Abstract
Several causes of infertility have been identified, and several papers have documented some compounds that cause infertility. One of the compounds reported to be toxic to the reproductive system is cyanide. In the management of infertility, various mechanisms ranging from synthetic drugs, natural products and supplements have been employed. Quercetin is an antioxidant supplement that has been used in the treatment of a variety of ailments. This work is aimed at investigating the role of quercetin in attenuating spermato-toxicity and testicular-histopathology induced by cyanide.
Seventy-two (72) male wistar rat (weight 190 g ± 10 g) were divided into nine groups (n = 8) except for groups 4 and 5 with (n = 16). Group 1 (control) received physiological saline while Groups 2 and 3 received 0.5 and 1 mg/kg body weight (bwt) cyanide respectively for 56 days, groups 4 and 5 received 0.5 and 1 mg/kg bwt cyanide respectively for 30 days. At day 30, eight animals were sacrificed from Groups 4 and 5 and the remaining eight (8) rats were subdivided into groups (6 and 7) and were given 20 and 40 mg/kg bwt of quercetin respectively for twenty-six days. Co-administration of cyanide and quercetin at a dose of 0.5 mg/kg cyanide +20 mg/kg quercetin and 1 mg/kg cyanide +40 mg/kg quercetin were given to group 8 and 9 respectively for 56 days.
Significant decreases in sperm parameters (count, motile and normal sperm) and increases in malondiadehyde concentration were observed in the cyanide treated groups. Testicular histoarchitecture showed few to no spermatozoa in the lumen of rats treated with cyanide. All these effects were attenuated by quercetin.
In conclusion, quercetin regulates testicular histopathology induced by cyanide in Wistar rats. Data from this work suggests potential preventive or therapeutic applications of quercetin for individuals subjected to cyanide environmental pollution.
Keywords: Quercetin, Cyanide, Testicular histoarchitecture, Sperm parameters
Quercetin; Cyanide; Testicular histoarchitecture; Sperm parameters.
1. Introduction
Interference with any toxic exogenous substance at any level of the reproductive cycle may result in reproductive toxicity, making the reproductive cycle one of the most complex processes in biological functions. Reproductive toxicity can be defined as the unfavorable effects of a chemical on any phase of the reproductive cycle, including the impairment of reproductive function in both males and females, as well as the effects on the fetus or progeny (Efstathios, 2017) Various compounds have been documented to induce reproductive toxicity, which may later confer infertility problems on an individual. One of the compounds reported to be toxic to the reproductive system is cyanide.
Cyanide is a compound that has a C≡ N group in its structure (Gensa, 2019). Cyanide can be found in a variety of forms in the environment (Gensa 2019). Cyanide, hydrocyanic acid, hydrogen cyanide (HCN), and prussic acid are different toxic forms of cyanide (Gensa 2019). Cyanide causes a variety of health problems in children, including Konzo illness, thyroid goiter, and tropical ataxic neuropathy, as well as cretinism, stunted growth in children and fatalities, epilepsy, and behavioral and emotional abnormalities (Mushumbusi et al., 2020). In sub-saharan Africa, especially in Nigeria, the major source of cyanide exposure is via cassava consumption. Nigeria is the world's largest producer of cassava, giving the world 21 % of the world's total cassava produced in 2016 (Githunguri et al., 2007; FAO, 2016). In Nigeria, cassava intended for human consumption is processed into three major forms which are gari, fufu and cassava flour. Cassava is generally classified into sweet and bitter varieties according to the cyanogenic glucoside content of their roots. While the sweet ones have ≤50 mg/kg cyanide content, the bitter ones have ≥100/mg/kg cyanide content (CAC, 2019).
In the management of infertility, various mechanisms ranging from synthetic drugs, natural products and supplements have been employed. Quercetin is an antioxidant supplement that has been used in the treatment of a variety of ailments. Quercetin, a flavonoid abundant in many commonly consumed fruits and vegetables, is one of the most potent free radical scavengers (McAnulty et al., 2013; Gao et al., 2014). It has been demonstrated to have potent antioxidant and cytoprotective effects in preventing endothelial apoptosis caused by oxidants (Choi et al., 2003). In addition, quercetin protects tissues and organs within the whole body and scales back oxidative injury to fats that reduces cholesterol and protects against artery preventative, cardiovascular, and neurodegenerative diseases. Moreover, it boosts the level of glutathione and prevents cell death through scavenging oxygen radicals (Inal et al., 2005; D'Andrea, 2015). Recently, quercetin has been shown to inhibit an enzyme that converts testosterone into a molecule known as testosterone glucuronide, restoring the testosterone level and testicular function (Bharti et al., 2014). This work is aimed at investigating the role of quercetin in attenuating spermato-toxicity and testicular-histopathology induced by cyanide.
