Abstract
Hepatocellular carcinoma (HCC) is among the most common malignancies and has an unfavorable prognosis. The hepatitis B virus-encoded X (HBx) protein is closely associated with hepatocarcinogenesis. Sorafenib is a unique targeted oral kinase inhibitor for advanced HCC. Long noncoding RNAs (lncRNAs) mediate HCC progression and therapeutic resistance by acting as competing endogenous RNAs (ceRNAs). However, the ceRNA regulatory mechanisms underlying sorafenib resistance in HBx-associated HCC remain largely unknown. In this study, we found that translation regulatory lncRNA 1 (TRERNA1) upregulation by HBx not only promoted HCC cell proliferation by regulating the cell cycle in vitro and in vivo but also correlated positively with poor prognosis in HCC. Importantly, TRERNA1 enhanced sorafenib resistance in HCC cells. RNA sequencing (RNA-seq) analysis indicated that NRAS proto-oncogene (NRAS) is a potential target of TRERNA1 that mediates aspects of hepatocellular carcinogenesis. TRERNA1 acts as a ceRNA to regulate NRAS expression by sponging microRNA (miR)-22-3p. In summary, we show that increased TRERNA1 expression induced by HBx reduces HCC cell sensitivity to sorafenib by activating the RAS/Raf/MEK/ERK signaling pathway. We reveal a novel regulatory mode by which the TRERNA1/miR-22-3p/NRAS axis mediates HCC progression and indicates that TRERNA1 might constitute a powerful tumor biomarker and therapeutic target in HCC.
Keywords: HCC, TRERNA1, miR-22-3p, NRAS, sorafenib
Graphical abstract

The regulatory mechanisms underlying sorafenib resistance in HBV-associated hepatocellular carcinoma (HCC) therapy remain largely unclear. In this study, lncRNA TRERNA1 induced by HBx presents impeding sorafenib sensitivity to HCC cells by activating the RAS/Raf/MEK/ERK signaling pathway. These findings may provide a novel potential tumor biomarker and therapeutic target in HCC.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide, with a high recurrence rate and poor prognosis.1 Although surgical resection, local ablation of the tumor site, and liver transplantation are helpful for the treatment of early liver cancer, the prognosis of HCC is still poor, due to late diagnosis, rapid progression, and recurrence.2,3 Therefore, it is essential to understand the molecular mechanism of HCC initiation and progression. The identification of new diagnostic biomarkers and therapeutic targets for HCC has become an urgent problem to be solved.
Accumulated evidence implies that chronic hepatitis B virus (HBV) infection is the major cause of HCC. The HBV-encoded X protein (HBx) exerts various biological effects via genetic aberrations and epigenetic dysregulation during the progression of HCC.4,5 Long noncoding RNAs (lncRNAs) are a class of endogenous non-protein-coding RNAs greater than 200 nucleotides in length.6 lncRNAs perform a broad range of functions in chromatin modification, transcription, and post-transcriptional regulation, acting as signals, decoys, guides, scaffolds, and competing endogenous RNAs (ceRNAs).7, 8, 9 lncRNAs have emerged as crucial biomarkers and regulators in many cancers. Aberrant expression of lncRNAs in the development of HCC has attracted extensive attention.10 We previously demonstrated that HBx elevated expression of the oncogenic lncRNA UCA1 to promote HCC by repressing p27Kip1.11 However, it remains unclear whether lncRNAs induced by HBx play an important role in HCC progression. Additional lncRNAs that are involved in the initiation and progression of HBx-related HCC need to be investigated.
The translation regulatory lncRNA 1 (TRERNA1), located on chromosome 20q13.13, was first identified as an enhancer-like oncogene in lung cancer,12 and a recent study revealed that TRERNA1 is involved in the metastasis of several cancers such as breast cancer and gastric cancer.13,14 Our previous studies have shown that TRERNA1 facilitates HCC metastasis by dimethylating H3K9 in the CDH1 promoter region via recruitment of the EHMT2/SNAI1 complex.15 In the current study, we report that HBx regulates TRERNA1 expression, indicating its potential function during HBV-related HCC carcinogenesis.
Sorafenib is a small molecule inhibitor of Raf kinase used to treat advanced HCC.16 The RAS-Raf-mitogen-activated protein kinase (MAPK) kinase (MEK)-extracellular signal-regulated kinase (ERK) cascade is a key oncogenic signal transduction pathway activated in various tumors in humans.17 The medical treatment of choice for advanced-stage HCC was initially developed to inhibit the RAS-Raf-MEK-ERK cascade in HCC cells.18 However, sorafenib resistance remains a major obstacle for a category of patients who are inherently nonresponsive to sorafenib.19,20 Several studies have demonstrated that aberrant lncRNA expression levels reduce the drug sensitivity of tumors; overall, the roles of lncRNAs underlying HCC in sorafenib resistance are poorly understood.21, 22, 23 Thus, exploration of the lncRNAs induced by HBx may help to elucidate the mechanism of HCC progression and evaluate therapeutic implications for sorafenib-resistant HCC, providing new strategies for the diagnosis and treatment of liver cancer.
Here, we reveal that upregulation of the lncRNA TRERNA1 by HBx not only correlates positively with the poor prognosis of HCC but also promotes cell proliferation and cell-cycle progression in vitro and in vivo. Furthermore, our study suggests that TRERNA1 regulates expression of NRAS by sponging microRNA (miR)-22-3p; thus, we demonstrate that TRERNA1 and miR-22-3p may be critical determinants of sorafenib tolerance by regulating the RAS-Raf-MEK-ERK pathway. Our data shed new light on understanding the therapeutic benefit of interactions between lncRNAs and microRNAs (miRNAs) in sorafenib treatment and cell proliferation in HCC.
Results
TRERNA1 upregulation induced by HBx correlates positively with HBx expression level and poor prognosis in HCC patients
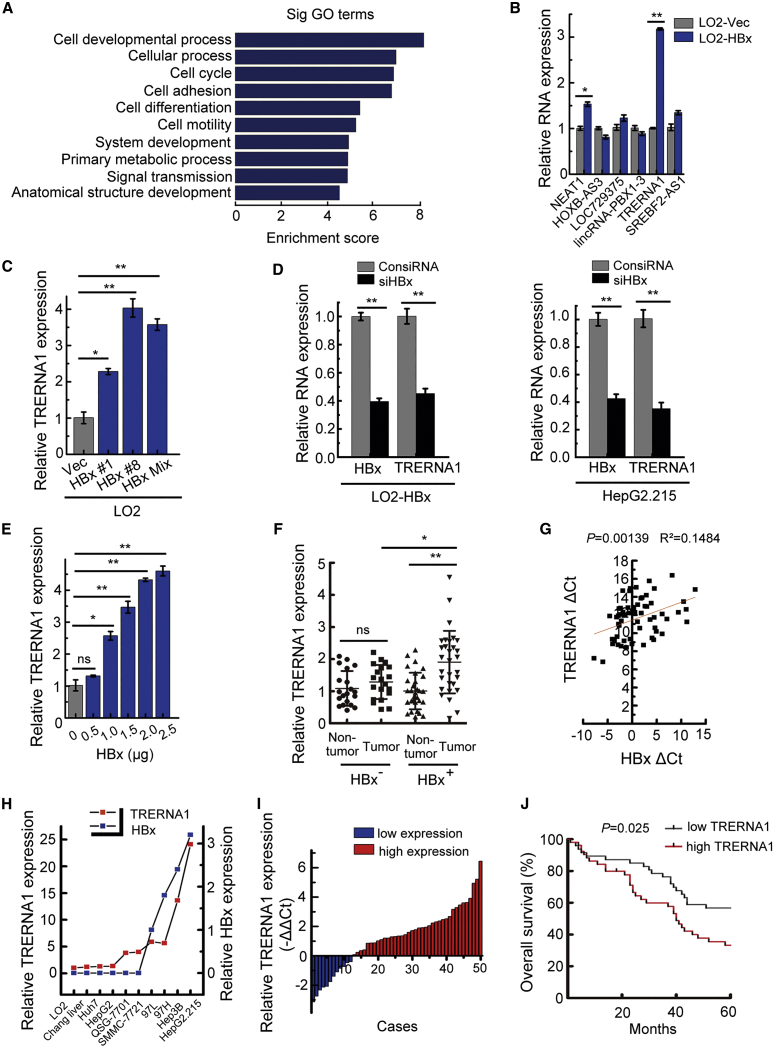
To identify differentially expressed ncRNAs regulated by HBx, we used a complementary DNA (cDNA) microarray to analyze lncRNA and mRNA expression profiles in LO2-HBx cells compared with the corresponding control LO2-vec cells. A total of 379 upregulated lncRNAs were classified by their position in the genome relative to protein-coding genes. Long intergenic ncRNAs (lincRNAs) accounted for 62% (234/379) of the lncRNAs identified (Figures S1A and S1B). The biological process category containing 456 upregulated protein-coding genes was subjected to Gene Ontology (GO) analysis to evaluate the top 10 functions of the most abundant genes after HBx overexpression. These abundant genes were enriched in processes of cell cycle and development (Figure 1A), implying that they may be significantly associated with tumor progression. Via co-expression network analysis of upregulated lncRNAs and mRNAs, 6 relevant lncRNAs were identified as candidates for further validation using quantitative reverse-transcriptase PCR (qRT-PCR). TRERNA1 was considered a potential candidate because of its relatively high expression level after transfection with HBx (Figure 1B). Next, we further confirmed the microarray data in LO2 cells subjected to different treatments and found that TRERNA1 expression was increased in LO2 cells stably transfected with HBx compared with the corresponding control cells (Figure 1C). When HBx was knocked down, as expected, TRERNA1 was significantly downregulated in LO2-HBx and HepG2.215 cells (Figure 1D). Additionally, TRERNA1 was upregulated by HBx in a manner dependent on the HBx level in the evaluated cells (Figure 1E).
