Abstract
Recent epidemiological studies analysing sex-disaggregated patient data of coronavirus disease 2019 (COVID-19) across the world revealed a distinct sex bias in the disease morbidity as well as the mortality – both being higher for the men. Similar antecedents have been known for the previous viral infections, including from coronaviruses, such as severe acute respiratory syndrome (SARS) and middle-east respiratory syndrome (MERS). A sound understanding of molecular mechanisms leading to the biological sex bias in the survival outcomes of the patients in relation to COVID-19 will act as an essential requisite for developing a sex-differentiated approach for therapeutic management of this disease. Recent studies which have explored molecular mechanism(s) behind sex-based differences in COVID-19 pathogenesis are scarce; however, existing evidence, for other respiratory viral infections, viz. SARS, MERS and influenza, provides important clues in this regard. In attempt to consolidate the available knowledge on this issue, we conducted a systematic review of the existing empirical knowledge and recent experimental studies following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The qualitative analysis of the collected data unravelled multiple molecular mechanisms, such as evolutionary and genetic/epigenetic factors, sex-linkage of viral host cell entry receptor and immune response genes, sex hormone and gut microbiome-mediated immune-modulation, as the possible key reasons for the sex-based differences in patient outcomes in COVID-19.
Key words: COVID-19, epigenetic mechanisms, evolution, genetics, SARS-CoV-2, sex, sex hormones
Introduction
The analysis of the sex-disaggregated data of the ongoing pandemic of coronavirus disease 2019 (COVID-19) at a global scale shows that development of severe disease symptoms along with the mortality count is higher in men. A recent study has demonstrated clear difference in sex-based immunological response in COVID-19 (Ref. 1). Scientists are still unravelling the unique properties of novel coronavirus virus strain causing this pandemic – severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). However, biological sex bias in the patient morbidity and mortality is not unique for the SARS-CoV-2, and similar antecedents have been known for previous virus infections, including from coronaviruses, such as severe acute respiratory syndrome (SARS) and middle-east respiratory syndrome (MERS) (Ref. 2). COVID-19-specific research explaining molecular mechanism(s) for sex-based differences in immunological responses is yet to be carried out. Key explanation for sex-biased presentations in COVID-19 is received from X-linkage of angiotensin-converting enzyme-2 (ACE2) gene, the human cell entry receptor for SARS-CoV-2 – which binds to viral spike protein. ACE2 is an interferon (IFN)-stimulated gene (ISG), which in turn has an oestrogenic regulation at the gene level (Refs 3, 4). Similarly, a human protease, transmembrane protease, serine 2 (TMPRSS2), which primes viral spike protein for host cell entry, has an androgenic regulation at the gene level (Refs 3, 4). Several immunoresponsive genes, such as Toll-like receptors 7 and 8 (TLRs-7 and -8), interleukins viz. IL-4, IL-10, IL-13, FoxP3 and CD-40L are X-linked, which are crucial in priming immune response against viral infections including coronaviruses (Refs 3, 4). However, literature cites multiple additional mechanisms in relation to other infectious diseases which may hold true for COVID-19, such as evolutionary existence of stronger immune response in the case of females (Refs 5, 6), immunomodulatory effect of sex hormones (Ref. 7) and gut microbiome (Ref. 8). In this paper, we have reviewed the existing empirical knowledge about the molecular mechanisms behind sex bias in immune responses against the respiratory viruses, with a focus on COVID-19.
Materials and methods
Objective
To identify plausible molecular mechanisms implicated in sex-based differences in patient outcomes in COVID-19.
Information sources and search strategy
The online literature sources including PubMed, Medline (EBSCO and Ovid), Google Scholar, ScienceDirect, Scopus, BioMedical and Web of Science (WoS) were explored extensively for the relevant data. Additionally, publication citations were also searched. The time period taken to review the COVID-19-specific data included from 1 December 2019 to 15 December 2020. The terms used for the search included COVID-19 and sex/sex differences, sex-based morbidity/mortality in COVID-19, molecular mechanisms, evolutionary basis, testis/ovary pathology in COVID-19, ACE2 expression in reproductive organs, immunological basis, genetic basis, epigenetics, role of smoking, microbiome, etc., plus months and year of study.
Protocol followed
A systematic review of recent studies was performed following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary file 1).
Data selection, extraction and qualitative synthesis
Full articles which provided information about sex-based morbidity/mortality, mechanism of entry of the SARS-CoV-2 virus and histological changes in reproductive tissue, sex hormone alterations, immunological responses in COVID-19 patients were included in the study. The original clinical/epidemiological/animal model studies showing significant statistical associations [in terms of odds ratios (ORs) and/or risk ratios] of biological sex of the patients/in vivo models with morbidity and mortality rates in COVID-19 or other respiratory viruses (SARS, MERS and influenza) were considered for qualitative analysis. Additionally, relevant non-COVID articles explaining evolutionary, genetic and epigenetics underlying sex-based differences in immunological response against respiratory viruses were also included. Full length original articles, case studies and meta-analysis papers were included. The databases presenting expression of SARS-CoV-2 host cell entry receptors and proteins in human tissues, and COVID-19 demographic details, were reviewed for the sex-differentiated data. In addition, articles presenting commentary and review of the current COVID-19 research were scrutinised for relevant descriptions and original data links. A pre-publication peer review was conducted for the original articles in preprint (without peer review) to consider any of them for inclusion. The articles with only abstract and newsletters were excluded from the study.
For the original studies included in this review, any case was considered having COVID-19 only if tested positive for SARS-CoV-2 m-RNA in reverse transcription-polymerase chain reaction irrespective of the severity of the clinical symptoms. Complete data collection and qualitative analysis was contributed and reviewed by all the members of the research team, and disagreements among the investigators were resolved by recheck for the errors (in the data collection and analysis process) and mutual discussion. Any study with odd results has been discussed separately. The final inferences were made purely based on the qualitative synthesis from the collected data and no further quantitative data analysis or statistical testing was performed.
Results and discussion
Overall, 158 articles and databases were identified that fitted to our selection criteria. After exclusion of duplicates, 140 research articles were finalised for screening, following which 39 articles were excluded because of various reasons (duplication of information, no relevant data related to sex differences, etc.) and only 91 articles were found eligible for the final analysis (Supplementary file 1). Additionally, two databases [Human Protein Atlas (https://www.proteinatlas.org/humanproteome/sars-cov-2) and Global Health 50/50 (https://globalhealth5050.org)] were used, which presented relevant sex-differentiated data. Articles were grossly categorised into following groups: providing details for (i) sex-specific prevalence, (ii) cell entry receptor-based mechanisms, (iii) evolutionary and genetic bases, (iv) role of epigenetics, (v) hormonal basis, (vi) immunological basis and (vii) and additive factors, such as smoking and gut microbiome. We discuss below, salient observations from the COVID-19-specific studies under each category, in light of similar evidence received for the other respiratory viruses, wherever applicable.
Sex-specific prevalence of morbidity/mortality in COVID-19
Most of the studies, including meta-analyses, have shown higher prevalence of severe COVID-19 and mortality in men in comparison with women. Jin et al. studied sex-based differences in disease severity and mortality in patients with COVID-19 in (1) a case series of 43 hospitalised patients and (2) a public data set of the first 37 cases of patients who died of COVID-19 and 1019 patients who survived. Investigators also studied the archived data of 524 patients with SARS, including 139 deaths, from Beijing in early 2003. These investigators observed that older age and a high number of comorbidities were associated with higher disease severity and mortality in men as well as in women patients with COVID-19 and SARS. In the case series of patients under treatment, men's cases tended to be more severe compared with women's (P = 0.035). In the public data set, men and women had the same prevalence for contracting infection; however, the number of men who died from COVID-19 was 2.4 times that of women (70.3 versus 29.7%, P = 0.016). Sex bias in mortality was also observed in SARS patients, wherein, the percentage of men were higher in the deceased group than that in the survived group (P = 0.015) (Ref. 9).
Williamson et al. conducted a cohort study using national primary care electronic health record data on 10 926 COVID-19-related deaths in the UK. Investigators found that, 90 days after the start of the study, the overall cumulative incidence of COVID-19-related death was less than 0.01% in those aged 18–39 years; however, in those aged 80 years or over, it was 0.67 and 0.44% in men and women, respectively. Men had a higher risk of death compared with women (fully adjusted hazard ratio 1.59 (1.53–1.65) (Ref. 10).
