Abstract
Background:
Coronavirus disease 2019 (COVID-19) is responsible for significant lung disease in adults. Despite mild manifestations in most children, multisystem inflammatory syndrome (MIS-C) associated with COVID-19 is well described in older children with cardiac manifestations. However, MIS-C-related cardiac manifestations are not as well described in younger children.
Methods:
The study is a retrospective analysis of MIS-C patients under the age of 5 years admitted between May and November 2020 to a single centre. Included cases fulfilled the case definition of MIS-C according to Royal College of Pediatrics and Child Health criteria with laboratory, electrocardiogram, or echocardiographic evidence of cardiac disease. Collected data included patients’ demographics, laboratory results, echocardiographic findings, management, and outcomes.
Results:
Out of 16 MIS-C cases under 5 years of age, 10 (62.5%) had cardiac manifestations with a median age of 12 months, 9 (90%) were previously healthy. Cardiac manifestations included coronary arterial aneurysms or ectasia in five (50%) cases, two (20%) with isolated myopericarditis, coronary ectasia with myocarditis in two (20%), and supraventricular tachycardia in one (10%). Intravenous immunoglobulins were given in all cases with coronary involvement or myocarditis. The median duration of hospitalisation was 7 (6–14) days; two (20%) cases with cardiac disease were mechanically ventilated and mortality in MIS-C cases below 5 years was 12.5%. Normalisation of systolic function occurred in half of the affected cases within 1 week and reached 100% by 30 days of follow-up.
Conclusions:
MIS-C associated with SARS-CoV-2 has a high possibility of serious associated cardiac manifestations in children under the age of 5 years with mortality and/or long-term morbidities such as coronary aneurysms even in previously healthy children.
Keywords: Cardiac, multisystem inflammatory syndrome, COVID-19, under 5 years
The coronavirus disease 2019 (COVID-19) or coronavirus-2 (SARS-CoV-2) has been declared a global pandemic by the World Health Organization and has affected millions of people worldwide.1 Initially, it was thought that COVID-19 was primarily an acute respiratory syndrome; however, with time, multisystem involvement has been increasingly recognised. In contrast to adults, COVID-19 in children usually leads to a mild illness and few children have been reported to have severe manifestations. However, children may develop a post-infectious cytokine storm, possibly due to the activation of macrophages.2 The condition has been termed “multisystem inflammatory syndrome in children (MIS-C)” or “pediatric multisystem inflammatory syndrome (PMIS)” and it is characterised by a hyperinflammatory syndrome with multi-organ dysfunction. Diagnostic criteria have been developed by different health organisations.3–5
At the beginning of the COVID-19 outbreak, several centres in the United States of America and Europe reported cases of MIS-C including cardiac involvement.6–8 Variable cardiac manifestations were reported.9 However, most reports or series of MIS-C cases documented that median age was greater than 5 years, and some researchers have considered the age at presentation as one of the differentiating features from Kawasaki disease.6,10,11 Only limited reports have documented MIS-C under the age of 5 years.12 In the current report, we present a series of MIS-C cases below the age of 5 years with an emphasis on cardiac involvement.
Subject and methods
This study is a retrospective analysis of a series of MIS-C patients admitted between May and November 2020 from a single centre. The research was approved by the IRB (Institutional Research Board) of the faculty of medicine, Mansoura University. Cases were children of up to 5 years of age with MIS-C who fulfilled the MIS-C case definition of the Royal College of Pediatrics and Child Health (RCPCH) criteria with cardiac clinical, laboratory, or echocardiographic findings as evidence of cardiac disease. Data were collected retrospectively included patients’ demographics, clinical presentations, laboratory results, and echocardiographic findings. Moreover, data on the course of the disease, treatments, and outcomes were evaluated.