Figure 1.
Increased TRERNA1 levels correlate with HBx expression and poor prognosis in HCC patients
(A) Bar plot showing the top ten most significantly enriched biological process Gene Ontology (GO) terms and their scores. Enrichment score, −log10 (p value). (B) Six representative differentially expressed long noncoding RNAs (lncRNAs) were selected and validated by quantitative real-time PCR. (C) The expression level of translation regulatory lncRNA 1 (TRERNA1) was measured by quantitative real-time PCR in LO2 cells stably transfected with HBx constructs or control (Ctrl) vector. LO2-HBx #1 and LO2-HBx #8 are two different stable HBx transfectant clones, and LO2-HBx Mix is the mixture of LO2 cells transfected with HBx. (D) TRERNA1 expression levels were measured by quantitative real-time PCR after knocking down HBx in LO2-HBx cells and in HepG2.215 cells. (E) The relative expression level of TRERNA1 was measured in LO2 cells after transient transfection with different doses of HBx expression constructs. (F) TRERNA1 expression was analyzed by quantitative real-time PCR in HBx-negative and HBx-positive HCC tissues (n = 50). (G) The correlation between the TRERNA1 transcript level and the HBx mRNA level was measured in HBx-positive tumor (n = 30) and paired nontumor (n = 30) tissues. Pearson correlation analysis was performed on the ΔCt values (normalized to β-actin) (p = 0.00139, R2 = 0.1484). (H) qRT-PCR analysis showing the correlation between the TRERNA1 level and the HBx mRNA level in 10 liver or HCC cell lines. (I) The relative expression patterns of TRERNA1 in paired HCC tissues and normal tissues as detected by quantitative real-time PCR are shown. Fifty patient samples were included in the analysis. β-actin was used as the internal control gene. (J) Kaplan-Meier curves showing the survival of HCC patients grouped by the TRERNA1 expression level in the HCC TMA (p = 0.025). The data are presented as the mean ± SD values; n = 3. ∗p < 0.05, ∗∗p < 0.01. ns, nonsignificant.
To investigate whether TRERNA1 expression is related to HBx expression in HCC, we next used qRT-PCR to analyze the relationship between the expression levels of TRERNA1 and HBx using HBx-negative (n = 20) and HBx-positive (n = 30) HCC tissues. TRERNA1 expression was increased in HBx-positive tumor tissues compared with HBx-negative control (NC) tumor tissues (Figure 1F). A positive correlation was observed between TRERNA1 and HBx levels in HBx-positive tumor (n = 30) and paired nontumor (n = 30) tissues based on the Pearson correlation coefficient (Figure 1G). In addition, a positive correlation between the expression level of TRERNA1 and HBx was observed in HCC cells (Figure 1H). We evaluated the value of elevated TRERNA1 for predicting HCC patient prognosis and found that TRERNA1 expression levels were higher in tumor tissues than in the paired adjacent nontumor tissues (Figure 1I). Next, we examined TRERNA1 expression in HCC tissue microarray (TMA) using in situ RNA hybridization. Based on the detailed scoring of TRERNA1 staining, patients were divided into a high-expression group (n = 55) and a low-expression group (n = 55) according to the median expression level (Figures S1C and S1D). As shown in Figure 1J, higher TRERNA1 expression indicated a poorer prognosis. Collectively, these data showed that aberrant TRERNA1 upregulation correlates with HCC development, implying that TRERNA1 may play an important role in the progression and prognosis of HCC.
TRERNA1 upregulation induced by HBx attenuates sorafenib sensitivity in HCC cells
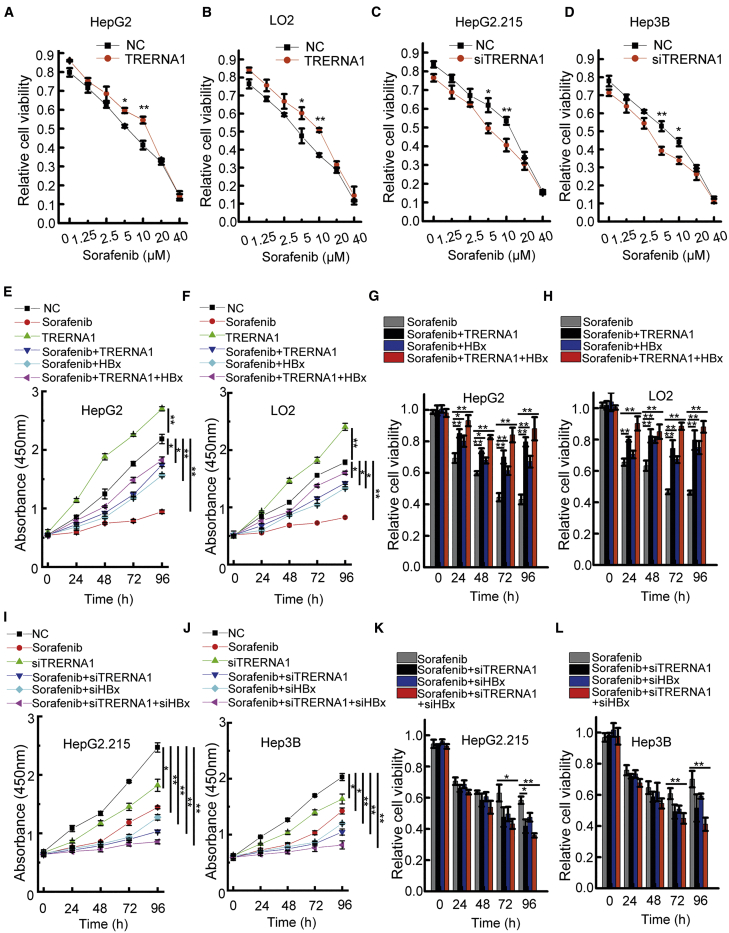
Sorafenib, a clinically approved kinase inhibitor, is currently the medical treatment of choice for patients with advanced-stage HCC. Sorafenib tolerance is a cause of treatment failure in patients with HCC. Several lncRNAs have been reported to mediate the regulation of drug resistance in cancer cells. We next examined whether TRERNA1 upregulation by HBx could confer sorafenib tolerance to increase the viability of HCC cells. A Cell Counting Kit-8 (CCK-8) assay was performed to evaluate the sensitivity of HCC cells to sorafenib. The combination of TRERNA1 transfection and sorafenib treatment (0–40 μM) for 48 h in HepG2 and LO2 cells significantly attenuated cell sensitivity to sorafenib compared with that in the corresponding control cells (Figures 2A, 2B, S2A, and S2B). Conversely, as shown in Figures 2C and 2D, TRERNA1 suppression in HepG2.215 and Hep3B cells increased sorafenib sensitivity across a concentration gradient of sorafenib (Figures S2C and S2D). Interestingly, we examined TRERNA1 expression levels in 10 hepatoma cell lines by qRT-PCR (Figure S1F) and found that HepG2.215 and Hep3B cell lines with high TRERNA1 expression were insensitive to sorafenib. However, LO2 and HepG2 cell lines with low TRERNA1 expression were sensitive to sorafenib.
Figure 2.
Effect of TRERNA1 on the sorafenib sensitivity of HCC cells
(A and B) TRERNA1 upregulation reduced the sorafenib sensitivity of HepG2 and LO2 cells after transfection with TRERNA1 or control treatment with different doses (0–40 μM) of sorafenib for 48 h. (C and D) TRERNA1 silencing enhanced the sensitivity of HepG2.215 and Hep3B cells to sorafenib after transfection with siTRERNA1 or control treatment with different doses (0–40 μM) of sorafenib for 48 h. (E and F) A CCK-8 assay was conducted in HepG2 (sorafenib 7.5 μM) or LO2 (sorafenib 5 μM) cells treated with sorafenib, TRERNA1, sorafenib + TRERNA1, sorafenib + HBx, sorafenib + TRERNA1 + HBx, or NC. (G and H) After transfection with NC, TRERNA1, HBx, or TRERNA1 + HBx, the viability of HepG2 (sorafenib 7.5 μM) and LO2 (sorafenib 5 μM) cells treated with sorafenib was assessed. (I and J) A CCK-8 assay was conducted in HepG2.215 (sorafenib 9 μM) and Hep3B (sorafenib 6 μM) cells treated with sorafenib, siTRERNA1, sorafenib + siTRERNA1, sorafenib + siHBx, sorafenib + siTRERNA1 + siHBx, or NC. (K and L) The viability of HepG2.215 (sorafenib 9 μM) and Hep3B (sorafenib 6 μM) cells transfected with NC, siTRERNA1, siHBx, or siTRERNA1 + siHBx and treated with sorafenib was assessed. The data are shown as the mean ± SD values; n = 3. ∗p < 0.05, ∗∗p < 0.01 (Student’s t test).
Our findings showed that TRERNA1 upregulation by HBx promoted sorafenib resistance in HCC cells. To evaluate the therapeutic value of agents targeting TRERNA1 on enhancing chemosensitivity, CCK-8 assays were conducted in HepG2 and LO2 cells transfected with TRERNA1 and HBx and then treated with sorafenib. As shown in Figures 2E and 2F, sorafenib decreased the viability of HepG2 and LO2 cells when administered alone. TRERNA1 significantly increased cell viability. However, in the other three groups (sorafenib + TRERNA1, sorafenib + HBx, and sorafenib + HBx + TRERNA1), TRERNA1 and HBx transfection combination with sorafenib treatment reduced the inhibition of cell proliferation in these two cell lines. Furthermore, cell viability in the TRERNA1 and HBx groups was not evidently reduced by combination treatment with sorafenib compared with that in the group treated with sorafenib alone (Figures 2G and 2H). In HepG2.215 and Hep3B cells, combination treatment with sorafenib and small interfering (si)TRERNA1 inhibited proliferation; in addition, cell proliferation was inhibited in the sorafenib + siTRERNA1, sorafenib + siHBx, sorafenib + siTRERNA1, and siHBx-treated groups compared with the control group (Figures 2I and 2J). Moreover, cell viability in the groups treated with combinations of sorafenib, siTRERNA1, and siHBx indicated that proliferation was reduced more significantly than in the group treated with sorafenib alone (Figures 2K and 2L). Taken together, these results indicated that TRERNA1 upregulation induced by HBx treatment attenuated the sensitivity of HCC cells to sorafenib.
