Abstract
Objectives
The purpose of this systematic review of the literature is to investigate which of the epoxy-based cements and those based on Tricalciumsilicate (MTA, Bioceramic) have the best sealing capacity through the analysis of studies that have provided a survey model in vitro of bacteria leakage.
Source
The articles were identified using electronic databases such as PubMed, Scopus, the search was conducted between 8.12.2020 and 31.12.2020 and a last search was conducted on 2.12.2021.
Study selection
678 records were identified and after removing the duplicates we obtain 481 records, with the first phase of screening and selection of records we reached 204 and with the application of the inclusion and exclusion criteria we selected 31 articles, only 9 studies made a direct comparison between the two endodontic cement categories and presented data that could be included in the metaanalysis.
Data
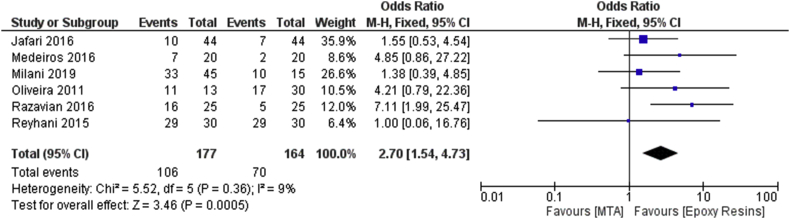
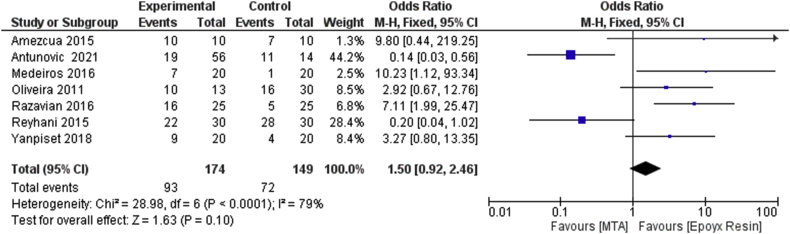
The meta-analysis of first outcome shows an odds ratio of 2.70 C.I.(Confidence Interval) [1.54, 4.73], the test for overall effect has a p value = 0.0005 with a heterogeneity index of I 2 of 9%; The second outcome meta-analysis shows an Odds Ratio of 1.50 C.I. (Confidence Interval) [0.92, 2.46] with a p value of 0.10 with an I 2 of 79%.
Conclusion
the sealing ability is higher for epoxy resins than for tricalcium silicate-based cements, for observation periods longer than 90 days.
Clinical relevance
The knowledge of the cement that determines the best sealing ability and resistance to microbial leakage, can be of help for the dentist who has to face clinical situations such as endodontic retreatments whose failure is determined by the persistence of bacteria in the endodontic canals.
Keywords: Sealing ability, Microbial leakage, Endodontic, Epoxy resin, MTA
sealing ability; microbial leakage; endodontic; epoxy resin; MTA
1. Introduction
One of the fundamental objectives of endodontic treatment is to establish a seal, which is as durable and predictable as possible over time, surrounding the apical region at the root of a tooth, ensuring the coronal seal is guaranteed by definitive restorations or crowns. Cements and resin or gutta percha cones are used to establish an apical seal, and it is essential that these materials have excellent sealing ability and resistance to microbial leakage [1, 2]. According to Komabayashi et al. (2020), the cementitious materials used in endodontics can be classified as follows: zinc oxide-eugenol, salicylate and tricalcium silicate (MTA and bioceramics), zinc oxide-fatty acid, glass ionomer, silicone, epoxy resin, and methacrylate resin. Recently, tricalcium silicate-based cements (MTA and bioceramics) have received a lot of attention for their high biocompatibility with osteo-inductive regenerative properties [3].
In addition to biocompatibility, effective sealing ability is fundamental for endodontic cements. The sealing ability of endodontic cements has been investigated using various methods including fluid infiltration [4, 5], radioisotope [6, 7], dye penetration [8], and loss of bacteria [8, 9]. Among the most commonly used methods, and the method clinically closest to the cause of endodontic failure, is a model based on the release of microorganisms such as Enterococcus faecalis, which is one of the microorganisms involved in secondary endodontic infections [10].
Recent studies on the sealing ability of tricalcium silicate-based cements (MTA and bioceramics) have shown non-superior performance compared to other endodontic cements. For example, Yanpiset et al. (2018) reported no statistically significant difference in bacterial leakage between a bioceramic sealant and epoxy resin, while Jafari et al. (2016) published an in vitro study on sealing ability and concluded that epoxy resin showed the lowest bacterial leakage as compared with MTA [11, 12]. Among the studies in the literature, there are some discrepancies in the results on the sealing ability of different cements. Since the epoxy- and tricalcium silicate-based cements (MTA and bioceramics) are different in their composition and biological properties, we decided to ask the following review question: Which of these two endodontic cements, epoxy- or tricalcium silicate-based cements, had the best sealing ability in an in vitro model of bacterial leakage?
The compositions of the two main epoxy resin-based cements, as reported by Komabayashi et al. (2020) are as follows: for AH-26 (bismuth oxide, hexamethyleneteramine, silver powder, titanium oxide, and bisphenol A diglycidyl ether) and for AH Plus (bisphenol A-based epoxy resin, zirconium oxide, bisphenol F based-epoxy resin, calcium tungstate, iron oxide, silica, N, N-dibenzyl-5-oxanonadiamin-1,9, amantiameamine, tricyclodecane-diamine, calcium tungstate, and zirconium oxide) [3].
