Highlights
-
•
The use of the ProFibro app for six weeks was not more effective than the use of a traditional paper book with similar content for health-related quality of life, symptoms, or self-care agency in individuals with fibromyalgia.
-
•
Improvements on severity of symptoms in both groups suggest that the self-care program using a mobile app or a paper book may be beneficial in individuals with fibromyalgia.
-
•
The Sleep Strategies was the most used function of the ProFibro app, followed by the Exercise Program, and the diary for the practice of gratitude.
Keywords: Chronic pain, Fibromyalgia, Mobile applications, Physical therapy, ProFibro, Self-care
Abstract
Background
The ProFibro application (app) was developed as a Mobile Health resource to promote self-care in fibromyalgia management.
Objective
This study aimed to assess the effects of the use of the ProFibro app for six weeks compared to the use of a traditional paper book of similar content to improve health-related quality of life, symptoms, and self-care agency in individuals with fibromyalgia.
Methods
Forty individuals with fibromyalgia were included in this randomized, single-blind, parallel trial. One group received intervention content using the ProFibro app on a smartphone while the other received similar information using a paper book. Participants were assessed at baseline and after six weeks. The primary outcome was the Revised Fibromyalgia Impact Questionnaire. Secondary outcomes were Widespread Pain Index, Pain Visual Analog Scale, Symptom Severity Scale, and Appraisal of Self-Care Agency Scale – Revised.
Results
No differences in changes were found between groups at the end of the treatment for any outcome. Both groups showed improvements in symptom severity.
Conclusions
The use of the ProFibro app for six weeks was not more effective than the use of a traditional paper book with similar content for health-related quality of life, symptoms, or self-care agency in individuals with fibromyalgia. Both groups showed improvements from baseline on severity of symptoms, suggesting that the self-care program using a mobile app or a paper book may be beneficial for individuals with fibromyalgia.
Introduction
Fibromyalgia syndrome (FMS) is a rheumatic disorder characterized by chronic widespread pain often associated with fatigue, unrefreshed sleep, and cognitive difficulties.1 The prevalence of FMS in Brazil ranges between 2.0 and 2.5%.2, 3 FMS is a debilitating condition that impairs quality of life (mean SF-36 physical and mental health component scores of 40.2 and 39.5, respectively), increases health care utilization (around 95% visited a health care provider in the past six months), and impairs work productivity and daily activities (around 41% reported overall work impairment, and 38% activity impairment).3 FMS is usually treated using a combination of pharmacological and non-pharmacological therapies, including education, exercise, sleep hygiene, and cognitive behavioural therapy. Effective management demands the patient's active participation, including taking responsibility for self-care and adherence to the treatment plan.4
The use of new technologies is growing in health communication for health promotion, disease prevention, and health care delivery.5, 6 Health communication tools are usually designed to improve lifestyle behaviours, reduce risk factors for disease, increase compliance with a treatment plan, better self-manage a condition, provide social support, or provide help with decision-making about health.7 Recently, Mobile Health (mHealth) has emerged as an important field of Electronic Health (eHealth) with the potential to improve the delivery of health-related services.8
A systematic review identified 85 trials that investigated the use of mobile technologies to improve disease management or change health behaviours, and concluded that there is suggestive evidence of benefit in health behaviour change and self-management of diseases.9 Another review summarized the evidence on the effects of eHealth and mHealth applications in chronic pain, including four studies with FMS. Moderate-quality evidence suggests that these interventions have significant effects on pain, depression, and self-efficacy.10
Based on scientific evidence and clinical experience, ProFibro mobile application (app) was developed as a mHealth resource for promoting self-care in FMS management while offering an engaging, dynamic, and interactive experience.11 It is available in Portuguese for free download on Google Play since March 2018: https://play.google.com/store/apps/details?id=br.com.projetoprofibro.profibro.
The objective of this study was to assess the effects of the use of the ProFibro app for six weeks compared to the use of a traditional paper book of similar content to improve health-related quality of life, symptoms, and self-care agency in patients with FMS. The frequency of use of the ProFibro app was also evaluated. We hypothesized that the ProFibro App group (PAG) would exhibit greater improvements than the Paper Book group (PBG).
Methods
A randomized, single-blind, parallel trial was conducted in the Physical Therapy, Speech Therapy and Occupational Therapy Department of the School of Medicine of the Universidade de Sao Paulo. The trial was prospectively registered at ClinicalTrials.gov No.: NCT03004911 (https://clinicaltrials.gov/ct2/show/NCT03004911).
Participants
An advertisement with general information about the trial and an email address for contact was published in the university newspaper and related social media. A screening questionnaire which covered basic inclusion and exclusion criteria was sent by email to who replied to the advertisement. Potential participants were identified through the results of the questionnaire and invited to a face-to-face assessment to confirm eligibility.
The study included adults and middle-aged individuals (19–59 years) with a medical diagnosis of FMS reconfirmed by the 2010 American College of Rheumatology (ACR) diagnostic criteria,1 who were smartphone users, and completed elementary education. Exclusion criteria were: individuals undergoing physical therapy or treated in the last three months; diagnosis of other conditions causing chronic pain (neuropathies, rheumatoid arthritis, osteoarthritis, spinal stenosis, or cancer); severe mental disorders (schizophrenia, psychosis, bipolar affective disorder, severe depression); hearing or visual impairment.
