Abstract
Insensitive high explosive materials (IHE) such as 3-nitro-1,2,4-triazol-5-one (NTO) and 2,4-dinitroanisole (DNAN) are increasingly being used in formulations of insensitive munitions alongside 1,3,5-trinitroperhydro-1,3,5-triazine (RDX). Load, assembly and packing (LAP) facilities that process munitions produce wastewater contaminated with IHE which must be treated before discharge. Some facilities can produce as much as 90,000 L of contaminated wastewater per day. In this review, methods of wastewater treatment are assessed in terms of their strengths, weaknesses, opportunities and threats for their use in production of IHE munitions including their limitations and how they could be applied to industrial scale LAP facilities. Adsorption is identified as a suitable treatment method, however the high solubility of NTO, up to 16.6 g.L−1 which is 180 times higher that of TNT, has the potential to exceed the adsorptive capacity of carbon adsorption systems. The key properties of the adsorptive materials along the selection of adsorption models are highlighted and recommendations on how the limitations of carbon adsorption systems for IHE wastewater can be overcome are offered, including the modification of carbons to increase adsorptive capacity or reduce costs.
Keywords: Adsorption, Insensitive high explosives, DNAN, NTO, Wastewater treatment
Adsorption, Insensitive High Explosives, DNAN, NTO, Wastewater treatment
1. Introduction
1.1. Insensitive munitions
Explosive formulations have been developed over time to give better performance as military requirements increase (Akhavan, 2011). However, increases in explosive power have led to increased sensitivity of stored and transported explosives, exemplified by the USS Forrestal aircraft carrier fire caused by a large amount of ordnance being unintentionally initiated which led to estimated damages of up to $72 million and the loss of 134 lives (Stewart, 2004). Following this disaster, governments and munitions manufacturers started to look for ways to ensure that munitions reliably fulfil their performance and operational requirements on demand whilst minimising the probability of inadvertent initiation, termed Insensitive Munitions (IM) (NATO, 2010). IM are designed to fail in a non-catastrophic way to ensure that the energy released is in a less dangerous manner than would otherwise be the case if it were to detonate, like burning or melting (Powell, 2016). In some situations, the formulation of the explosive filling is changed to make it more resistant to unintended initiation. There are two commonly manufactured IM fillings, polymer bonded explosives (PBX) which can be machined onto complex shapes and insensitive high explosives (IHE) which are more suited to melt casting and are compared in Table 1 (Akhavan, 2011).
Table 1.
A comparison of advantages and disadvantages of polymer bonded and melt cast explosive manufacture.
| Insensitive Munitions Material | Advantages | Disadvantages |
|---|---|---|
| Polymer Bonded Explosives |
|
|
| Melt Cast Explosives |
|
|
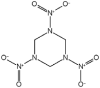
PBX use a polymer matrix, that can be energetic or non-energetic, to hold and coat solid explosive crystals. The matrix prevents the crystals impinging on each other and causing a reaction and so protects the explosive compositions from unintended initiation by shock (Zeman et al., 2019). IHE compositions consist of explosive materials that are not as sensitive to shock, such as 3-nitro-1,2,4-triazol-5-one (NTO) and 2,4-dinitroanisole (DNAN) which have similar energetic properties to the energetic materials they replace, like TNT, whilst providing better resistance to unintended initiation (Davies and Provatas, 2006; Vijayalakshmi et al., 2015). They can be used in isolation but are more often found in formulations alongside 1,3,5-trinitroperhydro-1,3,5-triazine (RDX). A comparison of performance characteristics for IHE materials compared to RDX and TNT can be seen in Table 2.
Table 2.
The structures and physicochemical properties of TNT and IHE materials.
| Energetic Material | TNT | RDX | DNAN | NTO |
|---|---|---|---|---|
| Empirical Formula | C7H5N3O6 | C3H6N6O6 | C7H6N2O5 | C2H2N4O3 |
| Structure | 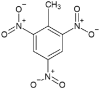 |
 |
 |
 |
| Density (g.cm−3) | 1.65 [1] | 1.82 [1] | 1.35 [2] | 1.93 [3] |
| Velocity of Detonation (m.s−1) | 6900 [4] | 8750 [4] | 5344 [5] | 7440 [6] |
| Melting Point (oC) | 80–82 [7] | 205.5 [8] | 94.5 [9] | 273 [10] |
| Figure of Insensitivity (Rotter Test) | 170 [2] | 80 [2] | 220 [2] | 90 [6] |
| Solubility (mg.L−1) | 89 at 20 °C [11] | 42 at 20 °C [11] | 276 at 25 °C [12] | 16,636 at 25 °C [6] |
1. (Wang et al., 2012), 2 (Davies and Provatas, 2006), 3 (Lee and Coburn, 1988), 4 (Meyer et al., 2015), 5 (Davies and Provatas, 2006), 6 (Spear et al., 1989), 7 (Chatterjee et al., 2017), 8 (Felt et al., 2013), 9 (Toghiani et al., 2008), 10 (Badgujar et al., 2008), 11, (Pennington and Brannon, 2002), 12 (Taylor et al., 2013).
In munitions design, the key performance properties of energetic materials are density, so that more energetic material can be loaded into the munition, and velocity of detonation (VoD), to ensure a high fragment velocity (Sikder and Sikder, 2004). A low melting point is desirable so that shells can be filed using a melt cast process for ease of manufacture but one still high enough to prevent the filling from melting inside the shell during storage causing voids within the shell. Due to their high melting points, RDX and NTO are held within a binder material to provide structure and enable the composition to be melt-cast into shells. Comparing the energetic materials in Table 2, it can be seen that NTO has a similar density to RDX and a slightly lower VoD, although there is evidence that particle morphology could increase this (Vijayalakshmi et al., 2015).
Comparison of TNT and DNAN shows that although DNAN does not have as high a density or VoD as TNT it is nonetheless similar enough to fulfil the same role. In addition, the higher melting point of DNAN may help to alleviate issues of void formation when munitions are stored in hot climates whilst still melting at a workable temperature for manufacture (Jackson and Zhang, 2017). The impact sensitiveness of an energetic material is assessed using the rotter impact test with a higher value denoting a lower chance of detonation due to impact (United Nations, 2009). It is these similarities, along with the increased figures of insensitivity over their predecessors, that prompted the US department of defence (DoD) to begin investigation of DNAN and NTO as a replacement IHE in munitions (Zunino et al., 2012).
1.2. Negative impacts of insensitive high explosives
IHE compositions containing mixtures of NTO, DNAN and RDX have been qualified as a main explosive fill in the M795 155 mm artillery shell, a munition that sees high usage rates on operations (Dawag and Morris, 2012). The standardisation of the IHE compositions to US military munitions means that it is more likely that they will be accepted into service in other NATO countries and around the world. With so much of this material being produced and used in the manufacturing of munitions and with further qualification of IHE formulations containing NTO and DNAN expected (Taylor et al., 2015), suitable risk management options of these materials are essential to ensure that unintentional long-term injury of personnel and/or environmental damage is minimised due to a lack of information. However as new formulations, there is minimal information available on their combined behaviour and long-term impact on the land and underlying water systems on which they will be used. The need for a proactive approach to assessing and managing the impact of energetic materials on the environment has been highlighted by the suspension of training on ranges such as the Massachusetts military reservation (MMR) on Cape Cod due to contamination of a groundwater aquifer (Clausen et al., 2004). The cessation of training has caused disruption to the training of future soldiers as well as the need for costly remediation (Fitzpatrick, 2001).
1.3. Production of contaminated wastewater
One of the ways IHE could enter the wider environment is through the discharge of contaminated water from a manufacturing facility. The most common source of IHE contaminated water is in the form of wastewater produced from the cleaning of equipment and finished munitions in load assemble pack (LAP) facilities. There are multiple types of contamination present in LAP wastewater resulting from different water usage in the plant. Activities include maintaining a steady temperature at the point of filling, wash-down of equipment between formulations and of finished munitions and steam washout of defective munitions so the shell body can be reused (Felt et al., 2013). Grit and metal particulates can be picked up during the wash-down process and carried to water storage tanks in addition to energetic materials in dissolved or solid form from the wash-down process.
There is a danger that the use of high temperature water and steam to wash equipment may result in precipitation of solid energetic material as the water cools, with the potential to block pipework and cause explosive hazards (Uhrmacher, 1983). In order to prevent this, large volumes of water are required. The volume and contamination of wastewater depends greatly on the size and type of the explosive facility producing it. Large explosive LAP facilities can produce over 90,000 L per day of wastewater at high concentrations; IHE wastewater has been measured at 34 mg.L−1 of RDX, 137 mg.L−1 of DNAN and 1413 mg.L−1 of NTO (Felt et al., 2013). The biological oxygen demand (BOD) measured in IHE wastewater is typically very low or at the point of non-detection (Felt et al., 2013). The pH is also low, typically around 3, due to the dissolved NTO in the solution (Felt et al., 2013). Smaller production facilities can produce as little as 100 L per quarter depending on the frequency of composition changes. In some, typically larger, facilities this wastewater is treated and reused whereas in smaller facilities it is disposed of by other means (Felt et al., 2013). Due to the relatively new adoption into service of IHE materials such as NTO and DNAN, methods that have previously been used to clean wastewater need to be revisited and re-evaluated to investigate whether they remain suitable for IHE contaminated wastewater (Bhanot et al., 2020).
In this review, an overview is given of the physical and chemical properties of IHE materials in which the challenges they present for removal from wastewater are identified. Methods of wastewater treatment are then critically assessed in terms of their strengths, weaknesses, opportunities and threats for their use in production of IHE munitions including their limitations and how they could be applied to industrial scale LAP facilities. Further to this, the way that adsorption is assessed using isotherms is discussed as well as desirable physical and chemical properties of adsorbent materials for sorption of explosives, including the limitations of using adsorbent materials. Finally, potential avenues of further research are put forward in order to overcome those limitations such as potential improvements to the activated carbon itself as well as process improvements in carbon manufacture to improve the sustainability and reduce the cost and of the final product.
2. Materials of interest
There are three main types of energetic materials that are commonly used in IM formulations. Nitroaromatics such as TNT and DNAN have a six membered carbon ring at their centre. Nitramines such as RDX have an alternating heterocyclic ring structure of carbon and nitrogen. Nitroazoles are characterised by the presence of a nitro group on a five membered heterocyclic azole structure (Table 2). When treating wastewater, the primary factors in developing a treatment system are the volume of wastewater produced and the concentration of contaminants in that wastewater (Rajagopal and Kapoor, 2001). This is a result of the amount of each energetic material used in the formulation, which are a result of its performance characteristics, and the solubility of that material in water both of which are presented in Table 2.
2.1. Nitroaromatics
Nitroaromatic compounds are among the largest and most important groups of industrial chemicals in use today (Ju and Parales, 2010). These compounds are organic molecules that consist of at least one nitro group (-NO2) attached to an aromatic ring. They are rarely found in nature and used in a wide range of industrial activities including plastics, dyes, pesticides and explosives (Ju and Parales, 2010).