The direct antimicrobial effects for epoxy resin-based cements, seem to be slightly lower as compared with those based on zinc oxide-eugenol [13]. In addition, higher cytotoxicity has been found towards fibroblasts as compared with other types of cements, along with a genotoxic effect for AH-26 due to the release of formaldehyde, which has not been found for AH Plus [14]; however, biocompatibility is higher than in zinc oxide-eugenol-based cements [15].
The composition of the main tricalcium silicate-based cements (MTA and bioceramics) are as follows: MTA Fillapex (Angelus, Brazil): methyl salicylate, butylene glycol, colophony, bismus trioxide, fumed silica, titanium dioxide, Paste B fumed silica, titanium dioxide, tricalcium silicate, dicalcium silicate, calcium oxide, tricalcium alminate, pentaerythritol rosinate, and p-toluenesulfide; Total Fill BC: zirconium oxide, calcium silicates, calcium phosphate, calcium hydroxide, filler, thickening agents; BioRoot RCS: tricalcium silicate, zirconium oxide, and aqueous solution of calcium chloride. Some authors do not consider MTA Fillapex to be a tricalcium silicate because its composition contains resin; in fact, Komabayashi places it among the silicates [3], in contrast to many other authors [16, 17].
Studies on the antimicrobial effects of tricalcium silicate-based cements (MTA and bioceramics) have reported differing results. Torabinejad et al. reported an antimicrobial effect on facultative bacteria but not on facultative anaerobes. Tanomaru-Filho et al. (2007) reported antimicrobial activity similar to other cements [18], while Estrela did not report any antimicrobial activity directed towards E. faecalis, S. aureus, C. albicans, and B. subtilis [19]. The antimicrobial effect seemed to depend on an increase in pH and the release of calcium hydroxide ions [20]. Most of the studies are in agreement in establishing an excellent biocompatibility superior to many other classes of tricalcium silicate-based cements with osteo-inductive properties [21].
Our hypothesis is that between the two types of cements (epoxy- and tricalcium silicate-based), there are differences in the ability to seal the apical region of a tooth and in the resistance to bacterial infiltration.
2. Materials and methods
This study was conducted with reference to the guidelines described by the preferred reporting items for systematic review and meta-analysis (PRISMA) [22].
The following PICO framework was followed: participants—root apexes of extracted teeth sealed with endodontic cements; intervention—sealing of the apical third in an in vitro model with bacterial infiltration; comparison—closure of the apical third of the tooth with 2 different types of endodontic cements (tricalcium silicate- and epoxy resin-based); outcome—sealing ability of cements measured through the odds ratio of infiltrated and non-infiltrated samples between the 2 types of endodontic cements (tricalcium silicate- and epoxy resin-based cements).
In this study, we aimed to answer the following PICO question: Which of the 2 types of endodontic cements, i.e., tricalcium silicate- or epoxy resin-based, has the best sealing ability in an in vitro model with bacterial infiltration, based on calculating the odds ratio between infiltrated and non-infiltrated samples, during the meta-analysis of the extracted data?
On the basis of the title and the abstract of numerous scientific studies, all in vitro and ex vivo studies on the sealing ability of endodontic cements based on a bacterial leakage model were considered to be potentially admissible.
The exclusion criteria applied to the studies were the following: not written in English, with infiltration measurement for a period less than 60 days, not ex vivo or in vitro models, and data not provided on the number of infiltrated samples at the end of the experimental observation period. Clinical cases, clinical trials, reviews (the reviews were considered as sources of bibliographic information, studied, and analyzed in a preliminary phase so as not to repeat a systematic review already performed by previous authors), were excluded from this systematic review.
The inclusion criteria applied to the studies were as follows: All in vitro and ex vivo studies that report data on the number of infiltrated samples for a period of at least 60 days for both types of endodontic cement and which present a low risk of bias. The inclusion and exclusion criteria are summarized in Table 1. The articles deemed suitable were read and analyzed in order to include them in the qualitative and quantitative analyses.
Table 1.
Inclusion and exclusion criteria.
| Category | Exclusion Criteria | Inclusion Criteria |
|---|---|---|
| Publication Language | Not English | English |
| Study types | Review, Systematic review, case report, case series, Clinical Study, study in vitro not ex vivo. | Study in vitro ex vivo1, performed on a bacterial leakage study model. |
| data characteristics | Report data over a period of less than 60 days. | Report data on the number of infiltrated samples in a period of over 60 days, report data on both types of cement (Tricalciumsilicate and epoxy resins). |
| Risk of Bias | High risk of bias. | Medium o low risk of bias. |
Performed on extracted teeth.
2.1. Research methodology
The articles were identified using electronic databases such as PubMed and Scopus. The search was conducted between 8 and 31 December 2020 and the final search was conducted on 1 February 2021.
All keywords used and the related database search details are explicitly shown in Table 2.
Table 2.