The trial protocol was approved by the Research Ethics Committee of the School of Medicine of the Universidade de Sao Paulo, Brazil (approval no. 20/08/2014), and followed the principles of the 1964 Declaration of Helsinki and its later amendments. Informed consent was obtained from all participants.
Sample size
The sample size was calculated to detect the minimal clinically important difference (MCID) of 14% between two groups in the primary outcome health-related quality of life, measured by the Revised Fibromyalgia Impact Questionnaire (FIQR). We assumed a standard deviation of 24.3.12, 13 Calculations indicated that a sample size of 30 participants would provide a power of 80% at a level of significance of 5% for a two-sided t-test. The sample size calculation was performed using the online software provided by the Massachusetts General Hospital Biostatistics Center (Boston, USA). Five participants were added to each group to account for possible dropouts, resulting in a total of 40 participants.
Outcomes
An outcome assessor blinded to the received interventions enrolled participants and evaluated them at baseline (Table 1) and after the six-week intervention.
Table 1.
Demographic and clinical data of the participants.
| ProFibro App group (n = 20) | Paper Book group (n = 20) | |
|---|---|---|
| Female sex | 19 (95%) | 20 (100%) |
| Age (years; mean ± SD) | 43.3 ± 8.4 | 42.1 ± 11.8 |
| Marital status | ||
| Single | 8 (40%) | 5 (25%) |
| Married | 6 (30%) | 11 (55%) |
| Divorced | 6 (30%) | 4 (20%) |
| Education | ||
| Primary – complete | 0 (0%) | 1 (5%) |
| Secondary – incomplete | 0 (0%) | 1 (5%) |
| Secondary – complete | 5 (25%) | 6 (30%) |
| University – incomplete | 0 (0%) | 2 (10%) |
| University – complete | 15 (75%) | 10 (50%) |
| Social status (%) | ||
| A + B | 4 (20%) | 4 (20%) |
| C | 11 (55%) | 4 (20%) |
| D + E | 5 (25%) | 12 (60%) |
| Disease duration (years, mean ± SD) | 8.9 ± 4.9 | 7.2 ± 5.8 |
Data are n (%) unless otherwise indicated. SD, standard deviation; A, household income >20 times minimum wage; B, household income 10–20 times minimum wage; C, household income 4–10 times minimum wage; D, household income 2–4 times minimum wage; E, household income <2 times minimum wage.
The primary outcome was health-related quality of life measured by the Brazilian Portuguese version of the FIQR.13, 14 The questionnaire consists of 21 questions, each scored on an 11-point numeric rating scale of 0 to 10, with 10 being ‘worst’. Questions are framed in the context of the past seven days. The FIQR is divided into three domains: Function, Overall Impact, and Symptoms. The Function score is the sum of nine questions divided by 3, the Overall Impact score is the sum of two questions, and the Symptoms score is the sum of 10 questions divided by 2. The total FIQR is the sum of the three domain scores (range 0–100).
Secondary outcomes were: pain measured with the Widespread Pain Index (WPI)1 and a Visual Analog Scale (VAS)15; severity of symptoms measured with the Symptom Severity (SS) Scale1; self-care measured by the Brazilian Portuguese version of the Appraisal of Self-Care Agency Scale – Revised (ASAS-R).16, 17
The WPI is one of the diagnostic variables of the 2010 ACR diagnostic criteria for FMS. It is a measure of the number of painful body regions (range 0–19) in the context of the past week.
The VAS was used to measure pain intensity at rest at the moment of the examination. It is a continuous scale consisting of a 10-cm horizontal line, anchored by two verbal descriptors: no pain (score of 0) and worst imaginable pain (score of 10).
The SS Scale is another diagnostic variable of the ACR diagnostic criteria. It is a 4-component scale (range 0–12) composed of assessor-rated cognitive problems, unrefreshed sleep, fatigue, and somatic symptom count. The timeframe for the assessment of the SS Scale is one week.
The ASAS-R is a multidimensional measure that evaluates the level of self-care agency, having, developing, or lacking the power for self-care (the score ranges from 15 to 75). It comprises 15 items using a five-point Likert scale ranging from 1 (totally disagree) to 5 (totally agree).
The ProFibro app was programmed to register the date and time of each access to its functions. The frequency of use of the app was assessed according to the number of times the participants accessed its functions after six weeks.
Randomization
Computer-generated random numbers were used for simple randomization of the participants. A physical therapist who was not involved in the trial concealed the random allocation sequence in sequentially numbered, opaque, sealed envelopes. After the baseline evaluation, a physical therapist researcher who was not involved in the outcome assessment and was responsible for delivering the intervention opened the sealed envelopes and assigned participants to PAG or PBG, accordingly.