Case definition of MIS-C according to the RCPCH
1. A child presenting with persistent fever more than 38.5°C, inflammation (neutrophilia, elevated CRP, and lymphopenia), and evidence of single- or multi-organ dysfunction (shock, cardiac, respiratory, renal, gastrointestinal, or neurological disorder) with additional features. This may include children fulfilling full or partial criteria for Kawasaki disease. 2. Exclusion of any other microbial cause, including bacterial sepsis, staphylococcal or streptococcal shock syndromes, infections associated with myocarditis such as enterovirus, and 3. SARS-CoV-2 polymerase chain reaction testing may be positive or negative.5
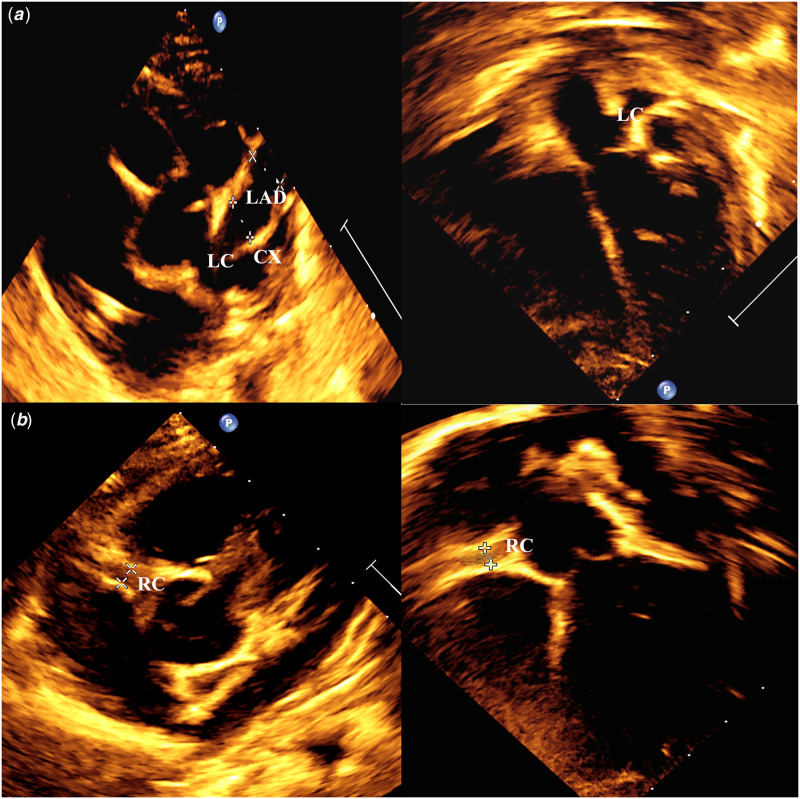
Figure 1.
Transthoracic echocardiogram of two cases ( a) Short parasternal view and a five-chamber view showing multiple coronary aneurysms are seen affecting left main coronary artery and its major branches; (b) short parasternal and five-chamber view showing diffuse ectatic dilatation of right coronary artery. LAD: left anterior descending artery, LC: left main coronary, LCX: left circumflex, RC: right coronary artery.
Laboratory investigations
Basic investigations were performed: complete blood count (CBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), serum ferritin, and troponin I.
Nose and throat swab viral polymerase chain reaction for SARS-CoV-2 was performed for all patients. Moreover, cases were tested for immunoglobulin M (IgM), and immunoglobulin G (IgG) to viral spike glycoprotein using an enzyme-linked Immunosorbent Assay (ELISA) test.
Electrocardiogram
At the initial presentation, 12-lead electrocardiogram was performed to exclude arrhythmia or conduction abnormality. It was repeated whenever required during the admission, especially if attacks of arrhythmias were suspected on monitoring.
Echocardiographic findings
Detailed transthoracic echocardiography for all the included children was performed using the Philips Epiq C7 X-matrix (Philips Medical Systems, Bothell, WA, United States of America; 2014). Images were recorded from the standard echocardiographic views, apical four-chamber, three-chamber, two-chamber, and parasternal short-axis and long-axis views based on the recommendations of the American Society of Echocardiography.13 For each patient, the following parameters were fulfilled:
Left ventricular systolic function was estimated through the assessment of fractional shortening using M-mode. Function was categorised according to fractional shortening as normal (28–45%), borderline (26–27%), mild (20–25%), moderate (15–19%), or severe reduction (≤14%).14
Coronary artery assessment with measurements of internal lumen diameter with the determination of z-scores of the right coronary artery, main left coronary artery, and its branches left circumflex and left anterior descending artery. Coronary aneurysm was defined using the American Heart Association z-score classification: no involvement (<2); dilation only (≥2 to <2.5); small aneurysm (≥2.5 to <5); medium aneurysm (≥5 to <10 and absolute dimension <8 mm); and giant aneurysm (≥10 or an absolute dimension ≥8 mm).15
Presence of mitral or tricuspid valvular regurgitation.
Presence of pericardial effusion.