Overview of the search methodology; Records identified by databases:678, 485 after removing overlaps. Articles included in meta-analysis: 9.
| Database - Provider | Key words | Search Details | Number of records | articles After removing overlaps articles | remaining articles that dealt with the issue of sealing ability for endodontic cements under review | Article remaining after applying the inclusion and exclusion criteria | Articles included in meta-analysis |
|---|---|---|---|---|---|---|---|
| Pub med | "epoxy resin sealer" | "epoxy resin sealer"[All Fields] | 53 | ||||
| Pub med | calcium silicate sealer | ("calcium silicate"[Supplementary Concept] OR "calcium silicate"[All Fields]) AND ("sealer"[All Fields] OR "sealers"[All Fields]) Translations calcium silicate: "calcium silicate"[Supplementary Concept] OR "calcium silicate"[All Fields] sealer: "sealer"[All Fields] OR "sealers"[All Fields] | 181 | ||||
| Scopus | epoxy resin sealer" | TITLE-ABS-KEY (“epoxy resin sealer”) | 77 | ||||
| Scopus | calcium silicate sealer | TITLE-ABS-KEY (“calcium silicate sealer”) | 17 | ||||
| Pub med | bioceramic AND endodontic | ("bioceramic"[All Fields] OR "bioceramics"[All Fields]) AND ("endodontal"[All Fields] OR "endodontic"[All Fields] OR "endodontical"[All Fields] OR "endodontically"[All Fields] OR "endodontics"[MeSH Terms] OR "endodontics"[All Fields]) Translations bioceramic: "bioceramic"[All Fields] OR "bioceramics"[All Fields] endodontic: "endodontal"[All Fields] OR "endodontic"[All Fields] OR "endodontical"[All Fields] OR "endodontically"[All Fields] OR "endodontics"[MeSH Terms] OR "endodontics"[All Fields] |
226 | ||||
| Scopus | bioceramic AND endodontic | TITLE-ABS-KEY (bioceramic AND endodontic) | 124 | ||||
| Web of science | epoxy resin sealer | You searched for: TOPIC: (epoxy resin sealer) Timespan: All years. Indexes: SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC. |
337 | ||||
| Web of science | You searched for: TOPIC: (bioceramic AND endodontic) Timespan: All years. Indexes: SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC. |
115 | |||||
| 1130 | 481 | 204 | 31 | 9 |
The research methodology was carried out in 4 phases.
In the first phase, the method for identifying the records was chosen taking into consideration the following points:
-
a.
Choice of 2 reviewers with the task of identifying records and a 3rd reviewer with the task of resolving doubtful situations;
-
b.
Choice of databases and providers;
-
c.
Choice of keywords;
-
d.
Decisions on inclusion and exclusion criteria.
The second phase involved the identification of the records on the databases (the duplications were removed through the use of EndNote 9 software), the screening of potentially eligible articles (through an analysis of the title and abstract), and the choice of articles to be included in the meta-analysis.
The third phase involved the comparison of the studies identified by the 2 independent reviewers and the choice of articles to be included in the meta-analysis (the k-agreement between the 2 reviewers was 0.84). During this phase, it was decided to also perform an additional meta-analysis data analysis which included an observation period of the tested samples of 90 days.
The fourth phase involved the extraction of data by the 2 reviewers independently with subsequent comparison of the extracted data.
The data sought in the studies by the two reviewers concerned the total number of samples with microbial leaking for the 2 types of endodontic cements (one type based on tricalcium silicate and the other type based on epoxy resins).
2.2. Statistical analysis protocol
The protocol for the meta-analysis was conducted based on the guidelines from the Cochrane Handbook for Systematic Reviews of Interventions. The program used to perform the meta-analysis was Reviewer Manager 5.3 (Cochrane Collaboration, Copenhagen, Denmark). The odds ratio between the two types of endodontic cements was measured taking into consideration the number of total samples and the number of samples with microbial leakage for each study included in the meta-analysis. The presence of heterogeneity was measured with the Higgins index (I2); values above 50% were considered to be heterogeneous. The risk of bias within the studies was assessed following the PRISMA guidelines for assessing the quality of studies in meta-analyses. The meta-analysis results were graphically depicted using a forest plot and the heterogeneity results using a funnel plot.
3. Results
From the searches in the PubMed, Scopus, and Web of Science databases, 1130 articles were initially identified. EndNote software was used to remove duplicates with 481 articles remaining. After the first phase of screening and selection of records, we identified 204 articles, and after the application of the inclusion and exclusion criteria, we selected 31 articles. There were only nine studies that made a direct comparison between the two types of endodontic cements and presented data that could be included in the meta-analysis.
We included the following nine articles in the meta-analysis:
-
•
six articles for Outcome 1, i.e., all studies reporting data on a number of infiltrated samples for a period of at least 90 days;
-
•
seven articles for Outcome 2, i.e., all studies reporting data on a number of infiltrated samples for a period of at least 60 days.
All selection and screening procedures are described in the flowchart shown in Figure 1.
Figure 1.
PRISMA 2009 flow diagram.
3.1. Study characteristics and data extraction
The included studies for the quantitative analysis were: For Outcome 1, Jafari (2016) [12], Medeiros (2016) [23], Milani (2019) [24], Oliveira (2011) [25], Razavian (2016) [26], and Reyhani (2015) [27]; for Outcome 2, Yanpiset (2018) [11], Amezcua (2015) [28], Medeiros (2016) [23], Oliveira (2011) [25], Razavian (2016) [26], Reyhani (2015) [27], and Antunovic (2021) [29].
The extraction of the data and the methods in which they have been reported follow the indications of the Cochrane Handbook for Systematic Reviews of Interventions.
The extracted data included the journal information (first author, data, and reference); the type of endodontic cement investigated, the number of samples examined, the number of samples showing bacterial infiltration with the elapsed time period, and the bacterial species used. If the data were reported as a percentage, the number was calculated through the use of proportions (Table 3).
Table 3.