2. Methodology
2.1. Experimental animals and care
Nine-week old seventy-two male Wistar rats (average weight of 190g ± 10g) were obtained, bred, and maintained in the central animal house of the University of Ilorin's College of Health Sciences. The animals were kept in the Department of Anatomy's animal room under regular laboratory conditions at temperatures ranging from 27° to 30° Celsius. The rats were randomly grouped into nine groups (1–9) with eight (8) animals per group, except for groups 4 and 5 with sixteen (16) rats each.
Group 1 was designated as the control group which received physiological saline while rats of Groups 2 and 3 received 0.5 and 1 mg/kg body weight cyanide respectively for 56 days, 4 and 5 received 0.5 and 1 mg/kg body weight cyanide respectively for 30 days. On the 30th day, eight rats from groups 4 and 5 were euthanized, and the remaining eight (8) rats were separated into groups (6 and 7) and administered 20 and 40 mg/kg body weight of quercetin for twenty-six (26) days, respectively. Co-administration of cyanide and quercetin at a dose of 0.5 mg/kg cyanide +20 mg/kg quercetin and 1 mg/kg cyanide +40 mg/kg quercetin was given to groups 8 and 9 respectively for 56 days. Water and food were supplied ad libitum and all compound administrations were administered orally using an orogastric canula. The University of Ilorin ethical review committee (UERC) granted ethical approval with the approval number (UERC/ASN/2017/1067), and all experimental procedures were carried out in accordance with the Institutional Ethical Review Committee of the University of Ilorin, Nigeria.
2.2. Animal sacrifice
The animals were sacrificed twenty-four hours after the last day of treatment by euthanizing them with 80 mg/kg of ketamine and using the transcardial perfusion method by fixing the entire rat's body with 4 % paraformaldehyde. Before transcardial perfusion was done, blood samples were collected via cardiac puncture into lithium-heparin bottles for biochemical assay while the caudal part of the epididymis was excised for sperm analysis. The testis was excised and quickly placed in Bouin's fluid and processed for histological examination.
2.3. Sperm analysis
2.3.1. Sperm count
The caudal end of the epididymis was minced in a buffer containing a small quantity of formaldehyde at a ratio of 1:5, respectively. The tip of the pipette was lowered into the hemacytometer's V-shaped groove, and a small sample was loaded into the chamber. The sample was allowed to settle for 2 min so that the cells would avoid drifting around the chamber and be in the same plane of view. To prevent the sample from drying out, the hemacytometer was mounted on straws inside a Petri dish containing a moistened filter pap.
The hemocytometer's central counting region has 25 large squares, each of which has 16 smaller squares. To prevent double counting, cells on the lines on the two sides of the large square were counted.
Since cells can be more concentrated on one side of the chamber, cell counting was performed in a non-biased manner (diagonal count). Cells were counted in 5 of the 25 broad squares, and the value obtained was then multiplied by 5 to obtain the number of cells per central counting area.
The count was done under a light microscope at ×400 magnification and was expressed as ×10ˆ6/ml (Rouge and Bowen, 2002).
2.3.1.1. Calculating sperm concentration
The cover-glass is 0.1 mm above the chamber's floor, and each of the nine squares on the grid has an area of 1 square mm, including the central counting area of 25 big squares. The core counting region has a volume of 0.1 mm3 or 0.1 μL. The number of sperm per ml of diluted sample was determined by multiplying the average number of sperm in each central counting region by 10,000, and the result was then multiplied by the dilution factor (Rouge and Bowen, 2002).
2.3.2. Sperm motility
The sperm sample was diluted in buffered saline, and 10 μL of this sample was pipetted onto a clean, pre-warmed microscope slide. A cover-slip was gently lowered onto the sample (so as to avoid the formation of air bubbles) and the slide was examined using a microscope with a 20x objective. At least ten widely-spaced fields were examined to provide an estimate of the percentage of motile cells (Rouge and Bowen, 2002).
2.3.3. Sperm morphology
The microscope slides and nigrosin-eosin stain had been pre-warmed to body temperature. A drop of stain was piped onto the end of a slide, followed by a droplet of sperm piped up next to the stain. The sperm and stain were combined by inserting the edge of another slide into the drops of stain and sperm and rocking the slide back and forth a few times. The surface of the first slide was then smeared with the second slide. Waving the slide back and forth in the air dried it quickly. With a 40x objective lens, the slide was examined under the microscope (Rouge and Bowen, 2002).