Trinitrotoluene (TNT) is the most commonly known example of a nitroaromatic compound. It is comprised of a benzene ring with a methyl group, and nitro groups in position 1, 3 and 5. TNT has been used in munitions since 1902 and is often used in conjunction with other energetic materials such as RDX in Composition B (Comp B) where it acts as a binder due to its low melting point. TNT has been studied extensively over its service life and is well characterised (Chatterjee et al., 2017). Indeed it is often used as a standard to compare other explosives against (Wharton et al., 2000).
2,4-Dinitroanisole (DNAN) is also a nitroaromatic compound that is considered an IHE with properties (Table 2) very similar to those of TNT (Taylor et al., 2017). DNAN has 90% of the explosive power of TNT, whilst being less susceptible to shock and requiring a much higher detonation temperature (Davies and Provatas, 2006). These physical properties make DNAN an attractive alternative to TNT, which was used as binder material in formulations such as Comp B due its low melting temperature and ability to be melt-cast meaning production lines would not need to be retooled in order to make use of it. DNAN is used in IM compositions such as IMX-101 and IMX-104.
Toxicologically, DNAN has a LD50 of 199 mg/kg−1 in rats less than a third that of TNT indicating its greater toxicity to mammals (Reddy et al, 2000, 2020). DNAN does not cause a visual discolouration of water whilst in solution, however, it can still have a serious ecotoxicological impact if allowed to enter a water course. Testing of the bacterium Vibrio fischeri resulted in an EC50 of 60.3 mg.L−1 compared to 0.7 mg.L−1 for TNT indicating a markedly lower effect on the growth but still cause for concern (Dodard et al., 2013). The aquatic toxicity of DNAN has been assessed for a number of model species including the fathead minnow (Pimephales Panelas), water fleas (Ceriodaphnia Dubia) and tadpoles (Rena Pipiens) which were all adversely affected at relatively low LC50's of 42 mg.g−1, 43 mg.g−1 and 21.3 mg.g−1 (Kennedy et al, 2013, 2017; Stanley et al., 2015).
2.2. Nitramines
Hexahydrotrinitrotriazine also known as Hexagen or RDX is a heterocyclic nitroamine and is considered the most important high explosive for military application in the United States (Koutsospyros et al., 2012). The full chemical structure and physicochemical properties for RDX can be seen in Table 2. RDX has an LD50 of 100 mg.kg−1 in rats and is considered a carcinogen (Meyer et al., 2005; US Department for Health and Human Services, 2012). In addition, although RDX does not cause visual discolouration in water, it has been shown to be acutely toxic to a number of aquatic species. One of these is the fathead minnow, with an LC50 of 12.7 mg.L−1 although adverse effects were observed in the test subjects ad concentration as low as 2.4mg.L−1 during a 96 h exposure (Burton et al., 1994). The widely documented negative toxicological effects of RDX have resulted in it being assigned a drinking water exposure limit (DWEL) by the United States environmental protection agency (EPA) of 0.1 mg.L−1 (United States Environmental Protection Agency, 2018). To date, RDX is the only one of the three energetic materials investigated in this review that has been assigned a DWEL or discharge limit in legislation.
2.3. Nitroazoles
3-nitro-1,2,4-triazol-5-one (NTO) is a white crystalline energetic compound that was first used in explosive formulations in 1987 (Lee et al., 1987). NTO is considered and IHE by virtue of having a very similar velocity of detonation to RDX (Table 2) whilst at the same time being much less sensitive to shock making it an attractive alternative. Indeed, some work conducted on spherical NTO crystals has suggested it could be a full replacement for RDX (Vijayalakshmi et al., 2015). This is due to the increased loading density of the resulting fill obtained by controlling the morphology of the NTO crystals as well as better flow properties at lower temperatures than comparable RDX fills attributed to the spherical nature of the particles (Vijayalakshmi et al., 2015).
It is therefore more important to understand how NTO behaves in the environment to ensure that the correct measures are put in place before production processes become entrenched and resistant to change. NTO is known to be acidic when in solution because it exhibits a negative charge at pH levels seen in the environment and has a low pKa (Mark et al., 2017). In addition, the high solubility of NTO in water means that it will likely be present in high concentrations in wastewater, depending on the formulation being used. This high solubility and the large percentage mass of NTO in formulations such as IMX-104 mean that a high mass of NTO has the potential to saturate adsorptive systems before the concentration is reduced to an environmentally safe level.
NTO has a high LD50 compared to other energetic materials is approximately 5000 mg.kg−1 in rats and studies of aquatic toxicity have produced similar results with toxicity levels so low that NTO could be classified as non-toxic (Haley et al., 2009; London and Smith, 1985; O'Bryan and Ross, 1988). However, contamination of water sources with NTO leads to visual discolouration at concentrations over 100 mg.L−1, which constitutes environmental degradation even if no ecotoxicological effect is observed (Tennant et al., 2019).
3. IHE wastewater treatment methods
Wastewater treatment technologies vary between the types of wastewater being treated. Some processes will use a single method whereas others use sewerage treatments that may have many steps, each containing a separate processing method. A list of common water treatment methods can be seen in Figure 1 based on their primary method of action. In addition, a comprehensive Strengths, Weaknesses, Opportunities and Threats (SWOT) analysis for each water treatment method is provided and summarised in Table 3.
Figure 1.
A list of wastewater treatment method organised by the primary method of action.
Table 3.
SWOT analysis for IHE wastewater treatment methods.
| Treatment method | Strengths | Weaknesses | Opportunities | Threats | References |
|---|---|---|---|---|---|
| Separation |
|
|
|
|
(Hammer and Hammer, 2001) |
| Electrolysis |
|
|
|
|
1 (Wallace et al., 2009) 2 (Cronin et al., 2007) |
| Photolysis |
|
|
|
|
3 (Bordeleau et al., 2013) 4 (Alnaizy and Akgerman, 1999) 5 (Mahbub and Nesterenko, 2016) |
| Bi-metallic reduction |
|
|
|
|
7 (Koutsospyros et al., 2012) 8 (Kitcher et al., 2017) 9 (Wanaratna et al., 2006) |
| Fenton oxidation |
|
|
|
|
10 (Felt et al., 2013) 11 (Liou et al., 2003) 12 (Zoh and Stenstrom, 2002) 13 (Bautista et al, 2007, 2008) |
| Microbial degradation |
|
|
|
|
14 (Weidhaas et al., 2018) 15 (Madeira et al., 2017) 16 (Platten et al, 2010, 2013) 17 (Karthikeyan and Spain, 2016) |
| Adsorption |
|
|
|
|
18 (Hoffsommer et al., 1977) 19 (V. M. Boddu et al., 2009) 20 (Morley and Fatemi, 2010) 21 (Todde et al., 2018) 22 (Fawcett-Hirst et al., 2020) |
3.1. Mechanical treatment
3.1.1. Separation
Lamella clarifiers, vertical chicanes and settling tanks are all separation methods that allow material held in suspension to collect so that they can be extracted before wastewater is moved on to the next stage of treatment (Hammer and Hammer, 2001). Due to the solubility of energetic materials such as NTO varying with temperature, it is likely that wastewater will be supersaturated with energetic material initially after washing down manufacturing equipment with steam. As the water cools, the energetic material will precipitate out, blocking other areas of the treatment process and causing an explosive hazard. Clarifiers, use the flow rate of the wastewater and the settling rate of the particles in suspension so promote settling onto inclined surfaces (tubes or plates) that collects and slides to the bottom of the container (Hammer and Hammer, 2001). In the case of NTO, precipitation from solution could further be enhanced with a basic washout medium followed by neutralisation during separation as the solubility of NTO increases with pH (Tennant et al., 2019).
A vertical chicane uses a similar process to the clarifier but the chicanes are arranged in such a way to force the water flow around them and negate turbulent flow, making settling more likely because the flow rate no longer needs to be overcome (Tarpagkou and Pantokratoras, 2014). A settler tank similarly stops the flow rate from being a factor by holding the water in place and allowing any particles in suspension to settle out of the fluid. However, this can take a lot of time as the process is not driven and must be allowed to occur naturally. Any disturbance of the water during the settling phase will disturb the material that has already settled.
In both cases the collection of energetic material during separation leads to an additional explosive hazard as a result of processing and are therefore not suited to use in munitions manufacture (Table 3).
3.1.2. Centrifugation
Centrifugation separates materials from one another according to their specific gravity. This is achieved by spinning the samples to exert a centripetal acceleration. The material in the sample stratifies according to its specific gravity with heavier materials experiencing a greater force and being pushed to the outside edge of rotation. As such, centrifugation may form part of a pre-treatment process that ensures all solid particles have been taken from the waste stream before they move to the next stage. As with the methods described in section 3.1.1 however, the generation of additional explosive hazards during the process make it an unattractive treatment method (Table 3).
3.1.3. Filtration
Filtration can only be used on wastewater streams that comprise of solid particles suspended in the water rather than those dissolved in it. All the processes outlined above have a common disadvantage for energetic material processing, in that they encourage the collection of solid explosives during water treatment (Table 3). This poses a potential safety concern to plant operators but could prove to be a boon if the material collected could be recycled back into the manufacturing process. Filtration methods are therefore useful as a first stage in a treatment process to ensure that any material that has precipitated out of solution, or any possible foreign matter that was swept up in the facility cleaning process, does not go on to later treatment methods.
3.2. Physicochemical treatment
3.2.1. Sorption
Sorption has long been the most used method for the treatment of wastewater containing energetic materials, ensuring they are not discharged to the environment (Ying et al., 2006). Sorption can take the form of a physical or chemical process depending on whether the contaminant attaches to the surface of the sorbent by intermolecular van de Waals forces or chemical bonding, respectively (Pitchtel, 2005). Sorption can further be divided into adsorption which is a surface phenomenon and absorption in which the contaminant migrates into the internal pore structure of the sorbent material (Dubinin, 1960; IUPAC, 2014). The main classes of materials used in adsorption-based water treatment processes are zeolites, ion-exchange resins, alumina and activated carbon.
An ion exchange resin or polymer acts as a medium to allow ion exchange to occur. They take the form of an insoluble matrix that is often shaped as porous microbeads in order to maximise the surface area available on which reactions can take place (Hoffsommer et al., 1977). There are two types of ion exchange resins, anionic and cationic. Anionic exchange resins are strongly or weakly basic and replace anions (or negatively charged ions) with hydroxyl groups (OH-) (Song et al., 2012). Cationic exchange resins are acidic and replace cations with proton (H+). Whether using anion or cation exchange resins, for any ion exchange to take place, the resin must have a stronger affinity for the ions present in the solution than the ones that they were initially charged with (Rohm and Haas, 2008).
Ion-exchange resins were initially introduced to water treatment and purification processes as an alternative to natural or artificial zeolites. A zeolite is a mineral comprised of different combinations of aluminium, silicon and oxygen. There are over 40 types of zeolite that occur naturally as well as synthetically produced ones that can be tailored to specific applications. Zeolites use the same ion exchange process as ion-exchange resins but are also able to filter dirt and other particulate matter from the liquid passed through them at the same time. Some natural alumino silicate types include, Analcime, Chabazite, Clinoptilolite, Heulandite, Natrolite, Phillipsite and Stilbite.
