Abstract
Objective:
Coronavirus disease 2019 (COVID-19) vaccination effectiveness in healthcare personnel (HCP) has been established. However, questions remain regarding its performance in high-risk healthcare occupations and work locations. We describe the effect of a COVID-19 HCP vaccination campaign on SARS-CoV-2 infection by timing of vaccination, job type, and work location.
Methods:
We conducted a retrospective review of COVID-19 vaccination acceptance, incidence of postvaccination COVID-19, hospitalization, and mortality among 16,156 faculty, students, and staff at a large academic medical center. Data were collected 8 weeks prior to the start of phase 1a vaccination of frontline employees and ended 11 weeks after campaign onset.
Results:
The COVID-19 incidence rate among HCP at our institution decreased from 3.2% during the 8 weeks prior to the start of vaccinations to 0.38% by 4 weeks after campaign initiation. COVID-19 risk was reduced among individuals who received a single vaccination (hazard ratio [HR], 0.52; 95% confidence interval [CI], 0.40–0.68; P < .0001) and was further reduced with 2 doses of vaccine (HR, 0.17; 95% CI, 0.09–0.32; P < .0001). By 2 weeks after the second dose, the observed case positivity rate was 0.04%. Among phase 1a HCP, we observed a lower risk of COVID-19 among physicians and a trend toward higher risk for respiratory therapists independent of vaccination status. Rates of infection were similar in a subgroup of nurses when examined by work location.
Conclusions:
Our findings show the real-world effectiveness of COVID-19 vaccination in HCP. Despite these encouraging results, unvaccinated HCP remain at an elevated risk of infection, highlighting the need for targeted outreach to combat vaccine hesitancy.
The coronavirus disease 2019 (COVID-19) pandemic has spread throughout the United States, with >33.5 million cases and 602,401 deaths as of July 1, 2021.1 Healthcare personnel (HCP) caring for patients with COVID-19 work in locations with potentially increased risk for severe acute respiratory syndrome coronavirus virus 2 (SARS-CoV-2), the virus that causes COVID-19, due to frequency of exposure to oronasal secretions, variable availability of personal protective equipment (PPE) throughout the pandemic, limited testing availability, and risk of occupational transmission.2–5 Decreasing the number of COVID-19 cases among HCP has been achieved through several approaches, including improved access to PPE, and testing of symptomatic and asymptomatic people for early identification and isolation of SARS-CoV-2–infected patients and HCP.5 However, with >514,464 HCP cases and 1,689 HCP deaths reported as of July 1, 2021, widespread COVID-19 vaccination programs, particularly for HCP, are needed.1
The CDC Advisory Committee on Immunization Practices (ACIP) recommended HCP be prioritized to the first vaccination group on December 1, 2020, in anticipation of the forthcoming FDA Emergency Use Authorization (EUA) of several COVID-19 vaccinations.6 Subsequently, BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines were approved for emergency use based on large, randomized controlled trials reporting efficacies for preventing COVID-19 in the range of 94% to 95%.7,8 With these encouraging trial results, it became important to establish the factors associated with vaccine effectiveness in real-world healthcare settings where risk of infection potentially differs from the populations in which vaccines were initially studied. To this end, multiple studies of HCP have demonstrated the effectiveness of vaccinating against SARS-CoV-29–11; however, understanding of its effectiveness based on occupation remains limited.
The University of California Davis Health (UCDH) began staff vaccination on December 15, 2020, with the BNT162b2 vaccine and on December 22, 2020, with the mRNA-1273 vaccine. The rollout of SARS-CoV-2 immunizations coincided with a local surge of COVID-19 cases reflected in an increase in HCP infections at our institution. As part of a quality improvement analysis of our vaccination program, we conducted a retrospective cohort study of vaccine-eligible HCP at UCDH to determine the real-world effect of SARS-CoV-2 vaccination on the rate of COVID-19 cases and as a function of occupational risk of infection. We examined the incidence and severity of COVID-19 before and after vaccination with respect to dose number, time from each vaccine dose, and occupation. Although some data exist regarding vaccine effectiveness in real-world healthcare settings, our center’s experience, including occupational risk stratification, could further identify important patterns to inform those most at risk of infection and highlight areas where concentrated vaccination efforts are needed to combat vaccine hesitancy.
