Abstract
Doxorubicin has been used as an anticancer drug and has already indicated effective in the treatment of cancer. The incidence of cardiotoxicity due to doxorubicin was approximately 11%, resulting in the limited use of doxorubicin. Cardiac protection during doxorubicin therapy is needed because it can reduce the incidence of heart failure. Vernonia amygdalina (VA) is traditionally used by Indonesians as a traditional medicine and contains many secondary metabolites, including vernolide, vernodalol, vernoamygdalin, vernolepin, luteolin, luteolin 7-O-beta-glucoronoside and luteolin 7-O-glucoside. The pharmacological activity of VA has been widely studied, including its antimalarial, antidiabetic, anticancer, hepatoprotective, nephroprotective, and antioxidant activities. This research aimed to determine the antioxidant 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity, total phenol, total flavonoid, and cardioprotective effects of Vernonia Amygdalina.
Negative control was only intraperitoneal injection of doxorubicin (20 mg/kgbw) on the eight day while quercetin (85 mg/kgbw) and ethanol extract of Vernonia amygdalina (EEVA) 100, 200, 400 mg/kgbw dose are orally administered for eight consecutive days. Both quercetin and EEVA groups were also injected with doxorubicin (20 mg/kgbw) on the same day. On the following day, rats were injected with ketamine HCL 75 mg/kgbw and were dissected for heart blood collected. The blood collected 3 ml from each rat was analyzed for biochemical parameters. The analyzed biochemical parameters were Aspartate transaminase (AST), Alanine transaminase (ALT), Ureum, Creatinine, Creatinine kinase-MB (CK-MB), Lactate dehydrogenase (LDH), Troponin T, Brain natriuretic peptide (BNP), and antioxidant parameter Superoxide Dismutase (SOD).
The result showed that EEVA antioxidant activity was 40.51 ± 4.89 μg/mL, total flavonoid was 3.79 ± 0.61 mg QE/g extract, and total phenol was 281.575 ± 1.069 mg GAE/g extract. Quercetin (85 mg/kgbw) and EEVA (400 mg/kgbw) reduce AST, ALT, Ureum, Creatinine, CK- MB, LDH, Troponin T, BNP significantly and increase rats’ SOD level compared with negative control. So that, this study explicates that EEVA potentials as cardioprotective agent against doxorubicin by reducing biochemical parameters.
Keywords: Vernonia amygdalina, Antioxidant, Cardioprotective
Vernonia amygdalina, Antioxidant, Cardioprotective.
1. Introduction
Doxorubicin has been used as an anticancer drug and has already proven effective in the treatment of cancer. The incidence of cardiotoxicity due to doxorubicin is approximately 11%, and because of this, the use of doxorubicin has been limited [1]. A study conducted by Van Hoff reported that of 4000 patients who received doxorubicin treatment, 2.2% had heart failure symptoms [2]. A higher accumulative dose of doxorubicin given to the patient indicates an increasing incidence of congestive heart failure [3]. The doxorubicin mechanism occurs by forming free radicals and superoxide. The free radicals formed will stimulate the formation of malonedialdehyde (MDA), which can bind to molecular targets [4]. Doxorubicin administration can reduce the activity of endogenous antioxidants, including superoxide dismutase (SOD), glutathione peroxidase (GSH), and catalase (CAT) [5]. The oxidative stress formed by doxorubicin activates apoptotic cardiomyocyte signalling and eventually causes congestive heart failure [6].
Cardiac protection during doxorubicin therapy is needed because it can reduce the incidence of heart failure. Heart failure is a clinical syndrome in which symptoms include shortness of breath, fatigue, oedema, and orthopnea, as well as signs of increased venous pressure and pulmonary sound changes, with evidence of abnormal cardiac function [7]. Most of the studies of heart failure use biomarkers to confirm and predict a diagnosis of heart failure. Clinically, the left ventricular ejection fraction (LVEF) provides a marker that can be reliably applied to patients for guidelines on medical therapy. Research and development of heart failure biomarkers, such as BNP, CK-MB, troponin T, and LDH, has undergone rapid growth [8, 9]. Until now, the guidelines for preventive therapy for cardiotoxicity due to doxorubicin have not yet been available; therefore, it is essential to research compounds that can be used as cardioprotective agents for doxorubicin therapy.
Vernonia amygdalina (VA) is known to be used from generation to generation as an herbal medicine and is commonly found in tropical areas such as Indonesia and Malaysia. VA is also widely referred to as bitter leaves. VA is traditionally used by Indonesians as a traditional medicine and contains many secondary metabolites, including sesquiterpene lactone, vernolide, vernodalol, vernoamygdalin, and vernolepin [10]. Flavonoids include luteolin, luteolin 7-O-beta-glucoronoside and luteolin 7-O-glucoside [11]. Steroid glycosides include vernonioside A1, vernonioside A2, vernonioside A3, vernonioside, A4, vernonioside B1, vernonioside B2, vernonioside, B3, vernonioside C, vernonioside D, vernonioside 3, vernoniamyoside A, vernoniamyoside B, vernoniaamyoside C, vernoniamyoside D, vernoamyoside A, vernoamyoside B, vernoamyoside C, vernoamyoside D, dan jugga veramyoside A, veramyoside B, veramyoside C, veramyoside D, veramyoside E, veramyoside F, veramyoside G, veramyoside H, veramyoside I, and veramyoside J [12, 13, 14, 15, 16]. The steroid glycoside compounds of VA are structurally similar to cardiac glycosides, which have a steroid core, a sugar group on carbon number 3 and an unsaturated lactone ring on atom C number 17 [17].