A US patent filed in 1977 (Hoffsommer et al., 1977) describes a method of using strongly basic ion exchange resins to remove non-aromatic nitro- and nitroso-substituted organic compounds from water. Specific mention is made of RDX, HMX, nitroglycerine and nitrocellulose. The process took place on a fluidised bed with contaminant levels between 0.001 parts per million in solution up to saturation with resin loading from 0.04-0.4 percent of solution mass claimed as effective in contaminant removal. Thus ion-exchange has been used for removal of explosive compounds from water, but issues of regeneration and disposal of the spent resin remain. Regeneration of ion exchange resins takes the form of replacing the active ion with a fresh source of OH- or H+ ions as appropriate. A comparison of the advantages and disadvantages of zeolites, ion-exchange resins and activated carbon adsorbents is shown in Table 3.
Carbon sorption is another method that has seen widespread use in removing contaminants from air and water streams. It does this by allowing contaminants to 'sorb' or stick to the internal and external surfaces of the carbon particle. This sorption process is driven through the Van der Waals forces of the individual molecules of contaminants involved as well as the pore size of the carbon used to maximise the available surface area for sorption to take place. Modification of the carbon surface can intensify this effect by making adsorption sites on the surface of the carbon polar in nature, increasing the Van der Waals attraction between the target contaminant and the carbon (Kyzas and Deliyanni, 2015). Wastewater will contain many different types of contaminant with different particle sizes and electrochemical properties. This can cause issues with carbon filtration as the larger particles will block the pores of the filtration material, reducing the available surface area and dramatically reducing the efficiency of the process. Despite these issues, carbon is a common sorbent material that has been used for the removal of energetic materials (Table 3) such as TNT (Rajagopal and Kapoor, 2001), RDX (Morley and Fatemi, 2010) and DNAN (Boddu et al., 2009).
When a carbon filter has been totally saturated with contaminant and can no longer adsorb any more material it is not completely spent. The carbon matrix can be regenerated thermally by heating the carbon in order to destroy the adsorbed contaminant or through the use of chemical processes such as the use of an acid wash to remove the contaminant from the carbon matrix (Cevallos Toledo et al., 2020). Additionally, biological processes can be used where microbial action breaks down the contaminants in-situ in the carbon matrix (EI Gamal et al., 2018). The breakdown products that are formed are often less toxic than the original parent compounds and so the environmental effect can be reduced (Reungoat et al., 2012).
3.2.2. Electrolysis
Electrolysis is the method of separating ions using a flow of electrons from a negatively charged anion to a positively charged cation to promote reactions at the interaction between two phases (Kirk-Othmer, 2000). Most commonly in chemical engineering this is a solid and liquid phase interaction. Electrolysis has only been shown to work on some explosive materials, most notably NTO and TNT (Cronin et al., 2007), (Wallace et al., 2009). As TNT is a nitro aromatic compound this would indicate that electrolysis would also be effective on other nitroaromatic compounds including TNT breakdown products and DNAN. The effectiveness of electrolysis on NTO as a nitro compound is far more interesting as it would indicate that the effect is not solely on nitro aromatics and, given further study, could be shown to work on other classes of energetic materials as well. Whilst electrolysis could be effective on a wider range of materials, there is currently no evidence that is the case (Table 3).
Direct anodic electrochemical oxidation and mineralisation of NTO using cheap carbon electrodes and without chemical loading may represent an economical and clean method of NTO wastewater treatment (Cronin et al., 2007). Electrochemical treatment is often clean, environmentally friendly and cost efficient (Wallace et al., 2009). The mechanism proposed by (Wallace et al., 2009) accounts for all products of the reaction in the form of aqueous ammonium nitrate and gasses with an almost quantitative yield compared to initial NTO loading of 13 gL-1 (Cronin et al., 2007). Condensation during the second intermediate step can cause the formation of an Azoxy derivative, Azoxytriazolone (AZTO) instead. This reduction to form AZTO was found to be effective as a single stage process as the AZTO precipitates out of solution (Cronin et al., 2007). The reduction of NTO to the still explosive AZTO means that there are additional safety and processing concerns during operation that do not occur with carbon sorption systems (Table 3).
Electrolysis has not yet been shown to remove all IHE materials from solution however it could still be utilised as part of a larger multi-stage system in some use cases such as filling shells with the composition known as IMX-104. In IMX-104, NTO is by far the most soluble component, the level of contamination that this causes in wastewater could be enough to quickly overwhelm systems that seek to remove all contaminants in one process. Coupled with the high setup costs and potential space requirements of an electrolysis system this makes it less attractive than sorption systems at pre-existing LAP facilities.
3.2.3. Photolysis
Photons are packets of energy that take the form of light in either visible or non-visible spectra with the amount of energy they carry is dependent on the wavelength. When a photon is incident on a molecule, some or all the energy is transferred to the part of the molecule that was hit. The addition of energy excites the electrons in the shells and bonds to a higher energy level than they were previously at and can cause bonds within the molecule to break; once broken, the now unbonded electrons will seek to stabilise themselves by either attracting or donating electrons from the surrounding area and reforming into a new molecule. UV radiation also promotes the creation of hydroxyl radicals in water, which degrade energetic materials in solution through a process called photolysis. This process can be enhanced with the addition of hydrogen peroxide or the addition of a catalyst (Table 3) (Alnaizy and Akgerman, 1999). Photolysis has been shown to be effective in speeding the breakdown of RDX (Bordeleau et al., 2013) and therefore should also be effective at speeding the degradation of other nitroamines.
Mahbub and Nesterenko (2016) reported that the intramolecular hydrogen bonding within the molecule that controlled the photo adsorption of the molecule. Nitro groups that are common in energetic materials have a high adsorption of photon energy from visible and ultraviolet wavelengths. The decomposition of nitroaromatic compounds was reported as occurring via ring cleavage as a result of the loss of a nitro group followed by subsequent decomposition into HCHO, HCOOH, NH2CHO, N2O, NO2- and NO3+ (Mahbub and Nesterenko, 2016).
In order to use photolysis as a treatment method at LAP facilities then specially trained personal and handling facilities for hydrogen peroxide would be required. This increases the running costs of photolysis as a treatment method compared to a less training/manpower intensive system like sorption.
3.2.4. Bi-metallic reduction
Zero Valent Iron (ZVI) has been used to degrade algaecides, fungicides and bactericides such as dichlorophen (Ghauch and Tuqan, 2009). ZVI has a full outer shell of electrons present and hence a valence of zero, to cause degradation of contaminants in wastewater. This technique has been expanded to look at using ZVI to degrade RDX (Wanaratna et al., 2006). Using the method of Kim et al. (2003), the metal pairings (bimetals) work together using the surface of two dissimilar metals to promote intermediate reactions on their surface by donating and receiving electrons to catalyse the degradation of a compound in solution (Kim et al., 2003).
A bimetal system was devised using a combination of iron and copper or iron and Nickel (Koutsospyros et al., 2012). It was found that although there was no difference in effectiveness of the two metal pairings on degradation of RDX, HMX or TNT; the iron-nickel pairing was discounted due to concerns over nickel regulations in aquatic systems (Table 3) (Koutsospyros et al., 2012). Furthermore, the ratio of copper to iron was not found to have a significant impact on the rates of reaction but the acid used did have a significant effect with organic acids producing much higher rates of reaction than mineral acids. This may be due to a ‘buffer effect’ that resists an increase in pH which would otherwise increase corrosion of the iron and lead to a reduced surface area for the reaction to take place (Kitcher et al., 2017).
Bi-metallic reduction has so far been shown to work at 1% solid loading ratio of 600mg of bimetal particles in a 60 ml sample on a number of energetic materials including TNT (48 mgL-1), RDX (50 mgL-1), NTO (410 mgL-1) and DNAN (430 mgL-1) in the laboratory (Koutsospyros et al., 2012). Experiments undertaken show to work on RDX (36.37 mg L−1) and TNT (46.20 mg L−1) in a combined 60 mL “pink water” wastewater, although the affinity of TNT for the metallic reduction sites meant a time lag occurred before they were degraded (Koutsospyros et al., 2012). Individual tests were carried out on NTO and DNAN where the nitroaromatic DNAN showed a similar affinity to that of TNT and NTO took the longest to degrade. This suggests that the structure of the molecule that drives affinity for the process and not solubility.
Extrapolation of this work has shown that in order for mineralisation of NTO to take place, the process must occur in an acidic environment (Kitcher et al., 2017). This is because an acidic environment increases the dissolution of iron and therefore provides a reducing environment to allow organic compounds to be destroyed. Complete mineralisation of the NTO in the sample is evidenced by the lack of build-up of a urea peak in the later stages of the reaction. Therefore even the breakdown products of NTO have been removed, possibly by hydrolysis to form relatively benign products NH3 and CO2 (Kitcher et al., 2017).
Used in conjunction with other treatment methods further downstream, bi-metallic reduction could be used to reduce the pressure and maintenance burden on the other treatment methods of a full treatment system by reducing the amount of contaminant, especially the highly soluble NTO, which later treatment processes must remove. However, there is the potential for the metallic particles themselves to be lost during the treatment process and be subsequently discharged with the treated water. This has the potential to cause environmental damage due to the build-up of metal particles in water courses and soil systems around the treatment facility unless combined with other treatment processes.
3.2.5. Fenton Oxidation
Fenton oxidation is one of the most powerful oxidation technologies available, using hydroxyl radicals HO· and HOO· generated from reaction between hydrogen peroxide (H2O2) and Iron (II) (Fe2+) (Ebrahiem et al., 2013; Liou et al., 2003). It is frequently used to pre-treat coloured wastewater from the cosmetic, pharmaceutical and textile industries as well as the energetic materials TNT, dinitrotoluene (DNT) and RDX (Badawy et al., 2009; Bautista et al, 2007, 2008; Liou et al., 2003; Martınez et al., 2003). For example, complete removal of RDX from wastewater can be achieved using the Fenton oxidation process in as little as two hours at up to 50 °C (Zoh and Stenstrom, 2002). The main oxidation products are nitrates, nitrogen and carbon dioxide, with formaldehyde and formic acid produced as intermediates using a reactant ratio of 5178:48:1 (H2O2: Fe2+:RDX) (Zoh and Stenstrom, 2002). However, some of these products such as nitrates discussed earlier, and formaldehyde pose just as much risk to the environment as the initial RDX and therefore do not solve the problem of contamination (Table 3).
The use of Fenton oxidation for the decontamination of IMX-104 industrial wastewaters has not been reported. Fenton oxidation on the laboratory scale has been successfully demonstrated for both DNAN and NTO, and industrial scale tests have been successful for the treatment of DNAN contaminated wastewaters (Felt et al., 2013; Le Campion et al., 1999). The main mineralised products are carbon dioxide and ammonia, although other unidentified toxic by-products may also be produced (Felt et al., 2013). For a water treatment facility, these by-products as well as the iron used as a catalyst would have to be removed from solution by additional treatment steps in order to allow the resulting water to be discharged safely.