The meta-analysis of sex-differentiated data at a global scale robustly confirms that men are more vulnerable for developing severe disease and share higher mortality in comparison with women when caught with COVID-19. Recently, Peckham et al. analysed clinical data of 3 111 714 cases of COVID-19 from the published reports across the globe. The investigators observed that although there was no difference in the proportion of men and women with confirmed COVID-19, men had almost three times the odds of requiring intensive care unit (ICU) admission [OR = 2.84; 95% confidence interval (CI) = 2.06, 3.92] and higher odds of death (OR = 1.39; 95% CI = 1.31, 1.47) compared with women (Ref. 11). Another meta-analysis study by Yanez et al. analysed 178 568 COVID-19 deaths from a total population of approximately 2.4 billion people across 16 countries. The investigators observed that mortality rates from COVID-19 were 77% higher in men compared with those in women [incident rate ratio (IRR = 1.77; 95% CI = 1.74, 1.79] (Ref. 12). A further meta-analysis study by Nasiri et al. evaluating 5057 cases showed that the pooled mortality rate was on average 3.4 times higher in men as compared with women (95% CI = 1.2, 9.1; P = 0.01) (Ref. 13).
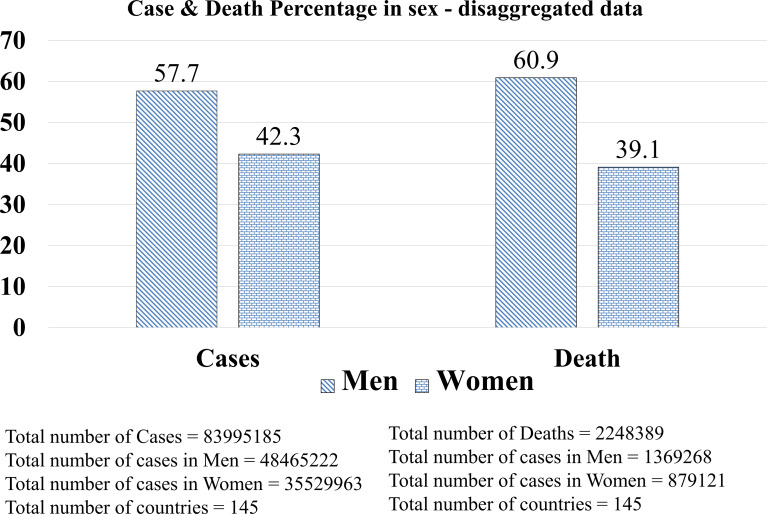
The analysis of sex-disaggregated epidemiological data at a global scale involving 83 995 185 confirmed cases of COVID-19 from 145 countries does support a male sex bias in fatality reflected across the studies which we have discussed above (Fig. 1) (on 21 April 2021, source: https://globalhealth5050.org) (Ref. 14). Of note, against the global trend, a report cited a higher rate of severity of symptoms in women in India and Vietnam (Ref. 15) and also official reporting of sex-differentiated data from both of the countries reflect this (Ref. 14). However, for the countries, such as Nepal and Slovenia, which were earlier showing this trend are now catching up to the global trend with increased reporting of the sex-differentiated data (Ref. 14). Possibly, rather than a biological factor, an early reporting based on smaller sample size, limited official reporting of the sex-differentiated data and country-specific socio-demographic factors might have been responsible for the aberrant reporting (Ref. 16).
Fig. 1.
COVID-19 sex-disaggregated data at a global scale. Data source: The COVID-19 Sex-Disaggregated Data Tracker, Global health 50/50 (https://globalhealth5050.org, an open access database. The data were reviewed until 21 April 2021).
Cell entry receptor-based molecular mechanism(s)
SARS-CoV-2 entry in human cells is mediated by a cell surface protein ACE2 (Ref. 17). SARS-CoV-2 binds to ACE2 with a receptor-binding domain present at its spike (S) protein (Ref. 17). For a successful host cell invasion priming of the S protein by the host proteases such as trans-membrane protease, serine 2 (TMPRSS2), cathepsin-L (CTSL), FURIN or ADAM-17 (a disintegrin and metalloproteinase domain-containing protein 17, also called as TACE) are essential (Refs 17, 18). Considering the essentiality of these host proteins for the entry of the virus inside the host cell, their distribution in the sex-specific tissue may be a deciding factor in creating a sex-specific bias in patient outcomes in COVID-19 (Refs 3, 4).
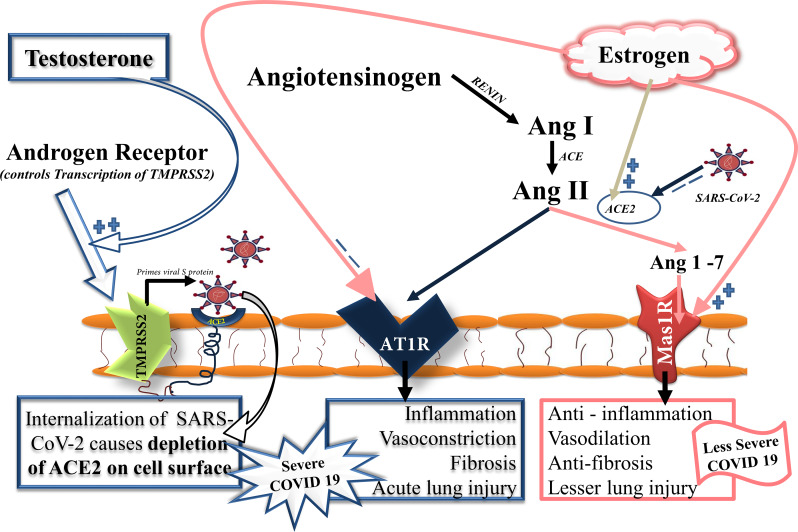
ACE2 and its analogue ‘angiotensin-converting enzyme (ACE)’ are key molecular regulators of renin–angiotensin system (RAS), or renin–angiotensin–aldosterone system, which regulates blood pressure, fluid and electrolyte balance, and systemic vascular resistance. ACE2 competes with its analogue ACE to keep a balance of the pathways regulating these functions (Fig. 2). SARS-CoV-2 can potentially downregulate ACE2 (but not ACE) in the infected epithelium of the lung (Refs 19, 20). A SARS-CoV-2-induced downregulation of ACE2 in lung epithelium (and perhaps in other tissues including vascular endothelium) can favour the ACE/Ang II/AT1R-mediated pathway leading to vasoconstriction, lung fibrosis and increased inflammation (Ref. 18).
Fig. 2.
Virus-mediated modulation of RAS in COVID-19 patients and influence of sex hormones. ACE2 and its analogue ACE are key molecular regulators of RAS, which regulates blood pressure, fluid and electrolyte balance and systemic vascular resistance. ACE2 competes with its analogue ACE to keep a balance of the pathways regulating these functions. ACE2 metabolises Ang II to Ang 1–7 which further act through Mas1R present in lung epithelial cells favouring vasodilatation, anti-inflammation and antifibrosis. Conversely, an ACE prevents metabolising Ang II which further acts on its cognate receptor AT1R favouring vasoconstriction, fibrosis and increased inflammation. SARS-CoV-2 potentially downregulates ACE2 (but not ACE) in the infected epithelial cells thus favours an ACE/Ang II/AT1R-mediated pathway leading to vasoconstriction, lung fibrosis and increased inflammation resulting in increased disease severity. Sex hormones can effectively modulate RAS axis influencing severity of disease in COVID-19 patients. Higher serum levels of oestrogen in females plausibly downregulates ACE and AT1R, and upregulates ACE2 and Mas1R, thus favours ACE2/Ang 1–7/Mas1R-mediated regulation of RAS resulting in less severe disease. Conversely, higher serum levels of testosterone in male produces an opposite effect. Testosterone by regulating expression of TMPRSS2 gene through AR causes increased internalisation of ACE2: SARS-COV-2 complex. The increased consumption of ACE2 by SARS-CoV-2 causes its depletion on epithelial cells thus activating the ACE/Ang II/AT1R-mediated pathway favouring vasoconstriction, increased tissue inflammation and fibrosis resulting in severe COVID-19. ACE, angiotensin converting enzyme; Ang II, angiotensin II; AT1R, angiotensin type II receptor 1; Mas1R, Mas1 proto-oncogene, G protein-coupled receptor.
