Highlights
-
•
Locoregional radiotherapy can prolong OS for patients with de novo metastatic nasopharyngeal carcinoma.
-
•
Nasopharyngeal carcinoma patients with oligometastic disease can benefit from locoregional radiotherapy rather than polymetastatic disease.
Keywords: Metastatic nasopharyngeal carcinoma, Locoregional radiotherapy, Oligometastatic disease, Polymetastatic disease
Abbreviations: LRRT, locoregional radiotherapy; PCT, palliative chemotherapy; NPC, nasopharyngeal carcinoma; dmNPC, de novo metastatic nasopharyngeal carcinoma; PCT, Palliative chemotherapy; OMD, Oligometastatic diseases; PMD, polymetastatic disease; OS, Overall survival; MVA, multivariate analyses; EBV, Epstein–Barr virus; LDH, Lactate dehydrogenase; KPS, Karnofsky performance score
Abstract
Background
To evaluate the value of locoregional radiotherapy (LRRT) in de novo metastatic nasopharyngeal carcinoma (dmNPC) and identify predictive factors for additional LRRT after palliative chemotherapy (PCT).
Methods
Overall survival (OS) was the primary endpoint. Patients who underwent PCT and LRRT were categorized as the PCT+LRRT group; patients who only received palliative chemotherapy were categorized as the PCT group. Oligometastatic diseases (OMD) was defined as ≤5 metastatic lesions and ≤2 metastatic organs.
Results
A total of 168 patients were included for this study. The median OS of patients in the PCT+LRRT group was significantly higher than those in the PCT group (57 months vs. 22 months, P<0.001). Multivariate analyses (MVA) showed that LRRT (HR=0.533, 95% CI: 0.319–0.889, P = 0.016) and OMD (HR=0.548, 95% CI: 0.331–0.907, P = 0.019) were independent prognostic factors for dmNPC. Furthermore, Kaplan–Meier analyses showed that the 3-year OS of patients who received LRRT was significantly better than those who did not receive LRRT in the OMD subgroup (66.3% vs. 25.2%, P<0.001). While, the 3-year OS of patients who received LRRT and without LRRT was no different in the polymetastatic disease (PMD) subgroup (38.9% vs.11.5%, P = 0.115). MVA showed that LRRT was a favorable prognosticator in the OMD subgroup (HR=0.308, 95% CI: 0.159–0.598; P<0.001), and not a favorable prognosticator in the PMD subgroup (HR=0.510, 95% CI: 0.256–1.014, P = 0.055).
Conclusions
LRRT has the potential to prolong OS in NPC patients with de novo OMD. These results suggest that OMD is a potential indicator for filtering beneficiaries from LRRT.
Introduction
Nasopharyngeal carcinoma (NPC) is a common malignant tumor in southeastern China, especially in Hong Kong, Guangdong, Guangxi, Fujian, and Jiangxi [1], [2], [3]. The incidence of de novo metastatic NPC (dmNPC) ranged from 6% to 15% [3, 4]. The prognosis of dmNPC is poor, and the median overall survival (OS) time is 12–21 months [5], [6], [7], [8], [9], [10]. At present, the internationally recommended treatment for dmNPC is systemic chemotherapy regardless of EBV-DNA status, and platinum-based combination chemotherapy is recommended [11]. In addition, several investigators found that dmNPC patients who receive locoregional radiation therapy (LRRT) on the nasopharynx and neck lesions may achieve better survival, by retrospective analyses [8, 12]. Recently, a prospective study confirmed that dmNPC patients who underwent LRRT plus palliative chemotherapy (PCT) achieved longer overall survival (OS) than those who received PCT alone [13]. However, it was unclear whether all patients benefited from the LRRT, and whether there is a subgroup of dmNPC that has a significant benefit from LRRT.
In 1995, Hellman and Weichselbaum jointly proposed the concept of "oligotransfer" [14]. The oligometastatic disease (OMD) is a period of relatively mild tumor biological invasiveness, which was in the transitional stage between the local-regional advance state and extensive metastasis. For OMD, the number of metastatic lesions and metastatic organs is limited. A series of subsequent studies found that the prognosis of OMD was significantly better than that of poly-metastatic disease (PMD) [15, 16]. In recent years, a number of studies, including prospective studies, have also confirmed that patients with OMD can benefit from local treatment (radiotherapy, surgery, or radiofrequency ablation) [8, 17, 18]. One study found that only patients with undetectable Epstein–Barr virus (EBV) DNA after PCT can benefit from post-PCT LRRT [19]. Unfortunately, the detection of EBV DNA has not been standardized in most institutions and popularized universally, which limits its clinical application. It is necessary to find indicators or classifications that can be widely used and accurately screen the population benefiting from local treatment. Recently, one retrospective study showed that not all dmNPC patients could benefit from LRRT; perhaps only OMD patients can benefit from it, and PMD patients do not necessarily benefit from LRRT [20]. More research evidence is needed to determine whether OMD is a marker for screening the population benefiting from LRRT.
Therefore, we carried out a real-world study to analyze the value of LRRT for dmNPC patients, and at the same time, explore whether OMD can be used as an indicator for screening patients who benefit from LRRT.