The pharmacological activity of VA has been widely studied, including its antimalarial [18], antidiabetes [19], anticancer [20], hepatoprotective [21], nephroprotective [22], analgesic and antipyretic [23], antibacterial [24], and antioxidant properties [25]. Ethanol extracts of VA have a toxic dose higher than 5000 mg/kg BW [26]. Luteolin compounds have a cardioprotective effect by increasing the expression of the SERCA2a receptor on cardiomyocytes in the presence of anti-calcium overload and anti-apoptosis [27]. Since Doxorubicin causes cardiotoxicity, a research is needed to prevent this serious incidence. Previous research has shown the evidence that Vernonia amygdalina has strong antioxidant activity. It can be used as a cardioprotective agent. Therefore, this study determines the Vernonia amygdalina cardioprotective activity in leaves of doxorubicin-induced rats.
2. Materials
Vernonia Amygdalina leaves were collected from Faculty of Pharmacy, Univesitas Sumatera Utara, Indonesia (coordinates 3033′36.5″ N 98039′12.5″ E). Doxorubicin (Merck), Ethanol (BrataChem),EthylAcetate (BrataChem), n-hexane (BrataChem), Methanol (BrataChem), natrium carbonat (BrataChem), 1,1-diphenyl-2picrylhidrazyl/DPPH (Merck), quercetin/QE (Sigma), vitamin C (Sigma), gallic acid/GAE (Sigma), sodium carboxymethyl cellulose/CMC-Na (Sigma), folin-Ciocalteu (Merck), natrium carbonat (BrataChem), aluminium foil (BrataChem), sodium acetate (BrataChem), distilled water (BrataChem), hydrochloric acid/HCl (BrataChem), aspartate transaminase/AST reagent, alanine transaminase/ALT regent, ureum regent, creatinine regent, Creatinine Kinase-MB/CK-MB Elisa kit, lactate dehydrogenase/LDH Elisa kit, Troponin T Elisa kit, and brain natriuretic peptide/BNP Elisa kit.
2.1. Animals
Rats were obtained from the animal house of the Faculty of Pharmacy, Universitas Sumatera Utara This research used 30 rats by average weight were 180–200 g and given food and water ad libitum for 12 h dark/light cycle. This research has received the approval of the Universitas Sumatera Utara Ethics Commission (registration number 0521/KEPH-FMIPA/2019).
2.2. Extract preparation
The total gram of dry VA is 700 g in powder that was macerated with 10 L n-hexane. Firstly the powder was dried and dissolved with Ethyl acetate in three days then stirred occasionally at a room-temperature. Lastly, the powder was dried and dissolved with Ethanol in three days stirred occasionally at a room-temperature. Each filtrate was collected and evaporated under pressure.
2.3. Experimental design
Rats were randomly divided into six groups (5 rats/group). Group 1 received CMC-Na orally for eight days, group 2 received single-dose doxorubicin (20 mg/kg BW) in day eighth, group 3 received quercetin (85 mg/kg BW) for eight days and intraperitoneal injection with single- dose doxorubicin (20 mg/kgbw) in day eighth, groups 4–6 received EEVA (100, 200, 400 mg/k BW PO) for eight days and intraperitoneal injection with doxorubicin (20 mg/kgbw) in day eighth. On the day ninth, rats were injected ketamine HCL (75 mg/kg BW IP) sacrificing 3 ml blood directly collected from the heart. Collected blood was centrifugate 1000 rpm (4 °C) for ten minutes. It will analyze biochemical parameters AST, ALT, Ureum, Creatinine, CK-MB, LDH, Troponin T, BNP, and endogenous antioxidant SOD.
2.4. 2,2-Diphenyl-1-Picrylhydrazyl/DPPH scavenging activity
The DPPH scavenging activity was following the method from Blois with slight modification [28]. About 25 mg of ethanol extract of African leaves is dissolved with 25 mL of methanol and sonicated for 30 min (40 °C) then centrifuged at 1000 rpm for 10 min. Then diluted to obtain a concentration (6.25 μg/mL, 12.5 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL). 20 mg DPPH dissolved with 100 ml methanol 200 μg/mL sonicated for 30 min (40 °C), then centrifuged 100 rpm for 10 min and diluted to obtain a concentration of 40 μg/mL as control. The extract solution was mixed with DPPH, then vortexed and left for 30 min at a temperature of 27 °C. Then it was measured using a spectrophotometer at 517 nm. Vitamin C was used as a reference.
| DPPH scavenging activity (IC50) = (Absorbance Control - Absorbance Sample)/Absorbance Control. |
2.5. Determination of total phenolic content (TPC)
Total phenolic content using Folin-Ciocalteu regent by following the Sanchez-Rangel method [29]. 10.5 mg of ethanol extract of VA dissolved in 10 mL methanol 0.5 ml ethanol extract of VA mixed with 2.3 mL of water + 0.2 mL of Folin-Ciocalteu's regent subsequently vortexed for ± 1 min, left for 5 min, then added 2 mL of 20% sodium carbonate and let stand for 70 min. Then it was measured using a spectrophotometer at 775 nm. Gallic acid was used as a reference. Total phenol is calculated as GAE/g of extract. The total phenol was repeated 5 times.