Number of total and leaked samples, based on the time elapsed (10–120 days). MTA-PG: Mineral trioxide aggregate, Propylene glycole; AH26 (Dentsply, DeTrey, Konstanz, Germany) and MTA Fillapex (Angelus, Londrina, Brazil), Apatite Root Canal Sealer (Sankin-Kogyo, Tokyo, Japan), CPM: Portland cement (EGEO SLR, MTM Argentina SA, Buenos Aires, Argentina), MTA (Angelus, Londrina, Paraná, Brazil), MBPc (University of São Paulo, Brazil, epoxy resin sealer containing calcium hydroxide), AH Plus (Dentsply DeTrey, Konstanz, Germany), Resilon (Real Seal®, Sybron Endo, Glendora, USA), Super-EBA (Harry J. Bosworth, Skokie, IL, USA), ProRoot MTA (Dentsply Sirona), AHP: AH Plus, ESE: Epiphany SE (Pentron Clinical Technologies, LLC., Wallingford, CT, USA); SEL: Sealapex (Kerr Corp., CA, USA); AGP: Activ GP (Brasseler USA, Dental Instrumentation, USA); EDF: Endofill, (Dentsply Industria e Comercio Ltda, Petropolis, RJ, Brazil); CPM: Endo CPM Sealer (EGEO S.R.L. under license of MTM Argentina S.A., Buenos Aires, Argentina); MTAS: MTA Sealer (Araraquara Dental School, UNESP, Brazil); BCS: bioceramic sealer (Totalfill BC Sealer, FKG Dentaire SA, La Chaux-de-Fonds, Switzerland); GP: gutta percha; BCC: bioceramic-impregnated gutta percha cone; BioRoot RCS: BioRoot root canal sealer (Septodont, Saint Maur-des-Fosses, France).
| First author, data, reference | endodontic cements tested | number of samples | number of samples with leakage |
Bacteria | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 120 days | |||||
| Milani, 2019 [4] | MTA-PG in dry canals | 15 | 45 | 7 | Enterococcus faecalis | |||||||||
| MTA-PG in wet canals | 15 | 12 | ||||||||||||
| MTA fillapex | 15 | 14 | ||||||||||||
| AH26 | 15 | 15 | 10 | |||||||||||
| Jafari 2016 [2] | AH26 | 44 | 44 | 7 | Enterococcus faecalis | |||||||||
| MTA fillapex | 44 | 44 | 10 | |||||||||||
| Apatite Root Canal Sealer | 44 | 11 | ||||||||||||
| Medeiros 2016 [3] | white MTA, | 20 | 7 | 7 | 7 | 7 | Enterococcus faecalis | |||||||
| CPM, | 20 | 4 | 4 | 4 | 4 | |||||||||
| MBPc | 20 | 1 | 1 | 2 | 2 | |||||||||
| Amezcua 2015 [9] | SuperEBA | 10 |
Staphylococcus aureus Enterococcus faecalis, Pseudomonas aeruginosa, Bacillus subtilis, Candida albicans. |
|||||||||||
| RealSeal® thermoplasticized | 10 | 9 | ||||||||||||
| ProRoot® MTA | 10 | 10 | ||||||||||||
| Thermoplasticized gutta-percha + AH Plus® | 10 | 7 | ||||||||||||
| Oliveira 2011 [5] | AHP | 15 | 30 | 5 | 7 | 8 | 9 | Enterococcus faecalis | ||||||
| S26 | 15 | 8 | 9 | 9 | 10 | |||||||||
| ESE | 15 | 4 | 9 | 11 | 12 | |||||||||
| SEL | 15 | 7 | 7 | 8 | 11 | |||||||||
| AGP | 15 | 6 | 11 | 14 | 14 | |||||||||
| EDF | 14 | 7 | 7 | 11 | 12 | |||||||||
| CPM | 15 | 9 | 10 | 12 | 13 | |||||||||
| MTAS | 13 | 13 | 8 | 10 | 11 | 12 | ||||||||
| Razavian 2016 [6] | AH 26 | 25 | 5 | Enterococcus faecalis | ||||||||||
| MTA Fillapex | 25 | 16 | ||||||||||||
| Reyhani 2015 [7] | AH Plus | 15 | 11 | 12 | 14 | 14 | 14 | 14 | 14 | 15 | 15 | Enterococcus faecalis | ||
| AH Plus post | 15 | 11 | 12 | 14 | 14 | 14 | 14 | 15 | 15 | 15 | ||||
| MTA Fillapex | 15 | 7 | 8 | 8 | 9 | 9 | 11 | 13 | 14 | 15 | ||||
| MTA Fillapex post | 15 | 6 | 8 | 9 | 10 | 10 | 11 | 14 | 15 | 15 | ||||
| Yanpiset 2018 [8] | GP/AH plus | 20 | 4 | Enterococcus faecalis | ||||||||||
| BCC/AH plus | 20 | 9 | ||||||||||||
| GP/BCS | 20 | 9 | ||||||||||||
| BCC/BCS | 20 | 5 | ||||||||||||
| Antunovic 2021 [10] | BCS | 14 | 56 | 0 | 2 | Enterococcus faecalis | ||||||||
| BioRoot RCS | 14 | 1 | 5 | |||||||||||
| MTA Fillapex | 14 | 2 | 5 | |||||||||||
| MTA Plus | 14 | 5 | 7 | |||||||||||
| AH Plus | 14 | 14 | 1 | 11 | ||||||||||
3.2. Risk of bias
The risk of bias was assessed based on the Checklist for Reporting In vitro Studies (CRIS) guidelines [30] proposed to evaluate in vitro dental studies. The results are shown in Table 4; each type of cement was assigned a value from one to five (where one = low quality and five = high quality). The questions that the reviewers answered by assigning a score were the following:
-
1.
For the sample size calculation, “Is the sample size adequate for obtaining statistically significant results?”
-
2.
For meaningful difference between groups, “Has the ‘meaningful difference’ measurement been set correctly in the groups taking into account the sample size and the type of measurement?”
-
3.
For sample preparation and handling, “Does the study describe information on the production or handling of the samples to be tested?”
