materials in Medicine, 20(1), 281–286. [DOI] [PubMed] [Google Scholar]
- Khvostenko D, Hilton TJ, Ferracane JL, Mitchell JC, & Kruzic JJ (2016). Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dental Materials, 32(1), 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansakara TI, Tong F, Bardeen CJ, & Tivanski AV (2020). Mechanical Properties and Photomechanical Fatigue of Macro- and Nanodimensional Diarylethene Molecular Crystals. Nano letters, 20(9), 6744–6749. [DOI] [PubMed] [Google Scholar]
- Monfoulet Laurent-Emmanuel, et al. (2014). The pH in the Microenvironment of Human Mesenchymal Stem Cells Is a Critical Factor for Optimal Osteogenesis in Tissue-Engineered Constructs. Tissue Engineering Part A, 20(13–14), 1827–1840. [DOI] [PubMed] [Google Scholar]
- Liu H, Yazici H, Ergun C, Webster TJ, & Bermek H (2008). An in vitro evaluation of the Ca/P ratio for the cytocompatibility of nano-to-micron particulate calcium phosphates for bone regeneration. Acta Biomaterialia, 4(5), 1472–1479. [DOI] [PubMed] [Google Scholar]
- Liu H, et al. (2013). The promotion of bone regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by effects on integrin-BMP/Smad signaling pathway in BMSCs. Biomaterials, 34(18), 4404–4417. [DOI] [PubMed] [Google Scholar]
- Lu Y, et al. (2018). Multifunctional Copper-Containing Carboxymethyl Chitosan/Alginate Scaffolds for Eradicating Clinical Bacterial Infection and Promoting Bone Formation. ACS Applied Materials & Interfaces, 10(1), 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miszuk JM, et al. (2018). Functionalization of PCL-3D electrospun nanofibrous scaffolds for improved BMP2-induced bone formation. Applied Materials Today, 10, 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugan R, & Ramakrishna S (2004). Bioresorbable composite bone paste using polysaccharide based nano hydroxyapatite. Biomaterials, 25(17), 3829–3835. [DOI] [PubMed] [Google Scholar]
- Oliveira CBS, et al. (2014). Comparative Study on the Antioxidant and Anti-Toxoplasma Activities of Vanillin and Its Resorcinarene Derivative. Molecules, 19(5), 5898–5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Wang Y, Tan S, & Cheng G (1998). Preparation of Chitosan Derivatives. Synthesis of N-Schiff Base Type and N-Secondary Amino Type Chitosan-Crown Ethers. Polymer Journal, 30(10), 843–845. [Google Scholar]
- Pinheiro A, Cooley A, Liao J, Prabhu R, & Elder S (2016). Comparison of natural crosslinking agents for the stabilization of xenogenic articular cartilage. Journal of orthopaedic research, 34(6), 1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariatinia Z, & Jalali AM (2018). Chitosan-based hydrogels: Preparation, properties and applications. International Journal of Biological Macromolecules, 115, 194–220. [DOI] [PubMed] [Google Scholar]
- Sileika TS, Barrett DG, Zhang R, Lau KHA, & Messersmith PB (2013). Colorless Multifunctional Coatings Inspired by Polyphenols Found in Tea, Chocolate, and Wine. Angewandte Chemie, 52(41), 10766–10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, et al. (2018). Current development of biodegradable polymeric materials for biomedical applications. Drug design, development and therapy, 12, 3117–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Zhu F, Hu Q, & Krebsbach PH (2014). Controlling stem cell-mediated bone regeneration through tailored mechanical properties of collagen scaffolds. Biomaterials, 35(4), 1176–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umashankar PR, Mohanan PV, & Kumari TV (2012). Glutaraldehyde treatment elicits toxic response compared to decellularization in bovine pericardium. Toxicol Int, 19(1), 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrier S, et al. (2016). Tissue engineering and regenerative approaches to improving the healing of large bone defects. European Cells and Materials, 32, 87–110. [DOI] [PubMed] [Google Scholar]
- Wang C-Q, Wu J-L, Zhuo R-X, & Cheng S-X (2014). Protamine sulfate–calcium carbonate–plasmid DNA ternary nanoparticles for efficient gene delivery. Molecular BioSystems, 10(3), 672–678. [DOI] [PubMed] [Google Scholar]
- Wang J, & Zhuang S (2017). Removal of various pollutants from water and wastewater by modified chitosan adsorbents. Critical Reviews in Environmental Science and Technology, 47(23), 2331–2386. [Google Scholar]
- Wers E, Oudadesse H, Lefeuvre B, Merdrignac-Conanec O, & Barroug A (2015). Evaluation of the kinetic and relaxation time of gentamicin sulfate released from hybrid biomaterial Bioglass-chitosan scaffolds. Applied Surface Science, 353, 200–208. [Google Scholar]
- Xu C, et al. (2018). Self-healing chitosan/vanillin hydrogels based on Schiff-base bond/hydrogen bond hybrid linkages. Polymer Testing, 66, 155–163. [Google Scholar]
- Yao Q, Fuglsby KE, Zheng X, & Sun H (2020). Nanoclay-functionalized 3D nanofibrous scaffolds promote bone regeneration. Journal of Materials Chemistry B, 8(17), 3842–3851. [DOI] [PubMed] [Google Scholar]
- Zeng Q, et al. (2016). Self-Healing Elastin–Bioglass Hydrogels. Biomacromolecules, 17(8), 2619–2625. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tao L, Li S, & Wei Y (2011). Synthesis of Multiresponsive and Dynamic Chitosan-Based Hydrogels for Controlled Release of Bioactive Molecules. Biomacromolecules, 12(8), 2894–2901. [DOI] [PubMed] [Google Scholar]
- Zhuang ST, Yin YN, & Wang JL (2018). Simultaneous detection and removal of cobalt ions from aqueous solution by modified chitosan beads. International Journal of Environmental Science and Technology, 15(2), 385–394. [Google Scholar]
- Zou Q, Li J, & Li Y (2015). Preparation and characterization of vanillin-crosslinked chitosan therapeutic bioactive microcarriers. International Journal of Biological Macromolecules, 79, 736–747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