2.6. Determination of total flavonoid content (TPF)
10.5 mg of ethanol extract of VA and dissolved with methanol up to 10 ml, then pipette 0.5 ml of solution and add 1.5 ml of methanol, 0.1 ml of 10% aluminum chloride solution, 0.1 ml of 1 M sodium acetate and 2.8 ml of distilled water and allowed to stand for 30 min. Then it was measured at a wavelength of 436 nm. Measurements were made in 5 repetitions. The concentration of flavonoids was calculated from the substitution in the linear regression equation and expressed as the equivalent number of milligrams of quercetin in 1 g of extract [30].
2.7. Serum biochemical parameters
2.7.1. AST measurement
AST measurement level followed Reitment method and the AST level was measured sphectrophotometrically at 505nm. The concentrationexpressed as U/L [43].
2.7.2. ALT measurement
ALT Measurement level followed Reitment method and the ALT level was estimated sphectrophotometrically at 505 nm. The concentration expressed as U/L [43].
2.7.3. Ureum measurement
Ureum Measurement level followed Beale method and the Ureum level was measured sphectrophotometrically at 540 nm. The concentration expressed as mg/dL [44].
2.7.4. Creatinine measurement
Creatinine measurement level followed Dunn method and the Creatinine level was measured sphectrophotometrically at 530 nm. The concentration expressed as mg/dL [45].
2.8. Cardiac biochemical parameters
2.8.1. Troponin T measurement
The Cardiac Troponin T rat measurement Elisa kit (Fine Test, China), the preparation followed the manufacture guideline. The troponin T concentration determined by microplated set at 450 nm. The concentration expressed as pg/ml.
2.8.2. CK-MB measurement
CK-MB rat cardiac measurement, Elisa kit (Abclonal, China). the preparation followed the manufacture guideline. The CK-MB concentration was determined by microplated set at 450 nm. The concentration expressed as ng/ml.
2.8.3. LDH measurement
LDH rat cardiac measurement, Elisa kit (Abclonal, China), the preparation followed the manufacture guideline. The LDH concentration determined by microplated set at 450 nm. The concentration expressed as ng/ml.
2.8.4. BNP measurement
BNP rat cardiac measurement Elisa kit (Abclonal, China). the preparation followed the manufacture guideline. The BNP concentration determined by microplated set at 450 nm. The concentration expressed as pg/ml.
2.9. Cardiac antioxidant
2.9.1. SOD measurement
SOD rat cardiac measurement Elisa kit (Abclonal, China), the preparation followed the manufacture guideline. The SOD concentration determined by micro plated set at 450 nm. The concentration expressed as pg/ml.
2.10. Cardiac histopathology
Cardiac tissue preparations based on procedure described by [31], organs were fixed with 10% formalin solution in 3–4 h, with Acetone three times (two hours in each). After that, the cleaning was done using toluene three times (1–2 h in each). Embedding the sample in paraffin liquid 60–70 °C three times (two hours in each). The paraffin blocks molding process are carried out. The paraffinblock's cutting stages were carried out using a microtome which obtains 5 μm sheet thickness. The layer placed in a water bath which is 30 °C, and then attached to a glass slide and heated in the oven for two-three minutes. The result was observed under a microscope light with 10 × 40 magnification; the number of necrosi sand healthy cells was observed.
2.11. Statistical analysis
Statistical package for social science (SPSS) program 21 was used to analysis of the data. Data are expressed as Mean ± SEM. Comparison for more than 2 groups by using one way ANOVA followed by post-hoc tukey. Statistical significance was set at p < 0,05.
3. Result
3.1. Antioxidant activity of extract
2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity was used to determine the IC50 of ethyl acetate of VA, ethanol extract of VA, and n-hexane extract of VA. Table 1 below shows that the lowest IC50 is 40.51 ± 4.89 μg/mL ethanol extract of VA. The lower the IC50, the stronger the antioxidant activity.
Table 1.
IC50 (μg/mL) activity of DPPH scavenging activity.
| Extract | IC50 μg/mL (Mean ± SEM) |
|---|---|
| Ascorbic acid | 2.693 ± 2.31 |
| Ethyl acetate of VA | 45.90 ± 4.98 |
| Ethanol extract of VA | 40.51 ± 4.89 |
| N-hexane extract of VA | 65.45 ± 3.51 |
Antioxidant activities use 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity method. Table 1 shows that ethanol extract of VA (40.51 ± 4.89 μ/mL) which has a strong scavenging activity compared with ethyl acetate of VA (45.90 ± 4.98 μ/mL) and n-hexane extract of VA (65.45 ± 3.51 μ/mL). Were IC50 under 50 μg/mL, then it would have strong antioxidant. The strength antioxidant ethanol extract of VA is caused of its secondary metabolite such luteolin which has strong antioxidant. Another study conducted by Hsieh 2018 reported that luteolin has IC50 (26.304 μg/mL). Therefore, this study enhances the reason that ethanol extract of VA can inhibit free radicals produced by doxorubicin in vivo method.
3.2. Total flavonoid content
Table 2 shows the total flavonoid of each extract, and the highest total flavonoid was in ethanol extract of VA 3.795 mg QE/g extract followed by ethyl acetate extract of VA 3.65 ± 0.56 mg QE/g extract and n-hexane extract of VA 1.59 ± 0,34 mg QE/g extract respectively.
Table 2.