Although the Fenton process is a powerful chemical method for the degradation of a wide variety of energetic materials it is not without its drawbacks. The waste products from the reactions can often be more of a hazard to the operating staff and the environment at large than the original contaminants that were removed. In addition, the process is a carefully controlled process that must be overseen by trained chemists and the reagents used also require special training and handling equipment to operate safely (Fischer Scientific, 2018) which would increase both staffing and infrastructure costs for any plant using it on an industrial scale.
3.2.6. Ozonation
Wastewater from the production of energetic materials contains compounds with highly toxic nitro groups (Gu et al., 2019). The electron configuration of ozone, which presents both positively and negatively charged oxygen atoms, is what allows it to interact directly with contaminants directly through nucleophilic substitution (Adam et al., 2007). Interactions also occur indirectly through the formation of more powerful hydroxyl radicals via interaction with water (Zappi et al., 2016). In the ozonation process, highly concentrated organic substances are rapidly removed leading to the build-up of small molecule organic acids. Which then react at a much slower rate (Gu et al., 2019).
Different energetic materials exhibit different degradation processes depending upon their original structure. Ozone reacts by way of an addition reaction with benzene rings such as those found in TNT and DNAN, the destruction of which reduces the pH of the resulting wastewater (Gu et al., 2019). Intermediate products such as 1,3,5-trinitrobenzene (TNB) are also formed during the ozonation process and need to be monitored (Zappi et al., 2016).
Degradation of RDX through ozonation occurs through the indirect pathway when hydroxyl ions are formed through interaction of ozone with water. The method of degradation is similar to the degradation pathways from other AOPs such as Fenton oxidation (Adam et al., 2007). In the case of RDX ozonation, nitro groups are reduced to nitrates whereas the heterocyclic ring structure was reduced to organic byproducts (Bose et al., 1998). Indeed, experiments that include acetonitrile in solution as a hydroxyl scavenger have shown that RDX reacts with these hydroxyl radicals instead of directly with the ozone itself (Bose et al., 1998).
However, the transformation of RDX using ozone is affected by the pH level of the solution with the uncontrolled pH decreasing over time. When the pH is stabilised to neutral the time taken to completely mineralize RDX was under 72 h meaning that effective monitoring and adjustment of pH levels would be required in order to keep processing conditions optimal for solutions containing a mix of energetic materials (Adam et al., 2007).
Combinations of oxidative treatment technologies that involves UV light at differing wavelengths, with peroxide or ozone dosing have been experimented on different wastewaters with Low Pressure mercury UV (LPUV) lamps showing an affinity for the removal of RDX and HMX (Zappi et al., 2016). Despite this, using current systems, none were able to treat RDX contaminated water to below threshold discharge limits with the desired time period of 40 min. Although ozonation systems show promise they are not yet at the required level (Zappi et al., 2016).
UV-Ozone systems have relatively high capital costs and complex systems design due to the complexity of the equipment involved (Table 3) however this is offset in lower operating and maintenance costs (Zappi et al., 2016). In addition, it has been shown that the biodegradability of energetic wastewaters can be enhanced after the ozonation process (Gu et al., 2019).
3.3. Biological treatment
Biological wastewater treatment processes are common for the treatment for industrial wastewater and include: (1) bioremediation of wastewater that includes aerobic treatment (oxidation ponds, aeration lagoons, aerobic bioreactors, activated sludge, percolating or trickling filters, biological filters, rotating biological contactors, biological removal of nutrients) and anaerobic treatment (anaerobic bioreactors, anaerobic lagoons); (2) phytoremediation of wastewater that includes constructed wetlands, rhizofiltration, rhizodegradation, phytodegradation, phytoaccumulation, phytotransformation, and hyperaccumulators; and (3) mycoremediation of wastewater(Bajaj et al., 2008; Işık and Sponza, 2006; LaPara et al., 2001; Şen and Demirer, 2003).
3.3.1. Treatment under aerobic conditions
Bioreactors have been used to successfully degrade DNAN and NTO in synthetic wastewater with aerobic removal efficiencies of 58 ± 22% and 45 ± 24%, respectively when using purely aerobic methods (Weidhaas et al., 2018). In addition, specific strains of microbial organism have been identified that are able to degrade DNAN completely despite the presence of other IHE (Karthikeyan and Spain, 2016). There have also been reports of DNAN, NTO and nitroguanadine (NQ) being degraded from mixed solution (Richard and Weidhaas, 2014). Biodegradation systems that use multiple, sequenced reactors have shown more promise in removing IHE from wastewater with removal rates of up to 100% when using aerobic/anaerobic sequences (Weidhaas et al., 2018). Alternative methods for increasing the removal efficiency of IHE from wastewater include the use of electron donors such as sodium pyruvate which was shown to increase the removal rate of NTO from solution in waster to 93.5% in an anaerobic-aerobic system (Madeira et al., 2017).
3.3.2. Treatment under anaerobic conditions
Bioreactor treatment for IMX-104 would have to be a two-step process with an anaerobic reactor followed by an aerobic bioreactor (Felt et al., 2013). The second step would be required to treat the noxious, and toxic sludge produced during DNAN and NTO digestion in the anaerobic stage (Platten et al, 2010, 2013). Treatment of IMX-104 wastewater require optimisation, and thorough testing before full-scale use. Bioreactors can take a long time to reach stable operating conditions and maintaining those condition can be an intensive process (Table 3). The microbial populations must be carefully selected and be kept at conditions that promote the desired behaviours for degradation to take place. Bioreactors are therefore highly susceptible to changes in the environment such as temperature, pH and changes in wastewater composition. A starvation test to a DNAN bioreactor showed that a re-acclimatisation period was required after DNAN was reintroduced to a bioreactor following a 3-week gap, suggesting that the throughput of any bioreactor systems could be significantly disrupted by changes in work patterns at an LAP plant (Platten et al., 2010). This means that bioreactor systems are more suited to large scale treatment facilities that are less likely to have extreme variations in wastewater composition compared to smaller facilities. In addition, large scale bacterial death in a reactor can shut down treatment for weeks while the population is re-established. This can be a problem where populations have adapted to treat specific waste streams and cannot be easily replaced.
3.4. Summary of treatment methods
A wide variety of water treatment methods have been assessed for their viability for removing energetic materials, specifically IHE from solution in water. Physical treatment methods were found to pose an explosive risk due to solid energetic materials collecting in the systems examined. For physicochemical methods, the main issues found include the requirement for, and cost of infrastructure and reagents for treatment method such as electrolysis, ozonation and photolysis (Alnaizy and Akgerman, 1999; Cronin et al., 2007; Liou et al., 2003). In addition, not all of these methods have so far been demonstrated to be effective at removal of IHE from solution in water. Similarly, biological treatment methods require very specific environmental parameters that must be maintained and are susceptible to disruption from changes in the feed material, in this case IHE wastewater (Platten et al., 2010). There are therefore additional process and infrastructure requirements to ensure that the biological treatment systems can be maintained. Other physicochemical methods have the potential to pose risks to disposal from chemical waste used in Fenton Oxidation and nanoparticles from bimetallic reduction (Koutsospyros et al., 2012). Adsorption therefore remains an attractive water treatment method for IHE having already been shown to remove individual and combined materials from solution as well as many manufacturing facilities already having adsorption type treatment methods in place (Hoffsommer et al., 1977). As carbon is the most used adsorbent material it makes sense to fully determine the limits of existing technology and attempting to improve upon them.
4. The limitations of sorption
Sorption has been identified as a water treatment method that is widely used in current explosive wastewater treatment systems and one that shows potential for the treatment of IHE contaminated wastewater. Although there are a number of sorptive materials that can be used, activated carbon is by far the most widespread yet is not without its disadvantages. Much of the activated carbon produced commercially uses a bituminous carbon source. This means that the ultimate carbon source for production is fossilised carbon which is non-renewable (Menya et al., 2018). In addition, centralised production facilities necessitate the transport of large volumes of carbon to the point of use with the associated costs and environmental impact entailed. Sorption of IHE may also pose an issue for some activated carbons due to saturation of the carbon caused by the high solubility of some IHE materials such as NTO which is present in LAP wastewaters at over 30 times the concentration of RDX and 10 times that of DNAN (Felt et al., 2013).
Despite activated carbon successfully removing energetic materials from wastewater streams for decades (Felt et al., 2013; Uhrmacher, 1983) the higher solubility of new IHE materials such as NTO mean that existing activated carbon sorption systems are unable to cope with the mass of energetic material now in the wastewater stream. Due the increased mass of energetic material in solution, activated carbon will become saturated much faster, resulting in faster breakthrough times and more frequent replacement of columns or reactor beds. The additional cost in terms of personnel and raw materials threatens to make activated carbon sorption uneconomical for LAP wastewater treatment.
The disadvantages of the other technologies available for the treatment of IHE contaminated wastewater compared to the use of sorption mean that they present a greater risk to LAP facilities that wish to implement them. Therefore, an alteration to the existing activated carbon sorption method could help to retain the economic efficiency of activated carbon sorption despite the additional loading presented by the IHE materials DNAN and NTO.
Instead of enhancing the sorptive capacity of existing activated carbon, cheaper materials could instead be used so that although the volume of carbon used increases costs are kept the same or even decreased. An area of increasing interest is the use of activated carbons that are made from a biological waste material instead of a bituminous, or fossil fuel, based feedstock and are known collectively as biochars. A biochar is produced from the thermal decomposition of organic material in a limited oxygen atmosphere at temperatures less than 700 °C (Leman and Joseph, 2009). Much of the early interest in biochars stemmed from the need for treatment methods in developing countries that were not dependent on imparted chemical products or technology that could not be repaired in the country of use (Gwenzi et al., 2017). Indeed biochars are well suited to this type of water treatment due to the readily availability of organic material to use as a feedstock making it cost-effective and therefore accessible for the local population (Chaukura et al., 2016).
Despite the limited operating conditions for processing, the resulting material properties vary dramatically based on the preparation method used (Ahmad et al., 2014). As such there has been an effort to explore the pyrolysis conditions and techniques that provide the largest surface area per gram of material. Methods explored include both fast and slow pyrolysis, gasification, hydrothermal carbonisation and flash carbonisation with each technique having its own advantages and disadvantages in terms of the energy used and carbon yield form the original feed material (Meyer et al., 2011). In general it has been shown that higher temperatures produce biochars with higher micro porosity, surface area and hydrophobicity which makes them more suitable for removal of contaminants from water (Ahmad et al., 2014). Biochars are also often modified in order to enhance their sorption potential by using a secondary treatment process that further develops the pore structure of the material, increasing its surface area. Modification of biochars can take a number of forms including acid wash (Njoku and Hameed, 2011), base wash (Kyzas and Deliyanni, 2015) or the application of microwave radiation (Njoku et al., 2013; Şahin et al., 2013).