Methods
Study population
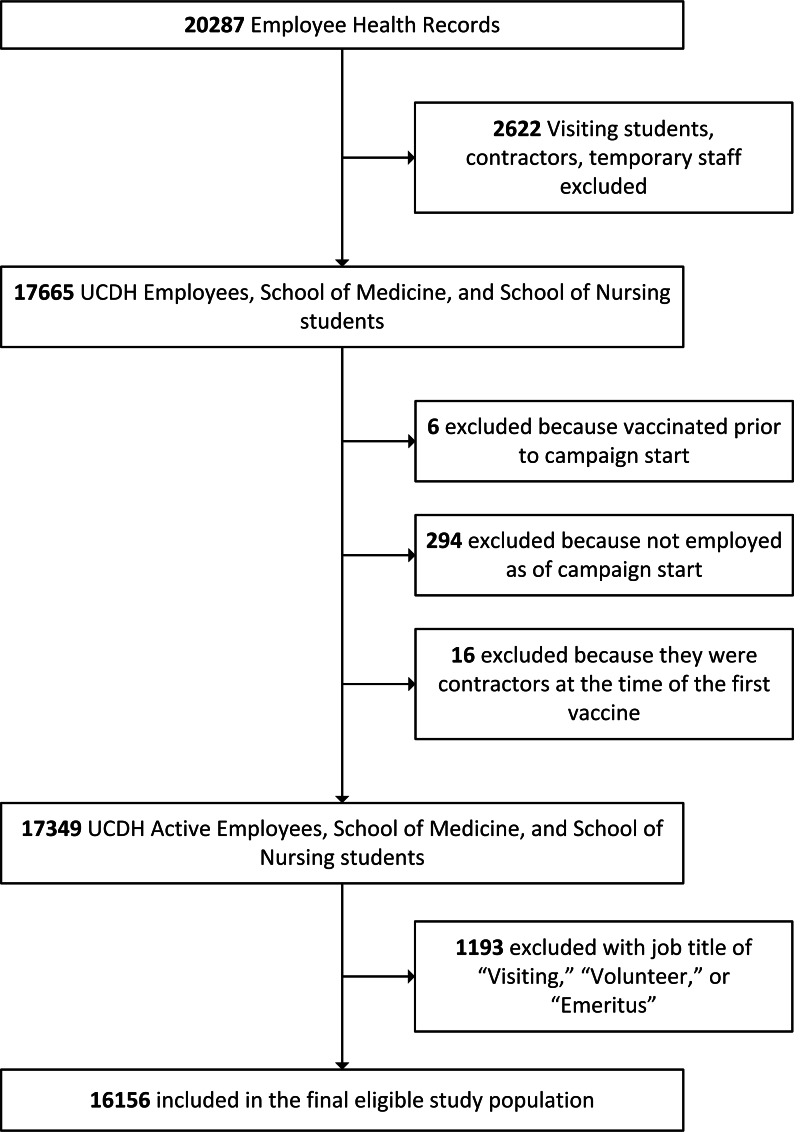
University of California Davis Health (UCDH), a large academic health system in Sacramento, California, includes a 627-bed acute-care teaching hospital, a physician’s practice group with >1,000 members, and a School of Medicine and School of Nursing with 480 and 187 students, respectively. The facility and surrounding community clinics serve a 33-county, 65,000 square-mile area of northern California providing tertiary and quaternary levels of care. The study cohort included 16,156 UCDH employees and students at the UC Davis Schools of Medicine and Nursing, aged ≥18 years, actively employed or enrolled as of October 20, 2020, including those previously infected with SARS-CoV-2. The study period was from October 20, 2020, to March 1, 2021, to capture an 8-week and an 11-week pre- and postvaccine HCP COVID-19 risk. We excluded records for employees terminated prior to the study start date. We also excluded the records of those who were vaccinated prior to the start of the vaccination campaign and participated in clinical trials because their vaccination date fell outside the study period, resulting in a potentially different risk of infection compared to other participants. Contractors, temporary employees, and volunteer, visiting, and emeritus faculty were excluded due to insufficient data in the employee medical record (EMR) to determine employment status (eg, active, inactive, or terminated) or job type and location. This quality improvement initiative was determined by the UCDH Institutional Review Board to not be human subjects research and thus did not require IRB review.
Study design and cohort
Data for this analysis were obtained from 3 sources: the UCDH employee EMR (Agility, NetHealth, Pittsburgh, PA), the UCDH patient EMR (Epic Systems, Verona, WI), and a separate employee case log that captured self-reported COVID-19 cases. Records were matched across these 3 sources using hierarchical matching logic to uniquely identify each employee. Employee vaccination data (doses and dates) were derived using the employee health EMR first, and then the patient EMR when those dates were missing or inconsistent. Data regarding SARS-CoV-2 positivity dates were obtained from the employee case log and from the patient EMR. If these dates were conflicting, the date of the first positive SARS-CoV-2 test was captured. UCDH offered voluntary asymptomatic SARS-CoV-2 testing to HCP as well as diagnostic testing for symptomatic employees.
We constructed a proxy measure for an individual’s likelihood of exposure to SARS-CoV-2 by examining personnel data in the employee EMR. The employee cost center and job family, function, and title were used to categorize employees into 5 main groups with varying risk of COVID-19: environmental/custodial services (EVS), nurses, physicians, respiratory therapists, and other. The “other” category was assumed to have a lower overall occupational infection risk and was composed of jobs that were deemed to have either no direct sustained patient contact (eg, informational technology, hospital administration, and human resources) or patient-focused employees with either small cohort size or heterogenous clinical roles (eg, dieticians, occupational therapists, and technicians). Students were classified based on their school of attendance. We used criteria defined by the ACIP to assign a subset of employees to phase 1a status (n = 12,104) based on job description, location, and involvement in direct patient care (Fig. 1).6 All EVS staff, nurses, physicians, and respiratory therapists were considered phase 1a employees. For the ‘phase 1a other’ cohort, we removed all nonclinical job types from the overall ‘Other’ category.