TRERNA1 promotes HCC cell proliferation in vitro and in vivo
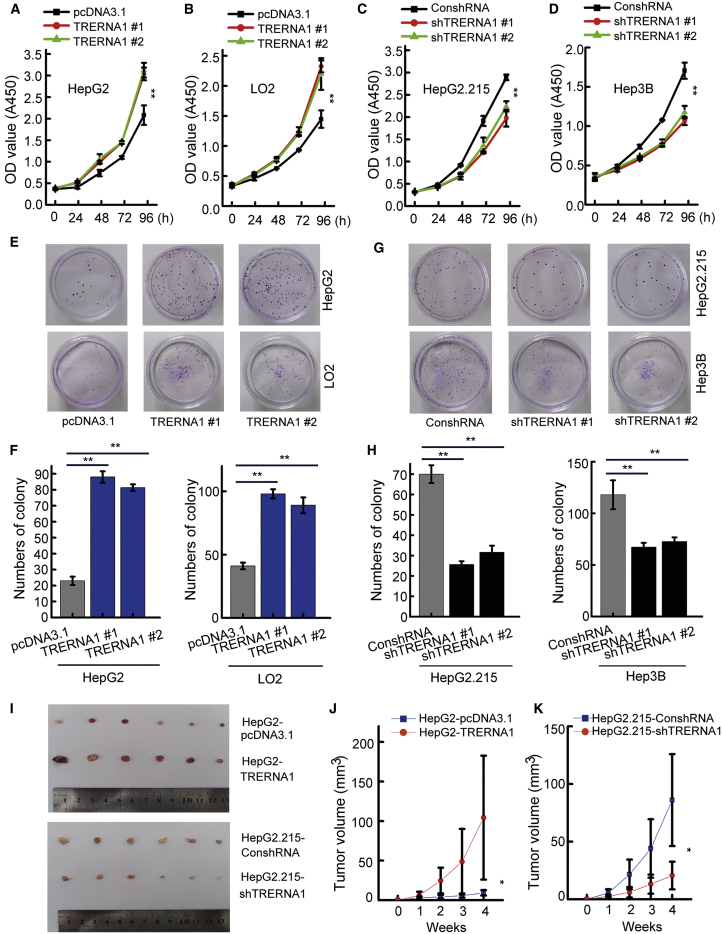
To reveal the potential function of TRERNA1 in HCC carcinogenesis, we overexpressed TRERNA1 in the HCC cell line HepG2 and the immortalized liver cell line LO2 (Figures S3A and S3B). Then, efficient short hairpin (sh)RNA targeting TRERNA1 was employed to knock down TRERNA1 in HepG2.215 and Hep3B cells (Figures S3C and S3D). CCK-8 and focus-formation assays showed that TRERNA1 overexpression increased the proliferative ability of LO2 and HepG2 cells. In contrast, TRERNA1 knockdown significantly reduced the proliferation of HepG2.215 and Hep3B cells (Figures 3A−3H). We further evaluated whether TRERNA1 regulates the tumorigenicity of HCC cells in vivo (Figures 3I, S3E, and S3F). In the tumor xenograft model, TRERNA1 overexpression significantly promoted tumor growth (Figure 3J). We also found that knockdown of TRERNA1 inhibited tumorigenicity compared with that in the control group (Figure 3K). Collectively, these results supported an oncogenic role for TRERNA1 in HCC.
Figure 3.
TRERNA1 promotes cell proliferation in vitro and in vivo
(A and B) TRERNA1 promoted the proliferation of HepG2 and LO2 cells, as assessed by a CCK-8 assay. (C and D) Knockdown of TRERNA1 reduced the proliferation of HepG2.215 and Hep3B cells, as assessed by a CCK-8 assay. (E and F) Focus-formation assays showed that the overexpression of TRERNA1 increased the colony numbers of HepG2 and LO2 cells. (G and H) Focus-formation assays showed that suppression of TRERNA1 reduced the colony numbers of HepG2.215 and Hep3B cells. (I) An orthotopic xenograft model was established in nude mice with cells transfected with TRERNA1 or shTRERNA1. (J) Tumor volume curves for nude mice in the TRERNA1 or control groups. (K) Tumor volume curves for nude mice in the shTRERNA1 and control groups. The data are presented as the mean ± SD values. ∗p < 0.05, ∗∗p < 0.01 (Student’s t test).
TRERNA1 promotes HCC cell growth by promoting cell-cycle progression
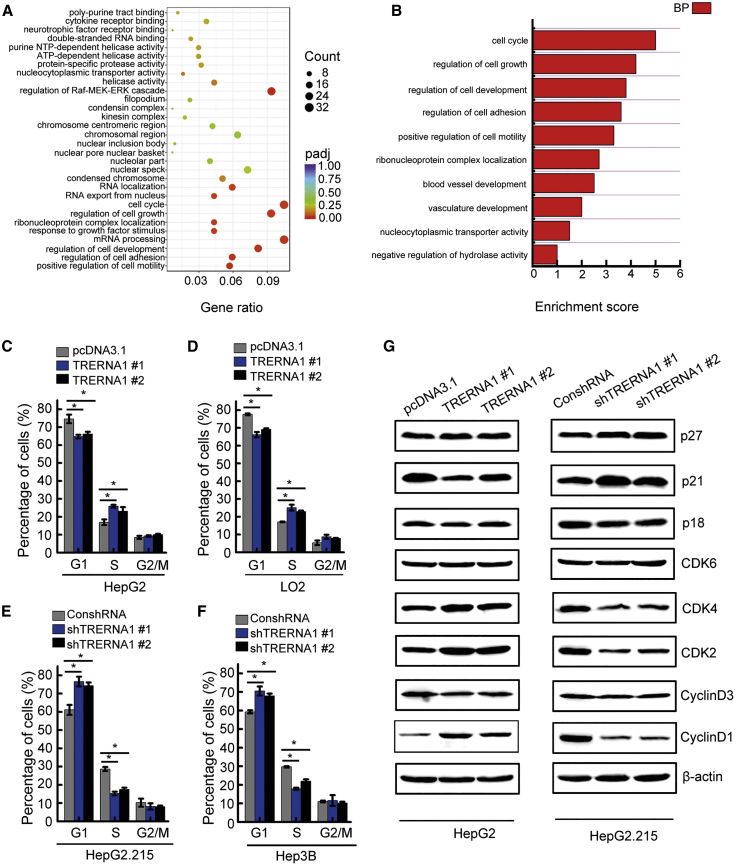
To explore the cause of the increased cell proliferation and molecular alterations in TRERNA1, we performed mRNA sequencing (mRNA-seq) in HCC cells after overexpression of TRERNA1. A total of 1,598 transcripts were upregulated (expression increased at least 2-fold) in TRERNA1-overexpressing cells, whereas 958 genes were downregulated as evaluated by the corresponding cutoff. Subsequently, GO analysis showed that the genes altered by TRERNA1 overexpression were enriched in the cell-cycle term, indicating that TRERNA1 promotes HCC cell growth by regulating the term “cell cycle” (Figures 4A and 4B). Next, flow cytometric analysis was performed to explore the alteration in cell-cycle progression in TRERNA1-overexpressing and TRERNA1-knockdown HCC cells. The results of the cell-cycle assay showed that TRERNA1 overexpression accelerated cell-cycle progression through the G1/S checkpoint in LO2 and HepG2 cells (Figures 4C and 4D), but downregulation of TRERNA1 induced cell-cycle arrest at the G1/S-phase boundary in HepG2.215 and Hep3B cells compared with the corresponding NC cells (Figures 4E and 4F). In addition, western blot analysis revealed that TRERNA1 overexpression upregulated expression of cyclin D1, cyclin-dependent kinase (CDK)2, and CDK4, which are G1/S regulators. Moreover, the expression levels of these cell-cycle-related proteins were decreased in cells with TRERNA1 downregulation (Figure 4G).
Figure 4.
TRERNA1 promotes HCC cell growth by promoting cell-cycle progression
(A) GO analysis of differentially expressed coding genes related to TRERNA1 overexpression in biological process categories. The top 20 GO terms are shown. (B) The biological process involving TRERNA1 was analyzed in TRERNA1-overexpressing HCC cells. (C and D) Flow cytometry shows the cell-cycle distribution upon TRERNA1 overexpression in HepG2 and LO2 cells. The bar charts indicate the relative percentages of cells in each phase. (E and F) Flow cytometry shows the cell-cycle distribution upon TRERNA1 knockdown in HepG2.215 and Hep3B cells. The bar charts indicate the relative percentages of cells in each phase. (G) Representative cell-cycle regulators, including cyclin-dependent kinases (CDKs) and CDK inhibitors (CDKIs), were examined in HepG2 cells with ectopic expression of TRERNA1 and in HepG2.215 cells with TRERNA1 knockdown. The data are presented as the mean ± SD values. ∗p < 0.05 (Student’s t test).