Metronidazole (MNZ) is an anti-bacterial that is often used in veterinary medicine (Ahmed and Theydan, 2013). MNZ shares some structural similarities with NTO as they are both 5 membered heterocyclic compounds although NTO has an additional nitrogen over MNZ. NTO and MNZ also both have attached Nitro groups and have a similar overall size meaning that biochars that prove suitable for MNZ could be applicable to NTO as well. Siris seed pods that were treated with a microwave and a potassium hydroxide chemical activator have been shown to remove MNZ from aqueous solution (Ahmed and Theydan, 2013). Alternatively, sugar cane bagasse has also been shown to remove MNZ from aqueous solution after carbonisation without further chemical activation (Sun et al., 2018).
Trinitrophenol, also known as picric acid, is an energetic material as well as a by-product in the synthesis of nitrobenzene. Trinitrophenol has been removed from aqueous solution using almond shells that had been pyrolyzed after chemical treatment with phosphoric acid (Mohan et al., 2011). Almond shells were chosen as a feedstock in this case due to their surface hardness and the fact that they release very little sulphur or ash when processed. Similarly, O-nitrophenol was shown to be removed from aqueous solution by AC that had been derived from coffee grounds, melon seeds or orange peels with a ratio of removal of 70–90% (Djilani et al., 2012). Waste products from agricultural and industrial processes were deliberately chosen for use in the study due to their perceived economic and environmental benefit. Toluene is a volatile organic compound that is produced by industrial processes such as the painting of vehicles and has harmful effects on human health including causing cancer. Biochars made from pine wood and wheat straw using microwave and potassium hydroxide have been shown to remove toluene from solution up to 200mg.L−1 (Mao et al., 2015).
There has to date been limited research into the sorption of explosives using biochars. Rice husks have been used for the sorption of TNT and RDX from solution with specific emphasis placed on the environmental and economic benefits of using a waste material as a feedstock (Lingamdinne et al., 2015). Both RDX and TNT have been shown to be removed from solution in water by biochar made from rice husk. The rice husk had been pyrolyzed in a low-oxygen environment at 700 °C for 4 h with removal efficiency of 97% and 55% for TNT and RDX, respectively (Lingamdinne et al., 2015). Although this shows that biochars could remove energetic materials from solution in water, there has so far been limited work in this area. The IHE constituents NTO and DNAN have been the subject of work into their sorption to chitins and cellulose materials (Todde et al., 2018) as well as bituminous activated carbons (V. M. Boddu et al., 2009; Chew et al., 2017) however work using biochars is yet to be published. It therefore becomes necessary to broaden the search for biochar adsorbents that could be suitable for the adsorption of other IHE. Pharmaceuticals, phenols and some dyes can have similarities to IHE in terms of their size, charge and structure that can help with the down selection of feedstock materials for biochar to be used in IHE adsorption. These include the pharmaceutical compounds paracetamol (Garcia-Mateos et al., 2015; Villaescusa et al., 2011), ibuprofen (Mestre et al., 2009) and metronidazole (Ahmed and Theydan, 2013; Sun et al., 2018).
Biochars have therefore been shown to be able to remove a wide variety of contaminants from aqueous solution via sorption. This includes contaminants that have similarities to IHE and therefore shows that biochars could be used to reduce the economic and environmental cost associated with IHE removal from wastewater. However, more research is required in order to prove that biochars can be a cost-effective adsorbent material for IHE wastewater.
4.1. Sorption characteristics
The characteristics of adsorbent materials are a result of their physical and chemical properties and can be heavily influenced by the feedstock material used as well as the processing methods. For example a biochar made from a plant lignin will have a very different microstructure compared to one prepared from an animal bone or shell (Gwenzi et al., 2017). The method of preparation used can likewise have a great impact on the properties of the final AC with temperature, processing time and activating agent having an impact as well as the physical and chemical properties of the feedstock material (Ahmad et al., 2014; Zhou et al., 2015). One of the most commonly used materials for sorption is activated carbon which itself encompasses a broad spectrum of materials that tend to have a focus on maximising physical sorption as opposed to chemical sorption methods used by other materials such as ion exchange resins and zeolites. This is due to the chemical nature of explosives such as TNT and RDX as well as IHEs like DNAN in solution in wastewater that do not dissociate to provide reactive sites for chemisorption.
4.1.1. Physical properties of activated carbons
Surface area is the primary driver of the sorption characteristics of an AC (Jain et al., 2016). The total surface area of a sorbent particle includes both the external surface of the article and the internal surface that is a result of the complex pore system inside the particle. The pore size distribution of the particle is also important as there are mixture of pore sizes present from macro (>50 nm), through meso (50 nm–2 nm) to micro (<2 nm) (Lee et al., 2006) (Ahmad et al., 2014). The particle size also has an effect on the surface area distribution of the granule as well as the packing density when it is used in sorption systems such as water treatment columns (Mohan et al., 2011). Smaller grains have a higher packing density and therefore a greater retention time than a column of the same geometry of larger particles. The single most important physical property of an activated carbon with regard to IHE sorption is the available surface area per kilogram of material (Loredo-Cancino et al., 2013). The relationship between an increase in GAC surface area and the lowering of equilibrium concentration of TNT in solution is clearly shown in Figure 2, reproduced from work carried out by (Rajagopal and Kapoor, 2001). The iodine number is often provided on the data sheets of sorptive materials and is used to give an indication of the porosity of the material being tested rather than its ability to adsorb any other specific contaminant; it is assessed using a three point isotherm in a standardised test methodology (ASTM, 2006).
Figure 2.
The relationship between an increase in GAC surface area and a lower equilibrium concentration of TNT in solution. Reproduced from (Rajagopal and Kapoor, 2001).
All of these properties must be considered when designing water treatment systems. The surface hardness of an AC grain becomes more important when using the AC in an industrial processing system such as a treatment column or fluidised bed reactor. This is because an individual grain of carbon must be able to withstand the pressure of the grains above it or the motion of the fluidised bed without breaking apart as this would change the size of the AC grain as well as having the potential to disrupt the flow of solution through a column, and therefore the retention time.
4.1.2. Chemical properties of activated carbons
In addition to the physical properties of the AC, the chemical structure or surface treatments can alter the sorption characteristics dramatically. Some ACs are impregnated with metal ions during the production process in order to in catalytic degradation properties (Choi et al., 2008). Alternatively pyridinic Nitrogen can be infused into the carbon matrix during manufacture such as in Calgon Centaur carbon (Baker, 2008; Calgon Carbon, 2015). Despite the method used, the theory behind changing the chemical properties of the carbon matrix is to introduce areas of different pH or electronegativity to the carbon grain and therefore offer a more attractive surface for adsorption for the target contaminant (Ahmed et al., 2016). Using the method, it could be possible to preferentially remove contaminants of concern from wastewater making this an interesting avenue of further research. For example, in the case of IHE wastewater with NTO present in a much greater concentration than DNAN or RDX, a specific carbon pre-treatment designed to preferentially remove NTO could be used. The resulting IHE wastewater could then pass through the pre-existing wastewater treatment system without saturating it and causing it to need replacing. In this way the other functions of the LAP facility can be kept operating as normal with minimal disruption.
4.2. Adsorption isotherms, modelling and practical considerations
Isotherms are a series of experiments conducted at a constant temperature to show the functional relationship between an adsorbent and adsorbate in reaction. They are used to assess the process of adsorption taking place in the reactions under the assumptions made by the isotherm model used. There are many isotherm models that can be used however this review has focussed on the commonly used Langmuir, Freundlich and Temkin isotherm models due to their diverse sets of underlying assumptions and therefore the information each can offer to the assessment of an adsorptive process. Each isotherm model is reviewed in the following paragraphs and a comparison of advantages and disadvantages can be seen in Table 4.
Table 4.
Advantages and limitations of each isotherm model.
| Isotherm model |
|||
|---|---|---|---|
| Langmuir | Freundlich | Temkin | |
| Advantages |
|
|
|
| Limitations |
|
|
|
4.2.1. Langmuir
The Langmuir isotherm is used extensively to model the adsorption of a wide variety of contaminants including dyes, pesticides, fertilisers, pharmaceuticals and energetics from aqueous solution using both commercial ACs and biochars (Mohan et al., 2011; Njoku et al., 2014; Sayğılı and Güzel, 2018). The ubiquity of the Langmuir isotherm makes it useful for drawing quick comparisons between ACs. If the Langmuir isotherm provides a good fit to the experimental data that is obtained from equilibrium experiments, then it shows the formation of an adsorbent monolayer is the most influential part of the adsorption process in that interaction.
4.2.2. Freundlich
The Freundlich isotherm is commonly used in the adsorption of contaminants from aqueous solution including pharmaceuticals and dyes (Zhou et al., 2015). The Freundlich isotherm has also been used in the adsorption of energetic materials from aqueous solution (Boddu et al., 2009; Morley and Fatemi, 2010; Rajagopal and Kapoor, 2001).
4.2.3. Temkin
Use of the Temkin isotherm therefore allows determination of whether an adsorption reaction is exothermic or endothermic. The resulting information is very useful as the equilibrium adsorptive capacity of an AC will change depending on the temperature (Sayğılı and Güzel, 2018). Thermal consideration for the implementation of a carbon adsorption system into practice can also be calculated from this data.
4.2.4. Practical considerations
The experimental parameters that effect the adsorptive capacity are dependent upon the relationship between the contaminant and the adsorbent. The purity of the contaminant used was found to have an effect of adsorptive capacity of RDX along with the natural organic content of water used in representative scenarios (Morley et al., 2005). The pH of the solution also has an impact on the adsorption of contaminants with adsorption of DNAN onto untreated GAC peaking at a pH of 6 compared to pH 7 when acid, base treated or chitosan covered GAC (V M Boddu et al., 2009). When considering the potential for treating wastewater containing multiple contaminants, the effect of each on the solution should be taken into effect. For example, NTO lowers the pH of water when in solution and so could negatively affect the adsorption of other contaminants in solution. In addition, it was found that the solubility of NTO in solution was dramatically enhanced when the Ph was adjusted from 6 to 14 with the addition of KOH (Tennant et al., 2019). For all of the contaminants of concern in this review, a higher initial concentration of contaminant in solution has led to a higher measured adsorptive capacity (Fawcett-Hirst et al., 2020). Therefore, any change in solubility as a result of a change of the pH of solution has the potential to change the outcome of any isotherm testing conducted.
5. Conclusions
Although there are many novel water treatment technologies that could be applied to the treatment of IHE contaminated wastewater, sorption is a proven technique that can be adapted to remove IHE contaminants from wastewater. However, there are a number of potential issues that need to be overcome before adsorption can be used in industrial scale IHE wastewater systems. Investigation is required into the sorptive capacity of commercially available ACs for NTO to determine if the theoretical saturation of carbon columns would occur in a real-world system before the NTO was removed from solution. If so, there are a number of potential avenues of research to consider.