Total flavonoid content.
| Extract | mg QE/g extract (Mean ± SEM) |
|---|---|
| Ethyl acetate of VA | 3.65 ± 0.56 |
| Ethanol extract of VA | 3.79 ± 0.61 |
| N-hexane extract of VA | 1.59 ± 0.34 |
Flavonoid is a secondary metabolite that commonly found in plants. The ethanol extract of VA has the highest flavonoid (3.79 ± 0,61 mg QE/g extract) compared with ethyl acetate of VA (3.65 ± 0,56 mg QE/g extract) and n-hexane extract of VA (1.59 ± 0,34 mg QE/g extract). Flavonoid has antioxidant activity, anti-cancer, and anti-diabetic. The higher Flavonoid content in the extract can indicate that the extract inhibits free radical. Some researches show that VA has 94,08 mg QE/g Flavonoid content in which the flavonoid are luteolin 7-O-beta-glucoside and luteolin 7-O-beta-glucuronoside as the most abundant [42].
3.3. Total phenol content
Table 3 shows the total phenol of each extract, and the highest flavonoid was in ethanol extract of VA 281.575 ± 1.0690 mg GAE/g followed by ethyl acetate of VA 61.154 ± 0.7278 mg GAE/g and n-hexane extract of VA 33.536 ± 0.7278 mg GAE/g respectively.
Table 3.
Total phenol content.
| Extract | mg GAE/g extract (Mean ± SEM) |
|---|---|
| Ethyl acetate of VA | 61.154 ± 0.7278 |
| Ethanol extract of VA | 281.575 ± 1.0690 |
| N-hexane extract of VA | 33.536 ± 0.7278 |
The highest phenol is in ethanol extract of VA 281.575 ± 1.0690 mg GAE/g. Other researches also showed that Vernonia amygdalina contains in high amount of phenol 113.76 mg GAE/g [42].
3.4. Serum biochemical parameters
Table 4 shows that negative control group increases AST, ALT, Ureum and Creatinine significantly compared with normal groups. The ethanol extract of VA (100 mg/kgbw and 200 mg/kgbw) lowers the AST, ALT, Ureum dan Creatinine is not significantly compared with negative control decline. However, ethanol extract of VA 400 mg/kg lowers AST, ALT, Ureum, and Creatinine significantly compared with negative control.
Table 4.
Serum biochemical parameters level.
| Treatment | AST (U/L) | AST (U/L) | Ureum (mg/dL) | Creatinine (mg/dL) |
|---|---|---|---|---|
| Normal | 52.89 ± 7.91 | 120.54 ± 18.3 | 44.67 ± 2,34 | 0.68 ± 0.02 |
| Control (−) | 120.65 ± 6.5∗ | 256.51 ± 16.2∗ | 112.56 ± 8,35∗ | 1.32 ± 0.15∗ |
| Control (+) | 48.33 ± 7.9 | 125.43 ± 12.71 | 48.67 ± 3,07 | 0.70 ± 0.18 |
| EEDA (100 mg/kgbw) | 86.91 ± 10.1 | 200.65 ± 3.31 | 84.53 ± 5,87 | 1.05 ± 0.45 |
| EEDA (200 mg/kgbw) | 70.54 ± 12.81 | 149.67 ± 9.76 | 56.29 ± 3,96 | 0.76 ± 0.17 |
| EEDA (400 mg/kgbw) | 49.31 ± 7.61# | 110.31 ± 8.91# | 45.76 ± 2,07# | 0.53 ± 0.11# |
Data are presented Mean ± SEM.
∗(p < 0,05) significant different from normal group.
#(p < 0,05) significant different from control (−) group.
Table 4 shows Amino-transaminase (AST), Alanine-transaminase (ALT), Ureum and Creatinine in each group. Doxorubicin causes the increase of AST 120.65 ± 6.5 U/L, ALT 256.51 ± 16.2 U/L, Ureum 112.56 ± 8.35 mg/dL, and Creatinine 1.32 ± 0.15 mg/dL. The first parameter used is AST to screen the cardiac injury. In contrast with now day parameter used, it was Troponin I, Troponin T, or BNP. The high ureum and creatinine compared with the normal group shows that doxorubicin causes not only cardiomyocyte injury but also nephron injury. The EEVA (400 mg/kgbw) lowers the biomarker to normal level while negative control (doxorubicin 20 mg/kgbw) increases AST, ALT, ALP, Ureum, and Creatinine significantly compared with normal group.
3.5. Cardiac biochemical parameters
Table 5 shows that negative control increases Troponin T, CK- MB, LDH and BNP significantly compared with normal group. Ethanol extract of VA 100 mg/kgbw and 200 mg/kgbw lowers Troponin T, CK-MB, LDH, and BNP not significantly compared with negative control. In contrast, EEVA 400 mg/kgbw lowers Troponin T, CK-MB, LDH, and BNP significantly compared with negative control.
Table 5.
Cardiac biochemical parameters level.
| Treatment | BNP (pg/ml) | TnT (pg/ml) | CK-MB (ng/ml) | LDH (ng/ml) |
|---|---|---|---|---|
| Normal | 87.81 ± 12.6 | 15.7 ± 2,8 | 25.67 ± 4.6 | 110.5 ± 12.9 |
| Control (−) | 150.5 ± 24.6∗ | 50.7 ± 2.1∗ | 90.89 ± 9.8∗ | 180.76 ± 3.5∗ |
| Control (+) | 98.67 ± 5.7 | 20.65 ± 4.67 | 30.87 ± 1.4 | 119.89 ± 0.76 |
| EEVA (100 mg/kgbw) | 167.7 ± 3.35 | 49.89 ± 11.9 | 87.77 ± 2.9 | 178.90 ± 2.1 |
| EEVA (200 mg/kgbw) | 110.45 ± 12.5 | 32.89 ± 2.9 | 60.87 ± 15.8 | 140.57 ± 2.9 |
| EEVA (400 mg/kgbw) | 80.89 ± 2.5# | 20.08 ± 4.09# | 39.98 ± 1.98# | 111.6 ± 11.51# |
Data are presented Mean ± SEM.