Material and methods
Patients
This study was approved by the Institutional Ethics Committee of the Nanchang University Cancer Hospital (2021ky078), China. Patients with histologically proven NPC who had distant metastasis at initial diagnosis at our institution between January 2010 to December 2019 were included. Other inclusion criteria were: 1) no history of previous treatment or prior malignancy; 2) a completed pretreatment evaluation according to our institutional protocol [21]; 3) availability of imaging data that permitted re-staging according to the TNM-8 [22]; receipt of platinum-based chemotherapy as a first-line treatment for a minimum of two-cycle with or without definitive radiotherapy at the nasopharynx and neck. Notably, patients who received definitive radiotherapy via conventional techniques, but not intensity-modulated radiation therapy (IMRT), would be excluded. In our center, the IMRT prescription dose for primary metastatic nasopharyngeal carcinoma and neck lesions was 60–70 Gy. OMD was defined as ≤5 metastatic lesions and ≤2 metastatic organs (such as the liver, lungs, bones, mediastinal lymph nodes, axillary lymph nodes, retroperitoneal lymph nodes, etc.) based on pathological examination and/or multiple radiologic imaging, while polymetastatic disease (PMD) referred to those who had >5 tumor lesions and/or >2 metastatic organs [23, 24].
Palliative chemotherapy
All mNPC patients received at least 2 cycles of platinum-based PCT regimes. PCT regimes used mostly in our institution were gemcitabine or paclitaxel, or docetaxel plus platinum every 3weeks per cycle. The dose regimen of every agent applied were as follows: (1) 1000 mg/m2 of gemcitabine on day 1 and 8; (2) 135–175 mg/m2 of paclitaxel on days 1; (3) 75 mg/m2 of docetaxel on day 1; (4) the most preferred platinum was cisplatin (a total of 80 mg/m2 for day 1–3), nedaplatin (80–100 mg/m2 on day 2) or lobaplatin (25–30 mg/m2 on day 2).
Local treatment
Patients who underwent LRRT on the primary site and cervical lymph nodes via IMRT technique were treated according to our institutional protocol(25). Detailed descriptions of IMRT plans and dose prescriptions have been published previously [25]. Briefly, the radiation dose prescribed in the protocol evolved: a total amount of 60–72 Gy to the PTVs of GTV, 60–66 Gy to the PTV of CTV-1, and 54–60 Gy to PTVs of CTV-2 and CTV-N. Patients with metastatic foci in the bones, lungs, and non-cervical lymph nodes amenable to local therapy were offered radiation therapy, surgery, and/or hyperthermia at the attending physician's discretion. The main organs of radiotherapy for metastatic lesions were bone and liver. In general, allowable doses for bone ranged from 30 to 54 Gy, such as 2 Gy/F × 20F, 2 Gy/F × 25F, 3 Gy/F × 10F, etc. The main dose of liver radiotherapy was 2 Gy/F × 20F, 2 Gy/F × 25F, or 5 Gy/F × 8F, depending on target size and location.
Follow-up
Pre-treatment evaluation included complete patient data, physical examination, complete blood count, renal and liver function tests, and magnetic resonance imaging (MRI) of the nasopharyngeal and neck regions, or tomography (CT) of the nasopharyngeal and neck regions, chest x-rays or computed tomography (CT), abdominal ultrasound or CT, single photon emission computed tomography whole-body skeletal scan, or PET/CT examination. The treatment evaluation is performed every 2 cycles of chemotherapy, and every 3 months after the treatment is completed. The solid tumor response evaluation criteria (RECIST 1.1) was used to evaluate tumor response [26]. This protocol complied with ethical standards and was approved by the ethics committee. All methods are carried out in accordance with relevant guidelines and regulations.
Statistical analysis
Overall survival (OS) was defined as the duration from the date of diagnosis to death from any cause or the last follow-up. Pearson χ2 test or Fisher's test was used to compare the basic clinical characteristics of patients in different groups. Kaplan–Meier survival analyses were used to estimate the OS with log-rank test for comparison of survival curves. Univariate and multivariate analyses (MVA) with Cox proportional hazard methods were used to estimate the risk of death. The MVA models were constructed using a backward step. The criterion for the backward step is for significance at the 0.05 level before a variable could stay in the model, while parameters with a significance of greater than 0.10 were removed from the model. A bilateral P value <0.05 was considered to be statistically significant. All data in the study were analyzed using R (version 3.6.1) and SPSS (SPSS 26.0 IBM Corp, Armonk, NY).
Results
Patient characteristics
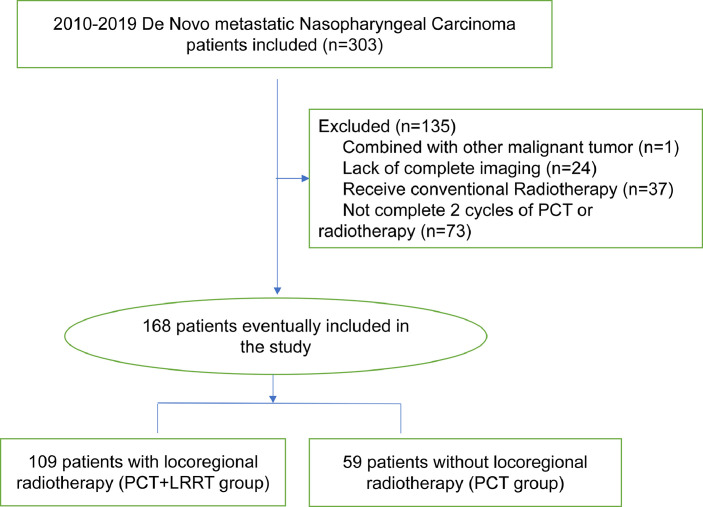
A total of 303 patients with nasopharyngeal carcinoma who were diagnosed with dmNPC from January 2010 to December 2019 were included in this study. A total of 135 were excluded, of which 73 patients did not complete 2 cycles of PCT, 24 patients lacked complete imaging images, 37 received conventional radiotherapy, and 1 had other primary malignant tumors (Fig. 1). In the end, 168 patients were included in the following analysis. One hundred and nine patients (64.9%) who underwent PCT and LRRT for nasopharyngeal and neck were divided into PCT+LRRT group and 59 patients (35.1%) who only received PCT were categorized as the PCT group. The baseline information and disease characteristics of the two groups were basically balanced, and detailed information was shown in Table 1.