Through the modification of the carbon surface or use of a resin for sorption, individual IHE materials could be preferentially adsorbed by providing adsorption sites targeted towards particular contaminants of concern before passing to another treatment step. Due to NTOs dissociation in water, this could take the form of a positive charge being introduced to the adsorbent surface or use of an anion exchange resin to promote chemisorption. This could be used alongside physical adsorption, in order to reduce the initial NTO concentration before a final carbon-based treatment step. Remaining RDX and DNAN could continue to be treated with current AC sorption methods and NTO disposed of in another way due to its significantly lower toxicity or pre-concentrated before being shipped to a specialist disposal site.
In addition, the use of biochars should be explored as an alternative to bituminous carbons in order to reduce the environmental impact of the use of AC. The reduction of costs and environmental impact are not the only positives from the use of biochars. Due to an increase in public awareness of environmental issues, a company making use of biochars instead of bituminous ACs could show a reduction in its carbon footprint and potentially look to explore elements of the circular economy if local materials proved suitable. Therefore, the use of alternative sorbent materials should be investigated for the removal of IHE from solution in order to make the process both more economically and environmentally sustainable.
Both avenues of research should be explored in order to improve the efficiency and reduce the impact of using adsorption as a treatment process for IHE contaminated wastewater. However, due to the differing needs and of large and small LAP facilities it would first be necessary to undertake an assessment of the needs to the specific facility and the needs of the operating organisation. In this way, the duty of the business to clean wastewater can be fulfilled with proposed solutions that are appropriate for, and proportional to, the activities undertaken on the site.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by Rheinmetall waffe munition Italia (RWMI).
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Adam M., Comfort S., Snow D., Cassada D., Morley M., Clayton W. Evaluating ozone as a remedial treatment for removing RDX from unsaturated soils. J. Environ. Eng. 2007;132:1580–1589. [Google Scholar]
- Ahmad M., Rajapaksha A.U., Lim J.E., Zhang M., Bolan N., Mohan D., Vithanage M., Lee S.S., Ok Y.S. Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere. 2014;99:19–33. doi: 10.1016/j.chemosphere.2013.10.071. [DOI] [PubMed] [Google Scholar]
- Ahmed M.J., Theydan S.K. Microporous activated carbon from Siris seed pods by microwave-induced KOH activation for metronidazole adsorption. J. Anal. Appl. Pyrolysis. 2013;99:101–109. [Google Scholar]
- Ahmed M.B., Zhou J.L., Ngo H.H., Guo W., Chen M. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour. Technol. 2016;214:836–851. doi: 10.1016/j.biortech.2016.05.057. [DOI] [PubMed] [Google Scholar]
- Akhavan J. Third. ed. Royal Society of Chemistry, c2011; Cambridge, UK: 2011. The Chemistry of Explosives. [Google Scholar]
- Alnaizy R., Akgerman A. Oxidative treatment of high explosives contaminated wastewater. Water Res. 1999;33:2021–2030. [Google Scholar]
- ASTM . ASTM Int; 2006. Standard Test Method for Determination of Iodine Number of Activated Carbon 1. [Google Scholar]
- Badawy M.I., Wahaab R.A., El-Kalliny A.S. Fenton-biological treatment processes for the removal of some pharmaceuticals from industrial wastewater. J. Hazard Mater. 2009;167:567–574. doi: 10.1016/j.jhazmat.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Badgujar D.M., Talawar M.B., Asthana S.N., Mahulikar P.P. Advances in science and technology of modern energetic materials: an overview. J. Hazard Mater. 2008;151:289–305. doi: 10.1016/j.jhazmat.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Bajaj M., Gallert C., Winter J. Biodegradation of high phenol containing synthetic wastewater by an aerobic fixed bed reactor. Bioresour. Technol. 2008;99:8376–8381. doi: 10.1016/j.biortech.2008.02.057. [DOI] [PubMed] [Google Scholar]
- Baker F.S. 2008. Catalytic Activated Carbon for Removal of Chloramines from Water. US 7,361,280 B2. [Google Scholar]
- Bautista P., Mohedano A.F., Gilarranz M.A., Casas J.A., Rodriguez J.J. Application of Fenton oxidation to cosmetic wastewaters treatment. J. Hazard Mater. 2007;143:128–134. doi: 10.1016/j.jhazmat.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Bautista P., Mohedano A.F., Casas J.A., Zazo J.A., Rodriguez J.J. An overview of the application of Fenton oxidation to industrial wastewaters treatment. J. Chem. Technol. Biotechnol. 2008;83:1323–1338. [Google Scholar]
- Bhanot P., Celin S.M., Sreekrishnan T.R., Kalsi A., Sahai S.K., Sharma P. Application of integrated treatment strategies for explosive industry wastewater—a critical review. J. Water Process Eng. 2020;35:101232. [Google Scholar]
- Boddu V.M., Abburi K., Fredricksen A.J., Maloney S.W., Damavarapu R. Equilibrium and column adsorption studies of 2,4-dinitroanisole (DNAN) on surface modified granular activated carbons. Environ. Technol. 2009;30:173–181. doi: 10.1080/09593330802422993. [DOI] [PubMed] [Google Scholar]
- Bordeleau G., Martel R., Ampleman G., Thiboutot S. Photolysis of RDX and nitroglycerin in the context of military training ranges. Chemosphere. 2013;93:14–19. doi: 10.1016/j.chemosphere.2013.04.048. [DOI] [PubMed] [Google Scholar]
- Bose P., Glaze W.H., Maddox D.S., Hill C. Degradation of RDX by various advanced oxidation processes: I. Reac. Rates. 1998;32 [Google Scholar]
- Burton D.T., Turley S.D., Peters G.T. The acute and chronic toxicity of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) to the fathead minnow (Pimephales promelas) Chemosphere. 1994;29:567–579. [Google Scholar]
- Calgon Carbon . 2015. CENTAUR ® 12x40 Catalytic Granular Activated Carbon. [Google Scholar]
- Cevallos Toledo R.B., Aragón-Tobar C.F., Gámez S., de la Torre E. Reactivation process of activated carbons: effect on the mechanical and adsorptive properties. Molecules. 2020;25 doi: 10.3390/molecules25071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Deb U., Datta S., Walther C., Gupta D.K. Common explosives (TNT, RDX, HMX) and their fate in the environment: emphasizing bioremediation. Chemosphere. 2017;184:438–451. doi: 10.1016/j.chemosphere.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Chaukura N., Gwenzi W., Tavengwa N., Manyuchi M.M. Biosorbents for the removal of synthetic organics and emerging pollutants: opportunities and challenges for developing countries. Environ. Dev. 2016;19:84–89. [Google Scholar]
- Chew S.C., Tennant M., Mai N., McAteer D., Pons J.-F. Practical remediation of 3-Nitro-1,2,4-triazol-5-one wastewater. Propellants, Explos. Pyrotech. 2017 [Google Scholar]
- Choi H., Al-Abed S.R., Agarwal S., Dionysiou D.D. Synthesis of reactive nano-Fe/Pd bimetallic system-impregnated activated carbon for the simultaneous adsorption and dechlorination of PCBs. Chem. Mater. 2008;20:3649–3655. [Google Scholar]
- Clausen J., Robb J., Curry D., Korte N. A case study of contaminants on military ranges: camp Edwards, Massachusetts, USA. Environ. Pollut. 2004;129:13–21. doi: 10.1016/j.envpol.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Cronin M.P., Day A.I., Wallace L. Electrochemical remediation produces a new high-nitrogen compound from NTO wastewaters. J. Hazard Mater. 2007;149:527–531. doi: 10.1016/j.jhazmat.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Davies P.J., Provatas A. Defence Science and Technology Organisation, Defence Science and Technology Organisation; Edinburgh, South Australia: 2006. Characterisation of 2,4-Dinitroanisole: an Ingredient for Use in Low Sensitivity Melt Cast Formulations. [Google Scholar]
- Dawag T.E., Morris J. 2012. Nitrotriazolone Process Optimization. [Google Scholar]
- Djilani C., Zaghdoudi R., Modarressi A., Rogalski M., Djazi F., Lallam A. Elimination of organic micropollutants by adsorption on activated carbon prepared from agricultural waste. Chem. Eng. J. 2012;189–190:203–212. [Google Scholar]
- Dodard S.G., Sarrazin M., Hawari J., Paquet L., Ampleman G., Thiboutot S., Sunahara G.I. Ecotoxicological assessment of a high energetic and insensitive munitions compound: 2,4-Dinitroanisole (DNAN) J. Hazard Mater. 2013;262:143–150. doi: 10.1016/j.jhazmat.2013.08.043. [DOI] [PubMed] [Google Scholar]
- Dubinin M.M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 1960;60:235–241. [Google Scholar]
- Ebrahiem E.E., Al-Maghrabi M.N., Mobarki A.R. Removal of organic pollutants from industrial wastewater by applying photo-Fenton oxidation technology. Arab. J. Chem. 2013 [Google Scholar]
- El Gamal M., Mousa H.A., El-Naas M.H., Zacharia R., Judd S. Bio-regeneration of activated carbon: a comprehensive review. Separ. Purif. Technol. 2018;197:345–359. [Google Scholar]
- Fawcett-Hirst W., Temple T.J., Ladyman M.K., Coulon F. Adsorption behaviour of 1,3,5-trinitroperhydro-1,3,5-triazine, 2,4-dinitroanisole and 3-nitro-1,2,4-triazol-5-one on commercial activated carbons. Chemosphere. 2020;255:126848. doi: 10.1016/j.chemosphere.2020.126848. [DOI] [PubMed] [Google Scholar]
- Felt D., Johnson J.L., Larson S., Hubbard B., Henry K., Nestler C., Ballard J.H. US Army Engineer Research and Development Center, Environmental Laboratory, ERDC/EL TR-13-20; Vicksburg: 2013. Evaluation of Treatment Technologies for Wastewater from Insensitive Munitions Production. [Google Scholar]
- Fischer Scientific Safety data sheet. Thermo. Fischer Sci. 2018:1–8. [Google Scholar]
- Fitzpatrick W.F. 2001. The lessons of massachusetts military reservation. [Google Scholar]