NRAS is a potential target of TRERNA1
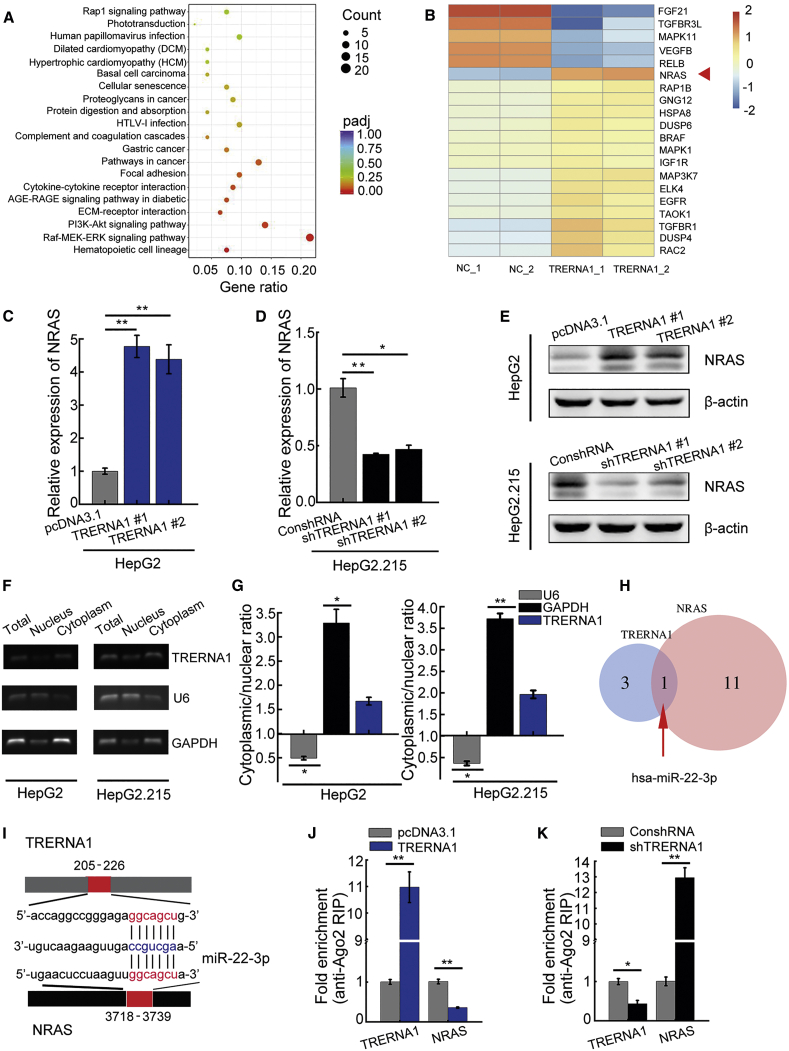
We found that the Raf-MEK-ERK signaling pathway was significantly enriched based on Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of the genes identified by RNA sequencing (RNA-seq) as being differentially expressed (Figure 5A). The heatmap suggested that NRAS is a potential target of TRERNA1 (Figure 5B), and subsequent qRT-PCR and western blot analyses confirmed NRAS expression to be suppressed after TRERNA1 knockdown in HepG2.215 cells (Figures 5C−5E). To explore the mechanisms underlying TRERNA1-mediated upregulation of NRAS, we separated the nuclear and cytosolic fractions and found that TRERNA1 was enriched in the cytoplasmic fraction (Figures 5F and 5G). lncRNAs can participate in post-transcriptional regulation as ceRNAs for miRNAs in the cytoplasm.24, 25, 26 We thus speculated that TRERNA1 may exert its effects by sponging miRNAs to upregulate expression of NRAS. Therefore, to investigate the effect of TRERNA1 and NRAS on miRNA expression, we performed bioinformatics analysis, which predicted that miR-22-3p harbors a common target binding site for both TRERNA1 and the NRAS 3′ UTR (Figures 5H and 5I). miRNAs degrade their binding targets in a manner dependent on Ago2, the core component of the RNA-induced silencing complex.27 We performed an anti-Ago2 RNA immunoprecipitation (RIP) assay to confirm this effect of miRNAs. Overexpression of TRERNA1 in HepG2 cells increased TRERNA1 enrichment but led to a significant decrease in Ago2 enrichment on NRAS transcripts (Figure 5J). In parallel, we observed increasing enrichment of Ago2 on NRAS transcripts in HepG2.215 cells with TRERNA1 knockdown compared with that in the corresponding control cells (Figure 5K). These results suggested that TRERNA1 can compete with NRAS in an Ago2-dependent manner.
Figure 5.
NRAS is a target of TRERNA1
(A) KEGG pathway analysis of coding genes with differential expression induced by TRERNA1 overexpression indicated that the Raf-MEK-ERK signaling pathway is the most enriched pathway. (B) Heatmap representation of RNA-seq results for transcription factors involved in the Raf-MEK-ERK pathway. The NRAS gene is indicated by the arrow. (C) The NRAS expression level was analyzed by qRT-PCR in the TRERNA1-overexpression HCC cell line HepG2-TRERNA1. (D) NRAS expression was analyzed by qRT-PCR in the TRERNA1-knockdown HCC cell line HepG2.215-shTRERNA1. (E) Western blot analysis showed increased expression of NRAS in HepG2-TRERNA1 cells and decreased expression of NRAS in HepG2.215-shTRERNA1 cells. (F and G) The subcellular fractions of TRERNA1 in HepG2 and HepG2.215 cells were analyzed by RT-PCR and qRT-PCR. U6 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used as the controls for the nuclear and cytoplasmic fractions, respectively (n = 3). (H) Venn diagram indicating the number of miRNAs altered after TRERNA1 and NRAS alteration as assessed by bioinformatics analysis. (I) miR-22-3p binding sites were predicted in the TRERNA1 and NRAS transcripts. (J) RIP assay of Ago2 enrichment relative to IgG on TRERNA1 and NRAS transcripts in HepG2 cells transfected with pcDNA3.1-TRERNA1 or control. (K) RIP assay of Ago2 enrichment relative to IgG on TRERNA1 and NRAS transcripts in HepG2.215 cells transfected with control or shTRERNA1. The data are presented as the mean ± SD values. ∗p < 0.05, ∗∗p < 0.01 (Student’s t test).
TRERNA1 activates the NRAS/Raf/MEK/ERK pathway by modulating miR-22-3p
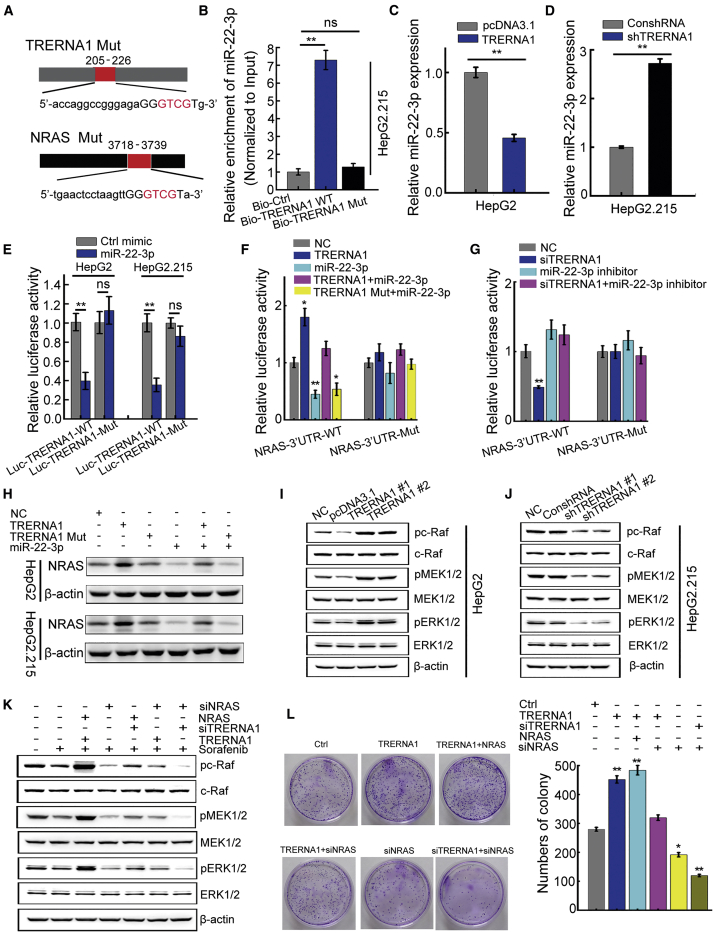
We established TRERNA1 and NRAS constructs with mutations in their miR-22-3p binding sites (Figure 6A). A pull-down assay with biotinylated constructs was performed to confirm the specific binding between TRERNA1 and miR-22-3p. miR-22-3p was significantly enriched in the TRERNA1 precipitate compared with the TRERNA1-mutated (Mut) precipitate (Figure 6B), demonstrating that TRERNA1 can directly and specifically sponge miR-22-3p. We then sought to determine the effect of TRERNA1 on miR-22-3p expression and found that TRERNA1 overexpression suppressed miR-22-3p expression (Figure 6C), whereas knockdown of TRERNA1 enhanced miR-22-3p expression (Figure 6D). Next, we constructed luciferase reporter vectors expressing wild-type TRERNA1 (Luc-TRERNA1-WT) and a Mut form of TRERNA1 (Luc-TRERNA1-Mut). The dual-luciferase reporter assay results revealed that the miR-22-3p mimics suppressed the luciferase activity of TRERNA1-WT more than that of TRERNA1-Mut (Figure 6E). These results indicated that TRERNA1 is a target of miR-22-3p. To explore whether TRERNA1-mediated sequestration of miR-22-3p is responsible for NRAS expression, we constructed luciferase reporters containing the NRAS 3′ UTR with a WT or a Mut miR-22-3p binding site. TRERNA1 transfection elevated the luciferase activity of the NRAS WT reporter but not the mutant reporter, whereas miR-22-3p mimics abolished this effect (Figure 6F). Conversely, the activity of the WT NRAS reporter was decreased upon TRERNA1 knockdown but was restored when cells were transfected with the miR-22-3p inhibitor, whereas the luciferase activity of the mutant NRAS reporter was unchanged (Figure 6G). Moreover, WT TRERNA1, but not mutant TRERNA1, sponged miR-22-3p and decreased NRAS protein expression (Figure 6H). We examine the expression levels of NRAS and miR-22-3p in 20 HBx-positive tissues, and a positive correlation was observed between TRERNA1 and NRAS levels in tumor and paired nontumor tissues based on the Pearson correlation coefficient. In addition, a negative correlation between the expression level of TRERNA1 and miR-22-3p was observed in HCC tissues. However, there was no correlation among HBx, NRAS, and miR22-3p (Figure S4). In addition, we downloaded RNA-seq data and clinical information of liver hepatocellular carcinoma (LIHC) patients from The Cancer Genome Atlas (TCGA) portal. The expression of TRERNA1 and NRAS in normal and HCC samples and their expression correlation were analyzed, respectively (Figures S5A−S5C). Besides, Kaplan-Meier analysis of month survival of cases that express high expression of TRERNA1 and NRAS compared with low expression was also shown in Figures S5D and S5E. Collectively, these data suggested that TRERNA1 functions as a ceRNA sponge for miR-22-3p to modulate NRAS expression.