∗(p < 0,05) significant different from normal group.
#(p < 0,05) significant different from control (−) group.
Table 5 shows Troponin T, Brain Natriuretic Peptide (BNP), Lactate Dehydrogenase (LDH), and Creatinine Kinase – MB (CK-MB) levels. This research result proves that negative control causes the increase of biomarker such as Troponin T, CK- MB, LDH and BNP. Doxorubicin forms reactive oxygen species (ROS) that induces cardmiomyocyte. Thus, cardmiomyocyte will release biomarkers such as Troponin T, CK-MB, LDH and BNP [6]. The EEVA 400 mg/kg BW can scavenge ROS that is produced by doxorubicin. Possible mechanism of luteolin in VA can inhibit carbonyl reductase-3 which is used to change doxorubicin to doxorubicinol which can cause cardiac injury [46]. The higher dose of extract given, the higher ability to lower biomakers level. Today the only compound approved by Food Drug and Administration (FDA) as cardioprotective is Dexrazoxane [47, 48].
3.6. Cardiac antioxidant
Table 6 showed that control (−) group was significance decreased SOD level compared to normal group. Ethanol extract of VA (100 mg/kgbw dan 200 mg/kgbw) increased SOD level but this increase was not significant compared to control (−). Ethanol extract of VA 400 mg/kgbw increased SOD level and this increase was significant compare to control (−).
Table 6.
Cardiac antioxidant.
| Treatment | SOD (ng/ml) |
|---|---|
| Normal | 43,21 ± 2,18 |
| Control (−) | 14,3 ± 2,8∗ |
| Control (+) | 45,12 ± 2,4 |
| EEVA (100 mg/kgbw) | 20,41 ± 11,3 |
| EEVA (200 mg/kgbw) | 35,76 ± 13,5 |
| EEVA (400 mg/kgbw) | 45,78 ± 12,6# |
Data are presented Mean ± SEM.
∗(p < 0,05) significant different from normal group.
#(p < 0,05) significant different from control (−) group.
Negative control shows the decrease of SOD significantly compared with normal group. Thus, doxorubicin causes the increase of ROS and it makes ROS and SOD levels imbalance. The EEVA 400 mg/kgbw increases SOD significantly compared with negative control. In conclusion VA has an ability to increase endogen antioxidant including SOD. Another previous research reported that VA had an ability to increase endogen antioxidant (Glutathione and Superoxide dismutase) [49].
3.7. Histopathology
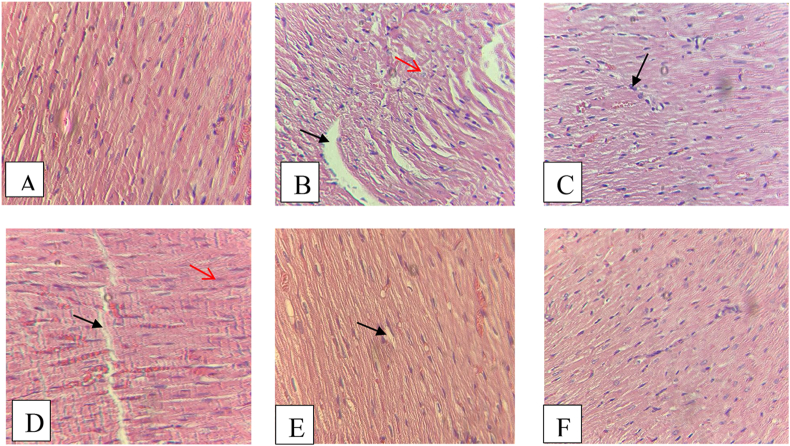
The characteristic of cardiac injury was found in the control negative group that only gave doxorubicin with hematoxylin-eosin staining, while in the normal group there were no changes on cardiac cell. Furthermore, in the group given EEVA dose 400 there were no changes on cardiac cells. Necrotic and aggregation were the main observed changes in the cardiac histopathology especially in the group control negative and EEVA (100 mg/kgbw). The histopathology of each group can be seen in the Figure 1
Figure 1.
Histopathology of heart tissue with H&E (10 × 40), (A) normal group showed a normal tissue without any necrotic cardiomyocytes, (B) control negative group that only give doxorubicin showed a necrotic (red arrow) and aggregation (black arrow) of cardiomyocytes, (C) control positive group showed a normal heart tissue, (D) EEVA (100 mg/kgbw) group showed a few necrotic (red arrow) and aggregation (black arrow) of heart tissue, (E) EEVA (200 mg/kgbw) group showed a few aggregation (black arrow) (F) EEVA (400 mg/kgbw) group showed a normal of heart tissue.