-
4.
For allocation sequence, randomization, and blinding, “Did the samples have equal and independent possibility of a sample entering any group?”
-
5.
For statistical analysis, “Are the statistical methods described?”
Table 4.
Assessment of the risk of bias within the studies, with scores 7 to 12 = low quality, 13 to 20 = intermediate quality, and 21 to 25 = high quality.
| First Author, Data | Sample size calculation | Meaningful difference between groups | Sample preparation and handling | Allocation sequence, randomization and blinding | Statistical analysis | Score | outcome |
|---|---|---|---|---|---|---|---|
| Milani, 2019 [4] | 3 | 3 | 4 | 3 | 4 | 17 | 1 |
| Jafari 2016 [2] | 4 | 3 | 3 | 3 | 4 | 17 | 1 |
| Medeiros 2016 [3] | 4 | 3 | 4 | 3 | 3 | 17 | 1, 2 |
| Amezcua 2015 [9] | 2 | 3 | 4 | 4 | 4 | 17 | 2 |
| Oliveira 2011 [5] | 3 | 3 | 3 | 2 | 3 | 14 | 1, 2 |
| Razavian 2016 [6] | 4 | 3 | 4 | 4 | 2 | 17 | 1, 2 |
| Reyhani 2015 [7] | 3 | 3 | 4 | 3 | 3 | 16 | 1,2 |
| Yanpiset 2018 [8] | 4 | 3 | 4 | 3 | 4 | 18 | 2 |
| Antunovic 2021 [10] | 3 | 3 | 4 | 3 | 4 | 17 | 2 |
Studies presenting a high risk of bias were not included in the meta-analysis and were eliminated during the inclusion phase (Table 4). The assessment of the risk of bias of the nine included articles was conducted by M.D.
The risk of bias among the studies for Outcome 1 is considered to be low; the heterogeneity showed a value represented by I2 (Higgins's index) of 9% (heterogeneity values greater than 50% are considered to be high) with a p-value < 0.36 from the Chi-squared test. The high heterogeneity is also confirmed by the funnel plot in Figure 2.
Figure 2.
Funnel plots of the evaluation of heterogeneity for first outcome.
The risk of bias among the studies for Outcome 2 is medium; the heterogeneity between the studies is considered to be high and the I2 index stands at a value of 79%b. A visual analysis of the funnel plot shows a study as a probable source of heterogeneity and bias between the studies (Reyhani (2015) [27] and Antunovic (2021) [29]) Figure 3. Therefore, we decided to perform a sensitivity analysis with an assessment of the confidence intervals of the individual studies to confirm the sources of heterogeneity.
Figure 3.
Funnel plot for the secondary outcome. The arrows highlight the sources of heterogeneity (I2 79%) Antunovic 2021, Reyhani 2015.
3.3. Meta-analysis
Statistical data analysis was performed using Rev Manager 5.4 (Cochrane Collaboration, Copenhagen, Denmark). The results were represented by forest plots.
The meta-analysis for Outcome 1 was conducted by applying fixed effects models given the low rate of heterogeneity (I2 = 9%). The meta-analysis shows an odds ratio of 2.70, with a confidence interval (CI) (1.54, 4.73). The test for the overall effect has a p-value = 0.0005 with an I2 of 9%. The forest plot presents a diamond positioned in favor of epoxy resin-based cements with a lower probability ratio of leakage for the tested samples after 90 days (Outcome 1) with 70 samples showing leakage as compared with a total of 164 samples (Figure 4).
Figure 4.
Forest plot of the fixed effects model of the meta-analysis of the first outcome.
The meta-analysis for Outcome 2 shows an odds ratio of 1.50, confidence interval (CI) (0.92, 2.46), a p-value of 0.10, with an I2 of 79%. The forest plot is in favor of epoxy resin-based cements as compared with the MTA group but not in a statistically significant way, in fact, the central rhombus intersects the line of no effect (Figure 5). As a result that the heterogeneity was high, we decided to perform a sensitivity analysis in search of heterogeneity sources. From the visual analysis of the confidence intervals of the forest plot, it emerges that there is a poor overlap of the confidence intervals for the Reyhani (2015) [27] and Antunovic (2021) [29] studies (confirmed by the funnel plot).
Figure 5.
Forest plot of the fixed effects model of the meta-analysis of secondary outcome.
By selectively removing the Reyahani (2015) and Antunovic (2021) studies, the heterogeneity (I2) goes from 79% to 0% and the forest plot still remains in favor of epoxy resin-based cements in a statistically significant way with an odds ratio of 5.05, CI(2 .46, 10.37), and p-value < 0.00001 (Figure 6).
Figure 6.
Sensitivity analysis: Forest plot of the meta-analysis of the secondary outcome; the arrows indicate the sources of heterogeneity that are identified by the funnel plot and are also evident on the forest plot.
4. Discussion
In the field of endodontics, one of the fundamental objectives for success is the achievement of an apical seal after a root canal, which must be guaranteed by the good seal of the material used.
All the studies that included a bacterial infiltration model involved the use of Enterococcus faecalis with the exception of Amezcua et al. [28], who also used other bacterial species.
The leakage model, in all included studies, involved the use of roots of mono-root elements sealed with endodontic cements, with the apex immersed from 2 to 4 mm in 5–10 mL of brain heart infusion (BHI), sterilized, sealed, and incubated at 37 °C from 24 h up to 3 days. Subsequently, Enterococcus faecalis was inserted and if there was leakage it was visualized through the variation of clarity of the BHI. The use of Enterococcus faecalis represents a proven and standardized model in several studies; furthermore, this bacterium has the ability to withstand concentrations of sodium hypochlorite at concentrations higher than 1% and is found mainly in persistent endodontic lesions [31, 32].