Figure 6.
TRERNA1 activates the NRAS/Raf/MEK/ERK pathway by modulating miR-22-3p
(A) Schematic outline of miR-22-3p binding-site mutations in TRERNA1 and NRAS. Mutant sequences were shown in red. (B) qRT-PCR was used to evaluate the enrichment of miR-22-3p in the products precipitated with the biotinylated TRERNA1, TRERNA1 mutated (Mut), and Bio-control probes. Input was used for normalization. (C) qRT-PCR analysis of miR-22-3p expression in HepG2 cells transfected with control vector and TRERNA1. (D) qRT-PCR analysis of miR-22-3p expression in HepG2.215 cells transfected with control vector and shTRERNA1. (E) Luciferase activity of TRERNA1 with transfection of control mimic or miR-22-3p in HepG2 and HepG2.215 cells. (F and G) Luciferase reporters of NRAS containing the wild-type or mutant miR-22-3p binding site. (H) Western blot analysis of NRAS in HepG2 and HepG2.215 cells transfected with NC, TRERNA1, TRERNA1 Mut, and miR-22-3p mimic. (I) Western blot analysis showed that TRERNA1 overexpression in HepG2 cells promoted the activation of Raf-MEK-ERK phosphorylation. (J) Western blot analysis showed that knockdown of TRERNA1 in HepG2.215 cells inhibited the activation of Raf-MEK-ERK phosphorylation. (K) Western blot analysis of Raf-MEK-ERK phosphorylation in cells transfected with TRERNA1, siTRERNA1, NRAS, and siNRAS and treated with sorafenib. (L) Focus-formation assay of HepG2.215 cells transfected with TRERNA1, siTRERNA1, NRAS, and siNRAS. The data are presented as the mean ± SD values. ∗p < 0.05, ∗∗p < 0.01 (Student’s t test).
NRAS functions as a key molecule in the Raf-MEK-ERK signal transduction pathway, leading to the phosphorylation of Raf family kinases and subsequently, MEK1/2 and ERK1, the final effectors of the cascade.28 The RAS-Raf-MEK-ERK cascade is a key oncogenic signal transduction pathway activated in HCC,29 and NRAS has emerged as a critical downstream effector of TRERNA1. It has reported that high expression of NRAS possessed poor prognosis of patients with HCC. In our study, we found that NRAS overexpression increased the proliferation of HepG2 cells (Figure S6A). In contrast, NRAS knockdown significantly reduced the proliferation of HepG2.215 cells by CCK-8 assay (Figure S6B). We also found that NRAS works as the essential factor for TRERNA1-mediated sorafenib resistance. NRAS attenuated sorafenib sensitivity in HCC cells (Figures S6C and S6D). With the analysis of the LO2-HBx microarray data, we also found that NRAS/Raf/MEK/ERK molecules had a relatively high expression in LO2-HBx cells compared with control (Figure S1E). To elucidate the molecular mechanisms, we analyzed the effect of TRERNA1 on RAS/Raf/MEK/ERK signaling pathway activation. In the HepG2 cell line, TRERNA1 overexpression promoted activation of the Raf-MEK-ERK phosphorylation cascade (Figure 6I). In contrast, Raf-MEK-ERK signaling was significantly inhibited in HepG2.215 cells with TRERNA1 knockdown compared with the corresponding control cells (Figure 6J). TRERNA1 was initially identified on the basis of its ability to inhibit the Raf-MEK-ERK cascade in HCC cells.18,30 Next, we determined whether NRAS is functionally involved in TRERNA1-mediated downstream signaling upon sorafenib treatment. Knockdown of NRAS abolished TRERNA1-mediated Raf-MEK-ERK phosphorylation. Conversely, restoration of NRAS expression restored downstream signaling in TRERNA1-silenced cells (Figure 6K). We further determined whether miR-22-3p/NRAS signaling contributes to the TRERNA1-induced malignant phenotypes of HCC cells. NRAS overexpression significantly reversed the inhibition of cell proliferation induced by silencing TRERNA1. Moreover, silencing NRAS obviously abolished the proliferation induced by TRERNA1 overexpression (Figure 6L). These results clarified that TRERNA1 promotes HCC proliferation by modulating miR-22-3p to activate the NRAS/Raf/MEK/ERK pathway.
TRERNA1 enhanced tumor sorafenib resistance in vivo
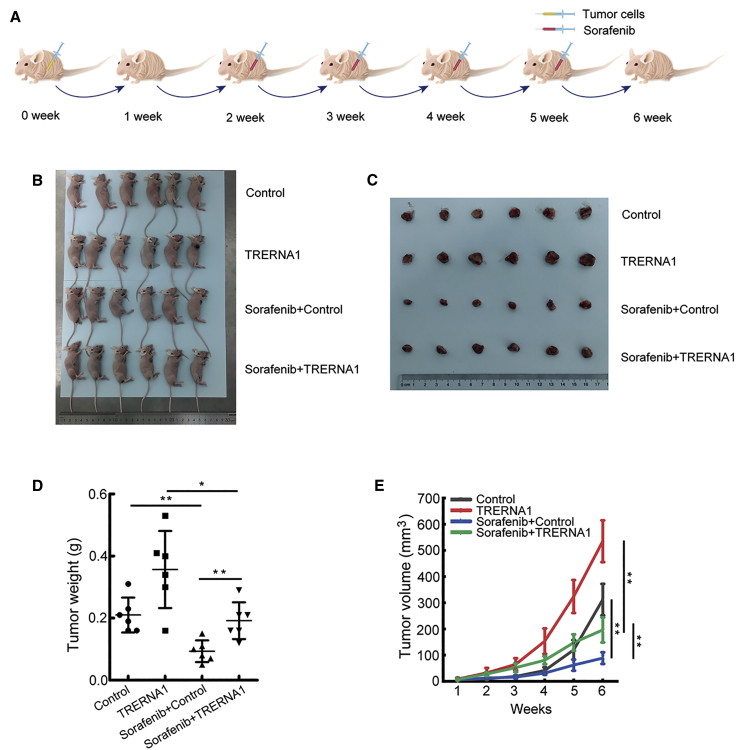
To assess whether the sorafenib treatment can be recapitulated in vivo, we established subcutaneous tumors in nude mice using HCC cells with or without TRERNA1 overexpression. We performed in vivo tumorigenesis with HepG2/pcDNA3.1 and HepG2/TRERNA1 cells to verify the relationship between TRERNA1-induced sorafenib resistance. We observed that TRERNA1 and NRAS expression was upregulated in the TRERNA1-overexpressed group derived from mouse tumor tissues using a qRT-PCR assay (Figure S7). As shown in the diagram in Figure 7A, HepG2/pcDNA3.1 and HepG2/TRERNA1 cells were injected into the right flank of mice. The tumors were allowed to grow for 2 weeks; then, the mice were treated with 30 mg/kg sorafenib diluted in sterile PBS by injection once weekly. Tumor volume was monitored during the treatment. After 4 weeks, the HepG2/TRERNA1-derived tumor had grown more than the HepG2/pcDNA3.1 tumor (Figures 7B and 7C). Sorafenib inhibited the tumor growth derived from HepG2/WT cells but had only a slight effect on the HepG2/TRERNA1 tumor as determined by tumor weight and volume (Figures 7D and 7E). This result indicated that TRERNA1 enhanced sorafenib resistance.
Figure 7.
TRERNA1 enhanced tumor sorafenib resistance in vivo
(A) The experimental protocol is shown in the diagram. (B) Tumor growth model showed that BALB/c nude mice were injected with stable TRERNA1 overexpression and control HCC cells via subcutaneous injection. The tumors were allowed to grow for 2 weeks; then, the mice were treated with 30 mg/kg sorafenib diluted in sterile PBS by injection once weekly. (C) Tumor cells excised from the flank of mice were examined. (D) Weights of the formed tumors. (E) Tumor volume growth curves. The data are presented as the mean ± SD. ∗p < 0.05, ∗∗p < 0.01.
Discussion
HCC is one of the most common malignant tumors worldwide and has a poor prognosis. The understanding of the mechanisms underlying the occurrence and development of HCC may provide new strategies for its diagnosis and treatment.31 Nearly 80% of HCC cases can be attributed to HBV infection; chronic HBV infection is a crucial risk factor for hepatocarcinogenesis.32 The HBx protein is considered the major regulator of HBV and acts as an oncogene in multiple biological functions via both epigenetic modifications and genetic regulation during oncogenesis and progression.5 HBx has been reported to promote the malignant progression of HCC via various epigenetic modifications, including DNA methylation, histone acetylation, and miRNA regulation.33 Moreover, emerging evidence has indicated that lncRNAs are involved in diverse biological processes and carcinogenesis as important epigenetic regulators.34 However, whether HBx can promote HCC by regulating lncRNA expression has not been elucidated. Our previous study demonstrated that various lncRNAs are aberrantly expressed via HBx.11 In the present study, with the use of cDNA microarray analysis in HBx-induced liver cells, we identified the lncRNA TRERNA1, which was upregulated by HBx in a manner dependent on HBx expression, as a candidate lncRNA. We further verified that TRERNA1 had higher expression in tumor tissues than in nontumor tissues. In addition, TRERNA1 overexpression correlated with poor a prognosis in HCC patients. These data showed that aberrant TRERNA1 expression correlates with the development of HCC, thus playing an important role in HCC progression and prognosis.