Interventions
The ProFibro app was developed in five stages, according to the prototyping paradigm.11, 18 In Stage 1, a panel of five physical therapists with expertise in FMS, five smartphone users with diagnosis of FMS, a digital interface designer, and a programmer analysed the requirements and content, and set the software objectives, based on current scientific evidence and clinical and patient experience. In Stage 2, the designer created preliminary screen layouts that were assessed by 10 smartphone users with FMS and then developed the final layouts. In Stage 3, the programmer developed the prototype. In Stage 4, 10 smartphone users with FMS pilot tested the prototype. In Stage 5, the designer improved the interface, according to the results of the pilot test, and the programmer built the final product. Its main functions are: patient education through animation19, 20; self-monitoring with the FIQR13, 14; sleep strategies with guided imagery relaxation technique, stimulus control therapy, and sleep hygiene21, 22, 23; scheduling function to facilitate the planning of daily routine; graded exercise program with aerobic, stretching, and strengthening exercises20, 24; a diary for the practice of gratitude25, 26; an eBook with nine chapters on family adjustments27; and hints through notifications.
A 64-page paper book was made after the mobile app was developed.28 Each app function was turned into a book chapter. The animation was turned into a comic strip. Paper versions of the FIQR for two months were provided. Guided imagery audios were converted to text to be read by or for the participant. The screen layouts of the exercise program with its written instructions and illustrations were transferred. The written instructions about stimulus control therapy and sleep hygiene, written content of the eBook on family adjustments and the hints were transferred. Instructions to manage daily schedule with a paper or digital planner and to keep a paper diary were provided.
Immediately after the baseline evaluation and intervention assignment, at a single individual session, the physical therapist researcher, who created the mobile app and the paper book, gave smartphones with the app installed to the PAG, and paper books to the PBG. Both groups received a 20-minute introductory tutorial leading the participants through the app functions or book chapters. For further instructions, the information icon of the app functions and tutorial video were presented to the PAG. All participants were instructed to explore and use the tools for six weeks with a view to self-care, while continuing their usual medical care.
Statistical methods
Data analysis was conducted using descriptive and inferential statistics.
Primary and secondary outcomes were compared to baseline in each group and between groups at the end of treatment. Analysis was performed on an intention-to-treat basis, with baseline observation carried forward in case of dropouts. Kolmogorov–Smirnov and Levene tests were used to verify normality and equality of variance of the data, respectively. Independent t tests were used with variables that were normally distributed, otherwise Mann–Whitney rank sum tests were used.
Cohen's d effect size for between and within-group differences was calculated. The magnitude of effect was considered small (d = 0.2), moderate (d = 0.5), and large (d = 0.8).29
In the analysis of clinically important changes, participants were classified as responders if they scored higher than the MCID for an outcome, and non-responders otherwise. The MCID for FIQR total score was considered a change of 8.1 points, and for VAS pain a change of 2 points.12, 30, 31 For the other variables, no MCID has yet been defined, and a change of 15% was used.32 The frequency of responders and non-responders was compared between groups with the Chi-Square test.
A p-value <0.05 was considered statistically significant. Statistical tests were performed using software SigmaStat 3.5 (Systat Software, Inc, Erkrath, Germany).
Results
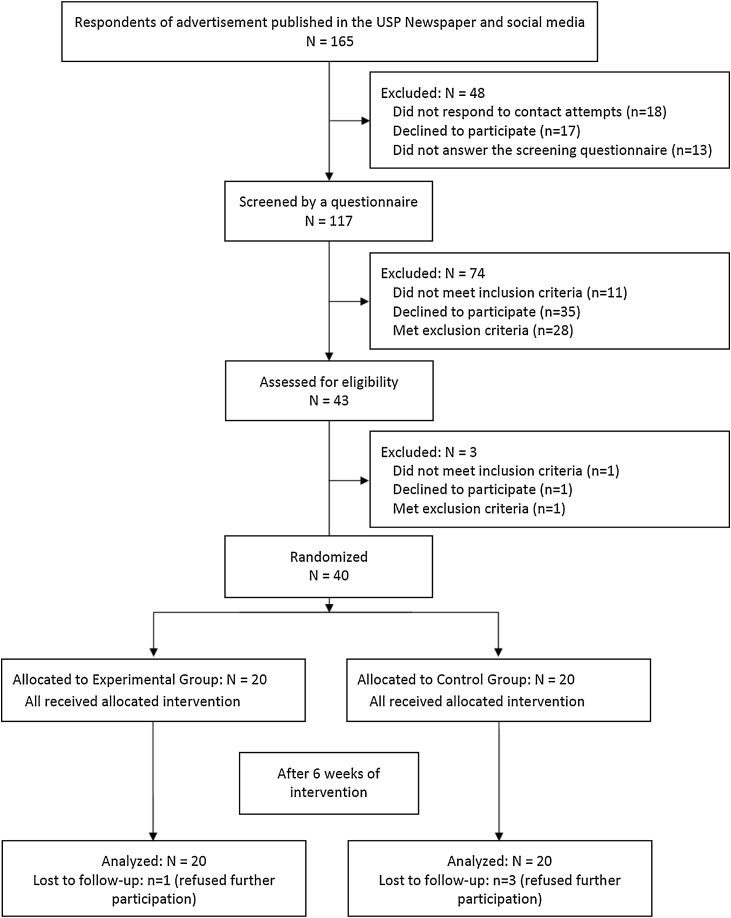
This trial was conducted between February and December 2017 (end of participant follow-up). There were 117 potential participants screened and/or assessed, from which 40 participants aged 43.0 ± 10.1 years were included in the study. Twenty participants were assigned to PAG (one dropout), and 20 to PBG (three dropouts). Fig. 1 is the flow diagram of the process of enrolment, intervention allocation, follow-up, and data analysis.