- Garcia-Mateos F., Ruiz-Rosas R., Marqués M.D., Cotoruelo L.M., Rodriguez-Miasol J., Cordero T. Removal of paracetamol on biomass-derived activated carbon : modeling the fixed bed breakthrough curves using batch adsorption experiments. Chem. Eng. J. 2015;279:18–30. [Google Scholar]
- Ghauch A., Tuqan A. Reductive destruction and decontamination of aqueous solutions of chlorinated antimicrobial agent using bimetallic systems. J. Hazard Mater. 2009;164:665–674. doi: 10.1016/j.jhazmat.2008.08.048. [DOI] [PubMed] [Google Scholar]
- Gu Z., Pan X., Guo S., Zhang A. 2019. Dinitrodiazophenol Industrial Wastewater Treatment by a Sequential Ozone Fenton Process; pp. 32666–32671. [DOI] [PubMed] [Google Scholar]
- Gwenzi W., Chaukura N., Noubactep C., Mukome F.N.D. Biochar-based water treatment systems as a potential low-cost and sustainable technology for clean water provision. J. Environ. Manag. 2017;197:732–749. doi: 10.1016/j.jenvman.2017.03.087. [DOI] [PubMed] [Google Scholar]
- Haley M.V., Kuperman R.G., Checkai R.T. Report: ECBC-TR-726. US Army Center for Health Promotion and Preventative Medicine, Directorate of Toxicology; 2009. Aquatic toxicity of 3-nitro-1,2,4-triazol-5-one. [Google Scholar]
- Hammer M.J., Hammer M.J.J. fourth ed. prentice Hall PTR; Upper Saddle River, NJ: 2001. Water and Wastewater Technology. [Google Scholar]
- Hoffsommer J.C., Kaplan L.A., Kubose D.A., Glover D.J. 1977. Removal of Explosive Materials from Water by Chemical Interaction on Strongly Basic Ion Exchange Resins; p. 4018676. [Google Scholar]
- Işık M., Sponza D.T. Biological treatment of acid dyeing wastewater using a sequential anaerobic/aerobic reactor system. Enzym. Microb. Technol. 2006;38:887–892. [Google Scholar]
- IUPAC . Absorption. In: McNaught A.D., Wilkinson A., editors. Compendium of Chemical Terminology. second ed. the “Gold Book”; IUPAC, Research Triagle Park, NC: 2014. [Google Scholar]
- Jackson T.L., Zhang J. Density-based kinetics for mesoscale simulations of detonation initiation in energetic materials. Combust. Theor. Model. 2017;21:749–769. [Google Scholar]
- Jain A., Balasubramanian A.R., Srinivasan M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016;283:789–805. [Google Scholar]
- Ju K.-S., Parales R.E. Nitroaromatic compounds, from synthesis to biodegradation. Microbiol. Mol. Biol. Rev. 2010;74:250–272. doi: 10.1128/MMBR.00006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikeyan S., Spain J.C. Biodegradation of 2,4-dinitroanisole (DNAN) by Nocardioides sp. JS1661 in water, soil and bioreactors. J. Hazard Mater. 2016;312:37–44. doi: 10.1016/j.jhazmat.2016.03.029. [DOI] [PubMed] [Google Scholar]
- Kennedy A.J., Laird J.G., Brasfield S.M., Lounds C.D., Melby N.L., Winstead B., Laird J.G., Winstead B., Brasfield S.M., Johnson M.S. 2013. Development of Environmental Health Criteria for Insensitive Munitions: Aquatic Ecotoxicological Exposures Using 2,4-Dinitroanisole. [Google Scholar]
- Kennedy A.J., Poda A.R., Melby N.L., Moores L.C., Jordan S.M., Gust K.A., Bednar A.J. Aquatic toxicity of photo-degraded insensitive munition 101 (IMX-101) constituents. Environ. Toxicol. Chem. 2017 doi: 10.1002/etc.3732. [DOI] [PubMed] [Google Scholar]
- Kim Y.H., Carraway E.R., Kim Y.H. Reductive dechlorination of tce by zero valent bimetals. Environ. Technol. (United Kingdom) 2003;24:69–75. doi: 10.1080/09593330309385537. [DOI] [PubMed] [Google Scholar]
- Kirk-Othmer . fourth ed. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2000. Kirk-Othmer Encyclopedia of Chemical Technology. [Google Scholar]
- Kitcher E., Braida W., Koutsospyros A., Pavlov J., Su T.-L. Characteristics and products of the reductive degradation of 3-nitro-1,2,4-triazol-5-one (NTO) and 2,4-dinitroanisole (DNAN) in a Fe-Cu bimetal system. Environ. Sci. Pollut. Res. 2017;24:2744–2753. doi: 10.1007/s11356-016-8053-7. [DOI] [PubMed] [Google Scholar]
- Koutsospyros A., Pavlov J., Fawcett J., Strickland D., Smolinski B., Braida W. Degradation of high energetic and insensitive munitions compounds by Fe/Cu bimetal reduction. J. Hazard Mater. 2012;219:75–81. doi: 10.1016/j.jhazmat.2012.03.048. [DOI] [PubMed] [Google Scholar]
- Kyzas G.Z., Deliyanni E.A. Modified activated carbons from potato peels as green environmental-friendly adsorbents for the treatment of pharmaceutical effluents. Chem. Eng. Res. Des. 2015;97:135–144. [Google Scholar]
- LaPara T.M., Nakatsu C.H., Pantea L.M., Alleman J.E. Aerobic biological treatment of a pharmaceutical wastewater:: effect of temperature on COD removal and bacterial community development. Water Res. 2001;35:4417–4425. doi: 10.1016/s0043-1354(01)00178-6. [DOI] [PubMed] [Google Scholar]
- Le Campion L., Giannotti C., Ouazzani J. Photocatalytic degradation of 5-Nitro-1,2,4-Triazol-3-one NTO in aqueous suspention of TiO2. Comparison with fenton oxidation. Chemosphere. 1999;38:1561–1570. doi: 10.1016/s0045-6535(98)00376-2. [DOI] [PubMed] [Google Scholar]
- Lee K.-Y., Coburn M.D. 1988. 3-Nitro-1,2,4-Triazol-5-one, A Less Sensitive Explosive; p. 4733610. [Google Scholar]
- Lee Kien-Yin, Chapman Lonnie, Codura Michael. 3-Nitro-1,2,4-triazol-5-one, a less sensitive explosive. J. Energetic Mater. 1987;5:27–33. [Google Scholar]
- Lee J., Kim J., Hyeon T. Recent progress in the synthesis of porous carbon materials. Adv. Mater. 2006;18:2073–2094. [Google Scholar]
- Leman J., Joseph S. first ed. Earthscan; London, UK: 2009. Biochar for Environmental Management. [Google Scholar]
- Lingamdinne L.P., Roh H., Choi Y.L., Koduru J.R., Yang J.K., Chang Y.Y. Influencing factors on sorption of TNT and RDX using rice husk biochar. J. Ind. Eng. Chem. 2015;32:178–186. [Google Scholar]
- Liou M.-J., Lu M.-C., Chen J.-N. Oxidation of explosives by Fenton and photo-Fenton processes. Water Res. 2003;37:3172–3179. doi: 10.1016/S0043-1354(03)00158-1. [DOI] [PubMed] [Google Scholar]
- London J.O., Smith D.M. 1985. A Toxicological Study of NTO. Los Alamos. [Google Scholar]
- Loredo-Cancino M., Soto-Regalado E., Cerino-Córdova F.J., García-Reyes R.B., García-León A.M., Garza-González M.T. Determining optimal conditions to produce activated carbon from barley husks using single or dual optimization. J. Environ. Manag. 2013;125:117–125. doi: 10.1016/j.jenvman.2013.03.028. [DOI] [PubMed] [Google Scholar]
- Madeira C.L., Speet S.A., Nieto C.A., Abrell L., Chorover J., Sierra-Alvarez R., Field J.A. Sequential anaerobic-aerobic biodegradation of emerging insensitive munitions compound 3-nitro-1,2,4-triazol-5-one (NTO) Chemosphere. 2017;167:478–484. doi: 10.1016/j.chemosphere.2016.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahbub P., Nesterenko P.N. Application of photo degradation for remediation of cyclic nitramine and nitroaromatic explosives. RSC Adv. 2016;6:77603–77621. [Google Scholar]
- Mao H., Zhou D., Hashisho Z., Wang S., Chen H., Wang H., Lashaki M.J. Microporous activated carbon from pinewood and wheat straw by microwave-assisted KOH treatment for the adsorption of toluene and acetone vapors. RSC Adv. 2015;5:36051–36058. [Google Scholar]
- Mark N., Arthur J., Dontsova K., Brusseau M., Taylor S., Šimůnek J. Column transport studies of 3-nitro-1,2,4-triazol-5-one (NTO) in soils. Chemosphere. 2017;171:427–434. doi: 10.1016/j.chemosphere.2016.12.067. [DOI] [PubMed] [Google Scholar]
- Martınez N.S., Fernández J.F., Segura X.F., Ferrer A.S. Pre-oxidation of an extremely polluted industrial wastewater by the Fenton?s reagent. J. Hazard Mater. 2003;101:315–322. doi: 10.1016/s0304-3894(03)00207-3. [DOI] [PubMed] [Google Scholar]
- Menya E., Olupot P.W., Storz H., Lubwama M., Kiros Y. Production and performance of activated carbon from rice husks for removal of natural organic matter from water: a review. Chem. Eng. Res. Des. 2018;129:271–296. [Google Scholar]
- Mestre A.S., Pires J., Nogueira J.M.F., Parra J.B., Carvalho A.P., Ania C.O. Waste-derived activated carbons for removal of ibuprofen from solution: role of surface chemistry and pore structure. Bioresour. Technol. 2009;100:1720–1726. doi: 10.1016/j.biortech.2008.09.039. [DOI] [PubMed] [Google Scholar]
- Meyer S.A., Marchand A.J., Hight J.L., Roberts G.H., Escalon L.B., Inouye L.S., MacMillan D.K. Up-and-down procedure (UDP) determinations of acute oral toxicity of nitroso degradation products of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) J. Appl. Toxicol. 2005;25:427–434. doi: 10.1002/jat.1090. [DOI] [PubMed] [Google Scholar]
- Meyer S., Glaser B., Quicker P. Technical, economical, and climate-related aspects of biochar production technologies: a literature review. Environ. Sci. Technol. 2011;45:9473–9483. doi: 10.1021/es201792c. [DOI] [PubMed] [Google Scholar]
- Meyer R., Kohler J., Homburg A. seventh ed. John Wiley and Sons; Weinheim, Germany: 2015. Explosives. [Google Scholar]
- Mohan D., Sarswat A., Singh V.K., Alexandre-Franco M., Pittman C.U. Development of magnetic activated carbon from almond shells for trinitrophenol removal from water. Chem. Eng. J. 2011;172:1111–1125. [Google Scholar]
- Morley M.C., Fatemi M. Adsorption of RDX and its nitroso metabolites onto activated carbon. Pract. Period. Hazard. Toxic, Radioact. Waste Manag. 2010;14:90–97. [Google Scholar]
- Morley M.C., Henke J.L., Speitel G.E. Adsorption of RDX and HMX in rapid small-scale column tests: implications for full-scale adsorbers. J. Environ. Eng. 2005;131:29–37. [Google Scholar]
- NATO . 2010. STANAG 4439. [Google Scholar]
- Njoku V.O., Hameed B.H. Preparation and characterization of activated carbon from corncob by chemical activation with H3PO4for 2,4-dichlorophenoxyacetic acid adsorption. Chem. Eng. J. 2011;173:391–399. [Google Scholar]