Razavian et al., among the studies included in the meta-analysis for both outcomes, reported statistically significant data in favor of epoxy resins; in fact, in the forest plot, the line representing the confidence intervals does not intersect the no effect line. Razavian concluded that AH-26 has a greater sealing ability as compared with MTA Fillapex. In agreement with the data of this study [26], Medeiros et al. [23] also reported statistically significant data in favor of epoxy resins (MBPc) as compared with MTA. Data in partial agreement with these studies but with no statistically significant results as reported by the forest plots of the two outcomes are the studies by Oliveira et al. (Outcomes 1 and 2) [25], Milani et al. and Jafary et al. (Outcome 1) [24], and Amezcua et al. and Yanpiset et al. (Outcomes 1 and 2) [11,28].
Oliviera et al. compared the 2 types of cements and also cements based on methacrylates and zinc oxide-eugenol, for a period up to 120 days, and concluded that the cements with the best sealing ability were AH Plus and Sealapex, while MTA was the cement that presented the worst sealing ability [25]. Data in partial contrast come from Reyhani et al. [27] who reported for both classes of cement an equal resistance to leakage.
Data from a 90-day sample observation period, from the studies included in the meta-analysis, report an odds ratio of 2.70 and CI [1.54, 4.73] with 70 samples showing leakage out of 164 samples for resin epoxy-based dements and 106 samples out of 177 samples for the MTA group.
These data are in line with Outcome 2, which measured the odds ratio of leaked and non-leaked samples of MTA and epoxy resins over an observation period of only 60 days, a shorter time period than Outcome 1, (odds ratio, 1.50; CI [0.92, 2.46]) with 72 leaked samples out of 149 samples for the epoxy resin-based cements and 93 leaked samples out of 174 samples for the MTA group.
Studies conducted on other models for testing the sealing ability have reported data in partial agreement with our review. Meidyawati et al. conducted a penetration study using inks on extracted teeth and compared mineral trioxide aggregate and resin epoxy sealer. They concluded that the sealing ability of MTA was lower than RES [33].
Microleakage studies using fluid infiltration models have presented data with non-statistically significant differences between epoxide resins and calcium silicate-based cements (MTA group) [34], in agreement with the data from the forest plot for Outcome 2.
Shourgashti (2018) [35] measured microleakage using a fluid transport model described by Wu et al. [36] and reported that the sealing ability of HealApex was comparable to that of AH-26, while, in the long term, the sealing of HealApex was based on epoxy sealant. Conclusions also in agreement with Amoroso-Silva et al., who compared resin-based cements (MBPc and S26) and calcium silicate-based cements (MTA and Portland cement), concluded that calcium silicate-based cements showed similar fluid filtration [34].
Asawaworarit et al. (2016) [37] reported that MTA Fillapex® had significantly more leakage than AH Plus® at 7 days, but at 4 weeks, MTA Fillapex® showed significantly better sealing ability than AH Plus® (p < 0.05). A study conducted by Ersahan et al. (2013) reported no difference between AH Plus and iRoot SP in terms of apical sealing capacity [38].
In addition, Gandolfi et al. (2010), in a study of sealing ability through the fluid flow meter apparatus described above, found no statistically significant difference between MTA and AH Plus [39].
Sönmez et al. (2012) reported that the sealing abilities of AH Plus and MTA were similar, while MTA Fillapex showed more micro-infiltrations than the other two materials [40].
The data on sealing ability are not clearly in favor of epoxy resin-based cements, especially if the study models that have foreseen the measurement by fluid filtration are taken into consideration, while for the bacterial models of microleakage, there is more agreement than the data above when the model foresees a measurement time of at least 90 days.
One limitation of this systematic review is the intermediate level (risk of bias) of the studies included in the review. The intermediate value was mainly caused by the unclear text of the articles in the allocation sequence, randomization, and blinding (risk of bias, Table 4 column 5) and gives a number of samples that are not always adequate to support a robust statistical analysis (risk of bias, Table 4 column 2).