TRERNA1 was first identified as lncRNA with an enhancer-like function, but it was found to play an unanticipated role in the activation of critical regulators of development and differentiation.12 Further study showed that TRERNA1 not only increased expression of its neighboring protein-coding genes to play this enhancer role but also resulted in broader trans-mediated effects. Indeed, TRERNA1 has been reported to contribute to breast cancer, gastric cancer, and liver cancer metastasis in cis or in trans.13, 14, 15 However, its function and mechanism in the malignant progression of HCC have not yet been elucidated. Here, we show that TRERNA1 promotes HCC cell proliferation in vivo and in vitro; cell-cycle assay results demonstrate that TRERNA1 accelerates cell-cycle progression through the G1/S checkpoint. These data further support our findings that TRERNA1 functions as an oncogenic molecule and plays a critical role in HCC progression.
Recent studies have shown that lncRNAs regulate gene expression by mechanisms involving epigenetic regulation, DNA damage, signal transduction pathways, and miRNA silencing.35 Indeed, miRNA silencing has been identified as a new post-transcriptional regulatory mechanism by which lncRNAs function as natural ceRNA sponges.36, 37, 38 In our study, the heatmap of RNA-seq results showed that NRAS is a target of TRERNA1. In addition, bioinformatic analysis predicted that as required for an effective miRNA sponging mechanism, miR-22-3p harbors a common binding site for both TRERNA1 and NRAS. Ago2 is the core component of the RNA-induced silencing complex.39,40 We confirmed through an anti-Ago2 RIP assay that TRERNA1 overexpression led to significantly decreased enrichment of Ago2 on NRAS transcripts. Importantly, the results of the pull-down assay with biotinylated constructs confirmed the specific binding between TRERNA1 and miR-22-3p. TRERNA1 increased the luciferase activity of WT NRAS but not mutant NRAS, whereas the miR-22-3p mimics abolished this effect. Our results thus reveal that TRERNA1 modulates NRAS expression by functioning as a ceRNA sponge for miR-22-3p. This report is the first to show the function of TRERNA1 and miR-22-3p in HCC.
Inhibition of RAS emphasizes the importance of RAS downstream signaling pathways such as the MAPK pathway.41 Moreover, it highlights the major clinical limitation of Raf inhibitors, namely, acquired resistance.42 The RAS-Raf-MEK-ERK cascade is a key signaling pathway that plays an essential role in inducing human carcinogenesis.43,44 The recent development of chemical Raf kinase inhibitors that are clinically efficacious has established that the Raf-MEK-ERK cascade is a valid therapeutic target for solid tumors.45,46 Our results are the first to indicate that TRERNA1 activates the Raf-MEK-ERK phosphorylation cascade and that NRAS participates in TRERNA1-mediated activation of Raf-MEK-ERK phosphorylation.
NRAS is significantly overexpressed in sorafenib-resistant HCC cells compared with nonresistant cells. Moreover, NRAS knockdown partially restores sorafenib sensitivity in resistant HCC cells.29 Sorafenib is the first-line treatment for advanced HCC and acts by inhibiting the Raf-MEK-ERK cascade, a key to the survival of HCC patients.17 Regardless, patients with recurrent HCC who develop resistance to sorafenib have limited clinical therapeutic options, and activation of escape pathways from RAS/Raf/MEK/ERK signaling is considered to be a crucial mediator of chemoresistance.47 The underlying mechanism by which lncRNAs regulate HCC progression and sorafenib tolerance is largely unknown. Interestingly, several lncRNAs have been reported to contribute to drug resistance, such as the lncRNA sorafenib resistance-associated lncRNA in RCC (SRLR), lncRNA activated in RCC with sunitinib resistance (ARSR), and UCA1.48, 49, 50 In this study, we found that increased TRERNA1 levels contribute to sorafenib insensitivity in HCC cells. We investigated the molecular mechanism by which TRERNA1 confers sorafenib resistance on HCC cells and observed that the lncRNA TRERNA1 enhanced sorafenib tolerance by regulating NRAS expression, suggesting that TRERNA1 could be a therapeutic target to overcome sorafenib resistance.
In summary, we found that TRERNA1 is upregulated by HBx, playing a crucial role in promoting malignant phenotypes of HCC. In addition, our study elucidated the functions of TRERNA1 as a miRNA sponge to positively and post-transcriptionally regulate NRAS expression. TRERNA1 increases sorafenib resistance by activating the RAS/Raf/MEK/ERK signaling pathway. Taken together, our results suggest that the TRERNA1/miR-22-3p/NRAS axis is involved in cell proliferation and cell-cycle regulation in HCC. TRERNA1 promotes sorafenib resistance in HCC cells and may serve as a powerful tumor biomarker, providing a novel diagnostic and therapeutic target in HCC.
Materials and methods
Cell lines
HepG2, HepG2.215, and Hep3B HCC cells and the immortalized normal human cell line LO2 were obtained from the TCC Cell Bank (Shanghai, China). All cells were maintained in RPMI 1640 or DMEM supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 mg/mL streptomycin (Invitrogen, Carlsbad, CA, USA) in 5% CO2 at 37°C.
Microarray data analysis
LO2 cells were stably transfected with the HBx construct or control vectors in biological triplicate. Two HBx transfectants (LO2-HBx #1 and LO2-HBx #8), LO2-HBx stable cell mix, and three LO2-vec control cell lines were employed for cDNA microarray analysis of the gene-expression profiles as described in a previous study.11 Differentially expressed lncRNAs were considered those with an absolute fold change of ≥2.0 and a p value of <0.05. GO analysis was performed to identify the GO terms associated with the differentially expressed genes.
RNA in situ hybridization
Labeled, locked nucleic acid TRERNA1 probes were designed and synthesized by Servicebio Technology (Wuhan, China). The probe signals were detected with an anti-digoxigenin (DIG)-horseradish peroxidase (HRP) antibody (Jackson ImmunoResearch, USA) according to the manufacturer’s instructions. Images were acquired using Pannoramic Viewer.
HCC tissue specimens and TMA
HCC tissues and paired adjacent nontumor tissues were collected from a total of 50 patients who underwent radical resections between 2009 and 2016 at The First Affiliated Hospital of Nanjing Medical University (China). The TMA was prepared from samples from 110 patients undergoing resection between 2012 and 2013 at The First Affiliated Hospital of Nanjing Medical University (China). Overall survival (OS) was evaluated as the time from the date of surgery to the date of death or the last follow-up visit. Written, informed consent was obtained from each patient. Ethical approval was granted by the Medical Ethics Committee of the Medical School of Southeast University.
Plasmid construction
The pcDNA4/TO-HBx plasmids were a gift from Professor Guan Xinyuan at the University of Hong Kong. The cDNA of the lncRNA TRERNA1 and NRAS was synthesized by GENEWIZ (Suzhou, China) and then cloned into the Hind III/EcoRI sites of pcDNA3.1. The TRERNA1-shRNA DNA fragments were synthesized by GENEWIZ (Suzhou, China) and ligated into the BglII/HindIII sites of the pSUPER-EGFP vector after annealing. The primer sequences are shown in Table S1.
siRNAs/shRNAs, DNA plasmid, and miRNA transfections
Transfection of DNA plasmids and siRNAs/shRNAs were performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. 48 h after transfection with siRNAs, RNA or protein was harvested from cells for further analysis. miR-22-3p mimics, miR-22-3p inhibitors, and NC miRNA were purchased (RiboBio, Guangzhou, China). The primer sequences used for plasmid construction and the siRNA sequences are listed in Table S1.
RNA isolation and qRT-PCR analysis
Total RNA was extracted from cell and tissue lysates using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). RNA was reverse transcribed using a Reverse Transcription Kit (Takara, Dalian, China). Quantitative real-time PCR was performed with SYBR Green (Takara) in a StepOne Plus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) and repeated independently at least three times. The mRNA and lncRNA expression levels were normalized to those of β-actin using the 2−ΔΔCt method. The primer sequences used for qRT-PCR are shown in Table S2.
Cell viability and focus-formation assays
HepG2, HepG2.215, LO2, and Hep3B cells and their corresponding control cells were seeded at 2,000 cells/well in 96-well plates. Cell viability was assessed with CCK-8 (Dojindo, Kumamoto, Japan), and the optical density 450 (OD450) was measured in an iMark Microplate Reader (Bio-Rad, Hercules, CA, USA). For the focus-formation assays, HCC cells were cultured for 10 to 15 days in six-well plates fixed with methanol and stained with 0.1% crystal violet. Focus formation was scored by counting the stained colonies. All experiments were performed in triplicate at least three times.
In vivo xenograft assays
4-week-old athymic BALB/c mice (Yangzhou University Medical Center, China) were housed under standard pathogen-free conditions. A total of 1 × 107 transfected HCC cells resuspended in 200 μL of PBS were injected into the upper-left flank of each nude mouse (n = 6 per group). Then, the tumor diameter was measured weekly, and the tumor volume was calculated weekly from the measured length (L) and width (W) of the tumor using the following formula: V = 0.5 × L × W2. After 4 weeks, the mice were sacrificed, and the xenografts were removed and weighed. For the sorafenib treatment experiment, cells from pcDNA3.1 or the TRERNA1-overexpression group were suspended in 200 μL of PBS and subcutaneously injected into the upper flank of each mouse. After 2 weeks, the mice were orally administered vehicle or sorafenib (30 mg/kg, daily gavage) following a standard schedule of 3 weeks on and 1 week off treatment. Tumor volume was monitored every week. The tumors were harvested at the end of the experiments. All animal experiments were approved by the Animal Care and Use Committee of Jiangsu Province. All procedures followed the standard guidelines of the Medical School of Southeast University.