2.4. Histological studies
Routine histological processing was performed using the hematoxylin and eosin staining procedure. After the testicle had been properly fixed in Bouin's solution, t he testicles were then dehydrated with ascending grades of alcohol, cleared in xylene, and infiltrated into molten paraffin wax before being embedded in molten paraffin wax to form a tissue block. The rotary microtome was used to section the paraffin block containing the tissue at a thickness of 4 μm. After floating in a water bath at 40 °C, t he sections were then transferred to a glass slide and stained with hematoxylin and eosin stains. The slides were examined under a light microscope (magnification 100x).
2.5. Stereological examination
Histological analyses of the testes were captured using an Olympus binocular research microscope (Olympus, New Jersey, USA) which was connected to a 5.0 MP Amscope Camera (Amscope Inc, USA). The total diameter and height of seminiferous epithelium, Sertoli and Leydig cells were counted from captured testicular images (n = 8 per group/per analysis) using imageJ software.
2.5.1. Tubular diameter and height of seminiferous epithelium
These were measured at x100 magnification using an Olympus binocular research microscope (Olympus, New Jersey, USA) which was connected to a 5.0 MP Amscope Camera. Thirty (30) tubular profiles in the second half of spermatogenesis (stage IX) were chosen randomly and measured for each animal (Segatell et al., 2004). Tubular lengths per testis and per gram of testis were calculated using the formula of Varadaraj et al. (2001), thus: Tubular length per testis = Volume of seminiferous tubule/πr 2, where r = radius. Tubular length per gram of testis = Tubular length per testis/Testis weight.
2.5.2. Sertoli and Leydig cells count
The number of Sertoli cells and Leydig cells per testis and per gram of testis were determined using the formula of Castro et al. (2002), thus: Number of cells per testis = Total nuclear volume/Volume of a single nucleus. Number of cells per gram of testis = Number of cells per testis/Testis weight.
2.6. Biochemical analysis
Serum lipid peroxidase concentration was estimated using commercial diagnostic ELISA kit (product code MAK085 and sensitivity of 1 nmol/ml) gotten from Monobind Inc. Lake forest, CA, U.S.A. The procedure was performed using the method of thiobarbituric acid, which measures MDA-reactive products as described by Todorova et al. (2005). The absorbance was measured colorimetrically.
2.7. Statistical analysis
The statistical analyses were performed using GraphPad Prism version 7.0. The data was presented as a mean ± SD. One-way ANOVA was performed to look at the differences among the groups, and Bonferroni correction was employed to account for multiple comparisons. Statistical significance was defined as a p < 0.05.
3. Results
3.1. Quercetin boosts sperm parameters reduced by cyanide
Mean values of total sperm count among experimental groups depicted in Figure 1 showed significant in cyanide treated groups (2, and 5) and also cyanide post treated with quercetin (group 7) when compared with the control group 1 rats. Also, a significant decrease in total sperm count was also observed in the 1 mg/kg cyanide treated for 56 (group 3) and 30 (group 5) days when compared with the group 9 rats treated with concurrent administration of cyanide (1 mg/kg) and quercetin (40 mg/kg) for 56 days.
Figure 1.
Chart showing the total sperm count among the experimental groups after the administration of Cyanide and Quercetin. Data were presented as mean and standard error of mean (Mean ± SEM)∗ = is the significant level of difference in comparison with Control Group 1; # = is the significant level of difference in comparison with Group 7, χ = is the significant level of difference in comparison with group 9. p value < 0.05 was considered to be statistically significant; (n = 8).
Groups treated with 1 mg/kg cyanide for 56 days (group 3) and 0.5 mg/kg cyanide for 30 days (group 4) showed significant decreases in their total sperm count when compared with groups treated with cyanide and post treated with quercetin (group 7). When compared to the control group of rats, there was no significant difference between groups 6, 7, 8, and 9 treated with cyanide and quercetin.
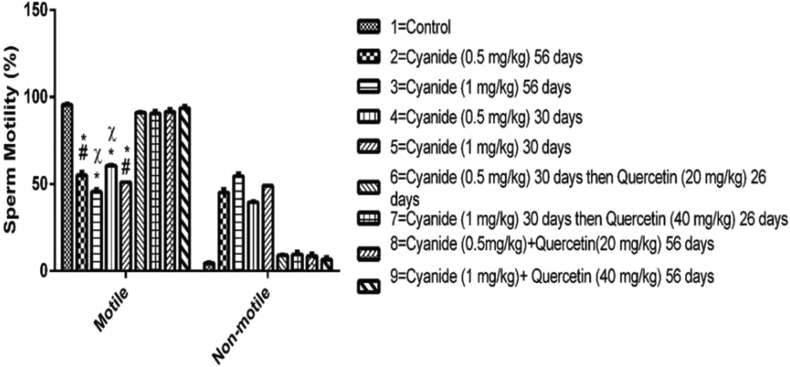
The results from Figure 2 showed mean values for sperm motility after administration of cyanide and quercetin. A high significant decrease in motile sperm was observed in all cyanide only treated groups (2, 3, 4 and 5) when compared with control group 1, while a moderate significant decrease was observed in 0.5 mg/kg cyanide treated for 56 days and 1 mg/kg cyanide treated for 30 days when compared with groups treated with cyanide and post treated with quercetin (group 7). Furthermore, a significant decrease in motile sperm was seen in groups 3 and 4 treated with 1 mg/kg cyanide for 56 days and 0.5 mg/kg cyanide for 30 days respectively, when compared with the group of 9 rats treated with concurrent administration of cyanide (1 mg/kg) and quercetin (40 mg/kg) for 56 days.