5. Conclusion
We can conclude that, even with the limitations of this study, the sealing abilities of epoxy resin-based cements, based on a bacterial micro-infiltration model, are higher than those of tricalcium silicate-based cements for observation periods longer than 90 days.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Nawal R.R., Parande M., Sehgal R., Rao N.R., Naik A. A comparative evaluation of 3 root canal filling systems. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011;111:387–393. doi: 10.1016/j.tripleo.2010.09.070. [DOI] [PubMed] [Google Scholar]
- 2.Toledano M., Osorio R., Vallecillo-Rivas M., Osorio E., Lynch C.D., Aguilera F.S., Toledano R., Sauro S. Zn-doping of silicate and hydroxyapatite-based cements: dentin mechanobiology and bioactivity. J. Mech. Behav. Biomed. Mater. 2021;114:104232. doi: 10.1016/j.jmbbm.2020.104232. [DOI] [PubMed] [Google Scholar]
- 3.Komabayashi T., Colmenar D., Cvach N., Bhat A., Primus C., Imai Y. Comprehensive review of current endodontic sealers. Dent. Mater. J. 2020;39:703–720. doi: 10.4012/dmj.2019-288. [DOI] [PubMed] [Google Scholar]
- 4.Asawaworarit W., Pinyosopon T., Kijsamanmith K. Comparison of apical sealing ability of bioceramic sealer and epoxy resin-based sealer using the fluid filtration technique and scanning electron microscopy. J. Dent. Sci. 2020;15:186–192. doi: 10.1016/j.jds.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitagawa H., Kitagawa R., Tsuboi R., Hirose N., Thongthai P., Sakai H., Ueda M., Ono S., Sasaki J.I., Ooya T. Development of endodontic sealers containing antimicrobial-loaded polymer particles with long-term antibacterial effects. Dent. Mater. : Offic. Publ. Acad. Dent. Mat. 2021 doi: 10.1016/j.dental.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Moloney L.G., Feik S.A., Ellender G. Sealing ability of three materials used to repair lateral root perforations. J. Endod. 1993;19:59–62. doi: 10.1016/S0099-2399(06)81195-7. [DOI] [PubMed] [Google Scholar]
- 7.Wu M.K., Wesselink P.R. Endodontic leakage studies reconsidered. Part I. Methodology, application and relevance. Int. Endod. J. 1993;26:37–43. doi: 10.1111/j.1365-2591.1993.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 8.Džanković A., Hadžiabdić N., Korać S., Tahmiščija I., Konjhodžić A., Hasić-Branković L. Sealing ability of mineral trioxide aggregate, biodentine and glass ionomer as root-end materials: a question of choice. Acta Med. Acad. 2020;49:232–239. doi: 10.5644/ama2006-124.312. [DOI] [PubMed] [Google Scholar]
- 9.Reddy N.V., Srujana P., Daneswari V., Konyala H.R., Mareddy A.R., Mohammad N. Sealing ability of MTA vs Portland cement in the repair of furcal perforations of primary molars: a dye extraction leakage model-an in vitro study. Int. J. Clin. Pediat. Dent. 2019;12:83–87. doi: 10.5005/jp-journals-10005-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abedi-Amin A., Luzi A., Giovarruscio M., Paolone G., Darvizeh A., Agulló V.V., Sauro S. Innovative root-end filling materials based on calcium-silicates and calcium-phosphates. J. Mater. Sci. Mater. Med. 2017;28:31. doi: 10.1007/s10856-017-5847-1. [DOI] [PubMed] [Google Scholar]
- 11.Yanpiset K., Banomyong D., Chotvorrarak K., Srisatjaluk R.L. Bacterial leakage and micro-computed tomography evaluation in round-shaped canals obturated with bioceramic cone and sealer using matched single cone technique. Restorat. Dent. Endodont. 2018;43:e30. doi: 10.5395/rde.2018.43.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jafari F., Sobhani E., Samadi-Kafil H., Pirzadeh A., Jafari S. In vitro evaluation of the sealing ability of three newly developed root canal sealers: a bacterial microleakage study. J. Clin. Exp. Dent. 2016;8:e561–e565. doi: 10.4317/jced.52992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carvalho N.K., Barbosa A.F.A., Coelho B.P., Gonçalves L.S., Sassone L.M., Silva E. Antibacterial, biological, and physicochemical properties of root canal sealers containing chlorhexidine-hexametaphosphate nanoparticles. Dent. Mater. : Offic. Publ. Acad. Dent. Mat. 2021;37:863–874. doi: 10.1016/j.dental.2021.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Athanassiadis B., George G.A., Abbott P.V., Wash L.J. A review of the effects of formaldehyde release from endodontic materials. Int. Endod. J. 2015;48:829–838. doi: 10.1111/iej.12389. [DOI] [PubMed] [Google Scholar]
- 15.Oh H., Kim E., Lee S., Park S., Chen D., Shin S.J., Kim E., Kim S. Comparison of biocompatibility of calcium silicate-based sealers and epoxy resin-based sealer on human periodontal ligament stem cells. Materials (Basel) 2020;13 doi: 10.3390/ma13225242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoshino R.A., Delfino M.M., da Silva G.F., Guerreiro-Tanomaru J.M., Tanomaru-Filho M., Sasso-Cerri E., Cerri P.S. Biocompatibility and bioactive potential of the NeoMTA Plus endodontic bioceramic-based sealer. Restorat. Dent. Endodont. 2020;46 doi: 10.5395/rde.2021.46.e4. e4-e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinho J.P., França S., Paulo S., Paula A.B., Coelho A.S., Abrantes A.M., Caramelo F., Carrilho E., Marto C.M., Botelho M.F. Effect of different irrigation solutions on the diffusion of MTA cement into the root canal dentin. Materials (Basel, Switzerland) 2020;13:5472. doi: 10.3390/ma13235472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanomaru-Filho M., aacute; rio, Tanomaru J.M.G., Barros D.B., Watanabe E., Ito I.Y. In vitro antimicrobial activity of endodontic sealers, MTA-based cements and Portland cement. J. Oral Sci. 2007;49:41–45. doi: 10.2334/josnusd.49.41. [DOI] [PubMed] [Google Scholar]