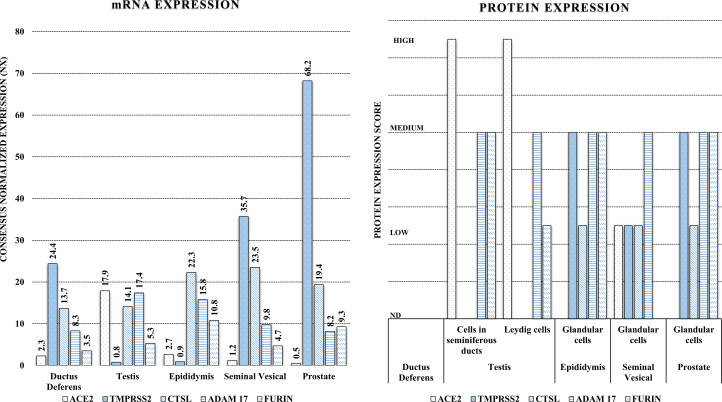
Multiple studies have shown a higher expression of ACE2 in men, specifically in reproductive tissues and more particularly in testis (testosterone producing Leydig cells, supportive Sertoli cells and seminiferous tubules which harbour spermatogonia) (Refs 21–23) (Fig. 3). Expression of TMPRSS2 is also significantly higher in reproductive tissues of men (Fig. 3). Accordingly, inflammation, leucocyte infiltration, damage of testicular tissue components and cell death, in post-mortem examinations and complaints of testicular pain in living COVID-19 patients were also reported. Similar evidence was also presented in SARS (Refs 23–25). However, no direct evidence for the presence of SARS-CoV-2 in testis of patients with COVID-19 could be shown yet. Conflicting reports are available for SARS-CoV-1 in human testis (but not for MERS) (Refs 23, 26).
Fig. 3.
Tissue-specific distribution (m-RNA and protein) of SARS-CoV-2 host cell entry receptor (ACE2) and related proteases (TMPRSS2, FURIN and ADAM17) in reproductive system components in men. A high expression (m-RNA and/or proteomic) of ACE2 and significantly increased expression of the one or more proteases is observable in multiple cellular/tissue components indicating their high susceptibility for the SARS-CoV-2 infection. Data source: Human Protein Atlas (https://www.proteinatlas.org/humanproteome/sars-cov-2, an open access database).
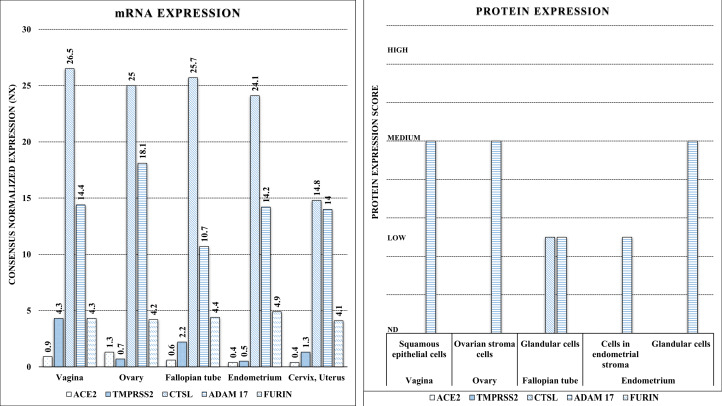
Conversely, expressions of other host proteases, CTSL, FURIN and ADAM17, were significantly higher in women reproductive tissue (Fig. 4) (Ref. 27). Of note, among the host proteases only TMPRSS2 has a known androgenic gene regulation which can influence its tissue expression hence may be a reason for its higher expression in men (Refs 4, 28). Testosterone by regulating expression of TMPRSS2 gene through androgen receptor (AR) causes increased internalisation of ACE2: SARS-CoV-2 complex (Ref. 29). The increased consumption of ACE2 by SARS-CoV-2 causes its depletion on lung epithelial cells and other tissue including vascular endothelium, thus activating the ACE/Ang II/AT1R-mediated pathway leading to vasoconstriction, increased tissue inflammation and fibrosis (Fig. 2). However, results from a recent preliminary study (Ref. 30) discouraged for androgen-mediated TMPRSS2 regulation in COVID-19 outcomes, at least for the pulmonary symptoms. Investigators of this study found no evidence for androgen regulation of pulmonary TMPRSS2 explaining sex-discordant COVID-19 outcomes in human as well as mice. However, they found sex-discordant expressions of AR and ACE2; AR showed limited expression in airway cells of human males but was absent in human females. In mice, males expressed significantly higher ACE2 protein in airways compared with females. They showed in mice, TMPRSS2 expression was unaffected and ACE2 modestly suppressed by potent AR blockade which led them to conclude that that sex differences in COVID-19 outcomes attributable to viral entry are independent of TMPRSS2 however ACE2 may have a role. Their results did not rule out a possible role of TMPRSS2-mediated mechanism in tissues other than lung (Ref. 30).
Fig. 4.
Tissue-specific distribution (m-RNA and protein) of SARS-CoV-2 host cell entry receptor (ACE2) and related proteases (TMPRSS2, FURIN and ADAM17) in reproductive system components in women. A low or non-detectable m-RNA and/or proteomic expression of ACE2, however, significant expression of one or more proteases is observable across the tissues, indicating their low susceptibility for SARS-CoV-2 infection. Data source: Human Protein Atlas (https://www.proteinatlas.org/humanproteome/sars-cov-2, an open access database).
Strong indications for the role of androgenic regulation of ACE2 in COVID-19 vulnerability in men and possible benefits from the use of anti-androgenic drugs were received from a recent study by Samuel et al. (Ref. 31). These investigators applied in vitro high-throughput drug screening to identify drugs that reduce ACE2 levels in human embryonic stem cell (hESC)-derived cardiac cells and lung organoids. Target analysis of hit compounds using bioinformatics revealed androgen signalling as a key modulator of ACE2 levels. Furthermore, they treated hESC-derived lung organoids with anti-androgenic drugs, which reduced ACE2 expression and protected the cells against SARS-CoV-2 infection. Investigators also examined clinical data on COVID-19 patients showing that prostate diseases, which are linked to elevated androgen, are significant risk factors and that the presence of genetic variants in the patients that increase androgen levels is associated with higher disease severity (Ref. 31).
Role of evolution and genetics, epigenetic mechanisms and sex hormones
Evolution and genetics
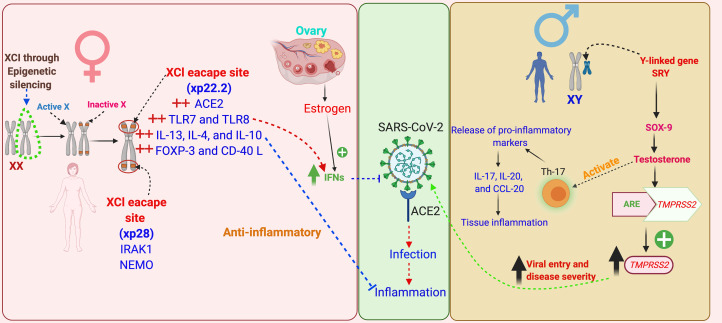
The good health of females is crucial for population growth of a species, as they raise offspring. Females may pass the protective immune response against the infectious agents to their babies in uterus and through breast milk, thus helping their survival. Stronger immune responses against infections in the case of females have an evolutionary trail which is evident across the species (Refs 5, 6). The underlying mechanisms favouring immunological gain for the females are not well understood; however, genetic landscape of immune genes of the organisms provides some significant clues, as they show a female dominance in their expressions (Ref. 32). First such clue is the existence of dual X-chromosome in females. However, the extra X-chromosome does not remain functional because of a phenomenon, known as X-chromosome inactivation (XCI), in females (Fig. 5). Some of the X-linked genes (approximately 15% of X genes in humans and 3% in mice) in the females escape XCI, providing double dosage of those genes (Refs 32, 33).
Fig. 5.