Figure 2.
Chart showing the sperm motility among the experimental groups after the administration of Cyanide and Quercetin. Data were presented as mean and standard error of mean (Mean ± SEM)∗ = is the significant level of difference in comparison with Control Group 1; # = is the significant level of difference in comparison with Group 7, χ = is the significant level of difference in comparison with group 9. p value < 0.05 was considered to be statistically significant; (n = 8).
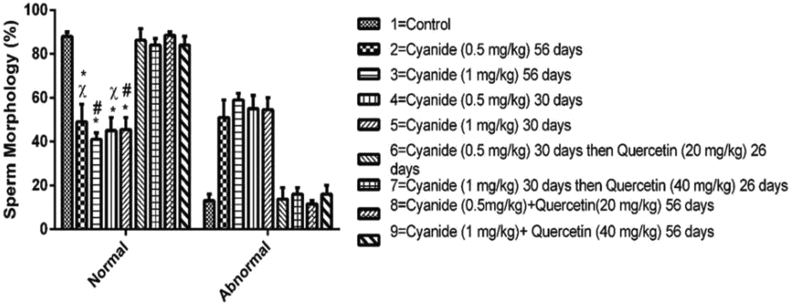
As revealed in Figure 3, the percentage of normal sperm was significantly reduced in all cyanide treated groups 2, 3, 4 and 5 when compared with control group 1. Furthermore, the percentage of normal sperm in groups 3 (1 mg/kg cyanide for 56 days) and 5 (0.5 mg/kg cyanide for 30 days) was significantly reduced when compared with groups treated with cyanide and post treated with quercetin (group 7). Groups 2 (0.5 mg/kg cyanide for 56 days) and 4 (0.5 mg/kg cyanide for 30 days) normal sperm were significantly decreased when compared with group 9 rats treated with concurrent administration of cyanide (1 mg/kg) and quercetin (40 mg/kg) for 56 days. When compared to the control group of rats, there was no significant difference between groups 6, 7, 8, and 9 treated with cyanide and quercetin.
Figure 3.
Chart showing sperm morphology among the experimental groups after the administration of Cyanide and Quercetin. Data were presented as mean and standard error of mean (Mean ± SEM)∗ = is the significant level of difference in comparison with Control Group 1; # = is the significant level of difference in comparison with Group 7, χ = is the significant level of difference in comparison with group 9. p value < 0.05 was considered to be statistically significant; (n = 8).
3.2. Stereological investigation
As shown in Table 1, there was a significant (p < 0.05) decrease in the seminiferous tubule diameter and epithelial height of animals treated with cyanide (groups 2, 3, 4 and 5) when compared with the control (group 1) and animals treated with quercetin (groups 6, 7, 8, and 9). Furthermore, when compared to the control and quercetin-treated groups, the Leydig cell area increased significantly (p < 0.05) in the cyanide-treated groups. The Sertoli cell count revealed a significant decrease in the total nuclear volume of sertoli cells in rats administered with cyanide when compared with the quercetin treated rats. There is no significant difference between groups 6, 7, 8 and 9 treated with quercetin when compared with control (group 1) rats.
Table 1.