Management
Patients with coronary artery involvement were treated with intravenous immunoglobulins at a dose of 2 g/kg and high-dose aspirin at a dose of 80–100 mg/kg/d. In patients with persistent fever or worsening inflammatory markers after 48 hours, the second dose of intravenous immunoglobulin was given. Thromboprophylaxis with antiplatelet therapy or anticoagulation was based on the extent of coronary involvement. A similar dose of intravenous immunoglobulin was given in cases of myocarditis. Moreover, inotropes (e.g., milrinone or dobutamine) were given in the cases of left ventricle dysfunction. In case of borderline or residual systolic function impairment by discharge, angiotensin-converting enzyme inhibitors were prescribed.
Statistical analysis
All statistical analyses were performed using SPSS software (version 25, IBM Corp., Armonk, NY, United States of America). Results are presented as mean ± SD or median (IQR), while categorical variables are presented as frequencies and percentages.
Results
Out of 16 patients with MIS-C under the age of 5 years, 10 (62.5%) had evidence of cardiac involvement. Table 1 demonstrates the demographic characteristics, clinical presentation, and laboratory findings of the study cohort. The median age at presentation was 12 months (IQR: 4.5–27 months, range 35 days to 4.5 years). Most cases were males (80% of cases) and the majority of patients (90%) were previously healthy with no comorbidities. One patient had unoperated transposition of the Great Arteries with ventricular septal defect. All patients presented with a fever, while gastrointestinal manifestations including vomiting and/or diarrhoea were present in six patients (60%). Mucocutaneous manifestations of Kawasaki-like disease were present in eight patients (80%). Variable respiratory symptoms were found in four patients (40%); of those, only one patient had positive polymerase chain reaction for COVID-19 in addition to pleural effusion and pulmonary infiltrations on CT chest consistent with category 6 of COVID-19 Reporting and Data (CO-RAD) System. Furthermore, an infant with Kawasaki-like disease had gangrenous toes, lymphadenopathy, and shock on admission. It was noticeable that all patients had a significant rise in C-reactive protein, Erythrocyte sedimentation rate, and serum ferritin levels with leucocytosis; however, leucopenia was not evident in our cohort with a median of 2.45 103/μL and only three patients (30%) had a mild decrease in the lymphocytic count for age. Serology for SARS-CoV-2 was positive in all cases for the combined IgG and IgM. One patient had positive SARS-CoV-2 polymerase chain reaction.
Table 1.
Demographics, clinical presentations, and basic laboratory results of MIS-C cases under the age of 5 years with cardiac findings.
| Variable | N(%) or median (IQR)* | |
|---|---|---|
| Age (months) | 12 (4.5–27) | |
| Sex | Male: 8(80%) | |
| Female: 2(20%) | ||
| Weight (Kg) | 8 (5.1–14) | |
| Comorbidity | Previously healthy | 9 (90%) |
| CHD | 1(10%) | |
| Clinical presentations | Fever | 10 (100%) |
| Respiratory | 4 (40%) | |
| Gastrointestinal (vomiting ± diarrhoea) | 6(60%) | |
| Mucocutaneous lesions | 7 (70%) | |
| Shock | 1 (10%) | |
| Others: | ||
| Gangrene | 1 (10%) | |
| Lymphadenopathy | 1 (10%) | |
| Jaundice | 1 (10%) | |
| Basic laboratory findings | CRP (mg/L) | 82.5(42.75–146.5) |
| ESR (mm/h) | 78.2(19.5–127.5) | |
| TLC (103/μL) | 14.2(11.2–22) | |
| Lymphocytes (103/μL) | 2.45(1.93–5.25) | |
| Hgb (g/L) | 7.65(6.98–8.75) | |
| Platelet (103/μL) | 310(245–354) | |
| Serum ferritin (ng/ml) | 371 (131.56–532.5) | |
| COVID-19-related laboratory findings | PCR positive | 1(10%) |
| COVID-19 antibodies (IgM and IgG) | 10(100%) | |
Data are expressed as median (interquartile range) or frequencies (percentage).
CRP = C-reactive protein; ESR = Erythrocyte sedimentation rate; Hgb = hemoglobin; PCR = polymerase chain reaction; TLC = total leucocytic count.