Figure 1.
Flow diagram of the randomized parallel trial.
Demographic and clinical characteristics are shown in Table 1. Groups were similar in terms of sex, age, education, and FMS duration. The PBG had more married participants and lower socioeconomic status.
Table 2 summarizes the results of each outcome at baseline and end of treatment for both groups, within-group changes and between-group change scores.
Table 2.
Health-related quality of life, pain, symptom severity, and self-care agency outcome data.
| Outcomes | ProFibro App group | Paper Book group | Between-group change score |
|---|---|---|---|
| FIQR total score (0–100) | |||
| Baseline | 62.6 ± 16.8 | 65.4 ± 18.0 | |
| Post-intervention | 53.7 ± 17.1 | 55.5 ± 18.2 | |
| Within-group change | −8.9 (−15.5, −2.4) | −9.9 (−18.1, −1.6) | 1.0 (−10.0, 11.9) |
| FIQR function (0–30) | |||
| Baseline | 16.5 ± 6.8 | 18.5 ± 6.9 | |
| Post-intervention | 14.9 ± 6.8 | 15.8 ± 7.6 | |
| Within-group change | −1.5 (−3.9, 0.8) | −2.7 (−5.7, 0.3) | 1.2 (−2.8, 5.1) |
| FIQR overall impact (0–20) | |||
| Baseline | 13.0 ± 4.2 | 12.3 ± 5.8 | |
| Post-intervention | 9.8 ± 5.3 | 9.5 ± 5.1 | |
| Within-group change | −3.2 (−5.0, −1.4)* | −2.8 (−4.9, −0.7) | 0.4 (−2.4, 3.2) |
| FIQR symptoms (0–50) | |||
| Baseline | 33.1 ± 8.1 | 34.6 ± 7.8 | |
| Post-intervention | 29.0 ± 7.1 | 30.3 ± 8.2 | |
| Within-group change | −4.2 (−7.4, −1.0) | −4.4 (−8.5, −0.3) | 0.2 (−5.2, 5.6) |
| WPI (0–19) | |||
| Baseline | 13.6 ± 4.0 | 13.6 ± 3.7 | |
| Post-intervention | 12.5 ± 4.6 | 12.3 ± 4.5 | |
| Within-group change | −1.1 (−2.8, 0.7) | −1.3 (−3.1, 0.5) | 0.3 (−2.3, 2.8) |
| VAS pain (0–10) | |||
| Baseline | 5.9 ± 2.2 | 5.7 ± 2.2 | |
| Post-intervention | 5.1 ± 2.6 | 5.3 ± 2.3 | |
| Within-group change | −0.9 (−2.0, 0.2) | −0.4 (−1.7, 0.9) | 0.5 (−1.3, 2.2) |
| SS (0–12) | |||
| Baseline | 9.4 ± 1.5 | 9.2 ± 1.9 | |
| Post-intervention | 7.6 ± 2.9 | 7.6 ± 2.4 | |
| Within-group change | −1.8 (−3.0, −0.6)a | −1.6 (−2.7, −0.5)a | 0.2 (−1.5, 1.9) |
| Self-care (15–75) | |||
| Baseline | 51.9 ± 9.7 | 53.5 ± 11.0 | |
| Post-intervention | 56.8 ± 10.2 | 54.3 ± 11.0 | |
| Within-group change | 4.9 (1.0, 8.8) | 0.8 (−2.4, 4.0) | 4.1 (−1.1, 9.3) |
FIQR, Revised Fibromyalgia Impact Questionnaire; WPI, Widespread Pain Index; VAS, Visual Analog Scale; SS, Symptom Severity; outcome values at baseline and post-intervention are mean ± standard deviation. Values for within-group change and between-group change scores are mean (95% confidence interval).
Statistically significant differences (p < 0.05).
In the comparison between groups, no statistically significant differences were found at the end of treatment for the primary outcome FIQR total score (mean difference [MD] = 1.0, 95% confidence interval [CI]: −10.0, 11.9) or the domains function (MD = 1.2, 95% CI: −2.8, 5.1), overall impact (MD = 0.4, 95% CI: −2.4, 3.2), and symptoms (MD = 0.2, 95% CI: −5.2, 5.6). No significant differences were found for the secondary outcomes WPI (MD = 0.3, 95% CI: −2.3, 2.8), VAS pain (MD = 0.5, 95% CI: −1.3, 2.2), SS (MD = 0.2, 95% CI: −1.5, 1.9), or self-care (MD = 4.1, 95% CI: −1.1, 9.3). Between-group effect sizes were small for self-care (d = 0.24). No effect or very small effect sizes were observed for the other outcomes (d = 0.17 or less).