Effect of cyanide and quercetin on stereological parameters (Seminiferous Tubules (ST) Diameter, height of seminiferous epithelium (SE), Sertoli cell count, and Leydig cell count) of Wistar rats in all experimental groups.
| Stereological Parameters | ST Diameter (DM) μm | Height of SE μm | Leydig cell area (μm2) | Total nuclear volume of sertoli cell (μm3) |
|---|---|---|---|---|
| Group 1 | 172.50 ± 3.54 | 53.08 ± 0.13 | 107.02 ± 1.90 | 32.15 ± 2.05 |
| Group 2 | 130.95 ± 1.80∗# | 42.05 ± 3.46∗# | 132.21 ± 6.13# | 27.36 ± 5.15∗# |
| Group 3 | 131.67 ± 9.30∗# | 39.77 ± 1.21∗# | 143.08 ± 1.02∗# | 24.44 ± 0.64∗# |
| Group 4 | 143.15 ± 1.89∗# | 40.05 ± 2.71∗# | 121.95 ± 2.18∗# | 23.14 ± 3.18∗# |
| Group 5 | 139.65 ± 5.63 | 40.65 ± 1.60∗# | 134.05 ± 3.03∗# | 24.04 ± 1.77∗# |
| Group 6 | 168.50 ± 2.94 | 51.20 ± 4.30 | 112.95 ± 6.08 | 30.34 ± 4.01 |
| Group 7 | 161.83 ± 3.18∗ | 50.45 ± 3.08 | 119.55 ± 4.00∗ | 32.84 ± 3.37 |
| Group 8 | 171.20 ± 3.24 | 52.95 ± 3.22 | 107.28 ± 1.37 | 33.22 ± 2.00 |
| Group 9 | 174.71 ± 4.14 | 52.15 ± 5.21 | 111.05 ± 2.08 | 32.38 ± 4.14 |
Data were represented as mean and standard error of mean (mean ± SEM). ∗(p < 0.05), significantly different when compared to control group 1, # (p < 0.05), significantly different when compared to comparison with Group 7.
3.3. Quercetin mop up malondiadehyde radicals generated as a result of cyanide
Figure 4 depicts the mean MDA concentrations in the experimental groups, revealing a significant increase in the cyanide treated groups (2, and 5) as well as the cyanide post treated with quercetin (group 7) when compared to the control group 1 rats. Groups treated with 0.5 mg/kg cyanide for 30 days (group 4) and 1 mg/kg cyanide for 30 days (group 5) showed a slight significant increase in MDA concentration when compared with groups treated with cyanide and post treated with quercetin (group 7).
Figure 4.
Chart showing the serum concentration of MDAamong the experimental groups after the administration of Cyanide and Quercetin. Data were presented as mean and standard error of mean (Mean ± SEM)∗ = is the significant level of difference in comparison with Control Group 1; # = is the significant level of difference in comparison with Group 7, χ = is the significant level of difference in comparison with group 9. p value < 0.05 was considered to be statistically significant; (n = 8).
Moreover, significant increase in MDA concentration was also observed in 0.5 mg/kg cyanide (group 2) and 1 mg/kg cyanide (group 5) both treated for 56 days when compared with the group 9 rats treated with concurrent administration of cyanide (1 mg/kg) and quercetin (40 mg/kg) for 56 days. No significant difference in groups 6, 7, 8 and 9 treated with cyanide and quercetin when compared with control group 1 rats.
3.4. Quercetin improves testicular cytoarchitecture
From Figures 5 and 6, the control group (Group 1) shows normal and organized cytoarchitecture. The lumen also contains mature spermatozoa. This observation is also consistent with the appearance of the testicular cytoarchitecture of animals that were treated with quercetin and cyanide concomitantly (groups 8 and 9). Furthermore, as depicted in Figures 5 and 6, the testicular cytoarchitecture of animals treated with cyanide (Groups 2 to 5) appears disorientated, with the wide lumen of the seminiferous tubules containing few spermatozoa. Animals post-treated with quercetin had normal testicular microarchitecture.
Figure 5.
Cytoarchitectural presentations of the rat testes stained with H&E (x100 Mag). Higher magnification of areas within the black square boxes are presented in Figure 6.
Figure 6.
Cytoarchitectural presentations of the rat testes stained with H&E (x400 Mag). Observed cells in the seminiferous tubules are Spermatogonia (Sg), Sertolic (Sc), Spermatid (Spd)while Spermatozoa are seen in the Lumen (L).
4. Discussion
The present study was designed to evaluate possible therapeutic effects of quercetin against cyanide-induced reproductive toxicity and testicular damage in adult male rats. Reproductive tissues are generally affected to a greater extent by endocrine disruptors and agents that increase the generation of reactive oxygen species. Many in vitro and in vivo studies have revealed its toxic effects on reproduction (Shivanoor and David, 2015a, Shivanoor and David, 2015b).
Data from this present study shows that quercetin efficiently modulated lipid peroxidation. It has been reported that Cd preferentially binds to sulfhydryl group (–SH)-containing cellular molecules such as GSH and SOD (Abdeen et al., 2019). The depletion of –SH is an important indirect mechanism of oxidative stress induced by Cd, especially those that participate in oxidative phosphorylation and detoxification. These events eventually induce the accumulation of ROS (such as superoxide radicals, hydroxyl radicals, and hydrogen peroxide) and further promote the formation of lipid peroxides.