Fig. 1.
Flowchart.
Table 1.
Clinical Characteristics of 168 de Novo Metastatic Nasopharyngeal Carcinoma Patients.
| Characteristics | No.(%) | P | |
|---|---|---|---|
| PCT+LRRT group(n = 109) | PCT group (n = 59) | ||
| Gender | 0.074 | ||
| Male | 86 (78.9) | 53 (89.8) | |
| Female | 23 (21.1) | 6 (10.2) | |
| Age, median(Range) | 50.0 (18.0–79.0) | 55.0 (29.0- 74.0) | 0.003 |
| KPS | 0.366 | ||
| >80 | 83 (76.1) | 45 (76.3) | |
| ≤80 | 26 (23.9) | 14 (23.7) | |
| T classification | 0.491 | ||
| T1–3 | 70 (64.2) | 41 (69.5) | |
| T4 | 39 (35.8) | 18 (30.5) | |
| N classification | 0.001 | ||
| N0–2 | 69 (63.3) | 22 (37.3) | |
| N3 | 40 (36.7) | 37 (62.7) | |
| LDH (IU/L) | <0.001 | ||
| Normal (≤250) | 85 (78.0) | 29 (49.2) | |
| Abnormal (>250) | 24 (22.0) | 30 (50.8) | |
| EBV DNA(Copies/ml) | <0.001 | ||
| ≤4000 | 64(58.7) | 13 (22.0) | |
| >4000 | 41 (37.6) | 40 (67.8) | |
| missing | 4 (3.7) | 6 (10.2) | |
| Chemotherapy cycles | 0.002 | ||
| 2–5 | 58 (53.2) | 46 (78.0) | |
| ≥6 | 51 (46.8) | 13 (22.0) | |
| Sites of metastases | |||
| Bone | 0.300 | ||
| No | 42 (38.5) | 18 (30.5) | |
| Yes | 67 (61.5) | 41 (69.5) | |
| Liver | 0.007 | ||
| No | 75 (68.8) | 28 (47.5) | |
| Yes | 34 (31.2) | 31 (52.5) | |
| Lung | 0.790 | ||
| No | 83 (76.1) | 46 (78.0) | |
| Yes | 26 (23.9) | 13 (22.0) | |
| Others | 0.504 | ||
| No | 93 (85.3) | 48 (81.4) | |
| Yes | 16 (14.7) | 11 (18.6) | |
| Number of metastases organ | 0.447 | ||
| 1–2 | 100 (91.7) | 52 (88.1) | |
| >2 | 9 (8.3) | 7 (11.9) | |
| Numbers of metastatic lesions | <0.001 | ||
| 1–5 | 78 (71.6) | 26 (44.1) | |
| ≥6 | 31 (28.4) | 33 (55.9) | |
| Radiotherapy for metastatic lesions | 0.001 | ||
| No | 76 (69.7) | 59 (100.0) | |
| Yes | 33 (30.3) | 0 (0.0) | |
| Oligometastastic disease | <0.001 | ||
| No | 31 (28.4) | 33 (55.9) | |
| Yes | 78 (71.6) | 26 (44.1) |
Abbreviation: LRRT, locoregional radiotherapy; PCT: palliative chemotherapy; KPS, Karnofsky performance status score; LDH, Lactate dehydrogenase; EBV, Epstein–Barr virus.
In this study, the median follow-up time was 44 months (1–110 months), the median OS of the whole group was 34 months (1–110 months). The median radiotherapy dose was 70.0 Gy (22.0–72.0 Gy), of which 99 patients (90.8%) were administered locoregional radiotherapy doses of 60.0 Gy and above (60.0–72.0 Gy). Ten patients (9.2%) were administered a locoregional radiotherapy dose of <60.0 Gy (2.2–54.0 Gy), of which 2 patients refused to continue radiotherapy due to personal reasons; three patients progressed during radiotherapy; three patients refused to continue radiotherapy due to sore throat; and the remaining 2 patients did not reach the radical dose due to economic reasons.