4. Discussion
This study aims to prove the cardioprotective effect of ethanol extracts of VA 100, 200, 400 mg/kg bw and doxorubicin 20 mg/kg bw. A previous study conducted by Asuquo showed that ethanol extract of VA (200 mg/kg bw) had a cardioprotective effect in streptozotocin (STZ)-induced rats (65 mg/kg bw) by reducing the incidence of hypertrophy and necrosis, while in this study found the incidence of necrotic and aggregation in cardiac histopathology on negative group (Figure 1) [31]. A study by Khang Wei reported that the ethanol extract of VA 400 mg/kg bw increased the activity of SOD, glutathione, and catalase in STZ-induced rats (65 mg/kg BW) [32]. VA is known to be rich in flavonoids that have potent antioxidant effects, including luteolin 7-O-β-glucuronoside and luteolin 7-O-β-glucoside [33]. VA contains many polyphenols, including 5-caffeoylquinic acid; luteolin-7-Oglucuronide; luteolin-7-O-glucoside; 1,5- 3,5- and 4,5-dicaffeoylquinic acids; luteolin; 4′-O-glucoside; apigenin 7-O-routineoside; apigenin 7-O-glucoside; and apigenin. Luteolin is mainly a polyphenol domain in Vernonia amygdalina [34]. According to Luo, luteolin is known to have anticancer, anti-inflammatory, and antioxidant activities. The cardioprotective mechanism of luteolin is to activate anti-apoptosis activity, increase cardiac function by regulating the MAPK family, block oxidative stress through haem oxygenase, increase the production of nitric oxide (NO) to prevent damage by free radicals, and improve systolic and diastolic function [35].
Doxorubicin is widely used for anticancer therapy, but its use is known to cause cardiotoxicity. Some of the doxorubicin mechanisms that cause this toxicity explicitly increase the production of reactive oxygen species (ROS) in mitochondria. ROS produce enzymes in the mitochondria that convert doxorubicin into semiquinone, and semiquinone reacts very quickly with oxygen and forms superoxide anion (O-). Doxorubicin is also known to cause decreased production of endogenous antioxidants, including GSH, catalase, and SOD. Some of the agents that have been used to prevent cardiotoxicity during doxorubicin use are dexrazoxane, carvedilol, omega 3 fatty acids, and natural products that can increase the production of antioxidants in cells, act as free radical scavengers, and lower lipid peroxidation [36]. Pongprot reported that patients treated with doxorubicin exhibit left ventricular dysfunction as well as elevated levels of cardiac biochemical parameters such as creatinine kinase-MB (CK-MB) and N-terminal pro-brain natriuretic peptides (N-proBNP) [37]. A rat cardiotoxicity model induced by doxorubicin revealed that the levels of CK-MB and lactate dehydrogenase were increased after treatment with doxorubicin [38]. Doxorubicin also increased the levels of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in rats [39].
The methanol extract at a VA dose of 500 mg/kg BW reduced AST and ALT levels in rats induced by carbon tetrachloride (CCL4 1.2 mg/kg bw), increased the concentrations of GSH, CAT, and SOD, and decreased MDA levels [40]. VA also contains steroid saponins, including vernoniamyoside A, vernoniamyoside B, vernoniamyoside C, vernoniamyoside D, vernoamyoside D, and vernonioside B2, which have cytotoxic activity on BT-549, MDA-MB-231, MCF, and HeLa cells [16]. Our research regarding the toxicity of VA shows that steroid glycosides in VA, such as vernonioside D, are estimated to have an LD50 of 8000 mg/kg [40]. Another acute oral toxicity study reported by Zakaria reported that the toxicity of African leaves is more than 5000 mg/kg BW [41].
5. Conclusion
This study proves that the ethanol extract of VA acts as a cardioprotective agent and reduces the levels of cardiac injury biomarkers such as BNP, Troponin T, CK-MB, and LDH. Furthermore, in the future VA can be developed into additional therapy while using doxorubicin to prevent cardiotoxicity.
Declarations
Author contribution statement
R.A. Syahputra: Performed the experiments; Wrote the paper.
U.Harahap: Conceived and designed the experiments.
A. Dalimunthe; M. Pandapotan: Contributed reagents, materials, analysis tools or data.
D. Satria: Analyzed and interpreted the data.
Funding statement
This work was supported by Universitas Sumatera Utara through DRPM 2020.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Anon Doxorubicin. React. Wkly. 2015;1580(1):157. [Google Scholar]
- 2.Bennink R.J., van den Hoff M.J., van Hemert F.J., de Bruin K.M., Spijkerboer A.L., Vanderheyden J.L.…van Eck-Smit B.L. Annexin V imaging of acute doxorubicin cardiotoxicity (apoptosis) in rats. J. Nucl. Med. 2004;45(5):842–848. [PubMed] [Google Scholar]
- 3.Hao G., Yu Y., Gu B., Xing Y., Xue M. Protective effects of berberine against doxorubicin-induced cardiotoxicity in rats by inhibiting metabolism of doxorubicin. Xenobiotica. 2015;45(11):1024–1029. doi: 10.3109/00498254.2015.1034223. [DOI] [PubMed] [Google Scholar]
- 4.Hamaguchi T., Azuma J., Awata N., Ohta H., Takihara K., Harada H.…Sperelakis N. Reduction of doxorubicin-induced cardiotoxicity in mice by taurine. Res. Commun. Chem. Pathol. Pharmacol. 1988;59(1):21. [PubMed] [Google Scholar]