Schematic representation of the role of evolution, genetics, epigenetic mechanisms and sex hormones in sex-based differences in COVID-19 outcomes. Females bear two X chromosomes, of which one gets inactivated during oogenesis through epigenetic silencing of the genes – this phenomenon is known as ‘X-chromosomal inactivation’ (XCI). Some of the X-linked genes escape XCI, among these are the key immune functions genes, such as ACE2, TLRs-7, -8, ILs-4, -10 and -13, FoxP3, CD40L, IRAK1 and NEMO, thus these genes express in females in double dosage in comparison with males. These immune genes not only prime the females for a stronger immune response against infectious agents, but also prevent against hyper-inflammatory responses, such as CS. Apart from this, certain immune genes have sex hormone-specific regulatory elements in their promoter region modulating their expression, such as oestrogen has for IFN, and TMPRSS2 has for TMPRSS2. A marked influence of sex hormones on the formation of cytokines has also been noted, such as testosterone induces greater syntheses of Th-17 cells and in turn release of pro-inflammatory markers in males, such as IL-17, IL-20 and CCL-20 – thus favour hyper-inflammatory responses and in turn poor clinical outcomes. XCI, X chromosome inactivation; ACE2, angiotensin-converting enzyme 2; TMPRSS2, transmembrane protease, serine 2; ARE, androgen receptor element; SRY, sex-determining region Y; SOX-9, SRY-box transcription factor-9; TLRs, Toll-like receptors; IFN, interferon; ILs, interleukins; FoxP3, forkhead box P3; CD40L, cluster of differentiation 40L; CCL-20, chemokine (C–C motif) ligand-20; IRAK1, interleukin-1 receptor-associated kinase 1; NEMO, NF-κB essential modulator.
X-chromosome is known to contain many immune response genes which escape XCI, notably, IL-4, IL-10, IL-13, FoxP3, CD-40L and TLRs-7 and -8 – the pattern recognition receptors – mediating host innate response against RNA viruses, including coronaviruses (Refs 4, 32). IL-4, -10 and -13 are anti-inflammatory ILs (Ref. 34) hence their abundance in females in comparison with the males might be a reason for less severe inflammatory disease in them. Interestingly, FoxP3, that is a lineage specification factor for the T-regulatory (T-reg) cells (a type of CD4+ T cells), has oestrogen response elements in the promoter gene region (Ref. 35). T-reg cells are essential for maintaining immuno-tolerance, preventing autoimmunity and limiting inflammations (Ref. 35). CD40L is involved in CD4+ T-cell-mediated help for B cells (Ref. 36). Thus, the abundance of FoxP3 and CD40L may contribute to the anti-inflammatory control preventing hyper inflammation, and stronger antibody-mediated response in females (Ref. 4).
Apart from these, interleukin 1 receptor associated kinase 1 (IRAK1), a key regulatory molecule in the TLR-dependent signalling pathway, and a number of genes coding for immune regulatory molecules functioning downstream in the cytokine receptor signalling, such as NF-κB essential modulator (NEMO) modulates NF-κB expression, are also X-linked (Ref. 32).
A more robust innate immune response in the early course of COVID-19 may give women a survival advantage over men. Additionally, the gene for the SARS-CoV-2 host cell entry receptor – ACE2 – is located on the X-chromosome, which provides a potential basis for sex-biased expression of this protein. Moreover, ACE2 is an ISG, which is in turn bears oestrogen regulatory elements in its promoter region, which can further explain a favourable innate immune response in females (Refs 3, 37).
Conversely, Y-linked genes, such as Sry (testis-determining factor), Sox9 (SRY-box 9), may be responsible for a dampening influence of testosterone on innate immune response in men (Ref. 33). Additionally, TMPRSS2 gene has a testosterone-inducible element in its promoter region, hence, it can be a key influencing factor in men (Refs 3, 38).
Sex linkage of viral cell entry receptor or immune molecule polymorphs might be a reason for higher vulnerability for the infection and increased disease severity in some individuals. Such variants have recently been reported for ACE2 (Refs 39, 40) and TLR7 (Ref. 41), both of which are X-linked molecules. In a case series study involving four young male patients, a rare mutation of TLR7 was associated with impaired IFN (types I and II) responses in patients with severe COVID-19 (Ref. 41). Polymorphs have also been reported for TMPRSS2 (Ref. 42), which has androgenic regulation at the gene level (Ref. 3). However, none of these studies could show a clear causal relationship for the patient outcomes favouring women.
Epigenetics mechanisms
Epigenetics is the study of genetic mechanisms which alter the expression of genes without altering the genetic code of an organism. Salient examples of epigenetic mechanisms are modifications in the chemical tags (such as methyl or hydroxyl groups) of the nucleotide bases in the DNA or modifications in the histone proteins covering the DNA, which silence or activate a gene. Additionally, the post-transcriptional silencing of gene expression through non-coding RNAs, such as microRNAs (miRNAs) and long-noncoding RNAs (lncRNAs), is also considered an epigenetic mechanism. Escape from XCI, influences of sex hormones and immunological tolerance are mediated through epigenetic mechanisms (Fig. 5) (Refs 4, 33).
Several miRNA and lncRNA expressions are involved in immune response activation specific to particular sex; particularly, miRNAs which regulated TLR-7 (Ref. 43). miRNAs are abundant on the X-chromosome and are known to be modulated by sex hormones. The X chromosome contains 10% of the ~800 miRNAs in the human genome, in comparison, the Y chromosome contains only two miRNAs. miRNAs – including miRNA-18 and miRNA-19, which are encoded on the X chromosome – have known roles in sex differences in immune responses against infectious agents, including viruses (Ref. 33). Pontecorvi et al., using a bioinformatics approach, identified a set of miRNAs involved in sex-specific modulation of SARS-CoV-2 host cell entry proteins (Ref. 44). Wet-lab studies investigating the sex-specific role of miRNAs and lncRNAs in COVID-19, are at the infantile stage now and further specific studies will reveal its insight mechanistic details.
Sex hormones
Lymphocytes and other immune cells express sex hormone receptors. Oestrogen response elements are present in majority of immune responsive genes, which facilitate a stronger immune response in adult females. Animal model studies showed that oestrogens promoted the development of antibodies against infectious agents, including respiratory viruses (Refs 33, 45, 46). Existing literature suggests that oestrogen plausibly downregulates ACE and angiotensin type II (Ang II) receptor 1 (AT1R), and upregulates expressions of ACE2 and Mas 1 receptors in epithelial cells (REF) thus favours the ACE2/Ang 1-7/Mas1R-mediated pathway leading to vasodilatation, anti-inflammation and antifibrosis (Fig. 2) (Refs 18, 47). Another female sex hormone progesterone also has anti-inflammatory and protective effects against viral infections of mucosal epithelium (Refs 48, 49).
Conversely, androgens, including testosterone and dihydrotestosterone, have an immunosuppressive influence (that extends over pro-inflammatory as well as anti-inflammatory marker genes). Studies showed that testosterone suppressed the development of antibodies in mice (Refs 33, 45–51). Lower immune responses to influenza vaccination were reported in men, particularly those with high levels of testosterone (Ref. 52).
Testosterone is also linked with higher expression of the Th-17 pro-inflammatory marker in men which is associated with increased disease severity (Refs 4, 33, 51). Men with androgen deficiencies have higher concentrations of inflammatory cytokines, such as IL-1β, IL-2 and tumour necrosis factor (TNF), antibody titres and CD4/CD8T cell ratios compared with men with basal testosterone levels (Refs 33, 52, 53). Furthermore, men treated with a gonadotropin-releasing hormone (GnRH) antagonist (which significantly reduces testosterone levels), have lower counts of T-reg cells and higher counts of natural killer cells in peripheral blood compared with placebo-treated men or men treated with both, GnRH antagonist and exogenous testosterone (Ref. 33).
In a recent prospective cohort study (Ref. 54), Cyan et al. reported significantly increased chance of ICU admissions (P = 0.001) and mortality (P = 0.002) with lower serum total testosterone levels in male patients with COVID-19. Investigators also noted post-infection significant decrease in total serum testosterone levels (from 458 ± 198 to 315 ± 120 ng/dl; P = 0.003). A decrease in the total serum testosterone was significantly correlated with an increase in age (r = 0.236; P = 0.000) and increased levels of pro-inflammatory markers, such as D-dimer (r = 0.213; P = 0.003), procalcitonin (r = 0.225; P = 0.000) and C-reactive protein (CRP) (r = 0.144; P = 0.003), which indicated the role of age and viral-induced pathogenesis in testosterone production in male patients with COVID-19 (Ref. 54).