Compared to baseline, both groups improved significantly on the SS Scale (PAG: MD = −1.8, 95% CI: −3.0, −0.6; PBG: MD = −1.6, 95% CI: −2.7, −0.5). There was also significant improvement of the FIQR overall impact domain in the PAG (MD = −3.2, 95% CI: −5.0, −1.4). Within-group effect sizes were moderate in both groups for FIQR total score (PAG: d = 0.52, PBG: d = 0.55), overall impact (PAG: d = 0.67, PBG: d = 0.51), symptoms (PAG: d = 0.54, PBG: d = 0.54), and SS (PAG: d = 0.78, PBG: d = 0.74). For self-care, the effect size was moderate for PAG (d = 0.49) and very small for PBG (d = 0.07). Very small or small effect sizes were observed for the other outcomes in both groups (d = 0.18–0.372).
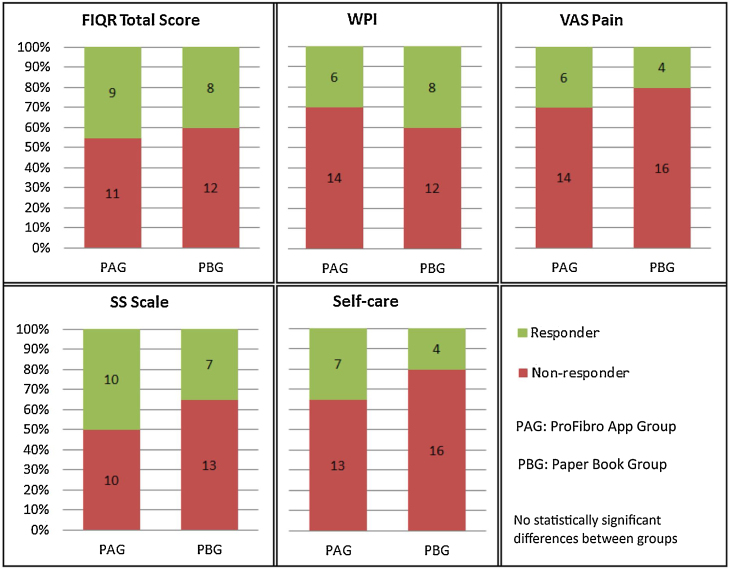
Fig. 2 shows the proportion of responders and non-responders to the interventions for each outcome. No statistically significant differences were found between groups.
Figure 2.
Analysis of clinically important changes of health-related quality of life, pain, symptom severity, and self-care agency for the ProFibro app and Paper Book groups.
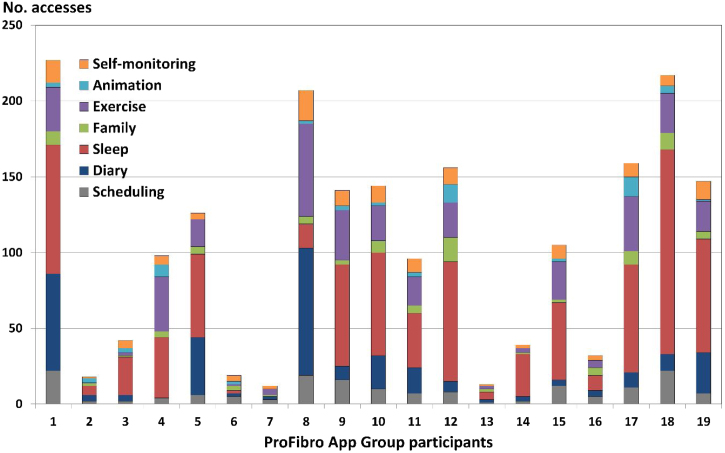
In the PAG, the total number of accesses to all functions of the ProFibro app ranged between 12 and 237 during the six-week intervention. Including the accesses to the tutorial, profile, and About ProFibro page, 12 of 20 participants accessed the mobile app more than 100 times. On the other hand, a very low number of accesses (lower than 50) was observed for seven participants (around 37%). The Sleep Strategies was the most used function, followed by the Exercise Program, and the Diary. Fig. 3 illustrates the frequency of use of the PAG of the app.
Figure 3.
Frequency of use of the participants of the ProFibro App group according to the number of accesses to the main ProFibro app functions during the six-week intervention.
Discussion
This randomized, single-blind, parallel trial found that the use of ProFibro app for six weeks did not result in better outcomes than a similar paper-based intervention. No significant differences were observed between PAG and PBG for any outcome. In the within-group analysis, both groups showed a significant reduction from baseline in symptom severity with a moderate effect size, and PAG showed also a significant moderate reduction in FIQR overall impact domain. However, these changes cannot be fully attributed to the interventions without a control group receiving usual clinical care only.
To our knowledge, there are no randomized trials comparing a smartphone application to a conventional paper-based intervention for self-care in fibromyalgia. Three randomized trials compared the use of electronic self-monitoring systems for weight loss in overweight or obese adults to paper records.33, 34, 35 Statistically significant reductions from baseline in body weight for both groups were found in two of these trials, but none of them found significant differences between groups. As in the present study, differences by method of self-monitoring were less pronounced than what was expected.