Survival analyses of LRRT in the whole group
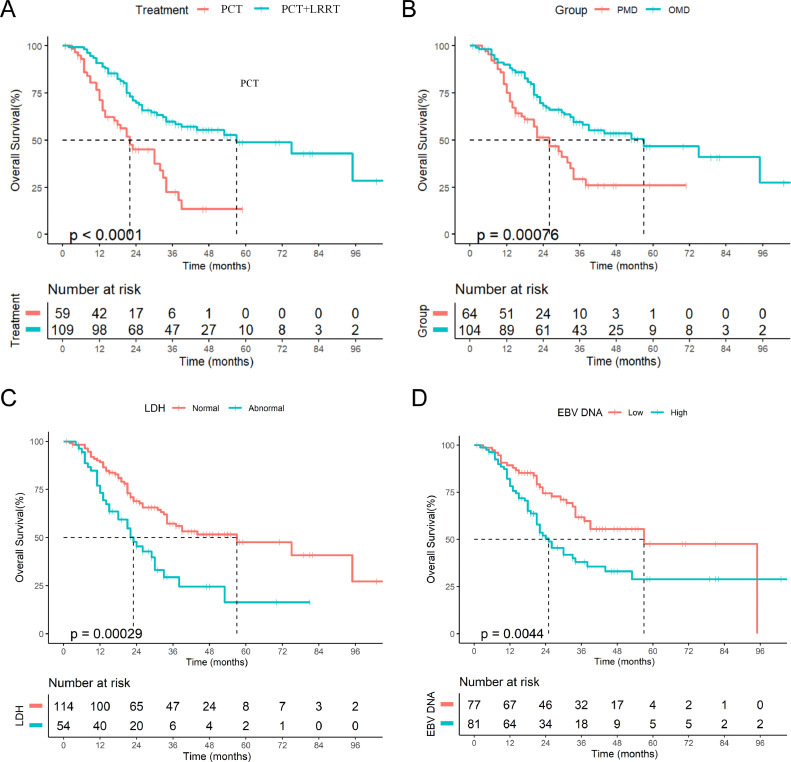
The median OS of patients in the PCT+LRRT group was significantly longer than that in the PCT group (57 months vs. 22 months, P<0.001, Fig. 2A). The 1-year, 2-year, and 3-year OS for patients in the PCT group were 71.1%, 44.0%, and 20.3%, respectively. The 1-year, 2-year, and 3-year OS for patients in the PCT+LRRT group were 89.8%, 69.8%, and 58.0%, respectively.
Fig. 2.
The Kaplan-Meier curves of OS in dmNPC patients: (A) OS for patients in locoregional radiotherapy and palliative chemotherapy groups; (B) OS for patients with oligometastatic and polymetastatic disease; (C) OS for patients with high or low level of EBV-DNA at pre-treatment; (D) OS for patients with normal and abnormal lactate dehydrogenase.
In addition, univariate analyses showed that OMD (HR=0.480, 95% CI: 0.309–0.746, P<0.001, Fig. 2B), LDH level (HR=2.212, 95% CI:1.418–3.453, P<0.001, Fig. 2C), plasma EBV DNA (HR=1.918, 95% CI:1.210–3.042, P = 0.004, Fig. 2D), total chemotherapy cycles (HR=0.567, 95% CI:0.355–0.905, P = 0.015), Karnofsky performance score (KPS) (HR=0.614, 95% CI:0.328–0.989, P = 0.041), and liver metastasis (HR=1.711, 95% CI:1.107–2.644, P = 0.013) were potential prognostic factors of OS (Table 2). While lung metastasis (HR=0.564, 95% CI:0.312–1.020, P = 0.052), bone metastases (HR=1.420, 95% CI:0.892–2.260, P = 0.133), other metastatic sites (HR=1.450, 95% CI:0.815–2.579, P = 0.199), T stage (HR=1.308, 95% CI:0.839–2.039, P = 0.235), gender (HR=0.596, 95% CI:0.315–1.128, P = 0.104), age (HR=0.941, 95% CI:0.613–1.444, P = 0.779), N staging (HR=1.013, 95% CI: 0.635–1.621, P = 0.980), and radiotherapy for metastatic sites (HR=0.786, 95% CI:0.448–1.377, P = 0.393) were not potential prognostic factors for OS (Table 2).
Table 2.
Univariate and Multivariate Analyses of OS by Locoregional Radiotherapy Adjusting for Other Potential Predictors in de Novo Metastatic Nasopharyngeal Carcinoma Patients. .