- 19.Estrela C., Sydney G.B., Bammann L.L., Felippe Júnior O. Mechanism of action of calcium and hydroxyl ions of calcium hydroxide on tissue and bacteria. Braz. Dent. J. 1995;6:85–90. [PubMed] [Google Scholar]
- 20.Rajasekharan S., Vercruysse C., Martens L., Verbeeck R. Effect of exposed surface area, volume and environmental pH on the calcium ion release of three commercially available tricalcium silicate based dental cements. Materials (Basel) 2018;11 doi: 10.3390/ma11010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gandolfi M.G., Iezzi G., Piattelli A., Prati C., Scarano A. Osteoinductive potential and bone-bonding ability of ProRoot MTA, MTA Plus and Biodentine in rabbit intramedullary model: microchemical characterization and histological analysis. Dent. Mater. : Offic. Publ. Acad. Dent. Mat. 2017;33:e221–e238. doi: 10.1016/j.dental.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medeiros P.L., Bernardineli N., Cavenago B.C., Torres S.A., Duarte M.A., Bramante C.M., Marciano M.A. Sealing ability of MTA, CPM, and MBPc as root-end filling materials: a bacterial leakage study. J. Appl. Oral Sci. : revista FOB. 2016;24:148–152. doi: 10.1590/1678-775720130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milani A.S., Firuzi S., Barhaghi M.H.S., Shahi S., Abdollahi A.A. Evaluation of sealing abilitiy of mineral trioxide aggregate mixed with propylene glycol as a root canal sealer: a in vitro study. Dent. Res. J. 2019;16:216–220. [PMC free article] [PubMed] [Google Scholar]
- 25.Oliveira A.C., Tanomaru J.M., Faria-Junior N., Tanomaru-Filho M. Bacterial leakage in root canals filled with conventional and MTA-based sealers. Int. Endod. J. 2011;44:370–375. doi: 10.1111/j.1365-2591.2011.01852.x. [DOI] [PubMed] [Google Scholar]
- 26.Razavian H., Barekatain B., Shadmehr E., Khatami M., Bagheri F., Heidari F. Bacterial leakage in root canals filled with resin-based and mineral trioxide aggregate-based sealers. Dent. Res. J. 2014;11:599–603. [PMC free article] [PubMed] [Google Scholar]
- 27.Reyhani M.F., Ghasemi N., Rahimi S., Milani A.S., Barhaghi M.H., Azadi A. Apical microleakage of AH Plus and MTA Fillapex(R) sealers in association with immediate and delayed post space preparation: a bacterial leakage study. Minerva Stomatol. 2015;64:129–134. [PubMed] [Google Scholar]
- 28.Amezcua O., Gonzalez A.C., Borges A.H., Bandeca M.C., Estrela C.R., Estrela C. Sealing ability of root-end filling materials. J. Contemp. Dent. Pract. 2015;16:210–214. doi: 10.5005/jp-journals-10024-1663. [DOI] [PubMed] [Google Scholar]
- 29.Antunovic M., Vukmanovic L., Budimir A., Kabil E., Anic I., Bago I. Evaluation of sealing ability of four bioceramic root canal sealers and an epoxy resin-based sealer: an in vitro study. Saudi Endodont. J. 2021;11:66–72. [Google Scholar]
- 30.Krithikadatta J., Gopikrishna V., Datta M. CRIS Guidelines (Checklist for Reporting In-vitro Studies): a concept note on the need for standardized guidelines for improving quality and transparency in reporting in-vitro studies in experimental dental research. J. Conserv. Dent. 2014;17:301–304. doi: 10.4103/0972-0707.136338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomes B.P., Ferraz C.C., Vianna M.E., Berber V.B., Teixeira F.B., Souza-Filho F.J. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int. Endod. J. 2001;34:424–428. doi: 10.1046/j.1365-2591.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- 32.Frough-Reyhani M., Ghasemi N., Soroush-Barhaghi M., Amini M., Gholizadeh Y. Antimicrobial efficacy of different concentration of sodium hypochlorite on the biofilm of Enterococcus faecalis at different stages of development. J. Clin. Exp. Dent. 2016;8:e480–e484. doi: 10.4317/jced.53158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meidyawati R., Suprastiwi E. Comparison sealing ability of MTA sealer and resin epoxy sealer. J. Int. Dent. Med. Res. 2017;10:134–138. [Google Scholar]
- 34.Amoroso-Silva P.A., Marciano M.A., Guimaraes B.M., Duarte M.A., Sanson A.F., Moraes I.G. Apical adaptation, sealing ability and push-out bond strength of five root-end filling materials. Braz. Oral Res. 2014;28 doi: 10.1590/1807-3107bor-2014.vol28.0043. [DOI] [PubMed] [Google Scholar]
- 35.Shourgashti Z., Keshvari H., Torabzadeh H., Rostami M., Bonakdar S., Asgary S. Physical properties, cytocompatibility and sealability of HealApex (a novel premixed biosealer) Iran. Endod. J. 2018;13:299–304. doi: 10.22037/iej.v13i3.20188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu M.K., De Gee A.J., Wesselink P.R. Leakage of AH26 and Ketac-Endo used with injected warm gutta-percha. J. Endod. 1997;23:331–336. doi: 10.1016/S0099-2399(97)80417-7. [DOI] [PubMed] [Google Scholar]
- 37.Asawaworarit W., Yachor P., Kijsamanmith K., Vongsavan N. Comparison of the apical sealing ability of calcium silicate-based sealer and resin-based sealer using the fluid-filtration technique. Med. Princ. Pract. 2016;25:561–565. doi: 10.1159/000450577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ersahan S., Aydin C. Solubility and apical sealing characteristics of a new calcium silicate-based root canal sealer in comparison to calcium hydroxide-, methacrylate resin- and epoxy resin-based sealers. Acta Odontol. Scand. 2013;71:857–862. doi: 10.3109/00016357.2012.734410. [DOI] [PubMed] [Google Scholar]
- 39.Gandolfi M.G., Prati C. MTA and F-doped MTA cements used as sealers with warm gutta-percha. Long-term study of sealing ability. Int. Endod. J. 2010;43:889–901. doi: 10.1111/j.1365-2591.2010.01763.x. [DOI] [PubMed] [Google Scholar]
- 40.Sönmez I.S., Oba A.A., Sönmez D., Almaz M.E. In vitro evaluation of apical microleakage of a new MTA-based sealer. Eur. Arch. Paediatr. Dent. 2012;13:252–255. doi: 10.1007/BF03262880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.