A higher level of testosterone in male may be activating the ACE/Ang II/AT1R pathway of RAS in lung epithelium (and perhaps also in other tissues including vascular endothelium) leading to an increased severity of COVID-19 (Fig. 2) (Ref. 29), although any concrete in vivo evidence is currently lacking in this regard.
Numerous studies clearly suggest that sex hormones have an impact on individual's immune response however, that is age-dependent, and above described patterns are mostly limited to reproductive age (Refs 4, 33). In the case of children and elderly (>80 years), the influence of sex hormones over immune response is not much distinguishable (Ref. 55).
Figure 4 presents a summarised account of the role of evolution, genetics, epigenetic mechanisms and sex hormones in sex-based differences in COVID-19 outcomes.
Immunological factors
An increasing body of evidence established immunological factors are key determinants of the disease severity and patient outcomes in COVID-19 (Ref. 56); hence it is likely that they play significant roles in observed sex bias in the presentation of the cases. A hyperactive innate immune response against the invading pathogen leading to systemic hyper-inflammatory state – ‘cytokine storm (CS)’ – is a well-known phenomenon deciding disease severity in respiratory viral infections, such as SARS and influenza (Refs 56, 57). Clinical studies demonstrated that CS plays a very critical role in developing severe COVID-19 (Refs 56, 57). CS in COVID-19 is characterised by increased levels of a unique set of cytokines (and chemokines), such as IL-2, IL-6, IL-7, IL-10, granulocyte colony stimulating factor and granulocyte-macrophage colony stimulating factor, interferon-gamma inducible protein 10 (IP10), monocyte chemo-attractant protein 1, macrophage inflammatory protein 1-α, CRP and ferritin, TNFα and IFNγ. Particularly, circulating concentrations of CXCL10, CCL2, IL-2R, IL-6, TNFα, CRP and ferritin are found significantly increased in the patients requiring ICU admission (Refs 56, 57). IL-6 and IL-10 were reported as the key prognostic markers indicating disease severity in COVID-19 (Refs 58, 59).
As we discussed above (in subsection ‘Evolution and genetics’), a sex-based difference in the immune response can be largely attributed to differential regulation of the protective genes, which seem to be evolutionarily biased in favour of females, supposedly for ensuring better survival of the species (Refs 3, 37). Females not only show a more robust protective immune response but also develop lower tissue injury and have better tissue repair (Refs 60–64); these factors taken together can be reason they develop milder disease forms in comparison with males, including lower chances of developing systemic hyper-inflammatory states, such as CS.
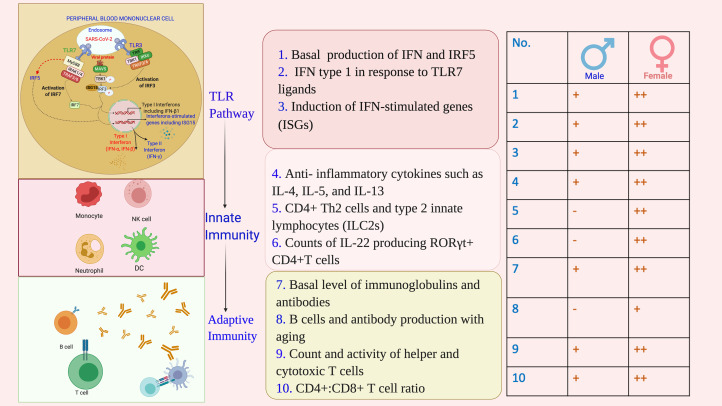
In vitro and in vivo studies suggest that females have relatively a higher number of innate immune cells as well as they form stronger innate immune responses (Refs 4, 33). A stronger TLR-7-mediated IFN response and higher basal production of IFN and IFN regulatory factor 5 (IRF5) were noted in vitro and animal model studies in the case of female organisms. In humans, IFN type I responses are differentially regulated between men and women (Ref. 65). Females also exhibited a stronger adaptive (cell and antibody mediated) immune responses in comparison with males. Basal levels of immunoglobulin as well as antibody responses were consistently higher in females compared with those in males (Fig. 6).
Fig. 6.
Immunological basis of sex-based differences in COVID-19 outcomes. A stronger protective immune response, for its each component: TLR-based viral sensing, and innate and adaptive immunity, is ensued against SARS-CoV-2 infection in females in comparison with males. TLR, Toll-like receptor; IFN, interferon; ISGs, interferon-stimulated genes; ILs, interleukins; IRF, interferon regulatory factor; CD, cluster of differentiation; RORγt, retinoic acid-related orphan receptor gamma-t; MAVS, mitochondrial antiviral signalling protein; IRAK, interleukin-1 receptor-associated kinase; TRAF, TNF receptor (TNFR) associated factor; TBK, TANK-binding kinase; MyD88, myeloid differentiation primary response gene 88.
The expressions of TLR-pathway and pro-inflammatory genes [e.g. TLR7, myeloid differentiation primary response gene 88 (MYD88), retinoic acid inducible gene-I (RIG-I), IRF7, IFN-β, Janus kinase 2 (JAK2), signal transducer and activator of transcription (STAT3), nuclear factor-κB (NFKB), IFN-γ and tumour necrosis factor (TNF)] were higher in female compared with those in male peripheral blood mononuclear cells from humans and tissues from rats (Ref. 66).
Remarkably, the presence of mutated forms of TLR7 was found associated with severe COVID-19 in male patients in a preliminary study (Ref. 41). Coronaviruses, including MERS-CoV and SARS-CoV-1, can induce type I IFN responses through TLR7 (Refs 67–69), and SARS-CoV-1 and SARS-CoV-2 have been shown to be sensitive to viral restriction by ISGs in vivo and in vitro (Refs 70, 71). Several studies have demonstrated that women produce more IFN type I in response to TLR7 ligands, including viral RNAs, resulting in higher induction of ISGs in women (Refs 72–75). The initial studies assessing type I IFN responses induced by SARS-CoV-2 infections suggested induction of ISGs by SARS-CoV-2 (Refs 76–78), but to a lower level than observed in SARS-CoV infection (Refs 76, 77), indicating a potential immune evasion mechanism (Fig. 6) (Ref. 4).
Human studies have shown, regardless of the age, females have not only higher expressions of immunoglobulins and antibodies, but they also have higher expression of immune function genes in the B cells, higher count and activity of helper and cytotoxic T cells and higher CD4+:CD8+ T cell ratio (Refs 3, 4, 75). However, as a drawback of stronger immunity, women also have the greater susceptibility to autoimmune diseases (Refs 3, 4, 75). Keeping in that line, in patients with COVID-19, a more favourable immune response has been observed in women. Takahashi et al. examined sex differences in viral loads, SARS-CoV-2-specific antibody titres, plasma cytokines and blood cell phenotyping in patients with COVID-19 (Ref. 1). Investigators found a significant sex-based difference in immune responses. In the patients with moderate disease who had not received immunomodulatory medications, including high dose steroid (prednisolone >40 mg), they observed that men had higher plasma levels of innate immune cytokines, such as IL-8 and IL-18, as well as stronger induction of non-classical monocytes. In contrast, female patients, including the aged, showed significantly stronger T cell activation (Ref. 1). They additionally found that a poor T cell response negatively correlated with patients' age and led to poor outcome, which was more prominent in men. However, higher innate immune cytokines were associated with worse disease progression in women, but not in men (Ref. 1).
Some investigators have also shown that SARS-CoV-2 may be detected in nasal swabs for longer periods in men compared with women indicating a delayed viral clearance in men (Refs 79, 80).