| Characteristics | Univariate analyses | Multivariate analyses | ||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Gender | 0.104 | |||
| Male | Reference 0.596 (0.315–1.128) | |||
| Female | ||||
| Age (years) | 0.212 | |||
| ≥52 | Reference 0.941 (0.613–1.444) | |||
| <52 | ||||
| KPS | 0.041 | 0.058 | ||
| ≤80 | Reference 0.614 (0.328–0.989) | Reference 0.614 (0.371–1.017) | ||
| >80 | ||||
| T classification | 0.230 | |||
| T1–3 | Reference 1.308 (0.839–2.039) | |||
| T4 | ||||
| N classification | 0.980 | |||
| N0–2 | Reference 1.013 (0.635–1.621) | |||
| N3 | ||||
| LDH (IU/L) | <0.001 | |||
| Normal (≤250) | Reference 2.212(1.418–3.4530 | |||
| Abnormal (>250) | ||||
| EBV DNA(Copies/ml) | 0.004 | |||
| ≤4000 | reference | |||
| >4000 | 1.918 (1.210–3.042) | |||
| Chemotherapy cycles | 0.015 | 0.094 | ||
| <6 | reference | reference | ||
| ≥6 | 0.567 (0.355–0.905) | 0.641 (0.381–1.078) | ||
| Radiotherapy for metastatic lesions | 0.393 | |||
| No | Reference 0.786 (0.448–1.377) | |||
| Yes | ||||
| Liver metastases | 0.013 | |||
| No | reference | |||
| Yes | 1.711(1.107–2.644) | |||
| Lung metastases | 0.052 | |||
| No | reference | |||
| Yes | 0.564 (0.312–1.020) | |||
| Bone metastases | 0.133 | |||
| No | reference | |||
| Yes | 1.420 (0.892–2.260) | |||
| Other metastases | 0.199 | |||
| No | reference | |||
| Yes | 1.450 (0.815–2.579) | |||
| Locoregional radiotherapy | <0.001 | 0.016 | ||
| No | Reference 0.373 (0.239–0.583) | Reference 0.533 (0.319–0.889) | ||
| Yes | ||||
| Oligometastatic disease | <0.001 | 0.019 | ||
| No | Reference 0.480 (0.309–0.746) | Reference 0.548 (0.331–0.907) | ||
| Yes | ||||
Abbreviation: KPS, Karnofsky performance status score; LDH, Lactate dehydrogenase; EBV, Epstein–Barr virus.
After adjusting for liver metastases, EBV DNA, LDH, chemotherapy cycles, KPS, and OMD, and LRRT, MVA showed that LRRT (HR=0.533, 95% CI:0.319–0.889, P = 0.016) and OMD (HR=0.548, 95% CI:0.331–0.907, P = 0.019) were independent prognostic factors for dmNPC, while KPS (HR=0.614, 95% CI:0.371–1.017, P = 0.058), and chemotherapy cycles (HR=0.641, 95% CI:0.381–1.078, P = 0.094) were not independent prognostic factors for OS (Table 2).
Survival analysis of LRRT in subgroup
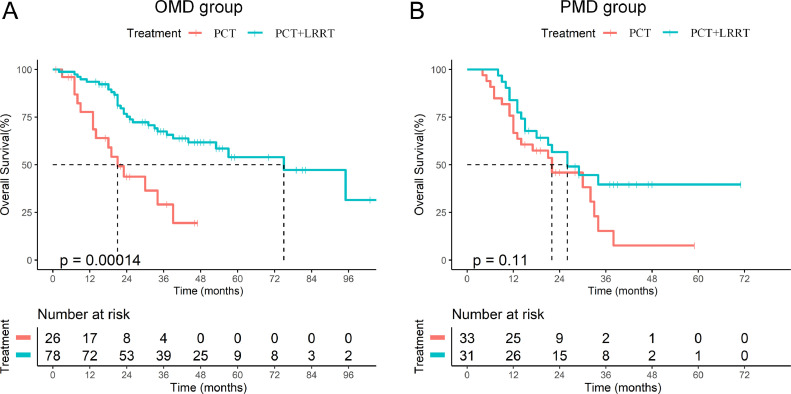
In order to further analyze the beneficiaries of LRRT, we conducted a stratified analysis of the value of LRRT based on the results of multivariate analyses. Kaplan–Meier analyses showed that the 3-year OS of patients with LRRT was significantly better than that of those without LRRT in the subgroup of OMD (66.3% vs. 25.2%, P<0.001, Fig. 3A). In comparison, the 3-year OS of patients who received LRRT and PCT in the subgroup of PMD showed no difference (38.9% vs. 11.5%, P = 0.115, Fig. 3B).
Fig. 3.
The Kaplan-Meier curves of OS in dmNPC of oligometastatic and polymetastatic subgroup: (A) OS of patients with or without locoregional radiotherapy in oligometastatic subgroup; (B) OS of patients with or without locoregional radiotherapy in polymetastatic subgroup.
After adjusting for KPS, chemotherapy cycles, LDH, and liver metastases, MVA showed that LRRT was a favorable prognosticator in the OMD subgroup (HR=0.308, 95% CI: 0.159–0.598, P<0.001) (Table 3), while MVA showed that LRRT was not a potential prognostic factor for OS in the PMD subgroup (HR=0.510, 95% CI: 0.256–1.014; P = 0.055, Table 3).
Table 3.
Multivariate analyses of Overall Survival in Subgroup of Oligometastatic and Polymetastatic Disease.
| Characteristics | OMD | PMD | ||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Nasopharyngeal | ||||
| Radiotherapy, No/Yes | 0.413 (0.198–0.861) | 0.018 | 0.934 (0.402–2.172) | 0.875 |
| KPS, ≤80/>80 | 0.742 (0.379–1.456) | 0.742 | 0.559 (0.239–1.312) | 0.182 |
| Chemotherapy cycles, <6/≥6 | 0.706 (0.335–1.490) | 0.361 | 0.419 (0.180–0.997) | 0.977 |
| LDH, Abnormal/Normal | 0.956 (0.402–2.274) | 0.956 | 1.593 (0.739–3.430) | 0.234 |
| EBV DNA,>4000 /<4000 | 1.591 (0.806–3.139) | 0.181 | 0.887 (0.349–2.257) | 0.802 |
Abbreviation: OMD, Oligometastatic Disea; PMD, Polymetastatic Disease; KPS, Karnofsky performance status score; LDH, Lactate dehydrogenase; EBV, Epstein–Barr virus.