A randomized trial for adults with diabetes compared a smartphone application to a paper diary, and found no differences between groups in the outcome measures.36 Finally, a randomized trial for sedentary adults compared a web-based physical activity intervention and a paper-based intervention. Significant improvements from baseline in the outcome measures were observed, but no differences between groups were found.37
Findings indicate that the ProFibro app is not superior to the paper book. Hence, the choice of one tool over the other depends on patient's preference and book availability. In clinical practice, despite the widespread use of digital devices, the traditional paper book for delivering a self-care program may still be considered in a shared decision-making process involving health professionals and patients. An exploratory study with 429 students investigated the preference for reading in print or digitally, and found that for short readings around 43% prefer print, 35% prefer digital screens, and 22% have no preference. With long texts, around 80% prefer print. Advantages reported for print included ease of annotation and paper's tactile properties, while disadvantages were lack of convenience and expenditure of environmental and monetary resources. The biggest advantage of screen reading was convenience, while disadvantages were eyestrain and distraction.38
After their participation in the trial, some participants shared their experiences with the research team, suggesting factors other than the type of tool used to deliver the self-care program potentially having more influence on clinical response: self-efficacy and family support. Self-efficacy is an essential component of undertaking and engaging in a new activity. Two determinant factors are involved: efficacy expectation and outcome expectation.39 In both groups of the trial, some participants demonstrated a lack of belief that they could introduce the proposed activities into daily life or continue to engage in them, and that these proposals could produce beneficial effects. On the other hand, regardless of using the app or the book, some participants demonstrated that they believed in the activities, they effectively introduced them in their daily life and were committed to continuing them.
Behavioural change is also influenced by the social environment. Several studies in rheumatic diseases have shown the benefits of supportive relationships, especially with family members. The involvement of people from the patient's social environment is recommended to increase the effectiveness of self-care programs.40 In the present study, participants from both groups associated their clinical improvement to family support for practicing the self-care program.
A longer intervention duration could have increased the participants’ clinical response because many app functions and book chapters in the self-care program imply the incorporation of healthy habits in the daily routine. An experimental study investigated the habit formation process in everyday life and observed that half of the participants took more than two months to reach their limit of automaticity.41
Providing health professional support could also have facilitated the use of the ProFibro app or the paper book. In the management of chronic conditions, the need to expand the role of the health professional from patient education to self-care support is being acknowledged. The concept of patient education is restricted to providing information with the expectation that the increase of patient's knowledge lead to behavioural change. In the concept of self-care support, besides providing information, the health professional helps the patient to build confidence and develop abilities to deal with the chronic condition.42 In FMS, the importance of the health professional-patient relationship is well established, especially for the increase of the patient's active participation in the treatment.4
The Sleep Strategies was the most used app function, reflecting the need of individuals with fibromyalgia to deal with this common complaint. The Exercise Program and the Diary were also frequently used, pointing out the participants’ acknowledgement of the benefits of such activities and their effort to incorporate them into daily life. Additionally, these three functions are recommended to be used several times a week. In contrast, Self-Monitoring, Animation, and Scheduling function do not require such a high frequency of use. A better use of the Family Adjustments could be of benefit, and the health professional could mediate discussions with the patient's family.
The findings of this trial suggest the need to further investigate the effects of the use of ProFibro app in different conditions. In future studies, the use of ProFibro app as a complementary resource in combination with standard care could be compared to a control receiving only standard care. The duration of the intervention should be longer considering the time people take to reach their limit of automaticity in the habit formation process.41 Patients may benefit from a closer monitoring by a health professional.42 The use of strategies to improve participants’ self-efficacy and the involvement of family members could be considered.
Limitations
Because the operating system of the participants’ smartphones could be incompatible with the ProFibro app, they received a smartphone with the app already installed. Even though they were instructed to insert their own SIM card into the new smartphone, many participants chose not to do so. Therefore, they used the ProFibro app in a similar way to the PBG: some would carry them as an additional item, and others would leave them at home. Therefore, the advantage of the mobile app of being accessible to the user anywhere could not be fully utilized. The use of the app in this condition is likely to cause a lower frequency of use than if the app was installed in the participants’ own phones. Future studies should consider including only participants whose smartphones have a compatible operating system and enough capacity to install the app.
Conclusion
The use of ProFibro app for six weeks was not more effective than the traditional paper book with similar content for health-related quality of life, symptoms, or self-care agency in patients with FMS. Both groups showed improvements from baseline on severity of symptoms, suggesting that the self-care program using a mobile app or a paper book may be beneficial in individuals with FMS. About 40% of the participants of the PAG had a low frequency of use of ProFibro app. The Sleep Strategies was the most used app function, followed by the Exercise Program, and the Diary.
Conflict of interest
None declared.
Acknowledgement
This work was supported by Sao Paulo Research Foundation (FAPESP grant number 2014/17547-5). The funding source had no involvement in conducting the study or preparing the article for publication.