- 5.P N.R., P S.R., M R.R. Studies on the effect of doxorubicin on MDA, NO2, NO3, Se-GSH peroxidase and SOD levels in albino rat tissues. Afr. J. Biotechnol. Acad. J. 2007 Oct 18;6(20):2303–2309. [Google Scholar]
- 6.Mitry M.A., Edwards J.G. Doxorubicin induced heart failure: phenotype and molecular mechanisms. IJC Heart Vasc. 2016;10:17–24. doi: 10.1016/j.ijcha.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Jr., Drazner M.H.…Johnson M.R. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 8.Liquori M.E., Christenson R.H., Collinson P.O., deFilippi C.R. Cardiac biomarkers in heart failure. Clin. Biochem. 2014;47(6):327–337. doi: 10.1016/j.clinbiochem.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 9.Braunwald E. Biomarkers in heart failure. N. Engl. J. Med. 2008;358(20):2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 10.Luo X., Jiang Y., Fronczek F.R., Lin C., Izevbigie E.B., Lee K.S. Isolation and structure determination of a sesquiterpene lactone (vernodalinol) from Vernonia amygdalina extracts. Pharmaceut. Biol. 2011;49(5):464–470. doi: 10.3109/13880209.2010.523429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alara O.R., Abdurahman N.H., Ukaegbu C.I., Hassan Z., Kabbashi N.A. Dataset on LC-Q-TOF/MS tentative identification of phytochemicals in the extract of Vernonia amygdalina leaf through positive ionization. Data in Brief. 2018;21:1686. doi: 10.1016/j.dib.2018.10.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jisaka M., Ohigashi H., Takegawa K., Hirota M., Irie R., Huffman M.A., Koshimįzu K. Steroid glucosides from Vernonia amygdalina, a possible chimpanzee medicinal plant. Phytochemistry. 1993;34(2):409–413. [Google Scholar]
- 13.Jisaka M., Ohigashi H., Takagaki T., Nozaki H., Tada T., Hirota M.…Koshimizu K. Bitter steroid glucosides, vernoniosides A1, A2, and A3, and related B1 from a possible medicinal plant, Vernonia amygdalina, used by wild chimpanzees. Tetrahedron. 1992;48(4):625–632. [Google Scholar]
- 14.Quasie O., Zhang Y.M., Zhang H.J., Luo J., Kong L.Y. Four new steroid saponins with highly oxidized side chains from the leaves of Vernonia amygdalina. Phytochem. Lett. 2016;15:16–20. [Google Scholar]
- 15.Igile G., Olenszek W., Jurzysta M., Aquino R., de Tommasi N., Pizza C. Vemoniosides D and E, two novel saponins from Vernonia amygdalina. J. Nat. Prod. 1995;58(9):1438–1443. [Google Scholar]
- 16.Wang J., Song H., Wu X., Zhang S., Gao X., Li F.…Chen Q. Steroidal saponins from Vernonia amygdalina Del. and their biological activity. Molecules. 2018;23(3):579. doi: 10.3390/molecules23030579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagrov A.Y., Shapiro J.I., Fedorova O.V. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol. Rev. 2009;61(1):9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abosi A.O., Raseroka B.H. In vivo antimalarial activity of Vernonia amygdalina. Br. J. Biomed. Sci. 2003;60(2):89–91. doi: 10.1080/09674845.2003.11783680. [DOI] [PubMed] [Google Scholar]
- 19.Asante D.B., Effah-Yeboah E., Barnes P., Abban H.A., Ameyaw E.O., Boampong J.N.…Dadzie J.B. Antidiabetic effect of young and old ethanolic leaf extracts of Vernonia amygdalina: a comparative study. J. Diabet. Res. 2016;2016 doi: 10.1155/2016/8252741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gresham L.J., Ross J., Izevbigie E.B. Vernonia amygdalina: anticancer activity, authentication, and adulteration detection. Int. J. Environ. Res. Publ. Health. 2008;5(5):342–348. doi: 10.3390/ijerph5050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adesanoye O.A., Farombi E.O. Hepatoprotective effects of Vernonia amygdalina (astereaceae) in rats treated with carbon tetrachloride. Exp. Toxicol. Pathol. 2010;62(2):197–206. doi: 10.1016/j.etp.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Iwara I.A., Otu E.A., Efiong E.E., Igile G.O., Mgbeje B.I.A., Ebong P.E. Evaluation of the nephroprotective effects of combined extracts of Vernonia amygdalina and Moringa oleifera in diabetes induced kidney injury in albino Wistar rats. Scholars J. Appl. Med. Sci. 2013;1:881–886. [Google Scholar]
- 23.Tijjani M.A., Mohammed G.T., Alkali Y.T., Adamu T.B., Abdurahaman F.I. Phytochemical analysis, analgesic and antipyretic properties of ethanolic leaf extract of Vernonia amygdalina Del. J. Herbmed Pharmacol. 2017;6(3):95–99. [Google Scholar]
- 24.Alo M.N., Anyim C., Igwe J.C., Elom M., Uchenna D.S. Antibacterial activity of water, ethanol and methanol extracts of Ocimum gratissimum, Vernonia amygdalina and Aframomum melegueta. Adv. Appl. Sci. Res. 2012;3(2):844–848. [Google Scholar]
- 25.Erasto P., Grierson D.S., Afolayan A.J. Evaluation of antioxidant activity and the fatty acid profile of the leaves of Vernonia amygdalina growing in South Africa. Food Chem. 2007;104(2):636–642. [Google Scholar]
- 26.Zakaria Y., Azlan N.Z., Nik N.F., Muhammad H. Phytochemicals and acute oral toxicity studies of the aqueous extract of Vernonia amygdalina from state of Malaysia. J. Med. Plants Stud. 2016;4(3):1–5. [Google Scholar]