Western blot analysis
The protein content in cell lysates was quantified using a bicinchoninic acid (BCA) protein assay kit (Beyotime, Jiangsu, China). Cell lysates were analyzed by immunoblot with primary antibodies specific for cell-cycle-related proteins (Cell Cycle Regulation Sampler Kit, #9932; Cell Signaling Technology [CST]), NRAS (D261977; Sangon Biotech), phospho-Erk1/2 pathway components (Phospho-Erk1/2 Pathway Sampler Kit, #9911; CST), and HRP-conjugated secondary antibodies (Sigma, USA). An anti-β-actin antibody (Sigma) was used as the internal reference. The fluorescence intensity was measured in a chemiluminescent imaging system (Tanon, Shanghai, China).
mRNA-seq
The mRNA-seq experiments were performed by Novogene (Beijing, China). The mRNA-seq library was prepared for sequencing using standard Illumina protocols. In brief, total RNA from HepG2 cells transfected with TRERNA1 or control RNA was isolated using TRIzol reagent (Invitrogen). RNA extraction was performed using Dynabeads Oligo(dT) (Invitrogen Dynal). Double-stranded cDNAs were synthesized using SuperScript II RT (Invitrogen) and random hexamer primers. The cDNAs were then fragmented by nebulization, and the standard Illumina protocol was subsequently followed to construct the mRNA-seq library. Differential expression analysis of the two groups was performed using the DESeq R package (1.10.1). DESeq provides statistical routines for determining differential expression in digital gene-expression data using a model based on the negative binomial distribution. The resulting p values were adjusted using the Benjamini and Hochberg approach for controlling for multiple testing by calculating the false discovery rates. Genes with an adjusted p value of <0.05, as determined by DESeq, were considered differentially expressed.
Subcellular fractionation
According to the PARIS Kit (Life Technologies, Carlsbad, CA, USA) instructions, 106 cells were washed in cold PBS and then resuspended in 500 μL of ice-cold cell fractionation buffer. The cytoplasmic fractions were carefully aspirated from the nuclear pellets after centrifugation. Next, the nuclear pellets were lysed in ice-cold cell-disruption buffer. Cytoplasmic and nuclear RNA were extracted and purified for subsequent RT-PCR or qRT-PCR.
Luciferase reporter assay
Reporter vectors expressing human lncRNA TRERNA1-WT, TRERNA1-Mut (containing a mutant binding site for miR-22-3p), NRAS-WT, and NRAS-Mut (containing a mutant binding site for miR-22-3p) were purchased from RiboBio (Guangzhou, China). Luciferase activity was assessed using a Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) 48 h after transfection. All experiments were performed in triplicate.
RNA pull-down assay
Biotinylated RNAs were reverse transcribed using Biotin RNA Labeling Mix (Roche, USA) and T7 RNA polymerase (Takara Biomedical Technology, Beijing, China). The products were treated with RNase-free DNase I (Roche, USA) and purified with an RNeasy Mini Kit (QIAGEN, USA). The bound RNAs were purified with TRIzol for qRT-PCR analysis.
RIP
RIP experiments were performed using the Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, USA). Protein-RNA complexes were immunoprecipitated using anti-Ago2 antibody (Millipore, USA), anti-SNRNP70 antibody (positive control), or normal rabbit immunoglobulin G (IgG; NC). The coprecipitated RNA was pulled down with magnetic beads and detected by RT-PCR or qRT-PCR.
Statistical analysis
Student’s t test (two-tailed) was performed to evaluate differences between two groups. One-way analysis of variance (ANOVA) was used to evaluate differences between more than two groups. Receiver operating characteristic (ROC) curve analysis was performed to confirm the value of TRERNA1 in predicting OS. The Pearson chi-square (χ2) test was used, and the correlation coefficients were calculated to evaluate correlations between two variables, namely, TRERNA1 expression and clinicopathological characteristics. All tests were performed at least three times, and the data are presented as the mean ± SD values. Differences were considered significant when the p value was <0.05 (∗p < 0.05, ∗∗p < 0.01). Statistical analyses were performed using SPSS software (version 17.0; Chicago, IL, USA).
Acknowledgments
All data generated or analyzed during this study are included in this published article and its additional files. Consent to publish has been obtained from all authors. The research protocol was reviewed and approved by the Human Research Ethics Committee of Zhongda Hospital, and written, informed consent was obtained from each patient included in the study. This work was supported by grants from the National Natural Science Foundation of China (81972664, 81672414, and 82002984) and Natural Science Foundation of Jiangsu Province (BK20200119).
Author contributions
H.F. conceived and designed the experiments. W.S. conducted the experiments and wrote the manuscript. Y.Q. performed the animal experiment. H.W. constructed the plasmids used in this study. C.Z., M.L., H.S., and Y.X. performed a portion of the cell experiments. Z.Z. provided the HCC clinical specimens. P.G. wrote a portion of the schematic diagram. Y.L. and X.L. analyzed the TMA. All authors reviewed the manuscript before submission.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2021.04.011.
Supplemental information
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Llovet J.M., Zucman-Rossi J., Pikarsky E., Sangro B., Schwartz M., Sherman M., Gores G. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 3.Allemani C., Matsuda T., Di Carlo V., Harewood R., Matz M., Nikšić M., Bonaventure A., Valkov M., Johnson C.J., Estève J., CONCORD Working Group Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin Y., Wu D., Yang W., Weng M., Li Y., Wang X., Zhang X., Jin X., Wang T. Hepatitis B virus x protein induces epithelial-mesenchymal transition of hepatocellular carcinoma cells by regulating long non-coding RNA. Virol. J. 2017;14:238. doi: 10.1186/s12985-017-0903-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang B., Han S., Feng B., Chu X., Chen L., Wang R. Hepatitis B virus X protein-mediated non-coding RNA aberrations in the development of human hepatocellular carcinoma. Exp. Mol. Med. 2017;49:e293. doi: 10.1038/emm.2016.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cech T.R., Steitz J.A. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157:77–94. doi: 10.1016/j.cell.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Prensner J.R., Iyer M.K., Sahu A., Asangani I.A., Cao Q., Patel L., Vergara I.A., Davicioni E., Erho N., Ghadessi M. The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat. Genet. 2013;45:1392–1398. doi: 10.1038/ng.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan J.H., Yang F., Wang F., Ma J.Z., Guo Y.J., Tao Q.F., Liu F., Pan W., Wang T.T., Zhou C.C. A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666–681. doi: 10.1016/j.ccr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Gong C., Maquat L.E. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3′ UTRs via Alu elements. Nature. 2011;470:284–288. doi: 10.1038/nature09701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim L.J., Wong S.Y.S., Huang F., Lim S., Chong S.S., Ooi L.L., Kon O.L., Lee C.G. Roles and Regulation of Long Noncoding RNAs in Hepatocellular Carcinoma. Cancer Res. 2019;79:5131–5139. doi: 10.1158/0008-5472.CAN-19-0255. [DOI] [PubMed] [Google Scholar]
- 11.Hu J.J., Song W., Zhang S.D., Shen X.H., Qiu X.M., Wu H.Z., Gong P.H., Lu S., Zhao Z.J., He M.L., Fan H. HBx-upregulated lncRNA UCA1 promotes cell growth and tumorigenesis by recruiting EZH2 and repressing p27Kip1/CDK2 signaling. Sci. Rep. 2016;6:23521. doi: 10.1038/srep23521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ørom U.A., Derrien T., Beringer M., Gumireddy K., Gardini A., Bussotti G., Lai F., Zytnicki M., Notredame C., Huang Q. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gumireddy K., Li A., Yan J., Setoyama T., Johannes G.J., Orom U.A., Tchou J., Liu Q., Zhang L., Speicher D.W. Identification of a long non-coding RNA-associated RNP complex regulating metastasis at the translational step. EMBO J. 2013;32:2672–2684. doi: 10.1038/emboj.2013.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu H., Hu Y., Liu X., Song W., Gong P., Zhang K., Chen Z., Zhou M., Shen X., Qian Y., Fan H. LncRNA TRERNA1 Function as an Enhancer of SNAI1 Promotes Gastric Cancer Metastasis by Regulating Epithelial-Mesenchymal Transition. Mol. Ther. Nucleic Acids. 2017;8:291–299. doi: 10.1016/j.omtn.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song W., Gu Y., Lu S., Wu H., Cheng Z., Hu J., Qian Y., Zheng Y., Fan H. LncRNA TRERNA1 facilitates hepatocellular carcinoma metastasis by dimethylating H3K9 in the CDH1 promoter region via the recruitment of the EHMT2/SNAI1 complex. Cell Prolif. 2019;52:e12621. doi: 10.1111/cpr.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finn R.S., Zhu A.X., Farah W., Almasri J., Zaiem F., Prokop L.J., Murad M.H., Mohammed K. Therapies for advanced stage hepatocellular carcinoma with macrovascular invasion or metastatic disease: A systematic review and meta-analysis. Hepatology. 2018;67:422–435. doi: 10.1002/hep.29486. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y., Huang J., Ma L., Shan J., Shen J., Yang Z., Liu L., Luo Y., Yao C., Qian C. MicroRNA-122 confers sorafenib resistance to hepatocellular carcinoma cells by targeting IGF-1R to regulate RAS/RAF/ERK signaling pathways. Cancer Lett. 2016;371:171–181. doi: 10.1016/j.canlet.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 18.Saidak Z., Giacobbi A.S., Louandre C., Sauzay C., Mammeri Y., Galmiche A. Mathematical modelling unveils the essential role of cellular phosphatases in the inhibition of RAF-MEK-ERK signalling by sorafenib in hepatocellular carcinoma cells. Cancer Lett. 2017;392:1–8. doi: 10.1016/j.canlet.2017.01.038. [DOI] [PubMed] [Google Scholar]