Fig. 1.
Study population and cohort selection process, October 20, 2020–March 1, 2021.
Flow diagram detailing the selection process used to define the final eligible study population of active UC Davis Health (UCDH) employees and students at the Schools of Medicine and Nursing. Absolute numbers are shown for each criterion.
In this study, we followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.12
Statistical methods
Categorical variables were summarized as counts and proportions. Proportional hazards models were used to evaluate the effect of vaccination dose on the hazard of COVID-19 after the start of the vaccination campaign. The event time was the number of days from the start of the campaign until a COVID-19 event. Employee infection status was monitored until March 1, 2021, or until the time of termination. Vaccination dose was modeled as a time-varying covariate. Schoenfeld residuals were graphically examined to assess the proportional hazard assumptions.
We first modeled infection rates among all employees relative to vaccination dose. We then restricted our analysis to phase 1a employees and evaluated differences in infection risk among job type (ie, nurses, physicians, respiratory therapists, environmental services workers, and ‘phase 1a other employees’), and primary employment location among nurses in combination with vaccine status. In each model, risk was evaluated relative to the category ‘phase 1a other employees’ for both job type and work location. Risk by employment location was studied only in nurses because other HCP job types are not consistently affiliated with an individual location at UCDH. Employees terminated prior to the start of the campaign were not included in the hazard modeling. All analyses were conducted in R statistical computing software, version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).13 Proportional hazard models were fit using the cph function in the rms package.14 All hypothesis tests were 2-sided and were evaluated at a significance level of 0.05.
Results
From December 15, 2020, through March 1, 2021, a total of 10,849 HCP received the first dose of vaccine, and 10,232 of these individuals (94%) received the second dose. The first dose of vaccine was administered to 44% of all HCP within the first 2 weeks. Many of the individuals who met criteria for phase 1a status (70%) as defined by the ACIP on December 1, 2020, were vaccinated as part of the campaign (Table 1).6 Of those vaccinated, 63% received the BNT162b2 (Pfizer-BioNTech) vaccine and 37% received the mRNA-1273 (Moderna) vaccine. The vaccinated HCP included physicians (23%), nurses (26%), respiratory therapists (1%), environmental services employees (2%), and other frontline workers with less or no direct patient contact (49%). Most (68%) of the vaccinated individuals were female, and 79% of the study cohort was aged 18–50 years.
Table 1.
Demographic and Clinical Characteristics of UCDH Employees Stratified by COVID-19 Test Positivity and Vaccination Status from March 1, 2020, to March 1, 2021 (N = 16,156)
| Demographic Factor | COVID-19 Test Positivity | COVID-19 Vaccination Status | |||
|---|---|---|---|---|---|
| Total UCDH, No. | Ever COVID-19 Positive, No. (%) | COVID-19 Positive During Vaccination Campaign, No. (%) | Unvaccinated, No. (%) | Received at Least 1 Dose of Vaccine, No. (%) | |