- 27.Luo Y., Shang P., Li D. Luteolin: a flavonoid that has multiple cardio-protective effects and its molecular mechanisms. Front. Pharmacol. 2017;8:692. doi: 10.3389/fphar.2017.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199–1200. [Google Scholar]
- 29.Sánchez-Rangel J.C., Benavides J., Heredia J.B., Cisneros-Zevallos L., Jacobo-Velázquez D.A. The Folin–Ciocalteu assay revisited: improvement of its specificity for total phenolic content determination. Anal. Methods. 2013;5(21):5990–5999. [Google Scholar]
- 30.Pękal A., Pyrzynska K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods. 2014;7(9):1776–1782. [Google Scholar]
- 31.Asuquo O.R., Igiri A.O., Akpan J.E., Akpaso M.I. Cardioprotective potential of Vernonia amygdalina and Ocimum gratissimum against streptozotocin (Stz)–Induced diabetes in wistar rats. Trop. Med. 2010;7(1) [Google Scholar]
- 32.Ong K.W., Hsu A., Song L., Huang D., Tan B.K.H. Polyphenols-rich Vernonia amygdalina shows anti-diabetic effects in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2011;133(2):598–607. doi: 10.1016/j.jep.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 33.Igile G.O., Oleszek W., Jurzysta M., Burda S., Fafunso M., Fasanmade A.A. Flavonoids from Vernonia amygdalina and their antioxidant activities. J. Agric. Food Chem. 1994;42(11):2445–2448. [Google Scholar]
- 34.Dagnon S., Novkova Z., Bojilov D., Nedialkov P., Kouassi C. Development of surrogate standards approach for the determination of polyphenols in Vernonia amygdalina Del. J. Food Compos. Anal. 2019;82:103231. [Google Scholar]
- 35.Luo Y., Shang P., Li D. Luteolin: a flavonoid that has multiple cardio-protective effects and its molecular mechanisms. Front. Pharmacol. 2017;8:692. doi: 10.3389/fphar.2017.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takemura G., Fujiwara H. Doxorubicin-induced cardiomyopathy: from the cardiotoxic mechanisms to management. Prog. Cardiovasc. Dis. 2007;49(5):330–352. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Pongprot Y., Sittiwangkul R., Charoenkwan P., Silvilairat S. Use of cardiac markers for monitoring of doxorubicin-induced cardiotoxicity in children with cancer. J. Pediatr. Hematol. Oncol. 2012;34(8):589–595. doi: 10.1097/MPH.0b013e31826faf44. [DOI] [PubMed] [Google Scholar]
- 38.Nugraha, S. E., Yuandani, E. S., & Syahputra, R. A. Investigation of Phytochemical Constituents and Cardioprotective Activity of Ethanol Extract of Beetroot (Beta Vulgaris. L) on Doxorubicin Induced Toxicity in Rat.
- 39.Barakat B.M., Ahmed H.I., Bahr H.I., Elbahaie A.M. Protective effect of boswellic acids against doxorubicin-induced hepatotoxicity: Impact on Nrf 2/HO-1 defense pathway. Oxidative Medicine and Cellular Longevity. 2018;2018 doi: 10.1155/2018/8296451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Syahputra, R. A., Harahap, U., Dalimunthe, A., Nasution, P., Haro, G., Widodo, D. H., & Satria, D. In-silico TOXICITY PREDICTION OF BIOACTIVE COMPOUNDS OF Vernonia amygdalina DELILE. AND DIGOXIN.
- 41.Zakaria Y., Azlan N.Z., Nik N.F., Muhammad H. Phytochemicals and acute oral toxicity studies of the aqueous extract of Vernonia amygdalina from state of Malaysia. J. Med. Plants Stud. 2016;4(3):1–5. [Google Scholar]
- 42.Alara O.R., Abdurahman N.H., Olalere O.A. Ethanolic extraction of bioactive compounds from Vernonia amygdalina leaf using response surface methodology as an optimization tool. J. Food Measur. Char. 2018;12(2):1107–1122. [Google Scholar]
- 43.Reitman S., Frankel F. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28:56. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 44.Beale R.N., Croft D. A sensitive method for the colorimetric determination of urea. J. Clin. Pathol. 1961;14(4):418–424. doi: 10.1136/jcp.14.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunn S.R., Qi Z., Bottinger E.P., Breyer M.D., Sharma K. Utility of endogenous creatinine clearance as a measure of renal function in mice. Kidney Int. 2004;65(5):1959–1967. doi: 10.1111/j.1523-1755.2004.00600.x. [DOI] [PubMed] [Google Scholar]
- 46.Kaiserová H., Šimůnek T., Van Der Vijgh W.J., Bast A., Kvasničková E. Flavonoids as protectors against doxorubicin cardiotoxicity: role of iron chelation, antioxidant activity and inhibition of carbonyl reductase. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2007;1772(9):1065–1074. doi: 10.1016/j.bbadis.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Kawasaki T., Sakai C., Harimoto K., Yamano M., Miki S., Kamitani T. Usefulness of high-sensitivity cardiac troponin T and brain natriuretic peptide as biomarkers of myocardial fibrosis in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2013;112(6):867–872. doi: 10.1016/j.amjcard.2013.04.060. [DOI] [PubMed] [Google Scholar]
- 48.Kane R.C., McGuinn W.D., Jr., Dagher R., Justice R., Pazdur R. Dexrazoxane (Totect™): FDA review and approval for the treatment of accidental extravasation following intravenous anthracycline chemotherapy. Oncologist. 2008;13(4) doi: 10.1634/theoncologist.2007-0247. [DOI] [PubMed] [Google Scholar]
- 49.Erukainure O.L., Chukwuma C.I., Sanni O., Matsabisa M.G., Islam M.S. 2019. Histochemistry, Phenolic Content, Antioxidant, and Anti-diab. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.