Discussion
Currently, more shreds of evidence prove that LRRT has apparent benefits. However, whether all dmNPC patients can benefit from LRRT remains to be clarified. Our study found that LRRT can significantly improve the OS of dmNPC patients. Further analyses found that the OS of OMD patients who received LRRT was significantly better than those who did not receive LRRT, while there was no significant difference in the OS of PMD patients who received LRRT and those who did not. These results suggest that OMD can be used as an indicator for screening LRRT benefit groups.
In our study, the median OS of patients receiving local treatment reached 57.0 months, and the 3-year OS rate exceeded 50% (58.0%), which is a remarkably good treatment effect. Our retrospective study showed that LRRT could effectively improve the OS of dmNPC patients in univariate analysis, and MVA showed that LRRT could improve the OS for the whole group of dmNPC. Our results were consistent with the results of a number of retrospective studies [12, 27, 28] and a prospective phase Ⅲ trial [13], which proved that LRRT could significantly improve the OS for dmNPC. Our study found that OMD (≤5 metastases and ≤2 metastatic organs) was an independent prognostic factor for dmNPC and also was an effective indicator to filter beneficiaries of LRRT from dmNPC patients. Coincidentally, data from another center also show that not all dmNPC patients can benefit from LRRT, and OMD can be used as an indicator to filter out beneficiaries [20]. Although the definition of OMD was not wholly consistent, our study and many previous studies have found that OMD was a prognostic factor for dmNPC [12, 13, 28]. OMD was considered to be at an intermediate stage between advanced tumors and PMD [29]. Patients with OMD had a better therapeutic effect, and some patients may be cured [8, 30]. Our study also found that patients with PMD cannot benefit from LRRT, while patients with oligometastatic dmNPC can benefit from LRRT. This suggests that OMD was an effective screening factor for beneficiaries from LRRT among dmNPC patients. To some extent, OMD and PMD in dmNPC patients are not the same disease, and their treatment strategies and programs should also be different.
From the concept of OMD put forward, a series of research confirmed that OMD has a better prognosis and benefits from local treatment [8, 17, 18]. In recent years, the improvement of radiotherapy technology, especially the gradual development of tomotherapy, greatly facilitates the implementation of local consolidative radiotherapy (LCR) for all OMD or PMD lesions [31]. LCR will further improve the prognosis of metastatic cancer and provide more possibility of curing for metastatic tumors [32]. In 2020, the National Comprehensive Cancer Network (NCCN) guidelines recommended LCR following systemic chemotherapy for patients with limited metastasis sites or a low tumor burden [33]. Unfortunately, in our retrospective analysis, we did not find that LCR for metastatic lesions improved the prognosis in dmNPC patients. The possible reason lies in the radiation therapy for relieving bone pain or reducing bone fractures of the weight-bearing bone. The biologically effective dose did not reach the actual therapeutic dose. For oligometastatic dmNPC, whether LCR of metastatic lesions based on LRRT can bring survival benefits needs to study. A phase II clinical trial (NCT04351282) is being explored, and the results are worth looking forward to.
Although our study found that PMD patients were not the beneficiaries of LRRT for dmNPC, this does not mean that LRRT is not feasible for PMD. For PMD patients, adequate chemotherapy may be needed. Only when the tumor burden is reduced, the LRRT treatment could prolong OS. Indeed, our study showed <6 cycles of chemotherapy was a poor prognostic factor for dmNPC. This suggests that adequate chemotherapy for dmNPC is necessary. Well-designed prospective clinical trials are expected to explore how to optimize the treatment strategy of PMD patients. Previous studies have also confirmed that the undetectable EBV DNA levels after 4–6 weeks of chemotherapy were a good prognostic factor and a biomarker to filter out beneficiaries of maintenance chemotherapy in metastatic NPC [34]. Unfortunately, there is no standard protocol for EBV DNA detection; only a few centers have a sensitivity and specificity of >90% for EBV DNA detection [35], [36], [37]. Besides, only 22.2% of patients were tested for EBV DNA during 4–6 cycles of chemotherapy in our study, which limits the feasibility of analyzing EBV DNA as a biomarker to filter out beneficiaries of LRRT.
The study has several potential limitations. First, because of the retrospective nature, selection bias may not be avoided. Second, this study is a single-center study, and more research center data are needed to confirm this conclusion. Finally, because EBV DNA was not fully tested at the end of 4–6 chemotherapy cycles, we could not explore the potential predictive value of LRRT for dmNPC patients.
Conclusions
The application of LRRT following PCT may prolong OS for patients with de novo mNPC. DmNPC patients with OMD may benefit from LRRT, while PMD patients may not benefit from LRRT. These results suggest that OMD may be used as an indicator for the screening of beneficiaries from LRRT.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author Contributions Statement
Fujuan Zeng & Tianzhu Lu: Conceptualization, Methodology, Software; Fei Xie, Lizhi Chen, Lin Zhang, Yong Su & Zhongren Yu: Investigation; Yun Xiao & Fan Ao: Resources; Guoqing Li & Zhiping Chen: Supervision; Jingao Li & Xiaochang Gong: Writing- Reviewing and Editing. All authors read and approved the final manuscript.