| Age group, y | |||||
| <30 | 2,875 | 221 (7.7) | 67 (2.3) | 938 (32.6) | 1,937 (67.4) |
| 30–39 | 5,368 | 423 (7.9) | 131 (2.4) | 1,925 (35.9) | 3,443 (64.1) |
| 40–49 | 3,929 | 270 (6.9) | 81 (2.1) | 1,313 (33.4) | 2,616 (66.6) |
| 50–64 | 3,580 | 207 (5.8) | 67 (1.9) | 1,024 (28.6) | 2,556 (71.4) |
| >65 | 386 | 8 (2.1) | 1 (0.3) | 92 (23.8) | 294 (76.2) |
| Unknown | 18 | 0 (0) | 0 (0) | 15 (83.3) | 3 (16.7) |
| Sex | |||||
| Female | 10,639 | 786 (7.4) | 245 (2.3) | 3,616 (34.0) | 7, 023 (66.0) |
| Male | 5,231 | 338 (6.5) | 99 (1.9) | 1,607 (30.7) | 3,624 (69.3) |
| Unknown | 286 | 5 (1.7) | 3 (1.0) | 84 (29.4) | 202 (70.6) |
| Race | |||||
| African American or Black | 822 | 68 (8.3) | 16 (1.9) | 294 (35.8) | 528 (64.2) |
| American Indian or Alaska Native | 39 | 3 (7.7) | 2 (5.1) | 7 (17.9) | 32 (82.1) |
| Asian | 2,799 | 146 (5.2) | 33 (2.0) | 341 (12.2) | 2,458 (87.8) |
| Native Hawaiian/ Pacific Islander | 658 | 37 (5.6) | 13 (2.0) | 46 (7.0) | 612 (93.0) |
| White | 5,738 | 445 (7.8) | 126 (2.2) | 934 (16.3) | 4,804 (83.7) |
| Other | 781 | 100 (12.8) | 36 (4.6) | 202 (25.9) | 579 (74.1) |
| Unknown race | 3,776 | 330 (8.7) | 121 (3.2) | 3,022 (80) | 754 (20) |
| Hispanic | 1,543 | 178 (11.5) | 60 (3.9) | 461 (29.9) | 1,082 (70.1) |
| Job position | |||||
| Physicians | 2,701 | 122 (4.5) | 16 (0.6) | 253 (9.4) | 2,448 (90.6) |
| Nurses | 3,877 | 347 (9.0) | 88 (2.3) | 1,089 (28.1) | 2,788 (71.9) |
| Respiratory therapists | 190 | 25 (13.2) | 9 (4.7) | 62 (32.6) | 128 (67.4) |
| EVS | 475 | 38 (8.0) | 16 (3.4) | 263 (55.4) | 212 (44.6) |
| Other staffa | 8,913 | 597 (6.7) | 209 (2.3) | 3,640 (40.8) | 5,273 (59.2) |
| Phase1a status 6 | |||||
| Yes | 12,104 | 951 (7.9) | 288 (2.4) | 3,592 (29.7) | 8,512 (70.3) |
| No | 4,052 | 178 (4.4) | 59 (1.5) | 1,715 (42.3) | 2,337 (57.7) |
Note. UCDH, University of California Davis Health; EVS, environmental/custodial services.
Other category was composed of jobs that were deemed to have either no direct sustained patient contact, such as informational technology, hospital administration, and human resources, or patient-focused employees with either small cohort size or heterogenous clinical roles, such as dieticians, occupational therapists, and technicians.
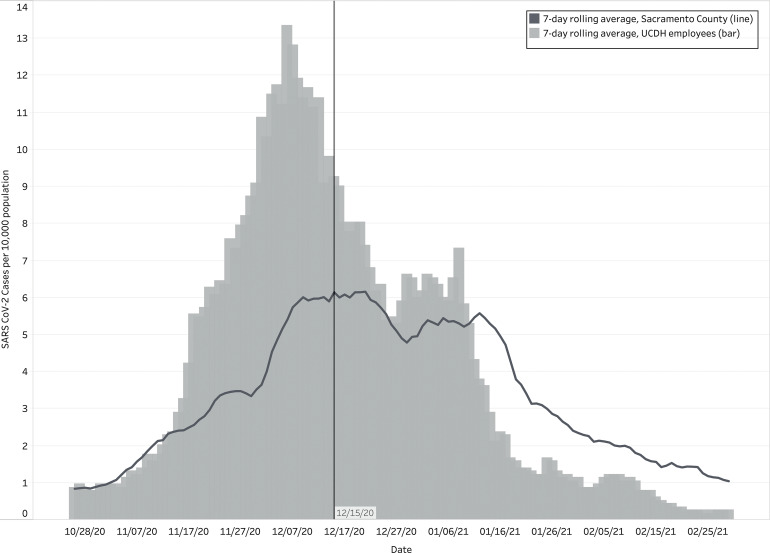
In total, 1,129 employees tested positive for SARS-CoV-2 by RT-PCR between March 1, 2020, and March 1, 2021. Also, 510 (45%) infections were identified in the 8 weeks prior to the start of the campaign (incidence rate, 0.56 per 1,000 person days) coinciding with a local surge of COVID-19 cases in northern California (Fig. 2). After December 15, 2020, the start of the vaccine campaign, 347 persons (31%) tested positive for SARS-CoV-2 (incidence rate, 0.28 per 1,000 person days). Among the 347 HCP who tested positive for SARS-CoV-2 after the campaign started, 85 (24%) had received at least 1 dose of vaccine, and most of those individuals (86%) tested positive between the first and second doses.
Fig. 2.
Sacramento County and UCDH employee SARS-CoV-2 incidence rate per 10,000 persons, by date. This figure shows the SARS-CoV-2 incidence rates per 10,000 persons as a seven-day rolling average for both Sacramento County (dark grey line) and UCDH employees (light grey bars) during the study period from October 20, 2020, to March 1, 2021.