Quercetin prevents antioxidant injury and cell death via several mechanisms, such as scavenging oxygen radicals (Coskun et al., 2005), protecting against lipid peroxidation and chelating metal ions (Murota et al., 2004). Previous studies have strongly emphasized that quercetin is considered as a surpass free-radical scavenging antioxidant (Gibellini et al., 2011) owing to a high number of hydroxyl groups and an ability to donate electrons or hydrogens, and scavenges hydroxyl groups, hydrogen peroxide, and superoxide anions (Heijnen et al., 2001).
Quercetin is able to chelate cyanide by forming a coordination bond with the lead ions through its orthophenolic groups located on the quercetin B ring (Bravo and Anacona, 2001).
The current study found that cyanide exposure resulted in significant decreases in epididymal sperm concentration, sperm motility (progressive), and the normal/abnormal sperm ratio. Many investigations on the reproductive system of male animals have shown that cyanide is toxic to testicular tissue and functions (Shivanoor and David, 2015a, Shivanoor and David, 2015b; Sanni et al., 2020), including significant reductions in the quantity of spermatozoa within the epididymis in cyanide-treated mice. Alterations in sperm parameters were observed following cyanide administration in previous studies (De Sousa et al., 2007; Shivanoor and David, 2015a, Shivanoor and David, 2015b). Previous studies have also shown that cyanide can cause depletion in ATP synthesis in the spermatozoa of crabs by inhibiting the enzyme necessary for ATP synthesis (Perchec et al., 1995). ATP plays a crucial role in the forward movement of sperm (Berg et al., 2002). Thus, perturbations in the ATP pool as a result of cyanide treatment may be responsible for reduced sperm motility observed in this study. Moreover, the decrease in sperm motility can be due to indirect effects of cyanide, like an increase in ROS generation in sperm cells. By causing lipid oxidation, ROS alters the integrity and the fluidity of cellular membrane structures, particularly of the cell membrane, which is essential for sperm motility, structural integrity, and ultimately for sperm viability (Wathes et al., Furthermore, morphometric evaluations show that these influences also resulted in the reduction of tubular diameter, epithelial height, and total nuclear Sertoli cell volume as seen in this study. Surprisingly, the Leydig cell area increased significantly in the cyanide treated animals when compared with the control group. This may be due to the intrinsic adaptive response to increased ROS generation, resulting in elevated biogenesis of mitochondria, as well as a recovery of testosterone-producing Leydig cells in cyanide treated rats. Different investigators have also noted that the male reproductive capacity was regularly decreased (Carlsen et al., 1992; Swan et al., 2000) due to chemical and physical endocrine disruptors, which supports our study (Kumar et al., Our study also proved the curative results of quercetin in preventing perturbations observed in the tubular diameter and epithelial height.
In the current study, treatments with quercetin resulted in a significant improvement in sperm parameters. The postulated roles of quercetin in prevention of cyanide toxicity can be explained by, firstly, their ability in free radical scavenging and secondly, the prevention of GSH depletion (Galati et al., 2001). Quercetin allows free radicals to attract a hydrogen atom from the antioxidant molecule rather than from polyunsaturated fatty acids, thus breaking the chain of free radical reactions, resulting in a marked decrease in the reactivity of free radicals (Galati et al., 2001). In this regard, Hämäläinen et al. (2007) hypothesized that constituents of quercetin inhibited lipid peroxidation and prevented glutathione depletion.
Due to a decrease in the number of germ cells, cyanide exposure caused morphological abnormalities in the testes, including distortion and shrinking of the seminiferous tubules. Similar results were observed by Acharya et al. (2006) after cyanide exposure in animal model. It has been reported that cyanide results in the generation of excessive oxidative stress (Gabbianelli et al., 2009). However, animals co-administered cyanide and quercetin and post-treated with quercetin had normal testicular microarchitecture. This finding is corroborated by the report of Sanni et al. (2020) on bioflavonoids. Quercetin has been found as a strong antioxidant and having antilipoperoxidative activity, which prevents cyanide induced oxidative damage.
5. Conclusion
In conclusion, this study showed that quercetin at 20 and 40 mg/kg bodyweight could produce protective effects in rats administered with cyanide, and this response was reflected in morphometric, seminal, antioxidant and histochemical parameters. Data from this work suggests potential preventive or therapeutic applications of quercetin for individuals subjected to cyanide environmental pollution.
Declarations
Author contribution statement
Dr. OYEWOPO Adeoye O.: Conceived and designed the experiments; contributed reagents, materials, analysis tools.