References
- 1.Wolfe F., Clauw D.J., Fitzcharles M.A. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62(May (5)):600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 2.Senna E.R., De Barros A.L., Silva E.O. Prevalence of rheumatic diseases in Brazil: a study using the COPCORD approach. J Rheumatol. 2004;31(March (3)):594–597. [PubMed] [Google Scholar]
- 3.Goren A., Gross H.J., Fujii R.K., Pandey A., Mould-Quevedo J. Prevalence of pain awareness, treatment, and associated health outcomes across different conditions in Brazil. Rev Dor. 2012;13(4):12. [Google Scholar]
- 4.Arnold L.M., Clauw D.J., Dunegan L.J., Turk D.C. A framework for fibromyalgia management for primary care providers. Mayo Clin Proc. 2012;87(May (5)):488–496. doi: 10.1016/j.mayocp.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lebleu J., Poilvache H., Mahaudens P., De Ridder R., Detrembleur C. Predicting physical activity recovery after hip and knee arthroplasty? A longitudinal cohort study. Braz J Phys Ther. 2019;(December) doi: 10.1016/j.bjpt.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dantas L.O., Barreto R.P.G., Ferreira C.H.J. Digital physical therapy in the COVID-19 pandemic. Braz J Phys Ther. 2020;(May) doi: 10.1016/j.bjpt.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suggs L.S. A 10-year retrospective of research in new technologies for health communication. J Health Commun. 2006;11(1):61–74. doi: 10.1080/10810730500461083. [DOI] [PubMed] [Google Scholar]
- 8.Vital Wave Consulting . UN Foundation-Vodafone Foundation Partnership; Washington, D.C. and Berkshire, UK: 2009. mHealth for Development: The Opportunity of Mobile Technology for Healthcare in the Developing World. [Google Scholar]
- 9.Free C., Phillips G., Galli L. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10(1):e1001362. doi: 10.1371/journal.pmed.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moman R.N., Dvorkin J., Pollard E.M. A systematic review and meta-analysis of unguided electronic and mobile health technologies for chronic pain – is it time to start prescribing electronic health applications? Pain Med. 2019;20(November (11)):2238–2255. doi: 10.1093/pm/pnz164. [DOI] [PubMed] [Google Scholar]
- 11.Yuan S.L.K., Marques A.P. Development of ProFibro – a mobile application to promote self-care in patients with fibromyalgia. Physiotherapy. 2018;(June) doi: 10.1016/j.physio.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Bennett R.M., Bushmakin A.G., Cappelleri J.C., Zlateva G., Sadosky A.B. Minimal clinically important difference in the fibromyalgia impact questionnaire. J Rheumatol. 2009;36(June (6)):1304–1311. doi: 10.3899/jrheum.081090. [DOI] [PubMed] [Google Scholar]
- 13.Paiva E.S., Heymann R.E., Rezende M.C. A Brazilian Portuguese version of the Revised Fibromyalgia Impact Questionnaire (FIQR): a validation study. Clin Rheumatol. 2013;32(August (8)):1199–1206. doi: 10.1007/s10067-013-2259-6. [DOI] [PubMed] [Google Scholar]
- 14.Bennett R.M., Friend R., Jones K.D., Ward R., Han B.K., Ross R.L. The Revised Fibromyalgia Impact Questionnaire (FIQR): validation and psychometric properties. Arthritis Res Ther. 2009;11(4):R120. doi: 10.1186/ar2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marques A.P., Assumpcao A., Matsutani L.A., Pereira C.A.B., Lage L. Pain in fibromyalgia and discriminative power of the instruments: Visual Analog Scale, Dolorimetry and the Mcgill Pain Questionnaire. Acta Reumatol Port. 2008;33(July–September (3)):345–351. [PubMed] [Google Scholar]
- 16.Damasio B.F., Koller S.H. The Appraisal of Self-Care Agency Scale – Revised (ASAS-R): adaptation and construct validity in the Brazilian context. Cad Saude Publica. 2013;29(October (10)):2071–2082. doi: 10.1590/0102-311x00165312. [DOI] [PubMed] [Google Scholar]
- 17.Sousa V.D., Zauszniewski J.A., Bergquist-Beringer S., Musil C.M., Neese J.B., Jaber A.F. Reliability, validity and factor structure of the Appraisal of Self-Care Agency Scale-Revised (ASAS-R) J Eval Clin Pract. 2010;16(December (6)):1031–1040. doi: 10.1111/j.1365-2753.2009.01242.x. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal R., Anderson C., Crowley K., Kannan P.K. Agency for Healthcare Research and Quality; Rockville, MD: 2011. Understanding Development Methods From Other Industries to Improve the Design of Consumer Health IT: Background Report (Prepared by Westat, under Contract No. HHSA290200900023I.) AHRQ Publication No. 11-0065-EF. [Google Scholar]
- 19.Heymann R.E., Paiva Edos S., Helfenstein M., Jr. Brazilian consensus on the treatment of fibromyalgia. Rev Bras Reumatol. 2010;50(January–February (1)):56–66. [PubMed] [Google Scholar]
- 20.Macfarlane G.J., Kronisch C., Dean L.E. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis. 2016;(July) doi: 10.1136/annrheumdis-2016-209724. [DOI] [PubMed] [Google Scholar]