The details of cardiac diagnoses including echocardiographic findings and serum troponin I level in cases of MIS-C under 5 years of age are shown in Table 2. The children were categorised according to their cardiac diagnosis: five in isolated coronary arterial disease (50%), two in combined coronary artery disease with myopericarditis(20%), one in myopericarditis(10%), and one in arrhythmia(10%). Variable coronary involvements were detected in seven patients including ectatic dilatation in at least one coronary artery in four cases, a small aneurysm in one case, moderate fusiform aneurysm with ectasia in another case, and multiple large aneurysms in one case. Coronaries affected included isolated right coronary artery in two cases, both main coronaries in two cases, right coronary with left main and left anterior descending involvement in two cases, and left main with left anterior descending and left circumflex in one patient. Regarding systolic myocardial impairment, four cases had low fractional shortening with three of those with mildly reduced function and one patient who had severe myocarditis with acute heart failure and severe reduction of fractional shortening (14%) and ejection fraction (31%). Troponin was increased in four cases in which systolic function was below normal and troponin was borderline in one case presenting with arrhythmia. A child in the series had moderate pericardial effusion with mild mitral regurgitation but without tamponade and did not require extensive intervention. No cases presented with more than mild atrioventricular valves regurgitation.
Table 2.
Details of cardiac diseases in MIS-C cases of the study.
| Cardiac diagnosis | N(%) | Age | Echo or ECG findings | Troponin I (ng/ml) | Initial FS | FS at 1 m |
|---|---|---|---|---|---|---|
| Isolated coronary artery disease | 5(50) | 5 months | Diffuse ectasia of RCA and LCA (z-score 2, 2.2) | <0.01 | 35% | 36% |
| 18 months | Diffuse Ectasia of RCA and LCA (z-score 2.2, 2.4) | <0.01 | 33% | 36% | ||
| 2 years | Small aneurysm in RCA (z-score 2.7) | <0.01 | 37% | 37% | ||
| 5 months | Medium aneurysm main LCA (z-score, 5), ectasia of LAD (z-score, 2.3). and diffuse ectasia of RCA (z-score, 3) | <0.01 | 29% | 33% | ||
| 3 months | Multiple medium and giant aneurysms in LAD, LCX, and LAD up to z-score of 11.5 | <0.01 | 28% | Died on second day of admission | ||
| Coronary artery disease with myopericarditis | 2(20) | 21 months | Ectasia of RCA (z-score, 2.2), mild pericardial effusion with decreased contractility | 0.70 | 24% | 30% |
| 6 months | Ectasia of RCA, LCA, and LAD (z-score, 2.2–2.4) with decreased contractility | 0.50 | 22% | 35% | ||
| Myocarditis or myopericarditis | 2(20) | 3 years | Dilated LV, subnormal contractility, mild mitral regurgitation, and mild pericardial effusion | 0.65 | 23% | 33% |
| 4.5 years | Dilated LV, poor contractility, mild mitral regurgitation, mild TR, and septal dyskinesia | 0.80 | 14% | Died on seventh day of admission | ||
| Arrhythmia | 1(10) | 36 days | Supraventricular tachycardia | 0.047 | 28% | 35% |
ECG = electrocardiogram; FS = fractional shortening; LAD = left anterior descending; LCA = left coronary artery; LCX = left circumflex; LV = left ventricle; RCA = right coronary artery.
Management and outcome of MIS-C cases under 5 years of age with cardiovascular involvement are presented in Table 3. Intravenous immunoglobulin was given to cases with coronary artery disease or myocarditis, while inotropes were used in 40% of cases. Discharge cardiac medication was needed in seven cases; five cases with an antiplatelet dose of aspirin and /or warfarin, one case received aspirin with angiotensin-converting enzyme inhibitor, and one patient on angiotensin-converting enzyme inhibitor alone. The median duration of hospitalisation was 7 days (6–14) and two patients died despite extensive inotropic and ventilatory support. The rest of the patients were discharged home clinically well with normalisation of systolic function within a week in half of all affected cases. All had a clinical review 1 week and 1 month after discharge with a repeat electrocardiogram and echocardiogram. There were no new coronary changes or functional deterioration with an improvement of coronary abnormalities in most cases, but the persistence of aneurysms in two cases (33% of survivors with residual coronary disease) at the one-month follow-up. All cases with coronary abnormalities had a long-term follow-up plan in the paediatric cardiology clinic and CT angiogram.
Table 3.