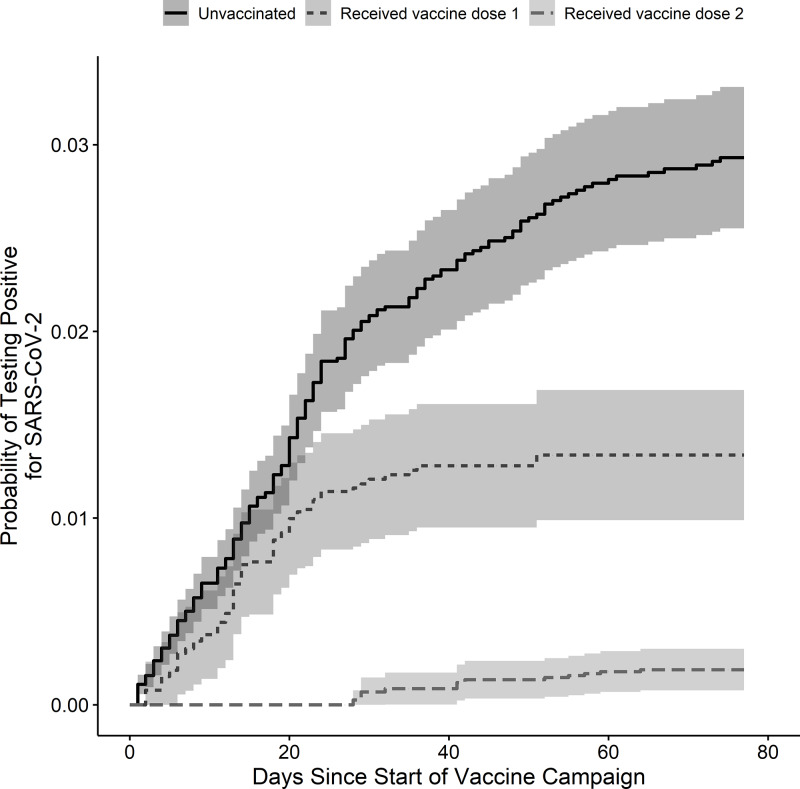
Receiving a single dose of vaccine decreased the hazard of testing positive for SARS-CoV-2 by 48% (HR, 0.52; 95% CI, 0.40–0.68; P < .001) and receiving the second dose decreased the hazard by 83% (HR, 0.17; 95% CI, 0.09–0.32; P < .001) compared to unvaccinated workers (Fig. 3). Of the 10,232 workers who had received both doses of mRNA vaccine by March 1, 2021, only 4 tested positive for SARS-CoV-2 two or more weeks after the second dose, consistent with a positivity rate of 0.04%. An additional 8 individuals tested positive for SARS-CoV-2 after receiving both vaccinations: 6 within 1–7 days after the second dose and 2 within 8–14 days. None of the vaccinated HCP who tested positive for SARS-CoV-2 were hospitalized, and there were no deaths.
Fig. 3.
Proportional hazards model of the effect of vaccination on the probability of testing positive for SARS-CoV-2. The figure shows the probability of a positive SARS-CoV-2 test in the study population after the start of a healthcare system-wide COVID-19 vaccination campaign using proportional hazards models. The risk was evaluated for 3 groups: (1) unvaccinated individuals (solid line), (2) individuals who received 1 dose of SARS-CoV-2 vaccine (short-dashed line), and (3) individuals who received both doses of SARS-CoV-2 vaccine (long-dashed line). Shaded areas represent 95% confidence intervals.
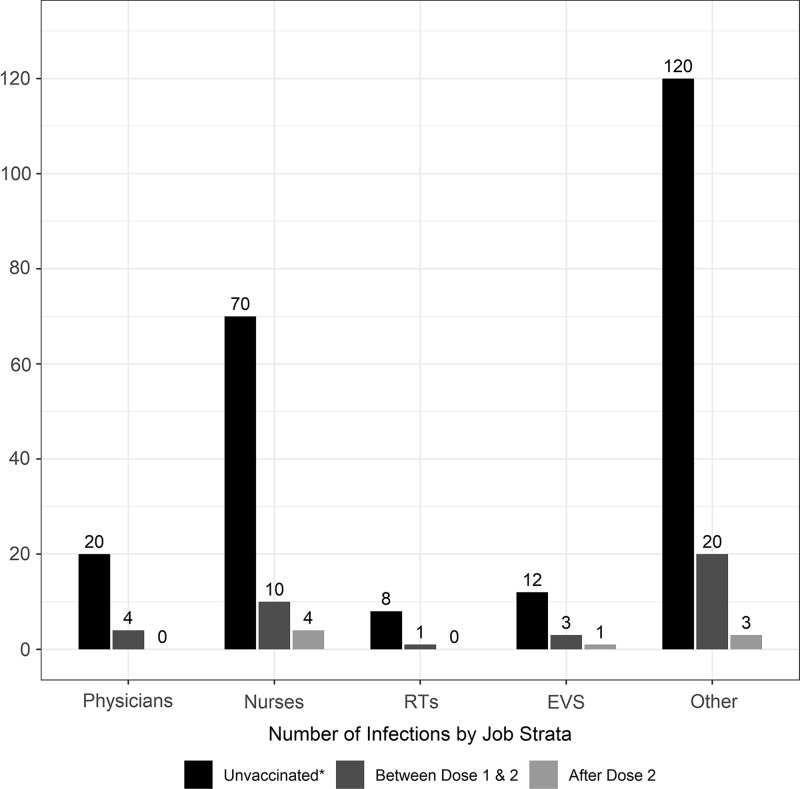
Among phase 1a occupational groups, the number of new infections decreased universally after vaccination (Fig. 4). Regardless of vaccination status, physicians had the lowest risk of infection (Table 2) among phase 1a HCP; respiratory therapists had the highest estimated risk, but the confidence intervals were wide, and the effect was not statistically significant. We examined the effect of work location in a subgroup of 3,877 nurses, of whom 347 (8.9%) tested positive for COVID-19 from March 1, 2020, to March 1, 2021. Of these, 88 tested positive on or after the start of the vaccination campaign. As with other groups, there was a protective effect of vaccination on risk of COVID-19, but the risk did not differ by work location (Table 2).