A faster clearance of the respiratory viral infection in females may be accounted for higher counts of CD4+ Th2 cells and type 2 innate lymphocytes (ILC2s) in them (Ref. 4). Viral clearance from the respiratory mucosa is enhanced by cytokines, such as IL-4, IL-5 and IL-13, which are secreted by type 2 immune cells (Ref. 81). The receptor for IL-13 (IL-13RA1) is encoded by the X chromosome, and may provide women with an advantage of higher gene dosage (Ref. 36). Conversely, androgens were shown to reduce ILC2 numbers and cytokine production in male mice (Refs 82, 83). Increased expression of IL-13 in females may also protect them through a SARS-CoV-2 host cell entry receptor-based mechanism. IL-13 was found to decrease the expression of ACE2 in epithelial cells in vitro, and respiratory epithelial cells of asthma patients with enhanced type 2 signalling exhibited reduced ACE2 expression compared with healthy controls (Ref. 84).
Apart from protection against the infection, immune cell profiles in women are skewed more towards tissue repair responses compared with men, which may have important implications in COVID-19. Women have higher counts of IL-22 producing retinoic acid-related orphan receptor gamma-t (RORγt) + CD4+ T cells which are known to be regulated by sex hormones (Refs 60–62). IL-22 promotes epithelial stem cell proliferation via STAT3 signalling (Ref. 63), contributing to tissue resilience upon damage. Tissue protective, including lung epithelial repair effects of IL-22, has also been demonstrated in several viral infections (Ref. 64).
How X-linkage of key immune response genes and oestrogenic regulation of IFN can influence the patient's outcomes in infectious diseases has been reflected by a recent COVID-19 study. Bastard et al. examined blood samples from 987 gravely ill COVID-19 patients from multiple ethnic backgrounds across the world. Surprisingly, in at least 101 (10.2%) of the patients who were aged 25–87 years, the investigators found auto-antibodies against IFN type I, of which 95 (94%) were men. However, auto-antibodies were not found in 663 individuals with asymptomatic or mild disease, and were present in only four out of 1227 (0.33%) healthy individuals (Ref. 85).
In addition to the severity of symptoms and mortality, sex-specific immunological responses will have a significant impact on incidence of post-survival health issues in patients with COVID-19, such as functional problems arising from residual organ damages and autoimmune diseases. Possible sex-based variation in the efficacy and adverse effects with vaccines is another crucial indication from the sex-specific immunological responses observed in patients with COVID-19. Sex-specific variations in response to the vaccine were reported in mice models for influenza (however, the same could not be concluded effectively in humans citing data constraints) and it is plausible that it may also hold true in COVID-19 (Refs 45, 86).
Figure 6 presents a summarised account of immunological basis of sex-based differences in COVID-19 outcomes.
Additive factors: smoking and gut microbiome
Smoking has been associated with severe pulmonary symptoms and higher mortality in COVID-19 (Refs 87–89). At the population scale, men are more likely to smoke compared with women, which plausibly can be a contributory reason for the higher overall COVID-19 mortality in men in comparison with women (Ref. 87). However, in the countries, such as Italy, where there is not wide gender-based difference in the prevalence of smoking, mortality in men remained greater compared with women (Ref. 14) indicating that smoking may have only a limited contribution to sex-specific mortality in COVID-19. Additionally, whether COVID-19 mortality varies between smoking men and women is not sufficiently studied, thus, warrant further study to primarily outline the role of smoking in disease mortality.
Sex-based differences in gut microbiome are considered an important determinant in priming immunological responses against infections, including viruses (Refs 8, 49, 90). Recently, it has been noted that sex hormones, specifically oestrogen, have a significant effect on the microbiome of the individuals (Refs 91–93). Prominent digestive symptoms are known in patients with COVID-19 and recent studies have shown gut mucosa as a potential site for SARS-CoV-2 infection (Ref. 94). Microbiome is considered to be a crucial factor in the development of gut mucosal immunity against the infections including viruses (Ref. 90), hence, may have significant implications for COVID-19 pathogenesis (Ref. 8). The role of gut microbiome in COVID-19-related morbidity and mortality, in reference of sex, has been narrowly studied and warrant further attention.
Non-biological factors
Apart from biological mechanisms, which are the key determinants of the sex-based differences, sex-based differences in COVID-19 mortality can depend on multiple environmental factors, such as gender-based socio-economic disparity and unequal reach to the health-care facilities (Ref. 16). An hostile environment added with the health policy discriminations may make a gender more vulnerable for contracting infections and having higher fatality rates, as we have observed a reverse trend in sex-differentiated data for some countries, which showed higher fatality rates in women in comparison with men, which are not being explained biologically (Ref. 16).
Concluding remarks
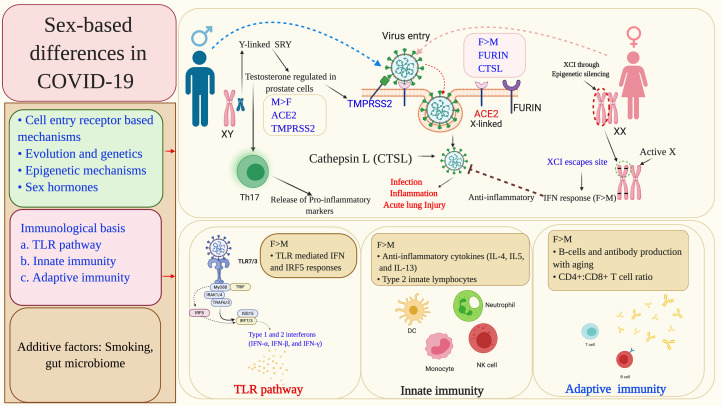
Existing literature and recent research reports provide coherent evidence for the immunological privilege of the women against severe disease symptoms and mortality in COVID-19. This review highlights multiple plausible molecular mechanisms mediating influence of biological sex on mortality in COVID-19 (summarised in Fig. 7 and Table 1), which can be broadly categorised as mechanisms leading to (i) escape of immune response genes present on X chromosome from inactivation, (ii) sex hormones, primarily oestrogen and testosterone, mediated regulation of multiple immune response genes, receptors and tissue proteases and (iii) related to smoking and gut microbiome. An understanding of these molecular mechanisms may help identifying the potential drug targets and developing a sex-differentiated therapeutic approach for COVID-19. Currently, evidence is limited and nascent; further in-depth research will be necessary to obtain a concrete understanding of this issue.
Fig. 7.
Summary of the molecular mechanisms leading to sex-differentiated outcomes in COVID-19 patients. The sex-based differences primarily occur at the level of viral cell entry receptors and immune response mechanisms against the infection. Sex-specific genetic and epigenetic factors, and also sex hormones differentially modulate viral cell entry receptors and associated host proteases, viral sensing receptors and multiple immune response genes including IFN. Some other factors, such as smoking and gut microbiome have also been found to influence the patient outcomes in a sex-based manner. XCI, X chromosome inactivation; ACE2, angiotensin-converting enzyme 2; TMPRSS2, transmembrane protease, serine 2; CTSL, cathepsin L; ARE, androgen receptor element; SRY, sex-determining region Y; SOX-9, SRY-box transcription factor-9; TLRs, Toll-like receptors; IFN, interferon; IRAK1, interleukin-1 receptor-associated kinase 1; ISGs, interferon-stimulated genes; IRF-interferon regulatory factor; TRAF, TNF receptor (TNFR) associated factor; TRIF, TIR-domain-containing adapter-inducing interferon-β; MyD88, myeloid differentiation primary response gene 88.
Table 1.