Declaration of Competing Interest
None
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81460409, 81860664 and 81660453), National Cancer Center Climbing Fund (NCC201814B044, and NCC201814B040). and the Youth fund of Science and Technology Department of JiangXi Province (20161BAB215255). This work was also supported by grant from the Fujian Provincial Natural Science Foundation of China (No. 2019J01194). We thank editage (http://www.fabiao@editage.cn) for editing this manuscript.
Contributor Information
Xiaochang Gong, Email: gxcanddw@163.com.
Jingao Li, Email: lijingao@hotmail.com.
References
- 1.Chen Y.P., Chan A.T.C., Le Q.T., Blanchard P., Sun Y., Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Wei W.I., Sham J.S. Nasopharyngeal carcinoma. Lancet. 2005;365(9476):2041–2054. doi: 10.1016/S0140-6736(05)66698-6. [DOI] [PubMed] [Google Scholar]
- 4.Lee A.W., Ng W.T., Chan Y.H., Sze H., Chan C., Lam T.H. The battle against nasopharyngeal cancer. Radiother. Oncol. 2012;104(3):272–278. doi: 10.1016/j.radonc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Li W.Z., Lv S.H., Liu G.Y., Liang H., Guo X., Lv X. Development of a prognostic model to identify the suitable definitive radiation therapy candidates in de novo metastatic nasopharyngeal carcinoma: a real-world study. Int. J. Radiat. Oncol. Biol. Phys. 2021;109(1):120–130. doi: 10.1016/j.ijrobp.2020.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao W., He J., Gou Q., Duan B., Liu L., Ai P. Synchronous metastatic nasopharyngeal carcinoma: characteristics and survival of patients adding definitive nasopharyngeal-neck radiotherapy to systematic chemotherapy. Cancer Manag. Res. 2020;12:10211–10219. doi: 10.2147/CMAR.S276286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma B.B., Hui E.P., Chan A.T. Systemic approach to improving treatment outcome in nasopharyngeal carcinoma: current and future directions. Cancer Sci. 2008;99(7):1311–1318. doi: 10.1111/j.1349-7006.2008.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rusthoven C.G., Lanning R.M., Jones B.L., Amini A., Koshy M., Sher D.J. Metastatic nasopharyngeal carcinoma: patterns of care and survival for patients receiving chemotherapy with and without local radiotherapy. Radiother. Oncol. 2017;124(1):139–146. doi: 10.1016/j.radonc.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Sun X.S., Liang Y.J., Chen Q.Y., Guo S.S., Liu L.T., Sun R. Optimizing the treatment pattern for de novo metastatic nasopharyngeal carcinoma patients: a large-scale retrospective cohort study. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.543646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verma V., Allen P.K., Simone C.B., 2nd, Gay H.A., Lin S.H. Addition of definitive radiotherapy to chemotherapy in patients with newly diagnosed metastatic nasopharyngeal cancer. J. Natl. Compr. Canc. Netw. 2017;15(11):1383–1391. doi: 10.6004/jnccn.2017.7001. [DOI] [PubMed] [Google Scholar]
- 11.Pfister D.G., Spencer S., Adelstein D., Adkins D., Anzai Y., Brizel D.M. Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2020;18(7):873–898. doi: 10.6004/jnccn.2020.0031. [DOI] [PubMed] [Google Scholar]
- 12.Chen M.Y., Jiang R., Guo L., Zou X., Liu Q., Sun R. Locoregional radiotherapy in patients with distant metastases of nasopharyngeal carcinoma at diagnosis. Chin. J. Cancer. 2013;32(11):604–613. doi: 10.5732/cjc.013.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.You R., Liu Y.P., Huang P.Y., Zou X., Sun R., He Y.X. Efficacy and safety of locoregional radiotherapy with chemotherapy vs chemotherapy alone in de novo metastatic nasopharyngeal carcinoma: a multicenter phase 3 randomized clinical trial. JAMA Oncol. 2020;6(9):1345–1352. doi: 10.1001/jamaoncol.2020.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hellman S. Weichselbaum RR. Oligometastases. J. Clin. Oncol. 1995;13(1):8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 15.Tian Y.H., Zou W.H., Xiao W.W., Zeng L., Yuan X., Bai L. Oligometastases in AJCC stage IVc nasopharyngeal carcinoma: a subset with better overall survival. Head Neck. 2016;38(8):1152–1157. doi: 10.1002/hed.24345. [DOI] [PubMed] [Google Scholar]
- 16.Chee J., Liu X., Eu D., Loh T., Ho F., Wong L.C. Defining a cohort of oligometastatic nasopharyngeal carcinoma patients with improved clinical outcomes. Head Neck. 2020;42(5):945–954. doi: 10.1002/hed.26061. [DOI] [PubMed] [Google Scholar]
- 17.Hui E.P., Leung S.F., Au J.S., Zee B., Tung S., Chua D. Lung metastasis alone in nasopharyngeal carcinoma: a relatively favorable prognostic group. A study by the Hong Kong Nasopharyngeal Carcinoma Study Group. Cancer. 2004;101(2):300–306. doi: 10.1002/cncr.20358. [DOI] [PubMed] [Google Scholar]
- 18.Lo S.S., Moffatt-Bruce S.D., Dawson L.A., Schwarz R.E., Teh B.S., Mayr N.A. The role of local therapy in the management of lung and liver oligometastases. Nat. Rev. Clin. Oncol. 2011;8(7):405–416. doi: 10.1038/nrclinonc.2011.75. [DOI] [PubMed] [Google Scholar]