ADELEKE Opeyemi: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
JOHNSON Olawumi; AKINGBADE Adebanji; OLANIYI Kehinde S.; AREOLA Emmanuel D; TOKUNBO Olorunfemi: Performed the experiments; Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abdeen A., Abou-Zaid O.A., Abdel-Maksoud H.A., Aboubakr M., Abdelkader A., Abdelnaby A., Abo-Ahmed A.I., El-Mleeh A., Mostafa O., Abdel-Daim M. Cadmium overload modulates piroxicam-regulated oxidative damage and apoptotic pathways. Environ. Sci. Pollut. Res. Int. 2019;26(24):25167–25177. doi: 10.1007/s11356-019-05783-x. [DOI] [PubMed] [Google Scholar]
- Acharya U.R., Mishra M., Tripathy R.R., Mishra I. Testicular dysfunction and antioxidative defense system of Swiss mice after chromic acid exposure. Reprod. Toxicol. 2006;22(1):87–91. doi: 10.1016/j.reprotox.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Berg J.M., Tymoczko J.L., Stryer L. Oxidative Phosphorylation. fifth ed. W. H. Freeman; New York: 2002. Biochemistry. ch. 18. [Google Scholar]
- Bharti S., Misro M.M., Rai U. Quercetin supplementation restores testicular function and augments germ cell survival in the estrogenized rats. Mol. Cell. Endocrinol. 2014;383(1–2):10–20. doi: 10.1016/j.mce.2013.11.021. [DOI] [PubMed] [Google Scholar]
- Bravo A., Anacona J.R. Metal complexes of the flavonoid quercetin: antibacterial properties. Transit. Met. Chem. 2001;26(1):20–23. [Google Scholar]
- CAC . Joint FAO/WHO Food Standards Programme CODEX Committee on Contaminants in Foods. 13th Session Yogyakarta, Indonesia. 2019. Discussion paper on the establishment of MLs for HCN in cassava and cassava-based products and occurrence of mycotoxins in these products. [Google Scholar]
- Carlsen E., Giwercman A., Keiding N., Skakkebæk N.E. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y., Kang J., Han J. Biochemical and molecular actions of nutrients polyphenolic flavonoids differ in their antiapoptotic efficacy in hydrogen peroxide – treated human vascular endothelial cells. J. Nutr. 2003;133:985–991. doi: 10.1093/jn/133.4.985. [DOI] [PubMed] [Google Scholar]
- Coskun O., Kanter M., Korkmaz A., Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol. Res. 2005;51(2):117–123. doi: 10.1016/j.phrs.2004.06.002. [DOI] [PubMed] [Google Scholar]
- D’Andrea G. Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia. 2015;106:256–271. doi: 10.1016/j.fitote.2015.09.018. [DOI] [PubMed] [Google Scholar]
- De Sousa A.B., Maiorka P.C., Gonçalves I.D., de Sá L.R.M., Górniak S.L. Evaluation of effects of prenatal exposure to the cyanide and thiocyanate in wistar rats. Reprod. Toxicol. 2007;23(4):568–577. doi: 10.1016/j.reprotox.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Efstathios N. Second Edition. 2017. Relevance of Animal Testing and Sensitivity of End Points in Reproductive and Developmental Toxicity. Reproductive and Developmental Toxicology. [Google Scholar]
- FAO . 2017. Statistics database.http://faostat3.fao.org/faostat%20gateway/go/to/browse/T/*/E [Google Scholar]
- Gabbianelli R., Falcioni M.L., Cantalamessa F., Nasuti C. Permethrin induces lymphocyte DNA lesions at both Endo III and Fpg sites and changes in monocyte respiratory burst in rats. J. Appl. Toxicol. 2009;(4):317–322. doi: 10.1002/jat.1412. [DOI] [PubMed] [Google Scholar]
- Galati G., Moridani M.Y., Chan T.S., O’Brien P.J. Peroxidative metabolism of apigenin and naringenin versus luteolin and quercetin: glutathione oxidation and conjugation. Free Radic. Biol. Med. 2001;30(4):370–382. doi: 10.1016/s0891-5849(00)00481-0. [DOI] [PubMed] [Google Scholar]
- Gao C., Chen X., Li J. Myocardial mitochondrial oxidative stress and dysfunction in intense exercise: regulatory effects of quercetin. Eur. J. Appl. Physiol. 2014;114(4):695–705. doi: 10.1007/s00421-013-2802-9. [DOI] [PubMed] [Google Scholar]
- Gensa U. Review on cyanide poisoning in ruminants. J. Biol. Agricul. Healthc. 2019;9(6):1–12. [Google Scholar]