Fig. 4.
Number of new COVID-19 cases after the start of the COVID-19 vaccination campaign by job strata among those who were unvaccinated or before vaccination, those between vaccine dose 1 and 2, and those after both doses of vaccine. *Those in the unvaccinated category were infected with SARS-CoV-2 prior to their first dose of COVID-19 vaccine or were never vaccinated during the study period. Note. EVS, environmental/custodial services. RTs, respiratory therapists.
Table 2.
Risk of COVID-19 in Phase 1a Employees and Nurses
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Risk of COVID-19 in Phase 1a Employeesa | ||
| Vaccine dose 1 | 0.53 (0.40–0.71) | <.001 |
| Vaccine dose 2 | 0.22 (0.12–0.42) | <.001 |
| EVS | 1.09 (0.65–1.83) | .744 |
| Nurse | 0.83 (0.63–1.09) | .186 |
| MD | 0.40 (0.26–0.62) | <.001 |
| RT | 1.75 (0.89–3.43) | .104 |
| Risk of COVID-19 Among Nurses by Work Locationa | ||
| Vaccine 1 | 0.80 (0.5–1.27) | .339 |
| Vaccine 2 | 0.26 (0.07–0.96) | .044 |
| Ambulatory | 1.61 (0.82–3.15) | .164 |
| ED | 1.77 (0.80–3.93) | .158 |
| ICU | 0.80 (0.34–1.84) | .593 |
| Medical-surgical unit | 1.50 (0.88–2.53) | .135 |
Note. EVS, environmental/custodial services; MD, physicians; RT, respiratory therapists; ED, emergency department; ICU, intensive care unit.
Risk in each category was calculated by comparing to ‘phase 1a other’ employee category for both job type and job location. Phase 1a other employees were classified as phase 1a employees not in the listed categories. Other work location was defined as locations not included in the listed categories.
Discussion
This study conducted in an acute-care hospital and surrounding community clinics demonstrates the effectiveness of vaccinating HCP with BNT162b2 or mRNA-1273, resulting in a significant decrease of COVID-19 cases despite a local surge of cases during the concurrent period. A single dose of either vaccine decreased the risk of COVID-19 by 48% after the first dose and 83% after the second dose. The case positivity rate dropped to 0.04% at 2 weeks after the second vaccine dose. Although the number of COVID-19 cases after vaccination were small, thus precluding definitive comparisons of postvaccine infection rates by occupation or location, we found a lower risk among physicians compared to other job types, and a similar risk by practice location among nurses.
The findings of this study are consistent with those from the phase 3 trials of mRNA vaccines7,8 as well as recent real-world observational studies of vaccination campaigns among HCP9–12 and mass vaccination of Israeli citizens.16 As expected, all studies, including this one, showed that vaccination resulted in a major reduction in new cases of COVID-19 as well as the rarity of a positive test result 2 weeks after administration of the second dose of vaccine. Our findings expand on these reports by demonstrating that COVID-19 risk was similar across job types and work locations, which complements a similar finding noted in the HEROES-RECOVER study. This recent prospective cohort of 3,950 HCP and frontline essential workers found no difference in COVID-19 vaccine effectiveness by occupation.17 Our finding of lower COVID-19 risk among physicians may be explained by the inclusion of all physicians in our analysis, rather than only those with high-risk exposures (eg, emergency department, intensive care, and COVID-19 hospitalists). UCDH also witnessed widespread adoption of video visits during the pandemic, and it is tempting to speculate that the use of telehealth may have provided some protection among non–hospital-based physicians. In addition, we observed a numerically higher risk of COVID-19 among respiratory therapists. Although the lack of statistical significance may be explained by the relatively small sample size of respiratory therapists, the findings are plausible and could be due to their greater exposure to droplets and aerosols during aerosol-generating procedures such as intubations and sputum inductions (hazard ratio, 1.75; 95% CI, 0.89–3.43; P = .104). The differential exposure to infectious virus across occupations could affect risk in the unvaccinated and drive breakthrough infections in vaccinated employees. Factors affecting breakthrough infections will need to be examined in future studies.