- Njoku V.O., Foo K.Y., Hameed B.H. Microwave-assisted preparation of pumpkin seed hull activated carbon and its application for the adsorptive removal of 2,4-dichlorophenoxyacetic acid. Chem. Eng. J. 2013;215–216:383–388. [Google Scholar]
- Njoku V.O., Islam M.A., Asif M., Hameed B.H. Utilization of sky fruit husk agricultural waste to produce high quality activated carbon for the herbicide bentazon adsorption. Chem. Eng. J. 2014;251:183–191. [Google Scholar]
- O’Bryan T.R., Ross R.H. Chemical scoring system for hazard and exposure identification. J. Toxicol. Environ. Health. 1988;25:119–134. doi: 10.1080/15287398809531193. [DOI] [PubMed] [Google Scholar]
- Pennington J.C., Brannon J.M. Environmental fate of explosives. Thermochim. Acta. 2002;384:163–172. [Google Scholar]
- Pitchtel J. first ed. CRC Press LLC; Boca Raton, Florida: 2005. Waste Management Practices Municipal, Hazardous, and Industrial. 33487-2742. [Google Scholar]
- Platten W.E., Bailey D., Suidan M.T., Maloney S.W. Biological transformation pathways of 2,4-dinitro anisole and N-methyl paranitro aniline in anaerobic fluidized-bed bioreactors. Chemosphere. 2010;81:1131–1136. doi: 10.1016/j.chemosphere.2010.08.044. [DOI] [PubMed] [Google Scholar]
- Platten W.E., Bailey D., Suidan M.T., Maloney S.W. Treatment of energetic wastewater containing 2,4-dinitroanisole and N-methyl paranitro aniline. J. Environ. Eng. 2013;139:104–109. [Google Scholar]
- Powell I.J. Insensitive munitions – design principles and technology developments. Propellants, Explos. Pyrotech. 2016;41:409–413. [Google Scholar]
- Rajagopal C., Kapoor J.C. Development of adsorptive removal process for treatment of explosives contaminated wastewater using activated carbon. J. Hazard Mater. 2001;87:73–98. doi: 10.1016/s0304-3894(01)00179-0. [DOI] [PubMed] [Google Scholar]
- Reddy G., Chandra S.A.M., Lish J.W., Qualls C.W. Toxicity of 2,4,6-trinitrotoluene (TNT) in hispid cotton rats (Sigmodon hispidus): hematological, biochemical, and pathological effects. Int. J. Toxicol. 2000;19:169–177. [Google Scholar]
- Reddy G., Williams M., Quinn M.J. 2020. Wildlife Toxicity Assessment for 2,4-Dinitroanisole (DNAN) [Google Scholar]
- Reungoat J., Escher B.I., Macova M., Argaud F.X., Gernjak W., Keller J. Ozonation and biological activated carbon filtration of wastewater treatment plant effluents. Water Res. 2012;46:863–872. doi: 10.1016/j.watres.2011.11.064. [DOI] [PubMed] [Google Scholar]
- Richard T., Weidhaas J. Biodegradation of IMX-101 explosive formulation constituents: 2,4-dinitroanisole (DNAN), 3-nitro-1,2,4-triazol-5-one (NTO), and nitroguanidine. J. Hazard Mater. 2014;280:561–569. doi: 10.1016/j.jhazmat.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Rohm & Haas . Rohm&Haas; 2008. Ion Exchange for Dummies - an Introduction. [Google Scholar]
- Şahin Ö., Saka C., Kutluay S. Cold plasma and microwave radiation applications on almond shell surface and its effects on the adsorption of Eriochrome Black T. J. Ind. Eng. Chem. 2013;19:1617–1623. [Google Scholar]
- Sayğılı H., Güzel F. Behavior of mesoporous activated carbon used as a remover in Congo red adsorption process. Water Sci. Technol. 2018:wst2018100. doi: 10.2166/wst.2018.100. [DOI] [PubMed] [Google Scholar]
- Şen S., Demirer G.N. Anaerobic treatment of real textile wastewater with a fluidized bed reactor. Water Res. 2003;37:1868–1878. doi: 10.1016/S0043-1354(02)00577-8. [DOI] [PubMed] [Google Scholar]
- Sikder A.K., Sikder N. A review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications. J. Hazard Mater. 2004;112:1–15. doi: 10.1016/j.jhazmat.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Song H.O., Zhou Y., Li A.M., Mueller S. Selective removal of nitrate by using a novel macroporous acrylic anion exchange resin. Chin. Chem. Lett. 2012;23:603–606. [Google Scholar]
- Spear R.J., Louey C.N., Wolfson M.G. Maribyrnong; Victoria, Australia: 1989. A Preliminary Assessment of NTO as an Insensitive High Explosive. [Google Scholar]
- Stanley J.K., Lotufo G.R., Biedenbach J.M., Chappell P., Gust K.A. Toxicity of the conventional energetics TNT and RDX relative to new insensitive munitions constituents DNAN and NTO in Rana pipiens tadpoles. Environ. Toxicol. Chem. 2015;34:873–879. doi: 10.1002/etc.2890. [DOI] [PubMed] [Google Scholar]
- Stewart H.P. The impact of the USS Forrestal’s 1967 fire on United States navy shipboard damage control. 2004. p. 112.https://apps.dtic.mil/sti/pdfs/ADA429103.pdf Master of Military art and science thesis. [Google Scholar]
- Sun L., Chen D., Wan S., Yu Z. Adsorption studies of dimetridazole and metronidazole onto biochar derived from sugarcane bagasse: kinetic, equilibrium, and mechanisms. J. Polym. Environ. 2018;26:765–777. [Google Scholar]
- Tarpagkou R., Pantokratoras A. The influence of lamellar settler in sedimentation tanks for potable water treatment - a computational fluid dynamic study. Powder Technol. 2014;268:139–149. [Google Scholar]
- Taylor S., Ringelberg D.B., Dontsova K., Daghlian C.P., Walsh M.E., Walsh M.R. Insights into the dissolution and the three-dimensional structure of insensitive munitions formulations. Chemosphere. 2013;93:1782–1788. doi: 10.1016/j.chemosphere.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Taylor S., Park E., Bullion K., Dontsova K. Dissolution of three insensitive munitions formulations. Chemosphere. 2015;119 doi: 10.1016/j.chemosphere.2014.06.050. [DOI] [PubMed] [Google Scholar]
- Taylor S., Walsh M.E., Becher J.B., Ringelberg D.B., Mannes P.Z., Gribble G.W. Photo-degradation of 2,4-dinitroanisole (DNAN): an emerging munitions compound. Chemosphere. 2017;167:193–203. doi: 10.1016/j.chemosphere.2016.09.142. [DOI] [PubMed] [Google Scholar]
- Tennant M., Chew S.C., Krämer T., Mai N., McAteer D., Pons J.F. Practical colorimetry of 3-nitro-1,2,4-triazol-5-one. Propellants, Explos. Pyrotech. 2019;44:198–202. [Google Scholar]
- Todde G., Jha S.K., Subramanian G., Shukla M.K. Adsorption of TNT, DNAN, NTO, FOX7, and NQ onto cellulose, chitin, and cellulose triacetate. Insights from Density Functional Theory calculations. Surf. Sci. 2018;668:54–60. [Google Scholar]
- Toghiani R.K., Toghiani H., Maloney S.W., Boddu V.M. Prediction of physicochemical properties of energetic materials. Fluid Phase Equil. 2008;264:86–92. [Google Scholar]
- Uhrmacher J.C. 1983. Granular Activated Carbon Performance Capability and Availability. Columbia, MD. [Google Scholar]
- United Nations . Fifth. ed. 2009. Recommendations on the Transport of Dangerous Goods: Manual of Tests and Criteria. New York, Geneva. [Google Scholar]
- United States Environmental Protection Agency . 2018. 2018 Edition of the Drinking Water Standards and Health Advisories. [Google Scholar]
- US Department for Health and Human Services . 2012. Toxicological Profile for RDX, ATSDR’s Toxicological Profiles. [Google Scholar]
- Vijayalakshmi R., Radhakrishnan S., Shitole P., Pawar S.J., Mishra V.S., Garg R.K., Talawar M.B., Sikder A.K. Spherical 3-nitro-1,2,4-triazol-5-one (NTO) based melt-cast compositions: heralding a new era of shock insensitive energetic materials. RSC Adv. 2015;5:101647–101655. [Google Scholar]
- Villaescusa I., Fiol N., Poch J., Bianchi A., Bazzicalupi C. Mechanism of paracetamol removal by vegetable wastes: the contribution of Π-Π interactions, hydrogen bonding and hydrophobic effect. Desalination. 2011;270:135–142. [Google Scholar]
- Wallace L., Cronin M.P., Day A.I., Buck D.P. Electrochemical method applicable to treatment of wastewater from nitrotriazolone production. Environ. Sci. Technol. 2009;43:1993–1998. doi: 10.1021/es8028878. [DOI] [PubMed] [Google Scholar]
- Wanaratna P., Christodoulatos C., Sidhoum M. Kinetics of RDX degradation by zero-valent iron (ZVI) J. Hazard Mater. 2006;136:68–74. doi: 10.1016/j.jhazmat.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Wang C., Fuller M.E., Schaefer C., Caplan J.L., Jin Y. Dissolution of explosive compounds TNT, RDX, and HMX under continuous flow conditions. J. Hazard Mater. 2012;217–218:187–193. doi: 10.1016/j.jhazmat.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Weidhaas J., Panaccione A., Bhattacharjee A.S., Goel R., Anderson A., Acharya S.P. Whole community transcriptome of a sequencing batch reactor transforming 2,4-dinitroanisole (DNAN) and 3-nitro-1,2,4-triazol-5-one (NTO) Biodegradation. 2018;29:71–88. doi: 10.1007/s10532-017-9814-9. [DOI] [PubMed] [Google Scholar]
- Wharton R.K., Formby S.A., Merrifield R. Airblast TNT equivalence for a range of commercial blasting explosives. J. Hazard Mater. 2000;79:31–39. doi: 10.1016/s0304-3894(00)00168-0. [DOI] [PubMed] [Google Scholar]
- Ying W.C., Zhang W., Chang Q.G., Jiang W.X., Li G.H. Improved methods for carbon adsorption studies for water and wastewater treatment. Environ. Prog. 2006;25:110–120. [Google Scholar]
- Zappi M.E., Hernandez R., Gang D., Bajpai R., Kuo C.H., Hill D.O. Treatment of groundwater contaminated with high levels of explosives using advanced oxidation processes. Int. J. Environ. Sci. Technol. 2016;13:2767–2778. [Google Scholar]
- Zeman S., Hussein A.K., Jungova M., Elbeih A. Effect of energy content of the nitraminic plastic bonded explosives on their performance and sensitivity characteristics. Def. Technol. 2019;15:488–494. [Google Scholar]
- Zhou Y., Zhang L., Cheng Z. Removal of organic pollutants from aqueous solution using agricultural wastes: a review. J. Mol. Liq. 2015;212:739–762. [Google Scholar]
- Zoh K.-D., Stenstrom M.K. Fenton oxidation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) Water Res. 2002;36:1331–1341. doi: 10.1016/s0043-1354(01)00285-8. [DOI] [PubMed] [Google Scholar]
- Zunino L., Singh S., Jelinek L., Samuels P., Di Stasio A., Zunino L. IMX-104 characterization for DoD qualification. Insens. Munitions Energ. Mater. Technol. Symp. 2012;18 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.