Management and outcome of MIS-C cases under the age of 5 years with cardiac involvement.
| Variable | N(%) or median (IQR)* | ||
|---|---|---|---|
| Management | During admission | Oxygen requirements | 3(30%) |
| Ventilation | 2(20%) | ||
| Intravenous immunoglobulin | 9(90%) | ||
| Inotropic support | 4(40%) | ||
| Anticoagulant | 2(20%) | ||
| Antiarrhythmic | 1(10%) | ||
| On discharge | ASA ± warfarin | 6(60%) | |
| ACEIs | 2(20%) | ||
| Outcome | Short term | Hospital stay duration (days) | 7(6–14) |
| Mortality | 2/10 (20%) | ||
| Myocardial systolic function normalisation within 1 week | 2/4 (50%) | ||
| At 1-month follow-up | Myocardial systolic function normalisation | 4/4(100%) | |
| Persistence of coronary artery aneurysm or ectasia | 2/6(33.3%) | ||
Data are expressed as median (interquartile range) or frequencies (percentage).
ACEIs = angiotensin-converting enzyme inhibitors; ASA = acetyl salicylic acid.
Discussion
MIS-C related to COVID- 19 is now a well-described syndrome, especially in school-aged children and adolescents.16 MIS-C includes many forms of cardiac involvement including cardiac dysfunction, pericardial effusion, and coronary abnormalities. Nevertheless, limited reports have described cases under the age of 5 years, especially from a cardiac perspective. In the current series, variable cardiac disorders are described in this younger patient population.
Dufort et al reported a large case series of MIS-C cases from New York, 31 were under 5 years of age. In contrast to our series in which we have one case with CHD, 22 of their series had pre-existing comorbidities, especially obesity in 10 out of 31 cases.12 In the current work, most of the patients were males, a finding that is consistent with most MIS-C series of older children.6,10 However, in the current work, the male to female ratio of 8:2 is significantly higher. In a study of MIS-C cases with acute cardiac decompensation with only one case was under the age of 5 years, males comprised 51% of cases.17
Fever, which is a common presentation of MIS-C, was documented in all our cases, consistent with previous reports on MIS-C.10,18 Fever was followed by the presence of atypical Kawasaki-like mucocutaneous manifestations (60%) and gastrointestinal symptoms in half of our cases. In the Ramcharan et al series, 87% of cases with cardiac findings had gastrointestinal symptoms, and nearly half the cases with Kawasaki-like features.10
Cardiac involvement in MIS-C in our series was 62.5% which compares with the Verdoni et al series reporting 60%,7 while cardiac findings approached 100% in the Ramcharan et al cohort with 93% of them diagnosed with coronary artery involvements, although only one with aneurysms. Moreover, they documented a high incidence of atrioventricular valve regurgitation, documented in 13 of 15 cases with a median age of 8.8 years.10 In Matsubara et al’s series, coronary involvement in MIS-C patients (median age 11.4 years) was 4% with one case of coronary ectasia and no documented aneurysms, while coronary aneurysms were present in 8.8% of Feldestien et al series.19,20 In another large MIS-C cohort from the United States of America, aneurysms were found in 13.4% of cases with regression in 79.1% by 1 month and in 100% by 3 months; therefore, they suggested that rapid resolution of coronary aneurysm might be due to vasodilatation rather than destruction by inflammatory cells.21 However, this theory does not explain the presence of significant-sized aneurysms which is clearly a result of more than a simple vascular dilatation. In the Dufort et al MIS-C cases under the age of 5 years, myocarditis occurred in 39% with coronary involvement in 13% but with no details on the cardiac outcome of the cohort.12 In the current series, we had a comparable rate of myocarditis; however, coronary involvement was higher reaching 70% of cases with cardiac disorders.
Cardiac troponin I could be a useful marker in detecting cardiac involvement in children with MIS-C. The level indicating cardiac diseases is above 0.045 ng/ml in children aged less than 3 months and above 0.005 ng/ml for children aged greater than 3 months.22 In our series, 40% of cases had a high troponin value for age.
The recovery of myocardial systolic function in our series reached 100% of surviving cases within 1 month. Similarly, all cases with reduced fractional shortening were normalised before discharge with a median of 3 days in another series from the United Kingdom10, while the recovery was 71% within 7 days in a series of MIS-C with acute heart failure and a median age of 10 years.17 Furthermore, it was described that left ventricle ejection fraction in MIS-C was decreased in 34.2% of cases in a large series from the United States of America with a median age of 9.7 years with 91% of them having normal functions within a month and 99.4% normalised within 3 months of follow-up.21 Thus, the resolution of myocardial dysfunction is expected when the acute decompensation stage is aggressively managed.