Molecular mechanisms for sex-based differences in patient outcomes in COVID-19
| Sections | Salient findings | References |
|---|---|---|
| Sex-specific prevalence of morbidity/mortality in COVID-19 | M > F | 9,10,11,12,13,14,15,16 |
| Cell entry receptor-based mechanisms | ACE2 and TMPRSS2 expression in M > F, whereas expression of CTSL, FURIN and ADAM 17 in F > M | 3, 4, 17–30 |
| Evolution and genetics | Evolution favours better immunity against pathogens in females across the species, including humans, as they raise offspring. Certain X-linked genes with immune functions, notably, IL-4, IL-10, IL-13, FoxP3, CD-40L and TLRs-7 and -8, escape constitutional inactivation of second X chromosome in females which can lead to double dosage of these genes, thus ensure stronger immune responses against pathogens. In males, Y-linked genes such as SRY and SOX9 dampen the innate immunity, and testosterone inducibility of TMPRSS2 may influence viral infectivity of the host cells. Severe male cases were found to be associated with a rare X-linked mutation of a viral sensor on host cells – TLR7 | 3--, 32–42 |
| Epigenetics mechanisms | miRNAs, which are abundantly present on X chromosome, regulate SARS-CoV-2 host cell entry proteins. The miRNA modulation by sex hormones is mediated through epigenetic mechanisms | 4, 43, 44 |
| Sex hormones | In females, oestrogen promotes stronger IFN-mediated innate immune responses as well as antibody development against viral infections. Progesterone also has protective effects against viral infections. In males, androgens, including testosterone, have an immune-suppressive influence and suppress development of antibodies against viral infections | 4, 45–55 |
| Immunological factors | Females have better innate and adaptive immune responses. They have higher expression of TLR-pathway, pro-inflammatory genes and immune function genes in the B cells, along with higher count and activity of helper and cytotoxic T cells and a higher CD4+:CD8+ T cell ratio | 1, 4, 33, 56–86 |
| Additive factors: smoking and gut microbiome | Smoking may have only limited contribution to sex-specific mortality in COVID-19. Oestrogen significantly affects gut microbiome influencing gut mucosal immunity against the infections including viruses | 8, 14, 87–94 |
Limitations
Our projections for possible molecular mechanisms explaining sex-based difference in patient outcomes in COVID-19 have multiple limitations which need to be considered. Most importantly, currently, original studies in COVID-19, addressing this aspect, are very limited, and available literature largely present speculations based on results from other respiratory viral infections. However, these are important projections which may hold true in COVID-19 if tested, hence have extreme importance for designing future studies. Second, most of the epidemiological and clinical studies involving COVID-19 patients, included in this review, did not ensure standardised control of the confounding factors, such as sex-based disproportions in the included population and sex-biased reach to the medical facilities, which could have affected proportion of the sex-based presentations. However, the general observation across the studies that women with COVID-19 face lower morbidity and mortality is in line with the trend reflected from the global data. Third, there remains possibility that our used key words could not retrieve all studies under targeted categories, especially, many recent preliminary studies or preprint articles, which may have relevant findings.
Acknowledgement
We acknowledge help of databases, Human Protein Atlas (https://www.proteinatlas.org/) and Global health 50/50 (https://globalhealth5050.org) for making available COVID-19-related data used in this study.
Author contributions
A.K. wrote the first draft. R.K.N., M.K., P.P., C.K., V.P., S.K., P. S. S., K.S., K.K., and S. K. revised the draft. R.K.N. performed data analysis, and P.P. and R.K.N. prepared the figures.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/erm.2021.9.
click here to view supplementary material
Conflict of interest
The authors declared ‘no conflicts of interest’.
References
- 1.Takahashi T, et al. (2020) Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 588, 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Channappanavar R, et al. (2017) Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. Journal of Immunology 198, 4046–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scully EP, et al. (2020) Considering how biological sex impacts immune responses and COVID-19 outcomes. Nature Reviews Immunology 20, 442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunders MJ and Altfeld M (2020) Implications of sex differences in immunity for SARS-CoV-2 pathogenesis and design of therapeutic interventions. Immunity 53, 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Úbeda F and Jansen VAA (2016) The evolution of sex-specific virulence in infectious diseases. Nature Communications 7, 13849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuk M and Stoehr AM (2010) Sex differences in susceptibility to infection: An evolutionary perspective. In Klein SL and Roberts CW (eds), Sex Hormones and Immunity to Infection. Berlin, Heidelberg: Springer-Verlag Berlin Heidelberg, pp. 1–17. [Google Scholar]
- 7.Taneja V (2018) Sex hormones determine immune response. Frontiers in Immunology 9, 1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhar D and Mohanty A (2020) Gut microbiota and COVID-19- possible link and implications. Virus Research 285, 198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin J-M, et al. (2020) Gender differences in patients with COVID-19: focus on severity and mortality. Frontiers in Public Health 8, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson EJ, et al. (2020) Factors associated with COVID-19-related death using OpenSAFELY. Nature 584, 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peckham H, et al. (2020) Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nature Communications 11, 6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanez ND, et al. (2020) COVID-19 mortality risk for older men and women. BMC Public Health 20, 1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasiri MJ, et al. (2020) COVID-19 clinical characteristics, and sex-specific risk of mortality: systematic review and meta-analysis. Frontiers in Medicine 7, 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dataset Global Health 50/50. Available at https://globalhealth5050.org/the-sex-gender-and-covid-19-project/dataset/ (Accessed 13 October 2020).
- 15.Kumar S, et al. (2020) Assessment of the prevalence of symptoms in patients under institutional isolation in COVID-19 pandemic in India. Indian Journal of Palliative Care 26, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dehingia N and Raj A (2020) Sex differences in COVID-19 case fatality: do we know enough? Lancet Global Health 9, e14–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann M, et al. (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280, e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zipeto D, et al. (2020) ACE2/ADAM17/TMPRSS2 interplay may be the main risk factor for COVID-19. Frontiers in immunology 11, 576745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glowacka I, et al. (2010) Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. Journal of Virology 84, 1198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A, et al. (2020) SARS-CoV-2 cell entry receptor ACE2 mediated endothelial dysfunction leads to vascular thrombosis in COVID-19 patients. Medical Hypotheses 145, 110320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z and Xu X (2020) scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, Leydig and Sertoli cells. Cells 9, 920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen Q, et al. (2020) The ACE2 expression in Sertoli cells and germ cells may cause male reproductive disorder after SARS-CoV-2 infection. Journal of Cellular and Molecular Medicine 24, 9472–9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, et al. (2006) Orchitis: a complication of severe acute respiratory syndrome (SARS). Biology of Reproduction 74, 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deshmukh V, et al. (2021) Histopathological observations in COVID-19: a systematic review. Journal of Clinical Pathology 74, 76–83. [DOI] [PubMed] [Google Scholar]
- 25.Cardona Maya WD, Du Plessis SS and Velilla PA (2020) SARS-CoV-2 and the testis: similarity with other viruses and routes of infection. Reproductive Biomedicine Online 40, 763–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao J, et al. (2003) Clinical pathology and pathogenesis of severe acute respiratory syndrome. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 17, 217–21 (in Chinese). [PubMed] [Google Scholar]
- 27.SARS-CoV-2 related proteins – The Human Protein Atlas. Available at https://www.proteinatlas.org/humanproteome/sars-cov-2 (Accessed 1 June 2020).