- 21.Edinger J.D., Wohlgemuth W.K., Krystal A.D., Rice J.R. Behavioral insomnia therapy for fibromyalgia patients: a randomized clinical trial. Arch Intern Med. 2005;165(November (21)):2527–2535. doi: 10.1001/archinte.165.21.2527. [DOI] [PubMed] [Google Scholar]
- 22.Morgenthaler T., Kramer M., Alessi C. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An american academy of sleep medicine report. Sleep. 2006;29(November (11)):1415–1419. [PubMed] [Google Scholar]
- 23.Martinez M.P., Miro E., Sanchez A.I. Cognitive-behavioral therapy for insomnia and sleep hygiene in fibromyalgia: a randomized controlled trial. J Behav Med. 2014;37(August (4)):683–697. doi: 10.1007/s10865-013-9520-y. [DOI] [PubMed] [Google Scholar]
- 24.Busch A.J., Schachter C.L., Overend T.J., Peloso P.M., Barber K.A. Exercise for fibromyalgia: a systematic review. J Rheumatol. 2008;35(June (6)):1130–1144. [PubMed] [Google Scholar]
- 25.Campbell B. 2015. Counting your blessings: how gratitude improves your health. http://www.cfidsselfhelp.org/library/counting-your-blessings-how-gratitude-improves-your-health [accessed 20.02.15] [Google Scholar]
- 26.Emmons R.A., McCullough M.E. Counting blessings versus burdens: an experimental investigation of gratitude and subjective well-being in daily life. J Pers Soc Psychol. 2003;84(February (2)):377–389. doi: 10.1037//0022-3514.84.2.377. [DOI] [PubMed] [Google Scholar]
- 27.Campbell B. 2015. Adjusting to Serious Illness: Strategies for Patients and Their Families. http://www.cfidsselfhelp.org/library/adjusting-serious-illness-strategies-patients-and-their-families [accessed 20.02.15] [Google Scholar]
- 28.Yuan S.L.K. School of Medicine, University of Sao Paulo; Sao Paulo: 2018. Development of a mobile application to promote self-care in patients with fibromyalgia. [Thesis] [DOI] [PubMed] [Google Scholar]
- 29.Cohen J. 2nd ed. L. Erlbaum Associates; Hillsdale, N.J.: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 30.Farrar J.T., Young J.P., Jr., LaMoreaux L., Werth J.L., Poole R.M. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(November (2)):149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 31.Salaffi F., Stancati A., Silvestri C.A., Ciapetti A., Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8(August (4)):283–291. doi: 10.1016/j.ejpain.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Philadelphia Panel evidence-based clinical practice guidelines on selected rehabilitation interventions: overview and methodology. Phys Ther. 2001;81(October (10)):1629–1640. [PubMed] [Google Scholar]
- 33.Burke L.E., Conroy M.B., Sereika S.M. The effect of electronic self-monitoring on weight loss and dietary intake: a randomized behavioral weight loss trial. Obesity (Silver Spring) 2011;19(February (2)):338–344. doi: 10.1038/oby.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carter M.C., Burley V.J., Nykjaer C., Cade J.E. Adherence to a smartphone application for weight loss compared to website and paper diary: pilot randomized controlled trial. J Med Internet Res. 2013;15(April (4)):e32. doi: 10.2196/jmir.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung L.M., Law Q.P., Fong S.S., Chung J.W. Teledietetics improves weight reduction by modifying eating behavior: a randomized controlled trial. Telemed J E Health. 2014;20(January (1)):55–62. doi: 10.1089/tmj.2013.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drion I., Pameijer L.R., van Dijk P.R., Groenier K.H., Kleefstra N., Bilo H.J. The effects of a mobile phone application on quality of life in patients with type 1 diabetes mellitus: a randomized controlled trial. J Diabetes Sci Technol. 2015;9(May (5)):1086–1091. doi: 10.1177/1932296815585871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell B.L., Smith A.E., Rowlands A.V. Promoting physical activity in rural Australian adults using an online intervention. J Sci Med Sport. 2019;22(January (1)):70–75. doi: 10.1016/j.jsams.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Baron N.S., Calixte R.M., Havewala M. The persistence of print among university students: an exploratory study. Telemat Inform. 2017;34(August (5)):590–604. [Google Scholar]
- 39.Jones K.D., Burckhardt C.S., Bennett J.A. Motivational interviewing may encourage exercise in persons with fibromyalgia by enhancing self efficacy. Arthritis Rheum. 2004;51(October (5)):864–867. doi: 10.1002/art.20684. [DOI] [PubMed] [Google Scholar]
- 40.Taal E., Rasker J.J., Wiegman O. Patient education and self-management in the rheumatic diseases: a self-efficacy approach. Arthritis Care Res. 1996;9(June (3)):229–238. doi: 10.1002/1529-0131(199606)9:3<229::aid-anr1790090312>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 41.Lally P., van Jaarsveld C.H.M., Potts H.W.W., Wardle J. How are habits formed: modelling habit formation in the real world. Eur J Soc Psychol. 2010;40(6):998–1009. [Google Scholar]
- 42.Coleman M.T., Newton K.S. Supporting self-management in patients with chronic illness. Am Fam Phys. 2005;72(October (8)):1503–1510. [PubMed] [Google Scholar]