- 19.Cheng A.L., Kang Y.K., Chen Z., Tsao C.J., Qin S., Kim J.S., Luo R., Feng J., Ye S., Yang T.S. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 20.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., de Oliveira A.C., Santoro A., Raoul J.L., Forner A., SHARP Investigators Study Group Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 21.Wei L., Wang X., Lv L., Liu J., Xing H., Song Y., Xie M., Lei T., Zhang N., Yang M. The emerging role of microRNAs and long noncoding RNAs in drug resistance of hepatocellular carcinoma. Mol. Cancer. 2019;18:147. doi: 10.1186/s12943-019-1086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi K., Yan I.K., Wood J., Haga H., Patel T. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol. Cancer Res. 2014;12:1377–1387. doi: 10.1158/1541-7786.MCR-13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang P.F., Wang F., Wu J., Wu Y., Huang W., Liu D., Huang X.Y., Zhang X.M., Ke A.W. LncRNA SNHG3 induces EMT and sorafenib resistance by modulating the miR-128/CD151 pathway in hepatocellular carcinoma. J. Cell. Physiol. 2019;234:2788–2794. doi: 10.1002/jcp.27095. [DOI] [PubMed] [Google Scholar]
- 24.Wang W., Hu W., Wang Y., An Y., Song L., Shang P., Yue Z. Long non-coding RNA UCA1 promotes malignant phenotypes of renal cancer cells by modulating the miR-182-5p/DLL4 axis as a ceRNA. Mol. Cancer. 2020;19:18. doi: 10.1186/s12943-020-1132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M., Mao C., Ouyang L., Liu Y., Lai W., Liu N., Shi Y., Chen L., Xiao D., Yu F. Long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA. Cell Death Differ. 2019;26:2329–2343. doi: 10.1038/s41418-019-0304-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou R.S., Zhang E.X., Sun Q.F., Ye Z.J., Liu J.W., Zhou D.H., Tang Y. Integrated analysis of lncRNA-miRNA-mRNA ceRNA network in squamous cell carcinoma of tongue. BMC Cancer. 2019;19:779. doi: 10.1186/s12885-019-5983-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X., Pritykin Y., Concepcion C.P., Lu Y., La Rocca G., Zhang M., King B., Cook P.J., Au Y.W., Popow O. High-Resolution In Vivo Identification of miRNA Targets by Halo-Enhanced Ago2 Pull-Down. Mol. Cell. 2020;79:167–179.e11. doi: 10.1016/j.molcel.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin C., Zhu B., Zhang T., Liu T., Chen S., Liu Y., Li X., Miao X., Li S., Mi X. Pharmacological Targeting of STK19 Inhibits Oncogenic NRAS-Driven Melanomagenesis. Cell. 2019;176:1113–1127.e16. doi: 10.1016/j.cell.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Dietrich P., Gaza A., Wormser L., Fritz V., Hellerbrand C., Bosserhoff A.K. Neuroblastoma RAS Viral Oncogene Homolog (NRAS) Is a Novel Prognostic Marker and Contributes to Sorafenib Resistance in Hepatocellular Carcinoma. Neoplasia. 2019;21:257–268. doi: 10.1016/j.neo.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu W.P., Liu J.P., Feng J.F., Zhu C.P., Yang Y., Zhou W.P., Ding J., Huang C.K., Cui Y.L., Ding C.H. miR-541 potentiates the response of human hepatocellular carcinoma to sorafenib treatment by inhibiting autophagy. Gut. 2020;69:1309–1321. doi: 10.1136/gutjnl-2019-318830. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H., Diab A., Fan H., Mani S.K., Hullinger R., Merle P., Andrisani O. PLK1 and HOTAIR Accelerate Proteasomal Degradation of SUZ12 and ZNF198 during Hepatitis B Virus-Induced Liver Carcinogenesis. Cancer Res. 2015;75:2363–2374. doi: 10.1158/0008-5472.CAN-14-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo W., Sun Y.-F., Shen M.-N., Ma X.-L., Wu J., Zhang C.-Y., Zhou Y., Xu Y., Hu B., Zhang M. Circulating Tumor Cells with Stem-Like Phenotypes for Diagnosis, Prognosis, and Therapeutic Response Evaluation in Hepatocellular Carcinoma. Clin. Cancer Res. 2018;24:2203–2213. doi: 10.1158/1078-0432.CCR-17-1753. [DOI] [PubMed] [Google Scholar]
- 33.Huang J.F., Guo Y.J., Zhao C.X., Yuan S.X., Wang Y., Tang G.N., Zhou W.P., Sun S.H. Hepatitis B virus X protein (HBx)-related long noncoding RNA (lncRNA) down-regulated expression by HBx (Dreh) inhibits hepatocellular carcinoma metastasis by targeting the intermediate filament protein vimentin. Hepatology. 2013;57:1882–1892. doi: 10.1002/hep.26195. [DOI] [PubMed] [Google Scholar]
- 34.Yu S., Li N., Huang Z., Chen R., Yi P., Kang R., Tang D., Hu X., Fan X. A novel lncRNA, TCONS_00006195, represses hepatocellular carcinoma progression by inhibiting enzymatic activity of ENO1. Cell Death Dis. 2018;9:1184. doi: 10.1038/s41419-018-1231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahu A., Singhal U., Chinnaiyan A.M. Long noncoding RNAs in cancer: from function to translation. Trends Cancer. 2015;1:93–109. doi: 10.1016/j.trecan.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang K., Liu C.Y., Zhou L.Y., Wang J.X., Wang M., Zhao B., Zhao W.K., Xu S.J., Fan L.H., Zhang X.J. APF lncRNA regulates autophagy and myocardial infarction by targeting miR-188-3p. Nat. Commun. 2015;6:6779. doi: 10.1038/ncomms7779. [DOI] [PubMed] [Google Scholar]
- 38.Liu D., Li Y., Luo G., Xiao X., Tao D., Wu X., Wang M., Huang C., Wang L., Zeng F., Jiang G. LncRNA SPRY4-IT1 sponges miR-101-3p to promote proliferation and metastasis of bladder cancer cells through up-regulating EZH2. Cancer Lett. 2017;388:281–291. doi: 10.1016/j.canlet.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Wu P., Cai J., Chen Q., Han B., Meng X., Li Y., Li Z., Wang R., Lin L., Duan C. Lnc-TALC promotes O6-methylguanine-DNA methyltransferase expression via regulating the c-Met pathway by competitively binding with miR-20b-3p. Nat. Commun. 2019;10:2045. doi: 10.1038/s41467-019-10025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang X., Xie X., Liu P., Yang L., Chen B., Song C., Tang H., Xie X. Adam12 and lnc015192 act as ceRNAs in breast cancer by regulating miR-34a. Oncogene. 2018;37:6316–6326. doi: 10.1038/s41388-018-0410-1. [DOI] [PubMed] [Google Scholar]
- 41.Huntzicker E.G., Hötzel K., Choy L., Che L., Ross J., Pau G., Sharma N., Siebel C.W., Chen X., French D.M. Differential effects of targeting Notch receptors in a mouse model of liver cancer. Hepatology. 2015;61:942–952. doi: 10.1002/hep.27566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishida N., Kitano M., Sakurai T., Kudo M. Molecular Mechanism and Prediction of Sorafenib Chemoresistance in Human Hepatocellular Carcinoma. Dig. Dis. 2015;33:771–779. doi: 10.1159/000439102. [DOI] [PubMed] [Google Scholar]
- 43.Wellbrock C., Karasarides M., Marais R. The RAF proteins take centre stage. Nat. Rev. Mol. Cell Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 44.Dhillon A.S., Hagan S., Rath O., Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 45.Lavoie H., Therrien M. Regulation of RAF protein kinases in ERK signalling. Nat. Rev. Mol. Cell Biol. 2015;16:281–298. doi: 10.1038/nrm3979. [DOI] [PubMed] [Google Scholar]
- 46.Mandal R., Becker S., Strebhardt K. Stamping out RAF and MEK1/2 to inhibit the ERK1/2 pathway: an emerging threat to anticancer therapy. Oncogene. 2016;35:2547–2561. doi: 10.1038/onc.2015.329. [DOI] [PubMed] [Google Scholar]
- 47.Zheng J.-F., He S., Zeng Z., Gu X., Cai L., Qi G. PMPCB Silencing Sensitizes HCC Tumor Cells to Sorafenib Therapy. Mol. Ther. 2019;27:1784–1795. doi: 10.1016/j.ymthe.2019.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Z., Yang F., Wei D., Liu B., Chen C., Bao Y., Wu Z., Wu D., Tan H., Li J. Long noncoding RNA-SRLR elicits intrinsic sorafenib resistance via evoking IL-6/STAT3 axis in renal cell carcinoma. Oncogene. 2017;36:1965–1977. doi: 10.1038/onc.2016.356. [DOI] [PubMed] [Google Scholar]
- 49.Qu L., Ding J., Chen C., Wu Z.J., Liu B., Gao Y., Chen W., Liu F., Sun W., Li X.F. Exosome-Transmitted lncARSR Promotes Sunitinib Resistance in Renal Cancer by Acting as a Competing Endogenous RNA. Cancer Cell. 2016;29:653–668. doi: 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Chen X., Wang Z., Tong F., Dong X., Wu G., Zhang R. lncRNA UCA1 Promotes Gefitinib Resistance as a ceRNA to Target FOSL2 by Sponging miR-143 in Non-small Cell Lung Cancer. Mol. Ther. Nucleic Acids. 2020;19:643–653. doi: 10.1016/j.omtn.2019.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.