The length of hospital stay in our series was comparable to the average of MIS-C cases described in a meta-analysis by Ahmed et al18 Nevertheless, mortality is considered higher in our series with 20% of cases with cardiac involvement and about 12.5% of total MIS-C under the age of 5 years in our centre. In the cases of under the age of 5 years, series by Dufort et al mortality was only 3%, while it was 1.9% in a higher median age cohort by Feldestien et al and no mortality was reported in the series of cardiac decompensated MIS-C series by Belhadjer et al17 This could be due to the unavailability of extracorporeal membrane oxygenation in our centre as an advanced management step for acute decompensation of severe cases.
One of the limitations of the current work is that we did not document details of diastolic function or strain to detect subclinical myocardial injury. Another limitation is the descriptive study design and lack of comparative statistics with Kawasaki cases in the same age group. However, our series demonstrates that cardiac complications could be significant even in the younger age group with a median age of 12 months. Thus, the exclusion of this paediatric age category from COVID-19 vaccine trials should be reconsidered due to the severity, mortality, and persisting cardiac implications. Moreover, long-term follow-up is required for resolving cases with cardiac MRI and advanced echocardiographic modalities to enhance the understanding of MIS-C cardiac effects.
Conclusion
MIS-C associated with COVID-19 under the age of 5 years has the potential for cardiac manifestations, including mortality or long-term morbidities such as coronary aneurysms even in previously healthy children.
Acknowledgements
None.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
None.
Ethics standards
The authors assert that all work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and the study was reviewed and approved by the IRB (Institutional Research Board) of Mansoura University, Faculty of Medicine.
References
- 1.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed 2020; 91: 157–160. [DOI] [PMC free article] [PubMed]
- 2.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ.COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395: 1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Multisystem inflammatory syndrome in children and adolescents with COVID-19. Available at: https://www.who.int/publications/i/item/ multisystem-inflammatory-syndrome-in-childrenand-adolescents-with-covid-19. Accessed August 23, 2020.
- 4.CDC Health Alert Network. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). Available at: https://emergency.cdc.gov/han/2020/han00432.asp. Accessed July 30, 2020.
- 5.RCPCH. Paediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS) – guidance for clinicians. https://www.rcpch.ac.uk/resources/paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims-guidance.
- 6.Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ 2020; 369: m2094. [DOI] [PMC free article] [PubMed]
- 7.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020; 395: 1771–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouldali N, Pouletty M, Mariani P, et al. Emergence of Kawasaki disease related to SARS-CoV-2 infection in an epicentre of the French COVID-19 epidemic: a time-series analysis. Lancet Child Adolesc Health 2020; 4: 662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sperotto F, Friedman KG, Son MBF, VanderPluym CJ, Newburger JW, Dionne A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr 2021; 180: 307–322. [DOI] [PMC free article] [PubMed]
- 10.Ramcharan T, Nolan O, Lai CY, et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol 2020; 41: 1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Consiglio CR, Cotugno N, Sardh F, et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell 2020; 183: 968–981.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med 2020; 383: 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez L, Colan SD, Frommelt PC, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc/Echocardiogr 2010; 23: 465–495. [DOI] [PubMed] [Google Scholar]
- 14.Tissot C, Singh Y, Sekarski N.Echocardiographic evaluation of ventricular function-for the neonatologist and pediatric intensivist. Front Pediatr 2018; 6: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 2017; 135: 927–999. [DOI] [PubMed] [Google Scholar]
- 16.Jiang L, Tang K, Levin M, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis 2020; 20: e276–e288. [DOI] [PMC free article] [PubMed]
- 17.Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation 2020; 142: 429–436. [DOI] [PubMed]
- 18.Ahmed M, Advani S, Moreira A, et al. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine 2020; 26: 100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsubara D, Kauffman HL, Wang Y, et al. Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID-19 in the United States. J Am Coll Cardiol 2020; 76: 1947–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldstein LR, Tenforde MW, Friedman KG, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 2021; 325: 1074–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020; 383: 334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dionne A, Kheir JN, Sleeper LA, Esch JJ, Breitbart RE.Value of Troponin testing for detection of heart disease in previously healthy children. J Am Heart Assoc 2020; 9:e012897. [DOI] [PMC free article] [PubMed] [Google Scholar]