- 19.Sun X.S., Liu L.T., Liu S.L., Guo S.S., Wen Y.F., Xie H.J. Identifying optimal candidates for local treatment of the primary tumor among patients with de novo metastatic nasopharyngeal carcinoma: a retrospective cohort study based on Epstein-Barr virus DNA level and tumor response to palliative chemotherapy. BMC Cancer. 2019;19(1):92. doi: 10.1186/s12885-019-5281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H., Lu L., Lu T., Xu Y., Zong J., Huang C. Identifying the optimal candidates for locoregional radiation therapy in patients with de novo metastatic nasopharyngeal carcinoma. Head Neck. 2021 doi: 10.1002/hed.26726. [DOI] [PubMed] [Google Scholar]
- 21.Lin S., Pan J., Han L., Zhang X., Liao X., Lu J.J. Nasopharyngeal carcinoma treated with reduced-volume intensity-modulated radiation therapy: report on the 3-year outcome of a prospective series. Int. J. Radiat. Oncol. Biol. Phys. 2009;75(4):1071–1078. doi: 10.1016/j.ijrobp.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Pan J.J., Ng W.T., Zong J.F., Chan L.L., O'Sullivan B., Lin S.J. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer. 2016;122(4):546–558. doi: 10.1002/cncr.29795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lievens Y., Guckenberger M., Gomez D., Hoyer M., Iyengar P., Kindts I. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother. Oncol. 2020;148:157–166. doi: 10.1016/j.radonc.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Van Cutsem E., Cervantes A., Adam R., Sobrero A., Van Krieken J.H., Aderka D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016;27(8):1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 25.Zeng L., Zhang Q., Ao F., Jiang C.L., Xiao Y., Xie H.H. Risk factors and distribution features of level IB lymph nodes metastasis in nasopharyngeal carcinoma. Auris Nasus Larynx. 2019;46(3):457–464. doi: 10.1016/j.anl.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Zou X., You R., Liu H., He Y.X., Xie G.F., Xie Z.H. Establishment and validation of M1 stage subdivisions for de novo metastatic nasopharyngeal carcinoma to better predict prognosis and guide treatment. Eur. J. Cancer. 2017;77:117–126. doi: 10.1016/j.ejca.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 28.Lin S., Tham I.W., Pan J., Han L., Chen Q., Lu J.J. Combined high-dose radiation therapy and systemic chemotherapy improves survival in patients with newly diagnosed metastatic nasopharyngeal cancer. Am. J. Clin. Oncol. 2012;35(5):474–479. doi: 10.1097/COC.0b013e31821a9452. [DOI] [PubMed] [Google Scholar]
- 29.Weichselbaum R.R., Hellman S. Oligometastases revisited. Nat. Rev. Clin. Oncol. 2011;8(6):378–382. doi: 10.1038/nrclinonc.2011.44. [DOI] [PubMed] [Google Scholar]
- 30.Fandi A., Bachouchi M., Azli N., Taamma A., Boussen H., Wibault P. Long-term disease-free survivors in metastatic undifferentiated carcinoma of nasopharyngeal type. J. Clin. Oncol. 2000;18(6):1324–1330. doi: 10.1200/JCO.2000.18.6.1324. [DOI] [PubMed] [Google Scholar]
- 31.Engels B., Gevaert T., Everaert H., De Coninck P., Sermeus A., Christian N. Phase II study of helical tomotherapy in the multidisciplinary treatment of oligometastatic colorectal cancer. Radiat. Oncol. 2012;7:34. doi: 10.1186/1748-717X-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iyengar P., Wardak Z., Gerber D.E., Tumati V., Ahn C., Hughes R.S. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2018;4(1) doi: 10.1001/jamaoncol.2017.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colevas A.D., Yom S.S., Pfister D.G., Spencer S., Adelstein D., Adkins D. NCCN guidelines insights: head and neck cancers, version 1.2018. J. Natl. Compr. Canc. Netw. 2018;16(5):479–490. doi: 10.6004/jnccn.2018.0026. [DOI] [PubMed] [Google Scholar]
- 34.Zhou H., Lu T., Guo Q., Chen Y., Chen M., Chen Y. Effects of oral maintenance chemotherapy and predictive value of circulating EBV DNA in metastatic nasopharyngeal carcinoma. Cancer Med. 2020;9(8):2732–2741. doi: 10.1002/cam4.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu K.H., Lo Y.M., Tse G.M., Chan K.C., Chan A.B., Chow K.C. Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nonnasopharyngeal head and neck carcinomas. Clin. Cancer Res. 2004;10(5):1726–1732. doi: 10.1158/1078-0432.ccr-0991-3. [DOI] [PubMed] [Google Scholar]
- 36.Lin J.C., Wang W.Y., Chen K.Y., Wei Y.H., Liang W.M., Jan J.S. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N. Engl. J. Med. 2004;350(24):2461–2470. doi: 10.1056/NEJMoa032260. [DOI] [PubMed] [Google Scholar]
- 37.Lu T., Guo Q., Lin K., Chen H., Chen Y., Xu Y. Circulating Epstein-Barr virus microRNAs BART7-3p and BART13-3p as novel biomarkers in nasopharyngeal carcinoma. Cancer Sci. 2020;111(5):1711–1723. doi: 10.1111/cas.14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.