- 28.Montopoli M, et al. (2020) Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Annals of Oncology 8, 1040–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohamed MS, et al. (2021) Sex differences in COVID-19: the role of androgens in disease severity and progression. Endocrine 71, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baratchian M, et al. (2020) No evidence that androgen regulation of pulmonary TMPRSS2 explains sex-discordant COVID-19 outcomes. bioRxiv [Preprint] 2020.04.21.051201. [Google Scholar]
- 31.Samuel RM, et al. (2020) Androgen signaling regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men. Cell Stem Cell 27, 876–889, e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schurz H, et al. (2019) The X chromosome and sex-specific effects in infectious disease susceptibility. Human Genomics 13, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein SL and Flanagan KL (2016) Sex differences in immune responses. Nature Review Immunology 16, 626–638. [DOI] [PubMed] [Google Scholar]
- 34.Kucharzik T, et al. (1998) IL-4, IL-10 and IL-13 down-regulate monocyte-chemoattracting protein-1 (MCP-1) production in activated intestinal epithelial cells. Clinical and Experimental Immunology 111, 152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudensky AY (2011) Regulatory T cells and Foxp3. Immunological Reviews 241, 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tukiainen T, et al. (2017) Landscape of X chromosome inactivation across human tissues. Nature 550, 244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziegler CGK, et al. (2020) SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181, 1016–1035, e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stopsack KH, et al. (2020) TMPRSS2 and COVID-19: serendipity or opportunity for intervention? Cancer Discovery 10, 779–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benetti E, et al. (2020) ACE2 gene variants may underlie interindividual variability and susceptibility to COVID-19 in the Italian population. European Journal of Human Genetics 28, 1602–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao Y, et al. (2020) Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discovery 6, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Made CI, et al. (2020) Presence of genetic variants among young men with severe COVID-19. JAMA 324, 663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hou Y, et al. (2020) New insights into genetic susceptibility of COVID-19: An ACE2 and TMPRSS2 polymorphism analysis. BMC Medicine 18, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng Y, et al. (2017) Extracellular microRNAs induce potent innate immune responses via TLR7/MyD88-dependent mechanisms. Journal of Immunology 199, 2106–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pontecorvi G, et al. (2020) microRNAs as new possible actors in gender disparities of COVID-19 pandemic. Acta Physiologica 230, e13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fink AL (2018) Biological sex affects vaccine efficacy and protection against influenza in mice. Proceedings of the National Academy of Sciences USA 115, 12477–12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flanagan KL, et al. (2017) Sex and gender differences in the outcomes of vaccination over the life course. Annual Review of Cell and Developmental Biology 33, 577–599. [DOI] [PubMed] [Google Scholar]
- 47.Bukowska A, et al. (2017) Protective regulation of the ACE2/ACE gene expression by estrogen in human atrial tissue from elderly men. Experimental Biology and Medicine 242, 1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viveiros A, et al. (2021) Sex differences in COVID-19: candidate pathways, genetics of ACE2, and sex hormones. American Journal of Physiology-Heart and Circulatory Physiology 320, H296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hall OJ and Klein SL (2017) Progesterone-based compounds affect immune responses and susceptibility to infections at diverse mucosal sites. Mucosal Immunology 10, 1097–1107. [DOI] [PubMed] [Google Scholar]
- 50.Ghazizadeh Z, et al. (2020) Androgen regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men. BioRxiv. the preprint server for biology 2020.05.12.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gadi N, et al. (2020) What's sex got to do with COVID-19? Gender-based differences in the host immune response to coronaviruses. Frontiers in Immunology 11, 2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Furman D, et al. (2014) Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proceedings of the National Academy of Sciences USA 111, 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohamad NV, et al. (2019) The relationship between circulating testosterone and inflammatory cytokines in men. The Aging Male 22, 129–140. [DOI] [PubMed] [Google Scholar]
- 54.Çayan S, et al. (2020) Effect of serum total testosterone and its relationship with other laboratory parameters on the prognosis of coronavirus disease 2019 (COVID-19) in SARS-CoV-2 infected male patients: a cohort study. The Aging Male 23, 1–11. [DOI] [PubMed] [Google Scholar]
- 55.Bhopal SS and Bhopal R (2020) Sex differential in COVID-19 mortality varies markedly by age. Lancet (London, England) 396, 532–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar A, et al. (2021) Pathogenesis guided therapeutic management of COVID-19: an immunological perspective. International Reviews in Immunology 40, 54–71. [DOI] [PubMed] [Google Scholar]
- 57.Coperchini F, et al. (2020) The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Review 53, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dhar SK, et al. (2021) IL-6 and IL-10 as predictors of disease severity in COVID-19 patients: results from meta-analysis and regression. Heliyon 7, e06155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang J, et al. (2020) Serum interleukin-6 is an indicator for severity in 901 patients with SARS-CoV-2 infection: a cohort study. Journal of Translational Medicine 18, 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fuseini H, et al. (2018) Testosterone decreases house dust mite-induced type 2 and IL-17A-mediated airway inflammation. Journal of Immunology 201, 1843–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fuseini H, et al. (2019) ERα signaling increased IL-17A production in Th17 cells by upregulating IL-23R expression, mitochondrial respiration, and proliferation. Frontiers in Immunology 10, 2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Newcomb DC, et al. (2015) Estrogen and progesterone decrease let-7f microRNA expression and increase IL-23/IL-23 receptor signaling and IL-17A production in patients with severe asthma. Journal of Allergy and Clinical Immunology 136, 1025–1034, e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lindemans CA, et al. (2015) Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 528, 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pociask DA, et al. (2013) IL-22 is essential for lung epithelial repair following influenza infection. American Journal of Pathology 182, 1286–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ziegler SM and Altfeld M (2017) Human immunodeficiency virus 1 and type I interferons – where sex makes a difference. Frontiers in Immunology 8, 1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Griesbeck M, et al. (2015) Sex differences in plasmacytoid dendritic cell levels of IRF5 drive higher IFN-production in women. Journal of Immunology 195, 5327–5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karnam G, et al. (2012) CD200 receptor controls sex-specific TLR7 responses to viral infection. PLoS Pathogen 8, e1002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scheuplein VA, et al. (2015) High secretion of interferons by human plasmacytoid dendritic cells upon recognition of middle east respiratory syndrome coronavirus. Journal of Virology 89, 3859–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li SW, et al. (2016) SARS coronavirus papain-like protease inhibits the TLR7 signaling pathway through removing Lys63-linked polyubiquitination of TRAF3 and TRAF6. International Journal of Molecular Sciences 17, 678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haagmans BL, et al. (2004) Pegylated interferon-α protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nature Medicine 10, 290–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mantlo E, et al. (2020) Antiviral activities of type I interferons to SARS-CoV-2 infection. Antiviral Research 179, 104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berghöfer B, et al. (2006) TLR7 ligands induce higher IFN-α production in females. Journal of Immunology 177, 2088–2096. [DOI] [PubMed] [Google Scholar]
- 73.Chang JJ, et al. (2013) Higher expression of several interferon-stimulated genes in HIV-1-infected females after adjusting for the level of viral replication. Journal of Infectious Diseases 208, 830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meier A, et al. (2009) Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nature Medicine 15, 955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seillet C, et al. (2012) The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor α signaling. Blood 119, 454–464. [DOI] [PubMed] [Google Scholar]
- 76.Blanco-Melo D, et al. (2020) Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181, 1036–1045, e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chu H, et al. (2020) Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clinical Infectious Diseases 71, 1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou Z, et al. (2020) Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host & Microbe 27, 883–890, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zheng S, et al. (2020) Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January–March 2020: retrospective cohort study. BMJ 369, m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu K, et al. (2020) Factors associated with prolonged viral RNA shedding in patients with coronavirus disease 2019 (COVID-19). Clinical Infectious Diseases 71, 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gause WC, Rothlin C and Loke P (2020) Heterogeneity in the initiation, development and function of type 2 immunity. Nature Reviews Immunology 20, 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laffont S, et al. (2017) Androgen signaling negatively controls group 2 innate lymphoid cells. Journal of Experimental Medicine 214, 1581–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ricardo-Gonzalez RR, et al. (2018) Tissue signals imprint ILC2 identity with anticipatory function. Nature Immunology 19, 1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peters MC, et al. (2020) COVID-19-related genes in sputum cells in asthma: relationship to demographic features and corticosteroids. American Journal of Respiratory and Critical Care Medicine 202, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bastard P, et al. (2020) Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science (New York, N.Y.) 65, eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tadount F, et al. (2020) Is there a difference in the immune response, efficacy, effectiveness and safety of seasonal influenza vaccine in males and females? – A systematic review. Vaccine 38, 444–459. [DOI] [PubMed] [Google Scholar]
- 87.van Zyl-Smit RN, Richards G and Leone FT (2020) Tobacco smoking and COVID-19 infection. Lancet Respiratory Medicine 8, 664–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alqahtani JS, et al. (2020) Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One 15, e0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.WHO (2020) Smoking and COVID-19. Available at https://www.who.int/news-room/commentaries/detail/smoking-and-covid-19 (Accessed 5 October 5 2020).
- 90.Sankaran-Walters S, et al. (2013) Sex differences matter in the gut: effect on mucosal immune activation and inflammation. Biology of Sex Differences 4, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shin JH, et al. (2019) Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Respiratory Microbiology 170, 192–201. [DOI] [PubMed] [Google Scholar]
- 92.Haitao T, et al. (2020) COVID-19 and sex differences: mechanisms and biomarkers. Mayo Clinic Proceedings 95, 2189–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baker JM, Al-Nakkash L and Herbst-Kralovetz MM (2017) Estrogen–gut microbiome axis: physiological and clinical implications. Maturitas 103, 45–53. [DOI] [PubMed] [Google Scholar]
- 94.Kumar A, et al. (2020) Relevance of SARS-CoV-2 related factors ACE2 and TMPRSS2 expressions in gastrointestinal tissue with pathogenesis of digestive symptoms, diabetes-associated mortality, and disease recurrence in COVID-19 patients. Medical Hypotheses 144, 110271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/erm.2021.9.
click here to view supplementary material