Although data regarding COVID-19 risk and mRNA vaccine effectiveness among HCP are limited, particularly related to risk stratification by job type or location, more is known about influenza risk and vaccine acceptance in HCP. HCP have an increased risk of symptomatic and asymptomatic influenza infection18–20 though vaccination significantly reduces infection incidence.21 Job-related risk of influenza among HCP varies by study. One case-control study of the 2009 H1N1 pandemic found that physicians had 6 times higher odds of infection compared to other professions (eg, technicians) (OR, 6.03; 95% CI, 2.11–17.82),22 and another study noted that nurses had a similarly elevated risk compared to allied health staff.23 Compared to the mRNA COVID-19 vaccines, the influenza vaccine has a much lower adjusted effectiveness, ranging from 19% to 60% depending on the year.24,25
Vaccine hesitancy remains a persistent barrier to combating COVID-19, both in HCP and the general public.26 Our data show the highest acceptance of COVID-19 vaccination was among physicians and lowest among nonclinical HCP, although the latter group (non–phase 1a employees) in our study was below 57.7% acceptance. The rates of SARS-CoV-2 vaccination acceptance follow a similar pattern to the rates of influenza vaccination, of which more is known. Although influenza vaccine is less effective, HCP vaccination rates have been high, as noted in annual opt-in Internet panel surveys conducted for the Centers for Disease Control and Prevention, ranging from 78.4% to 81.1% during the last 5 years.27 During 2019–2020, influenza vaccination coverage was highest among physicians (98%) followed by nurses (92%) and was lowest among nonclinical HCP (77%).27
Although reasons for uncertainty over COVID-19 vaccination are complex, recent studies have found that substantial proportions of HCP have concerns about unknown long-term risks and acute side effects, as well as mistrust in the regulatory process.26,28 A recent survey conducted by the Kaiser Family Foundation and The Washington Post examined vaccination rates and concerns in 1,327 frontline HCP and 971 non-HCP.29 The survey documented concerns over side effects and lack of trust in vaccine safety and efficacy as major barriers to vaccination. Substantial differences in trust differed by race, level of education, and political affiliation. We hope that the high real-world COVID-19 vaccine effectiveness that we and others have reported will ultimately be able to overcome HCP concerns. It is clear from the available literature to date that targeted education and outreach, and ensuring easy employee access, will be critical for maximizing HCP vaccination rates among those not yet vaccinated.
This study has several limitations. We estimated through epidemiological investigations that 70% of UCDH HCP COVID-19 cases were due to community or unknown transmission during the study period rather than occupational transmission, potentially limiting interpretation of infection by job type and work location. Furthermore, we made the assumption that the risk of SARS-CoV-2 exposure outside the workplace was similar among all employees, but we acknowledge that there may be differences as a result of various factors, including socioeconomic status and an individual’s ability to take measures to avoid exposures outside work. This factor could have confounded our comparison of differential risk across HCP groups. Despite a relatively large total cohort size, the small number of infections seen after vaccination also limits interpretation of our between-group comparisons. As seen in other observational cohort studies, confounding factors due to differences between vaccinated and unvaccinated persons and their personal behaviors may have influenced this study. Those who adhere more to social distancing practices and wear personal protective equipment both in the community and at work may be more likely to be vaccinated and/or to seek routine asymptomatic or prompt diagnostic testing. The number of SARS-CoV-2 infections after vaccination was likely underestimated since routine asymptomatic testing is available but not required at UCDH, and our results could differ in the setting of mandatory asymptomatic screening. Other factors may have contributed to decreased rates of infection among HCP: a limited visitor policy, reinforcement of appropriate PPE while caring for COVID-19–positive or –suspected patients, emphasizing social distancing in the workplace, and a national information campaign decreasing contacts with families during December that occurred synchronously during the COVID-19 vaccination campaign.
Despite a decrease in local cases of COVID-19 during December 2020–March 2021, there was a significant difference in infection rates among vaccinated and unvaccinated HCP during a healthcare system-wide vaccination campaign. Our results indicate that vaccination can lead to a major reduction in transmission of SARS-CoV-2 in HCP. Our study highlights the importance of targeted outreach efforts to improve vaccination rates in HCP with low vaccination acceptance.
Acknowledgments
We thank Christopher Hilscher, MBA, and Irene Cortes-Puch, MD, for important contributions to the provisioning of data for this project. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support
J.Y.A., S.L.M., C.V.H., and A.Y.L received support from the UC Davis Health Innovation Technology Data Provisioning Core. S.L.T received support from the National Center for Advancing Translational Sciences, National Institutes of Health (grant no. UL1 TR001860).
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
References
- 1.COVID data tracker. Centers for Disease Control and Prevention website. https://covid.cdc.gov/covid-data-tracker. Accessed July 1, 2021.