- Gibellini L., Pinti M., Nasi M., Montagna J.P., De Biasi S., Roat E., Bertoncelli L., Cooper E.L., Cossarizza A. Quercetin and cancer chemoprevention. Evid. Based Compl. Alternat. Med. 2011:591356. doi: 10.1093/ecam/neq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Githunguri C.M., Mwiti S., Migwa Y. Vol. 8. 2007. Cyanogenic potentials of early bulking cassava planted at Katumani, a semi-arid area of Eastern Kenya; pp. 925–927. (Proceedings of the 8th African Crop Science Society Conference, El-Minia, Egypt). [Google Scholar]
- Hämäläinen M., Nieminen R., Vuorela P., Heinonen M., Moilanen E. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007 doi: 10.1155/2007/45673. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijnen C.G., Haenen G.R., Vekemans J.A., Bast A. Peroxynitrite scavenging of flavonoids: structure activity relationship. Environ. Toxicol. Pharmacol. 2001;10(4):199–206. doi: 10.1016/s1382-6689(01)00083-7. [DOI] [PubMed] [Google Scholar]
- Inal M.E., Akgün A., Kahraman A. Radioprotective effects of exogenous glutathione against whole-body gamma-ray irradiation: age- and gender-related changes in malodialdehyde levels, superoxide dismutase and catalase activities in rat liver. Meth. Find ExpClin. Pharmacol. 2005;24(4):209. doi: 10.1358/mf.2002.24.4.678452. [DOI] [PubMed] [Google Scholar]
- McAnulty L.S., Miller L.E., Hosick P.A., Utter A.C., Quindry J.C., McAnulty S.R. Effect of resveratrol and quercetin supplementation on redox status and inflammation after exercise. Appl. Physiol. Nutr. Metab. 2013;38(7):760–765. doi: 10.1139/apnm-2012-0455. [DOI] [PubMed] [Google Scholar]
- Murota K., Mitsukuni Y., Ichikawa M., Tsushida T., Miyamoto S., Terao J. Quercetin-4 ‘-glucoside is more potent than quercetin-3-glucoside in protection of rat intestinal mucosa homogenates against iron ion-induced lipid peroxidation. J. Agric. Food Chem. 2004;52(7):1907–1912. doi: 10.1021/jf035151a. [DOI] [PubMed] [Google Scholar]
- Mushumbusi C.B., Max R.A., Bakari G.G., Mushi J.R., Balthazary S.T. Cyanide in cassava varieties and people's perception on cyanide poisoning in selected regions of Tanzania. J. Agric. Stud. 2020;8(1):180–193. [Google Scholar]
- Perchec G., Jeulin C., Cosson J., André F., Billard R.J. Vol. 108. 1995. Cell Science; pp. 747–753. [DOI] [PubMed] [Google Scholar]
- Rouge M., Bowen R. 2002. Collection and evaluation of semen.http://www.vivo.colostate.edu/hbooks/%20pathphys/reprod/semeneval/collection.html Reproductive index Glossary. [cited 2021 May 3]. Available at: [Google Scholar]
- Sanni A.M., Idaguko C.A., Abdulazeez D.O., Adeleke O.S., Falana B.A. Naringen ameliorates cyanide-induced testicular and epididymal changes in Swiss albino mice (musmusculus) Res. J. Health Sci. 2020;8(4):291–302. [Google Scholar]
- Segatell T.M., Franca l.R., Inheiro F.P., Alemida C.C.D., Martinez M., Martinez F.E. Spermatogenic cycle length and spermatogenic efficiency in the Gerbil (Meriones unguiculatus) J. Androl. 2004;2(6):13. doi: 10.1002/j.1939-4640.2004.tb03156.x. [DOI] [PubMed] [Google Scholar]
- Shivanoor S.M., David M. Fourier transform infrared (FT-IR) study on cyanide induced biochemical and structural changes in rat sperm. Toxicol. Rep. 2015;2:1347–1356. doi: 10.1016/j.toxrep.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivanoor S.M., David M. Subchronic cyanide toxicity on male reproductive system of albino rat. Toxicol. Res. 2015;4(1):57–64. [Google Scholar]
- Swan S.H., Elkin E.P., Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ. Health Perspect. 2000;108:961. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorova I., Simeonova G., Kyuchukova D., Dinev D., Gadjeva V. Reference values of oxidative stress parameters (MDA, SOD, CAT) in dogs and cats. Comp. Clin. Path. 2005;13:190–194. [Google Scholar]
- Varadaraj C., Bartke A., Awoniyi C.A., Tsai-morris C.H., Dufau M.L., Rossell L.D., Kopchick J.J. Testicular endocrine function in GH receptor gene disrupted mice. Endocrinology. 2001;142(8):3443–3450. doi: 10.1210/endo.142.8.8298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.