- 2.Cohen J.The line starts to form for a coronavirus vaccine. Science 2020;369:15–16. [DOI] [PubMed] [Google Scholar]
- 3.Kambhampati AK, O’Halloran AC, Whitaker M, et al. COVID-19–associated hospitalizations among healthcare personnel—COVID-NET, 13 states, March 1–May 31, 2020. Morb Mortal Wkly Rep 2020;69:1576–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stubblefield WB, Talbot HK, Feldstein L, et al. Seroprevalence of SARS-CoV-2 among frontline healthcare personnel during the first month of caring for COVID-19 patients— Nashville, Tennessee. Clin Infect Dis 2021;72:1645–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Self WH, Tenforde MW, Stubblefield WB, et al. Seroprevalence of SARS-CoV-2 among frontline healthcare personnel in a multistate hospital network—13 academic medical centers, April–June 2020. Morb Mortal Wkly Rep 2020;69:1221–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 vaccine—United States, December 2020. Morb Mortal Wkly Rep 2021;69:1657–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Eng J Med 2021;384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N Engl J Med 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benenson S, Oster Y, Cohen MJ, Nir-Paz R.BNT162b2 mRNA COVID-19 vaccine effectiveness among healthcare workers. N Engl J Med 2021;384:1775–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keehner J, Horton LE, Pfeffer MA, et al. SARS-CoV-2 infection after vaccination in healthcare workers in California. N Engl J Med 2021;384:1774–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniel W, Nivet M, Warner J, Podolsky DK.Early evidence of the effect of SARS-CoV-2 vaccine at one medical center. N Engl J Med 2021;384:1962–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angel Y, Spitzer A, Henig O, et al. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among healthcare workers. JAMA 325:2457–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. The Equator Network website. https://www.equator-network.org/reporting-guidelines/strobe/. Published 2021. Accessed July 20, 2021.
- 14.R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2020.
- 15.rms: regression modeling strategies. version R package version 6.1-12021. R Project website. https://cran.r-project.org/web/packages/rms/index.html. Accessed July 20, 2021.
- 16.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021;384:1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson MG, Burgess JL, Naleway AL, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among healthcare personnel, first responders, and other essential and frontline workers—eight US locations, December 2020–March 2021. Morb Mortal Wkly Rep 2021;70:495–500. [DOI] [PMC free article] [PubMed]
- 18.Elder AG, O’Donnell B, McCruden EA, Symington IS, Carman WF.Incidence and recall of influenza in a cohort of Glasgow healthcare workers during the 1993–1994 epidemic: results of serum testing and questionnaire. BMJ 1996;313:1241–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lietz J, Westermann C, Nienhaus A, Schablon A.The occupational risk of influenza a (H1N1) infection among healthcare personnel during the 2009 pandemic: a systematic review and meta-analysis of observational studies. PloS One 2016;11:e0162061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilde JA, McMillan JA, Serwint J, Butta J, O’Riordan MA, Steinhoff MC.Effectiveness of influenza vaccine in healthcare professionals: a randomized trial. JAMA 1999;281:908–913. [DOI] [PubMed] [Google Scholar]
- 21.Imai C, Toizumi M, Hall L, Lambert S, Halton K, Merollini K.A systematic review and meta-analysis of the direct epidemiological and economic effects of seasonal influenza vaccination on healthcare workers. PloS One 2018;13:e0198685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lobo RD, Oliveira MS, Garcia CP, Caiaffa Filho HH, Levin AS.Pandemic 2009 H1N1 influenza among healthcare workers. Am J Infect Control 2013;41:645–647. [DOI] [PubMed] [Google Scholar]
- 23.Chen MI, Lee VJ, Barr I, et al. Risk factors for pandemic (H1N1) 2009 virus seroconversion among hospital staff, Singapore. Emerg Infect Dis 2010;16:1554–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CDC seasonal flu vaccine effectiveness studies. Centers for Disease Control and Prevention website. https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm. Accessed June 24, 2021.
- 25.Tenforde MW, Kondor RJG, Chung JR, et al. Effect of antigenic drift on influenza vaccine effectiveness in the United States—2019–2020. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa1884. [DOI] [PMC free article] [PubMed]
- 26.Rosenbaum L.Escaping catch-22—overcoming COVID vaccine hesitancy. N Engl J Med 2021;384:1367–1371. [DOI] [PubMed] [Google Scholar]
- 27.Acero C, Razzaghi H, Black CL, Wesley MG, Jeddy Z, Lindley MC. Influenza vaccination coverage among healthcare personnel—United States, 2019–20 influenza season. 2020. Centers for Disease Control and Prevention website. https://www.cdc.gov/flu/fluvaxview/hcp-coverage_1920estimates.htm. Accessed June 24, 2021.
- 28.Meyer MN, Gjorgjieva T, Rosica D.Trends in healthcare worker intentions to receive a COVID-19 vaccine and reasons for hesitancy. JAMA Netw Open 2021;4:e215344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirzinger A, Kearney A, Hamel L, Brodie M. KFF/The Washington Post frontline healthcare workers survey—vaccine intentions. Kaiser Family Foundation website. https://www.kff.org/report-section/kff-washington-post-frontline-health-care-workers-survey-vaccine-intentions/. Published 2021. Accessed April 19, 2021.